Abstract
Pyridoxal-phosphate-6-azophenyl-2′-4-disulfonate (PPADS), a purinergic 2 (P2) receptor antagonist, has been shown to attenuate the exercise pressor reflex in cats. In vitro, however, PPADS has been shown to block the production of prostaglandins, some of which play a role in evoking the exercise pressor reflex. Thus the possibility exists that PPADS blocks the exercise pressor reflex through a reduction in prostaglandin synthesis rather than through the blockade of P2 receptors. Using microdialysis, we collected interstitial fluid from skeletal muscle to determine prostaglandin E2 (PGE2) concentrations during the intermittent contraction of the triceps surae muscle before and after a popliteal arterial injection of PPADS (10 mg/kg). We found that the PGE2 concentration increased in response to the intermittent contraction before and after the injection of PPADS (both, P < 0.05). PPADS reduced the pressor response to exercise (P < 0.05) but had no effect on the magnitude of PGE2 production during contraction (P = 0.48). These experiments demonstrate that PPADS does not block the exercise pressor reflex through a reduction in PGE2 synthesis. We suggest that PGE2 and P2 receptors play independent roles in stimulating the exercise pressor reflex.
Keywords: exercise pressor reflex, groups III and IV muscle afferents, neural control of the circulation, muscle microdialysis, pyridoxal-phosphate-6-azophenyl-2′-4-disulfonate
arterial pressure, heart rate, and breathing increase at the onset of exercise in part due to the exercise pressor reflex, the afferent arm of which is comprised of group III and IV muscle afferents. The exercise pressor reflex is evoked by two types of stimuli, namely, metabolic and mechanical. Several lines of evidence suggest that the metabolic component of the exercise pressor reflex is triggered in part by prostaglandins and adenosine 5′-triphosphate (ATP). For example, the concentration of prostaglandins and ATP in muscle are both increased during static contraction (3, 6, 10, 11, 19). In addition, the pressor response to static contraction was attenuated when prostaglandin synthesis was prevented with indomethacin (18). Likewise, the pressor response to static contraction was attenuated when purinergic 2 (P2) receptors were blocked with pyridoxal-phosphate-6-azophenyl-2′-4-disulfonate (PPADS) (8). Finally, both indomethacin and PPADS attenuated the responses of thin fiber muscle afferents to static contraction (7, 15, 16). These findings suggest that prostaglandins and ATP are metabolites that contribute to the exercise pressor reflex through the stimulation of thin fiber muscle afferents.
PPADS has been shown to block prostaglandin synthesis by canine kidney cells in vitro (17). Consequently, the possibility exists that the attenuation of the exercise pressor reflex by PPADS is, in fact, caused by the inhibition of prostaglandin synthesis instead of by blocking P2 receptors on thin fiber muscle afferents. To test this possibility we examined the effect of PPADS on prostaglandin E2 (PGE2) concentrations in the interstitium of the triceps surae muscles while this muscle group was contracted in decerebrate cats. We measured interstitial PGE2 because it accounts for the largest proportion of prostaglandins formed from cyclooxygenase metabolism in skeletal muscle (1). Furthermore, Stebbins et al. (18) showed that an injection of PGE2 into the arterial supply of skeletal muscle partially restored the exercise pressor reflex that was attenuated by indomethacin, a cyclooxygenase blocker. In addition, PGE2 concentrations are increased during muscular contraction as shown by the present study and others (3, 6, 19). These findings suggest that PGE2 is a prostaglandin that contributes to the exercise pressor reflex.
METHODS
All procedures were reviewed and approved by the Institutional Care and Use Committee of the Pennsylvania State University, Hershey Medical Center. Adult cats of either sex (n = 30; 3.1 ± 0.2 kg; range, 2.5–4.4 kg) were initially anesthetized with a mixture of 5% isoflurane and oxygen. The right jugular vein and common carotid artery were cannulated for the delivery of drugs and fluids as well as for the measurement of arterial blood pressure. The carotid arterial catheter was connected to a pressure transducer (model P23 XL, Statham) to monitor blood pressure. Heart rate was calculated beat to beat from the arterial pressure pulse by a Gould Biotach amplifier. The trachea was cannulated, and the lungs were ventilated mechanically (Harvard Apparatus). Arterial blood gases and pH were measured by an automated blood gas analyzer (model ABL-700, Radiometer). Pco2 and arterial pH were maintained within the normal range either by adjusting ventilation or by intravenous administration of sodium bicarbonate (8.5%). A temperature probe was passed through the mouth to the stomach. Temperature was continuously monitored and maintained at 37–38°C by a water-perfused heating pad.
The left common iliac artery and vein were isolated, and snares were placed around these vessels to trap PPADS in the leg. The left triceps surae muscles, left tibial nerve, and left popliteal artery were isolated, after which the cat was placed in a Kopf stereotaxic and spinal unit. The calcaneal bone was cut, and its tendon was attached to a force transducer (model FT-10C, Grass) for measurement of the tension developed during contraction of the left triceps surae muscles. The knee joint was secured to a post to lock the left lower limb.
The cats were decerebrated at the midcollicular level under isoflurane anesthesia. Just before the decerebration procedure, dexamethasone (4 mg) was injected intravenously to minimize brain edema, and the left common carotid artery was tied off to reduce bleeding. All neural tissue rostral to the midcollicular section was removed, and the cranial vault was filled with agar.
Microdialysis can be used to introduce and remove ions, molecules, and drugs of interest to or from the interstitial space of skeletal muscle (5). We manufactured microdialysis probes by gluing both ends of a 4-cm length of capillary microdialysis membrane (0.20 mm in diameter, with a 13-kDa molecular cutoff) into nylon tubing. The nylon tubing was attached to a Luer tip adapter stub that connected the probe and the perfusate-filled syringe. Each cat had four microdialysis fibers placed in its triceps surae muscles; the fibers were separated by ∼0.5–1 cm. The probes were inserted into the muscles via a 20-gauge cannula inserted parallel to muscle fiber orientation. The insertion and exit points were ∼6 cm apart. The microdialysis probe was threaded through the internal lumen of the needle. The needle was withdrawn, leaving the membrane in place. The Luer tip adapter stub was attached to a syringe for the administration of saline through a perfusion pump (model 402, CMA) at 5 μl/min. We inserted the microdialysis probes and then waited 2 h for equilibration.
In 15 cats, the effect of PPADS on the reflex pressor, cardioaccelerator, and interstitial concentrations of PGE2 were assessed before and during the intermittent static contraction of the left triceps surae muscles. Intermittent contraction was initiated for 10 min by electrical stimulation of the left tibial nerve (40 Hz, 25 μs, 2 times motor threshold). Each contraction lasted 1 s and was followed by 3 s of rest. Baseline tension was set at 0.3–0.5 kg. The reason we used intermittent static contraction for 10 min was that this enabled us to collect enough microdialysis fluid (150 μl) to measure PGE2 concentrations by ELISA. The arterial blood pressure and heart rate were recorded for 10 min before and during the intermittent contraction period. Immediately before injecting PPADS, we tightened the snare placed around the left common iliac artery and vein. PPADS (10 mg/kg), dissolved in saline, was injected by inserting a 30-gauge needle into the popliteal artery and then injecting the compound in a volume of 0.3–0.5 ml. The snares were maintained for 15 min, after which they were released and the resting hind limb was freely perfused for another 15 min. At this point, the baseline measurements were obtained and then the triceps surae muscles were contracted for 10 min. The microdialysate samples for baseline and contraction data were collected for 10 min before and during each contraction.
In two control experiments, we used the same protocol as that described above for PPADS (i.e., trapped the drugs in the vasculature of the triceps surae muscles for 15 min, after which the muscles were freely perfused for 15 min before we initiated contraction). In the first, we injected indomethacin (1 mg/ kg) into the left popliteal artery in six cats. Indomethacin, a cyclooxygenase blocker, was dissolved (5 mg/ml) in 100 mM sodium carbonate. The volume injected into the popliteal artery was 0.5–0.8 ml. Indomethacin was injected to demonstrate that our method of measuring PGE2 concentrations was capable of detecting decreases in this cyclooxygenase metabolite. In the second control experiment, we injected saline (0.4 ml) into the left popliteal artery in nine cats. We performed the second control experiment to demonstrate that the increase in PGE2 concentration induced by muscle contraction was repeatable.
Muscle microdialysate samples were taken during both the baseline and contraction periods before and after injecting PPADS. Dialysate samples were stored at −80°C until analysis with a commercially available enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI). PGE2 concentrations are expressed in picograms per milliliter (12, 14).
Mean arterial pressure, heart rate, and PGE2 concentrations are expressed as means ± SE. Baseline mean arterial pressure and heart rate were assessed immediately before intermittent contraction started and peak values were taken during the contraction period. The lag time for the dialysate to travel into the microdialysis fiber in the belly of the muscle to the collection tube was 2 min and 45 s. We took into account this lag time to achieve 10 min of contracting muscle dialysate. PGE2 concentrations were calculated from microdialysate fluid collected for 10 min immediately before the intermittent contraction period and for 10 min during the contraction period. The tension-time index was calculated by integrating the area between the tension trace during contraction and the baseline level (Spike 2) and is expressed in kilograms times seconds (13). Statistical comparisons were performed either with a one-way repeated-measures ANOVA or two-way repeated-measures ANOVA. Post hoc tests between individual means were performed with the Tukey test if significant main effects were found with an ANOVA. The criterion for statistical significance was P < 0.05.
RESULTS
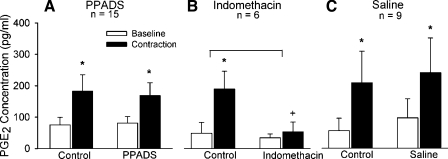
Intermittent contraction increased PGE2 concentrations in the microdialysate fluid both before and after PPADS injection (both, P < 0.05). PPADS had no effect on the magnitude of this increase (P = 0.48; Fig. 1A). In contrast, indomethacin markedly decreased PGE2 concentrations in the microdialysis fluid during intermittent contraction in each of the six cats tested (P < 0.05; Fig. 1B). Intermittent contraction increased PGE2 concentrations in the microdialysate fluid both before and after saline injection (both P < 0.05). Like PPADS, saline had no effect on the magnitude of this increase (P = 0.79; Fig. 1C).
Fig. 1.
Effects of intermittent static contraction on prostaglandin E2 concentrations ([PGE2]) before and after injection of pyridoxal-phosphate-6-azophenyl-2′-4-disulfonate (PPADS; A), indomethacin (B), or saline (C). White bars represent baseline values, and black bars represent values during contraction. *P < 0.05 baseline vs. peak. The symbol + indicates that PGE2 concentration during contraction after indomethacin was significantly less (P = 0.005) than PGE2 concentration during contraction before indomethacin (i.e., control). Horizontal bracket signifies that the increase in PGE2 concentration after indomethacin was significantly less (P = 0.009) than the increase in PGE2 concentration before indomethacin (i.e., control). n, Number of cats.
Intermittent contraction before PPADS increased mean arterial pressure and heart rate (Table 1). PPADS attenuated the pressor response to contraction (P < 0.05; Table 1) but had no effect (P = 0.11; Table 1) on the cardioaccelerator response. Intermittent contraction before indomethacin increased mean arterial pressure (Table 1). Indomethacin attenuated the pressor response to contraction (P < 0.05; Table 1). Before and after saline, the intermittent contraction increased mean arterial pressure (P < 0.05; Table 1). Saline had no effect on the pressor response to exercise (P = 0.59; Table 1). The tension time index did not differ between contractions before or after PPADS (P = 0.72), indomethacin (P = 0.60), or saline (P = 0.44; Table 1).
Table 1.
Change in MAP and HR from baseline to peak during intermittent contraction before and after PPADS, indomethacin, or saline
| n | Before Treatment | After Treatment | |
|---|---|---|---|
| PPADS | 15 | ||
| Baseline MAP, mmHg | 131±6 | 129±8 | |
| ΔMAP, mmHg | 21±4* | 12±3*† | |
| Baseline HR, beats/min | 149±11 | 151±8 | |
| ΔHR, beats/min | 10±2* | 7±3* | |
| TTI, kg·s | 407±42 | 404±40 | |
| Indomethacin | 6 | ||
| Baseline MAP, mmHg | 137±10 | 152±13† | |
| ΔMAP, mmHg | 30±7* | 16±5*† | |
| Baseline HR, beats/min | 141±11 | 137±21 | |
| ΔHR, beats/min | 9±4 | 7±3 | |
| TTI, kg·s | 420±47 | 427±47 | |
| Saline | 9 | ||
| Baseline MAP, mmHg | 121±9 | 116±10 | |
| ΔMAP, mmHg | 18±3* | 21±3* | |
| Baseline HR, beats/min | 142±10 | 135±11 | |
| ΔHR, beats/min | 4±6 | 8±2 | |
| TTI, kg · s | 346±54 | 335±53 |
Values are means ± SE; n, number of cats. Tension time index (TTI) during each 10-min contraction period before and after treatment with pyridoxal-phosphate-6-azophenyl-2′-4-disulfonate (PPADS), indomethacin, or saline. MAP, mean arterial pressure; HR, heart rate.
P < 0.05, baseline vs. peak;
P < 0.05, before drug vs. after drug.
DISCUSSION
This study shows that PPADS does not block the exercise pressor reflex by inhibiting PGE2 synthesis. PPADS, injected into the popliteal artery of decerebrate cats, had no effect on the production of PGE2 by the triceps surae muscles while they were either at rest or while they were contracting intermittently. In contrast, indomethacin, injected into the popliteal artery, decreased the production of PGE2 while the triceps surae muscles were at rest and while they were contracting. Our findings that indomethacin prevented much of the prostaglandin production induced by contraction, but that PPADS did not, demonstrate that it does not reduce prostaglandin synthesis in skeletal muscle. It also shows that our methods were capable of detecting a reduction in PGE2 concentration.
The lack of effect of PPADS on PGE2 production by the triceps surae muscles sheds important light on the issue concerning whether PPADS blocks both prostaglandin synthesis and P2 receptors (2). The attenuating effect of PPADS on the exercise pressor reflex cannot be explained by a decrease in prostaglandin production by the contracting muscles (2), leading us to conclude that in previous studies PPADS attenuated the exercise pressor reflex by blocking P2 receptors on the endings of group III and IV muscle afferents (4, 7–9). We note with interest that our findings in vivo contrast with those reported in vitro (17). This contrast may be attributable to either differences in PPADS concentrations between the two preparations or it may be an example of the unpredictable relationship between in vitro experiments and their in vivo counterparts.
GRANTS
This work was supported by National Institutes of Health Grants AR-051503 and HL-30710.
Acknowledgments
We thank Jennifer Probst and Yue Zhang for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Berlin T, Cronestrand R, Nowak J, Sonnenfeld T, Wennmalm A. Conversion of arachidonic acid to prostaglandins in homogenates of human skeletal muscle and kidney. Acta Physiol Scand 106: 441–445, 1979. [DOI] [PubMed] [Google Scholar]
- 2.Biaggioni I Autonomic/metabolic interactions modulating the exercise pressor reflex: the purinergic hypothesis. J Physiol 578: 5–6, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M, Kjaer M. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol 543: 691–698, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanna RL, Kaufman MP. Role played by purinergic receptors on muscle afferents in evoking the exercise pressor reflex. J Appl Physiol 94: 1437–1445, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Hickner RC Applications of microdialysis in studies of exercise. Exerc Sport Sci Rev 28: 117–122, 2000. [PubMed] [Google Scholar]
- 6.Karamouzis M, Karamouzis I, Vamvakoudis E, Ampatzidis G, Christoulas K, Angelopoulou N, Mandroukas K. The response of muscle interstitial prostaglandin E2 (PGE2), prostacyclin I2 (PGI2) and thromboxane A2 (TXA2) levels during incremental dynamic exercise in humans determined by in vivo microdialysis. Prostaglandins Leukot Essent Fatty Acids 64: 259–263, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Kindig AE, Hayes SG, Hanna RL, Kaufman MP. P2 antagonist PPADS attenuates responses of thin fiber afferents to static contraction and tendon stretch. Am J Physiol Heart Circ Physiol 290: H1214–H1219, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Kindig AE, Hayes SG, Kaufman MP. Blockade of purinergic 2 receptors attenuates the mechanoreceptor component of the exercise pressor reflex. Am J Physiol Heart Circ Physiol 293: H2995–H3000, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Kindig AE, Hayes SG, Kaufman MP. Purinergic 2 receptor blockade prevents the responses of group IV afferents to post-contraction circulatory occlusion. J Physiol 578: 301–308, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol 95: 577–583, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Mo FM, Ballard HJ. The effect of systemic hypoxia on interstitial and blood adenosine, AMP, ADP, and ATP in dog skeletal muscle. J Physiol 536: 593–603, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newberry RD, Stenson WF, Lorenz RG. Cyclooxygenase-2-dependent arachidonic acid metabolites are essential modulators of the intestinal immune response to dietary antigen. Nat Med 5: 900–906, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Gonzalez JF Factors determining the blood pressure responses to isometric exercise. Circ Res 48: I-76–I-86, 1981. [PubMed] [Google Scholar]
- 14.Pradelles P, Grassi J, Maclouf J. Enzyme immunoassays of eicosanoids using acetylcholine esterase as label: an alternative to radioimmunoassay. Anal Chem 57: 1170–1173, 1985. [DOI] [PubMed] [Google Scholar]
- 15.Rotto DM, Hill JM, Schultz HD, Kaufman MP. Cyclooxygenase blockade attenuates the responses of group IV muscle afferents to static contraction. Am J Physiol Heart Circ Physiol 259: H745–H750, 1990. [DOI] [PubMed] [Google Scholar]
- 16.Rotto DM, Schultz HD, Longhurst JC, Kaufman MP. Sensitization of group III muscle afferents to static contraction by products of arachidonic acid metabolism. J Appl Physiol 68: 861–867, 1990. [DOI] [PubMed] [Google Scholar]
- 17.Shehnaz D, Torres B, Balboa MA, Insel PA. Pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS), a putative P2Y1 receptor antagonist, blocks signaling at a site distal to the receptor in Madin-Darby canine kidney-D1 cells. J Pharmacol Exp Ther 292: 346–350, 2000. [PubMed] [Google Scholar]
- 18.Stebbins CL, Maruoka Y, Longhurst JC. Prostaglandins contribute to cardiovascular reflexes evoked by static muscular contraction. Circ Res 59: 645–654, 1988. [DOI] [PubMed] [Google Scholar]
- 19.Symons JD, Theodossy SJ, Longhurst JC, Stebbins CL. Intramuscular accumulation of prostaglandins during static contraction of the cat triceps surae. J Appl Physiol 71: 1837–1842, 1991. [DOI] [PubMed] [Google Scholar]