Abstract
Endothelium-derived hyperpolarizing factor (EDHF) plays a crucial role in modulating vasomotor tone, especially in microvessels when nitric oxide-dependent control is compromised such as in diabetes. Epoxyeicosatrienoic acids (EETs), potassium ions (K+), and hydrogen peroxide (H2O2) are proposed as EDHFs. However, the identity (or identities) of EDHF-dependent endothelial dilators has not been clearly elucidated in diabetes. We assessed the mechanisms of EDHF-induced vasodilation in wild-type (WT, normal), db/db (advanced type 2 diabetic) mice, and db/db mice null for TNF (dbTNF−/dbTNF−). In db/db mice, EDHF-induced vasodilation [ACh-induced vasodilation in the presence of NG-nitro-l-arginine methyl ester (l-NAME, 10 μmol/l) and prostaglandin synthase inhibitor indomethacin (Indo, 10 μmol/l)] was diminished after the administration of catalase (an enzyme that selectively dismutates H2O2 to water and oxygen, 1,000 U/ml); administration of the combination of charybdotoxin (a nonselective blocker of intermediate-conductance Ca2+-activated K+ channels, 10 μmol/l) and apamin (a selective blocker of small-conductance Ca2+-activated K+ channels, 50 μmol/l) also attenuated EDHF-induced vasodilation, but the inhibition of EETs synthesis [14,15-epoxyeicosa-5(Z)-enoic acid; 10 μmol/l] did not alter EDHF-induced vasodilation. In WT controls, EDHF-dependent vasodilation was significantly diminished after an inhibition of K+ channel, EETs synthesis, or H2O2 production. Our molecular results indicate that mRNA and protein expression of interleukin-6 (IL-6) were greater in db/db versus WT and dbTNF−/dbTNF− mice, but neutralizing antibody to IL-6 (anti-IL-6; 0.28 mg·ml−1·kg−1 ip for 3 days) attenuated IL-6 expression in db/db mice. The incubation of the microvessels with IL-6 (5 ng/ml) induced endothelial dysfunction in the presence of l-NAME and Indo in WT mice, but anti-IL-6 restored ACh-induced vasodilation in the presence of l-NAME and Indo in db/db mice. In dbTNF−/dbTNF− mice, EDHF-induced vasodilation was greater and comparable with controls, but IL-6 decreased EDHF-mediated vasodilation. Our results indicate that EDHF compensates for diminished NO-dependent dilation in IL-6-induced endothelial dysfunction by the activation of H2O2 or a K+ channel in type 2 diabetes.
Keywords: acetylcholine, coronary disease, endothelium, microcirculation, inflammation, endothelium-derived hyperpolarizing factor
endothelial cells release various relaxing and contracting factors (14, 15) that are responsible for the control of blood vessel tone. The relaxation factors include nitric oxide (NO) and prostacyclin (PGI2) as well as additional endothelial pathway(s) that involve the hyperpolarization of the vascular smooth muscle (10, 28). The last factor, termed endothelium-derived hyperpolarizing factor (EDHF), is observed in numerous blood vessels from different species (12). Different candidates have been proposed, such as potassium ions (K+), epoxyeicosatrienoic acids (EETs), and hydrogen peroxide (H2O2), to function as EDHFs (12). However, the specific identity of EDHF involved in the coronary microcirculation in type 2 diabetes is unknown.
The reduction in NO bioavailability impairs endothelium-dependent relaxation in diabetic vascular beds (7, 12). However, EDHF may play an important role in regulating the vascular tone and reactivity, especially in small resistant vessels when NO-mediated control is compromised (7, 35). Despite some studies in diabetic rats (13, 24, 38) and humans (1), many issues remain unresolved. Thus we propose that EDHF plays a pivotal role in type 2 diabetes-induced endothelial dysfunction. We and others recently reported that increased TNF-α and other cytokines in type 2 diabetes induce the activation of reactive oxygen species (ROS), leading to endothelial dysfunction in diabetic coronary arterioles, but no direct study has been conducted to show the role of IL-6 in type 2 diabetes-induced endothelial dysfunction (6, 16, 31). Moreover, the mechanisms of EDHF-mediated endothelial dysfunction in type 2 diabetic coronary arterioles have not been investigated. To test this we 1) evaluated the pivotal contribution of EDHF in type 2 diabetes-induced endothelial dysfunction, 2) evaluated the identity of EDHF candidates in coronary microcirculation in normal and type 2 diabetic mice, and 3) determined the role of IL-6 in EDHF-induced vasodilation in type 2 diabetes.
METHODS
Animal models.
The procedures followed were in accordance with approved guidelines set by the Laboratory Animal Care Committee at University of Missouri. Our animal use protocols were approved by Animal Care and Use Committee of the University of Missouri in Columbia. Wild-type (WT, C57BL/6J) controls, type 2 diabetic (db/db, BKS.Cg-m+/+ Leprdb/J) mice, and db/db mice null for TNF (dbTNF−/dbTNF−) were purchased from Jackson Laboratory (Bar Harbor, Me) and maintained on a normal rodent chow diet. Our studies used 24–30-wk-old, 27–31 g WT and 55–66 g db/db and dbTNF−/dbTNF− mice of either sex.
mRNA expression of IL-6 by real-time PCR.
Total RNA was extracted from isolated coronary arterioles using TRIzol reagent (Life Technologies) and was processed directly to cDNA synthesis using the SuperScript III Reverse Transcriptase (Life Technologies). cDNA was amplified using qRT-PCR Kit with SYBR Green (Life Technologies). Data were calculated by the 2−ΔΔCT method (23) and presented as fold change of transcripts for IL-6 gene in db/db mice normalized to β-actin, compared with WT mice (defined as 1.0-fold).
Treatment with IL-6 neutralization.
Neutralizing antibody to IL-6 (anti-IL-6) is a functional grade purified anti-mouse IL-6 (eBioscience). At 24–30 wk of age, both WT and db/db mice received the neutralizing anti-IL-6 (0.28 mg·ml−1·kg−1·day−1 ip for 3 days); dosage was based on our estimates of IL-6 expression (in the low ng or pg range), and this is able to neutralize 10–100-fold of this amount of IL-6.
Functional assessment of isolated coronary arterioles.
The techniques for identification and isolation of coronary microvessels were previously described in detail (40, 41). The heart was excised and immediately placed in cold (4°C) saline solution. Each coronary arteriole (50 to 100 μm in internal diameter) from WT, db/db, and dbTNF−/dbTNF− mice was carefully isolated and then used in the functional studies. To determine the response of coronary arterioles to EDHF stimulation, the vessels were cannulated with glass micropipettes and pressurized to 60 cmH2O intraluminal pressure without flow. After basal tone developed, the experimental interventions were performed. The concentration-diameter relationships for an activator of endothelium-dependent NO-mediated vasodilation, ACh (0.1 nmol/l to 10 μmol/l), and NO donor sodium nitroprusside (SNP, 0.1 nmol/l to 10 μmol/l) were then established. To determine EDHF-induced vasodilation, ACh-dependent vasodilation was performed in the presence of NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME, 10 μmol/l) and cyclooxygenase inhibitor indomethacin (Indo, 10 μmol/l) incubated for 30 min before beginning the protocols. In some experiments, a higher dose of l-NAME (100 μmol/l) was given with Indo to evaluate whether the full inhibition was achieved.
To distinguish which EDHF candidate(s) play a significant role in coronary microcirculation in WT and db/db mice, we studied 1) K+ channel blockade using a combination of a nonselective blocker of intermediate-conductance Ca2+-activated K+ channels (IKCa), charybdotoxin (CTX, 10 μmol/l), and a selective blocker of small-conductance Ca2+-activated K+ channels (SKCa), apamin (Apa, 50 μmol/l), incubated for 30 min; 2) epoxyeicosatrienoic acids (EETs) synthesis blocker, 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE, 10 μmol/l) incubated for 30 min; or 3) catalase, an enzyme that selectively dismutates H2O2 to water and oxygen (1,000 U/ml, 60-min incubation). Following the incubation, the responses to EDHF-induced vasodilation were examined. We studied the role of IL-6 (incubated for 30 min with IL-6, 5 ng/ml) in ACh-induced vasodilation in WT mice. All drugs were administered extraluminally in the chamber bathing solution during these functional experiments.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) except for 14,15-EEZE (from Dr. John R. Falck, University of Texas Southwestern Medical School, Dallas, TX).
Protein expression of IL-6 by Western blot analyses.
Hearts were separately homogenized and sonicated in lysis buffer (Cellytic MT Mammalian Tissue Lysis/Extraction Reagent, Sigma). Protein concentrations were assessed (BCA Protein Assay Kit; Pierce), and equal amounts of protein (40 μg) were separated by SDS-PAGE and transferred to nitrocellulose membranes (Hybond, Amersham). Horseradish peroxidase-conjugated goat anti-mouse was used as the secondary antibody (1:2,000 dilution) (Abcam). Signals were visualized by enhanced chemiluminescence (Amersham) and quantified by Quantity One (Bio-Rad Versadoc imaging system).
Data analysis.
At the end of each experiment, the vessel was exposed to 100-μmol/l SNP to obtain its maximal diameter at 60 cmH2O intraluminal pressure (41). All diameter changes to pharmacological agonists were normalized to the control diameter. All data are presented as means ± SE, except as specifically stated (e.g., as means ± SD for molecular studies). Statistical comparisons of vasomotor responses under different treatments were made with one-way or two-way ANOVA, and intergroup differences were tested with a least significance difference test. Significance was accepted at P < 0.05.
RESULTS
Body weight, abdominal girth, and glucose concentration.
Table 1 shows that body weight, abdominal girth, and glucose concentration were significantly higher in db/db mice, dbTNF−/dbTNF− mice, and db/db mice treated with anti-IL-6 compared with WT mice.
Table 1.
Body weight, abdominal girth, and glucose concentration
| Groups | WT | db/db | dbTNF−/dbTNF− | db/db + Anti-IL-6 |
|---|---|---|---|---|
| Body weight, g | 28±1.0 | 61±0.4* | 63±4* | 56±3* |
| Abdominal girth, cm | 8.5±0.2 | 13.3±0.3* | 13.3±0.4* | 12.6±0.3* |
| Glucose, mg/dl | 158±14 | 415±32* | 468±25* | 475±25* |
Values are means ± SE. Body weight, abdominal girth, and glucose concentration are higher in db/db, db/db mice null for TNF (dbTNF−/dbTNF−), and db/db mice treated with anti-IL-6 compared with wild-type (WT) mice (P < 0.05; n = 12). Body weight, abdominal girth, and glucose concentration of dbTNF−/dbTNF− and db/db mice treated with anti-IL-6 are not different from that of db/db mice.
P < 0.05 vs. WT.
Role of EDHF-mediated vasodilation in type 2 diabetes.
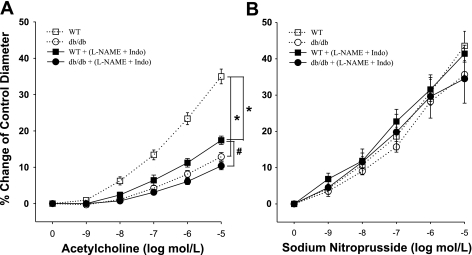
To measure EDHF-dependent dilation, we studied ACh-induced vasodilation in the presence of l-NAME and Indo. Vasodilation to ACh was significantly attenuated following the administration of l-NAME and Indo in WT mice, whereas ACh-induced vasodilation was resistant to l-NAME and Indo in db/db mice (Fig. 1A). A higher dose of l-NAME (100 μmol/l) did not affect this response (data not shown). This result indicates that EDHF-induced vasodilation is preserved in diabetic mice. ACh-induced vasodilation was significantly lower in db/db mice than that in WT control mice, and ACh-induced EDHF-dependent vasodilation was also attenuated in db/db mice compared with WT mice (Fig. 1A). However, the endothelium-independent vasodilator SNP-induced vasodilation was identical in db/db versus WT mice (Fig. 1B). The incubation with l-NAME and Indo did not affect the basal tone in these functional studies.
Fig. 1.
A: isolated coronary arterioles from wild-type (WT; n = 14) and db/db (n = 10) mice dilated in a concentration-dependent manner to ACh. ACh-induced, endothelium-dependent vasodilation was attenuated in db/db mice (n = 10) compared with WT mice (n = 14). Endothelium-derived hyperpolarizing factor (EDHF)-induced vasodilation [ACh-induced vasodilation in the presence of NG-nitro-l-arginine methyl ester (l-NAME) and indomethacin (Indo)] was lower compared with ACh-induced, endothelium-dependent vasodilation in WT (n = 14) and db/db mice (n = 10). B: sodium nitroprusside (SNP)-induced, endothelium-independent vasodilation was normal in both WT (n = 6) and db/db mice (n = 4), and l-NAME and Indo did not affect SNP-induced vasodilation in WT and db/db mice. *P < 0.05 vs. WT; #P < 0.05 vs. db/db.
Role of EDHF in coronary arterioles from diabetic mice.
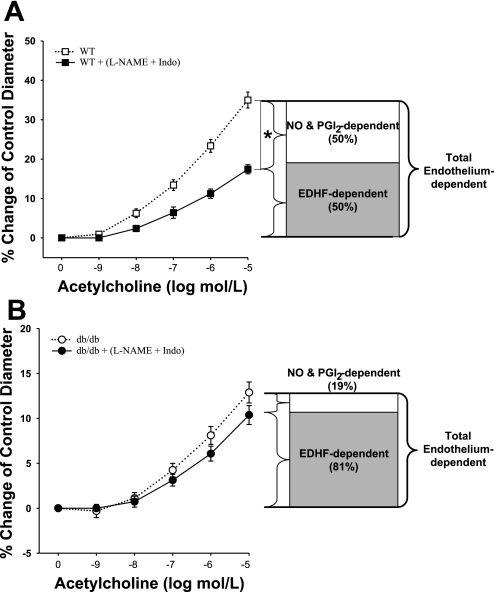
At 10 μmol/l of ACh, ∼50% of ACh-induced vasodilation is EDHF dependent and the other 50% is NO and PGI2-induced vasodilation in WT coronary arterioles (Fig. 2A), whereas in diabetic coronary arterioles, the portion of EDHF-dependent vasodilation is significantly increased to ∼81% of total endothelium-dependent vasodilation (Fig. 2B).
Fig. 2.
EDHF-dependent vasodilation (ACh-induced vasodilation in the presence of l-NAME and Indo) is 50% of total ACh-induced, endothelium-dependent vasodilation in WT (A, n = 10), whereas it is 81% in db/db mice (B, n = 10). The percentage shown is calculated at the highest dose of ACh (10 μmol/l). NO, nitric oxide; PGI2, prostacyclin. *P < 0.05 vs. WT.
Identity of EDHF in type 2 diabetes-induced endothelial dysfunction.
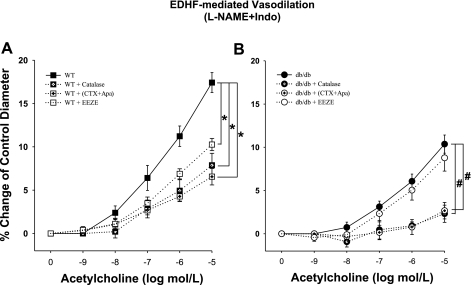
To establish the identity of EDHF, we administered the inhibitors of each EDHF pathway: 1) K+ channel blockade combined incubation of a specific blocker of IKCa CTX and a selective blocker of SKCa Apa; 2) EETs synthesis blocker, 14,15-EEZE; or 3) catalase dismutase of H2O2 to water and oxygen in WT and db/db mice. In WT mice, EDHF-induced vasodilation was significantly decreased with each inhibitor of the corresponding EDHF pathway, which indicates that K+, EETs, and H2O2 are involved in EDHF-induced vasodilation in normal control mice (Fig. 3A). In db/db mice, however, the incubation of 14,15-EEZE did not change the EDHF-induced vasodilation although catalase or the combination of CTX and Apa significantly attenuated the EDHF-dependent vasodilation, indicating that K+ and H2O2, but not EETs, are involved in the EDHF-induced vasodilation in type 2 diabetes (db/db mice, Fig. 3B). No significant differences in the basal tones of the isolated coronary arterioles incubated with the inhibitors were found.
Fig. 3.
A: incubation of coronary arterioles isolated from WT mice with K+ channel blockade [administration of the combination of a nonselective blocker of intermediate-conductance Ca2+-activated K+ channels (IKCa) charybdotoxin (CTX) and a selective blocker of small-conductance Ca2+-activated K+ channels (SKCa) apamin (Apa)], epoxyeicosatrienoic acids blocker, 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE), or catalase reduced EDHF-induced vasodilation. *P < 0.05 vs. WT; n = 7. B: incubation of coronary arterioles isolated from db/db mice with K+ channel blockade [administration of the combination of a nonselective blocker of IKCa CTX and a selective blocker of SKCa Apa] or catalase attenuated EDHF-induced vasodilation; however, incubation of coronary arterioles isolated from db/db mice with 14,15-EEZE did not change EDHF-induced vasodilation. #P < 0.05 vs. db/db (n = 7).
Role of IL-6 in type 2 diabetes-induced vascular dysfunction.
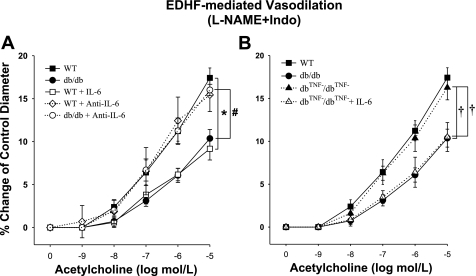
The incubation of arterioles isolated from WT mice with IL-6 impaired EDHF-induced vasodilation, whereas the administration of anti-IL-6 in diabetic db/db mice partially restored EDHF-mediated vasodilation comparable with the vasodilation in WT control mice. However, anti-IL-6 did not affect EDHF-mediated vasodilation in WT mice (Fig. 4A). In dbTNF−/dbTNF− mice, EDHF-induced vasodilation was similar to WT mice but greater than that in db/db mice. The treatment with IL-6 in dbTNF−/dbTNF− mice impaired EDHF-mediated endothelial function similar to that in db/db mice (Fig. 4B). In another series of experiments, the coronary vessels from db/db mice were treated with anti-IL-6 and then incubated with the inhibitors of the EDHF pathways. Regardless of the pathway affected, the improved response of db/db mouse coronary arterioles was impaired by the EDHF inhibitors (data not shown).
Fig. 4.
A: EDHF-induced vasodilation was significantly attenuated in db/db mice (n = 10) compared with WT mice (n = 14). IL-6 attenuated EDHF-induced vasodilation in WT (n = 5) mice to the level of db/db mice (n = 10). Neutralizing antibodies to IL-6 restored EDHF-induced vasodilation in db/db mice (n = 4). *P < 0.05 vs. WT; #P < 0.05 vs. db/db. B: EDHF-induced vasodilation in db/db mice null for TNF (dbTNF−/dbTNF−) mice (n = 6) was similar to that in WT mice (n = 14) but was significantly higher than in db/db mice (n = 10). IL-6 attenuated EDHF-induced vasodilation in dbTNF−/dbTNF− mice (n = 6) to the level of db/db mice (n = 10). †P < 0.05 vs. dbTNF−/dbTNF−.
Expression of IL-6 in type 2 diabetes.
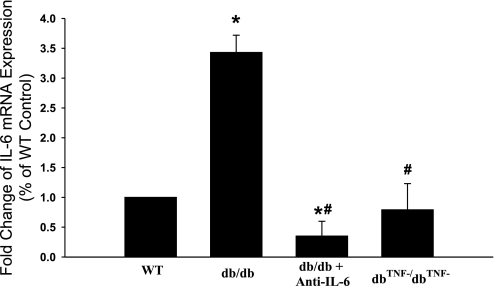
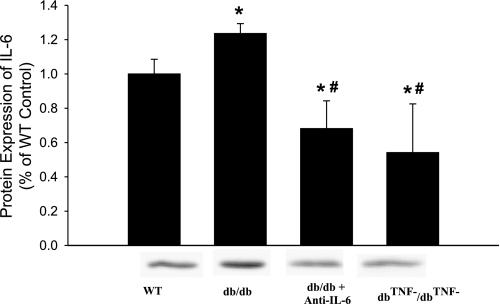
The mRNA expression of IL-6 in the heart tissue of WT, db/db, db/db mice treated with anti-TNF, and dbTNF−/dbTNF− mice was significantly elevated in db/db mice, but it was markedly attenuated in db/db mice treated with anti-IL-6 or in dbTNF−/dbTNF− mice (Fig. 5). Likewise, the protein expression of IL-6 was higher in db/db mice, but the anti-IL-6 treatment attenuated the protein expression of IL-6 in db/db mice. The protein expression of IL-6 is normal in dbTNF−/dbTNF− mice versus WT mice (Fig. 6).
Fig. 5.
mRNA expression of IL-6 was higher (3.5-fold) in db/db vs. WT mice. However, mRNA expression of IL-6 was attenuated in db/db mice treated with anti-IL-6 and in dbTNF−/dbTNF− vs. db/db mice. *P < 0.05 vs. WT; #P < 0.05 vs. db/db (n = 4).
Fig. 6.
The protein expression of IL-6 was higher in db/db vs. WT mice, but anti-IL-6 attenuated protein expression of IL-6 in db/db mice. IL-6 protein expression was attenuated in dbTNF−/dbTNF− vs. db/db mice. Moreover, the protein expression of IL-6 was lower in db/db mice treated anti-IL-6 and dbTNF−/dbTNF− mice compared with WT mice. *P < 0.05 vs. WT (n = 10); #P < 0.05 vs. db/db (n = 10).
DISCUSSION
Our results indicate that endothelium-mediated vasodilation is NO dependent in coronary arterioles in WT mice. However, we found that a portion of the NO-dependent, endothelium-dependent vasodilation is significantly reduced in db/db mice, supporting the view that EDHF plays a pivotal role in type 2 diabetes-induced endothelial dysfunction. Also, three EDHF candidates, H2O2, K+, and EETs, may play roles in dilating the coronary arterioles in response to ACh in WT control mice. The impairment of the H2O2 response and/or the abnormalities in K+ channels in advanced diabetes may be the possible mechanisms for the reduction in EDHF-mediated vascular function in db/db mice. Our studies indicated that the EETs were not involved in the endothelium-dependent, EDHF-mediated vasodilation in db/db mice. Our findings also support the concept that IL-6 plays a pivotal role in the EDHF-dependent endothelial dysfunction in type 2 diabetes, based on observing normalized coronary vascular function in the presence of anti-IL-6-neutralizing antibody. Also, the addition of IL-6 to the bath of WT coronary vessels produced a similar degree of dysfunction as seen in the db/db mice. The molecular evidence also supports our findings in that the expression of IL-6 was significantly increased in db/db mice. The administration of anti-IL-6 attenuated IL-6 expression in db/db mice compared with WT mice. Lastly, the expression of IL-6 was similar in dbTNF−/dbTNF− versus WT mice. These findings provide a further understanding of the mechanism(s) that contribute to the role of EDHF in endothelial dysfunction in type 2 diabetes in the coronary microcirculation.
Impaired NO-dependent coronary control and contribution of EDHF in type 2 diabetes.
In the present study, both NO- and EDHF-mediated vasodilation are attenuated in coronary arterioles from db/db mice compared with WT controls, whereas the endothelium-independent vasodilation is not altered. Our data support previous studies that diabetes-induced endothelial dysfunction is mainly NO-dependent in the coronary arterioles of diabetic mice (16) and prediabetic rat coronary resistance vessels (31). Inflammatory cytokine TNF-α plays an important role in the impaired NO-mediated vasodilation in diabetic coronary arterioles by attenuating endothelial NO synthase expression and increasing peroxynitrite production. These actions can result in decreasing NO bioavailability. Our findings provide the first evidence that EDHF-mediated vasodilation is impaired in coronary arterioles from advanced type 2 diabetic db/db mice. This is consistent with previous studies showing that EDHF-induced vasodilation is impaired in diabetic rat mesenteric arteries (13, 24).
Although NO-dependent vascular responses were not altered in diabetic femoral and mesenteric arteries (38), accumulating data have confirmed that decreased NO bioavailability is the major mechanism of endothelial dysfunction in diabetic coronary microcirculation. Endothelial dysfunction in advanced diabetes is due to the decreased endothelial NO synthase expression and is exaggerated by a diabetes-induced increase in intracellular sources for oxygen free radicals [e.g., mitochondria, xanthine oxidase, NAD(P)H oxidase]. However, there is still some vasodilation present in coronary microcirculation providing blood flow to the diabetic heart. Although NO has been considered as the major factor regulating endothelium-dependent relaxation, EDHF also appears to be an important mediator of vascular tone and reactivity, especially in small resistance vessels (11). Our data (Fig. 2) show that endothelium-dependent vasodilation is substantially attenuated with NO blockade in coronary arterioles in WT mice (50% of ACh-induced vasodilation is EDHF dependent at 10 μM of ACh), whereas it is preserved with NO blockade in diabetic vessels (81% of ACh-induced vasodilation is EDHF dependent at 10 μM of ACh), indicating that the contribution of EDHF is significantly increased in the coronary microcirculation in type 2 diabetes. This is consistent with the concept that a preserved EDHF function in the diseased state may represent a compensatory response to a reduction in NO bioavailability to sustain endothelial function and tissue perfusion (20, 25, 29).
Identity of EDHF in type 2 diabetes-induced endothelial dysfunction.
Several EDHF candidates have been proposed: 1) K+, 2) EETs, a product of cytochrome P-450 oxygenase, and/or 3) H2O2. These major candidates for EDHF play a crucial role in endothelium-dependent vasodilation when NO- and PGI2-mediated mechanisms are diminished or eliminated. The increased intracellular Ca2+ stimulated by ACh activates both SKCa and IKCa in the endothelial cell. The increase in extracellular K+ hyperpolarizes and relaxes vascular smooth muscle through the activation of inward-rectifying K+ channels and the Na+/K+-ATPase (9). We used a combination of CTX (a nonselective blocker of IKCa) and Apa (a selective blocker of SKCa) to investigate the role of the K+ as an EDHF candidate in the endothelial cell. Others have shown that these combined inhibitors block the efflux of K+ from the intracellular compartment to the extracellular space in the endothelial cell and that the relaxation of vascular smooth muscle was inhibited (18, 19, 27). EETs are synthesized in the endothelium from arachidonic acid via the action of cytochrome P-450 epoxygenase hyperpolarize vascular smooth muscle through activating the large-conductance Ca2+-activated K+ channels in the smooth muscle membrane (5). H2O2 is released from the endothelial cell and has been shown to hyperpolarize smooth muscle via IKCa activation (26). Catalase selectively dismutates H2O2 to water and oxygen and exogenous catalase inhibits the effects of exogenous H2O2 (33, 36). However, the limitation of the present study is that we do not know how catalase affects ROS or whether the ROS is acting from within the cell.
Our data show that the blockade of each pathway results in a significant reduction in the EDHF-mediated vasodilation from the coronary arterioles in WT mice, suggesting that each mechanism is involved in vasodilation in the normal healthy coronary microcirculation. In coronary arterioles isolated from db/db mice, EDHF-mediated vasodilation is not altered by the inhibition of EETs synthesis, the product of cytochrome P-450 oxygenase, whereas the antagonism of other factors, namely K+ and H2O2, results in a significant decrease in EDHF-dependent vasodilation in the same vascular beds. These results suggest that vasodilation induced by cytochrome P-450 oxygenase-derived EETs synthesis may be totally impaired in advanced type 2 diabetes. H2O2 and K+ have been introduced as the primary EDHFs, especially in the coronary circulation, and our results support this view (2, 4, 26). A highly selective and competitive inhibitor of EETs synthesis, 14,15-EEZE, was used in this study since nonspecific inhibitors of cytochrome P-450 may affect other vasoactive factors such as action of the K+ channel blocker (17). Previous studies reported that bradykinin-induced, EDHF-dependent relaxation in small mesenteric arteries from diabetic mice is regulated through cytochrome P-450. However, ACh-induced, EDHF-mediated relaxation involves neither cytochrome P-450 product nor H2O2, suggesting that a K+-dependent EDHF response may play a role in the endothelium-dependent vascular function in diabetic small mesenteric arteries (30). In contrast, the K+-mediated, EDHF response is impaired in small mesenteric arteries in Zucker diabetic fatty rats (3). This discrepancy may be due to the different vascular beds (arteries vs. small resistance vessels) and/or different species or strains of the animals.
H2O2 is an interesting factor in endothelium-dependent vasodilation. Our laboratory (16) previously reported that H2O2 was involved in the impairment of endothelium-dependent vasodilation since catalase, which dismutates H2O2 to water and oxygen, partially protected impaired vasodilation induced by ACh in type 2 diabetes. However, our present study shows that H2O2 is one of the EDHF candidates to induce vasodilation in coronary arterioles in WT and db/db mice when NO is blocked by l-NAME and Indo as supported by others (34, 39). Based on these results, we suggest that H2O2 plays a role in the stimulation of vasodilation as EDHF when NO is absent.
IL-6 and endothelial dysfunction in type 2 diabetes.
Accumulating evidence shows that endothelial dysfunction is associated with inflammation. Our laboratory has reported that TNF-α plays a pivotal role in endothelial dysfunction in type 2 diabetes through activating the advanced glycation end (AGE) products/receptor of AGE (RAGE) and nuclear factor-κB (NF-κB) signaling pathway (16). Like TNF-α, another proinflammatory cytokine, the interleukin family is important and is also closely associated with type 2 diabetes. Elevated plasma concentration of IL-6 is an indicator of the development of type 2 diabetes (8, 32), and the chronic administration of IL-6 causes insulin resistance (22). Moreover, the neutralization of IL-6 reduces hepatic insulin resistance in obese (Lepob) mice (21). However, no studies have reported a direct effect of IL-6 on endothelial function. The present results provide direct evidence that IL-6 plays a key role in EDHF-mediated endothelial dysfunction in type 2 diabetes. We found that EDHF-mediated vasodilation was impaired with the incubation of IL-6 in coronary arterioles from WT control mice, whereas this was restored with the treatment of IL-6-neutralizing antibody in coronary arterioles from db/db mice. These findings indicate that IL-6 plays a pivotal role in the diabetes-induced endothelial dysfunction. The present molecular data also support this view, since mRNA and protein expression of IL-6 are significantly higher in the db/db mice heart and the neutralization of IL-6 decreases both mRNA and protein expression in db/db mice heart, resulting in a lower level of expression than that in WT control mice.
Although the relationship between TNF-α and IL-6 has not been clearly established, Turner et al. (37) recently reported that IL-6 mRNA expression was stimulated via TNF-α receptor I and mediated through the p38 mitogen-activated protein kinase, phosphoinositide 3-kinase/Akt, and NF-κB pathway. Our current results also suggest that TNF-α is a mediator of IL-6, since our molecular data indicate that IL-6 mRNA and protein expressions are significantly reduced in dbTNF−/dbTNF− mice. Moreover, the functional data show that the EDHF-mediated vasodilatory function is restored in dbTNF−/dbTNF− mice to the level of WT control mice and that the EDHF-mediated vasodilation was impaired with the incubation of IL-6 in dbTNF−/dbTNF− mice.
In conclusion, EDHF contributes to endothelial-dependent vasodilation in maintaining coronary blood flow when the bioavailability of NO is substantially reduced in type 2 diabetes. Three EDHF candidates, K+, EETs, and/or H2O2, are involved in the EDHF-mediated vasodilation in normal coronary circulation, but the EETs are not involved in the diabetic condition. We also found that the overexpression of IL-6 (protein and mRNA) impairs the EDHF-mediated vasodilation in coronary arterioles in type 2 diabetic mice, and this impaired EDHF-mediated endothelial function can be restored to the level of normal control by the administration of neutralizing antibody to IL-6. These findings provide important new insights into the identity and mechanisms of EDHF-mediated vasodilation in the coronary circulation and may help identify novel therapeutic targets for cardiovascular disease associated with elevated levels of IL-6.
GRANTS
This study was supported by American Heart Association Scientist Development Grant 110350047A; Pfizer Atorvastatin Research Award 2004-37; and National Heart, Lung, and Blood Institute Grants RO1-HL-077566 and RO1-HL-085119 (to C. Zhang).
Acknowledgments
We thank Dr. John R. Falck for the generous proprietary gift of 14,15-EEZE.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anguloa J, Cuevasb P, Fernándezb A, Gabanchoa S, Allonaa A, Martín-Moralesa A, Moncadaa I, Videlac S, Sáenz de Tejada I. Diabetes impairs endothelium-dependent relaxation of human penile vascular tissues mediated by NO and EDHF. Biochem Biophys Res Commun 312: 1202–1208, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Burnham MP, Bychkov R, Feletou M, Richards GR, Vanhoutte PM, Weston AH, Edwards G. Characterization of an apamin-sensitive small-conductance Ca2+-activated K+ channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol 135: 1133–1143, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnham MP, Johnson IT, Weston AH. Impaired small-conductance Ca2+-activated K+ channel-dependent EDHF responses in Type II diabetic ZDF rats. Br J Pharmacol 148: 434–441, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bychkov R, Burnham MP, Richards GR, Edwards G, Weston AH, Feletou M, Vanhoutte P. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+ channel in porcine coronary endothelium: relevance to EDHF. Br J Pharmacol 137: 1346–1354, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell WB, Harder DR. Endothelium-derived hyperpolarizing factors and vascular cytochrome P450 metabolites of arachidonic acid in the regulation of tone. Circ Res 84: 484–488, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Dandona P, Aljada A, Chaudhuri A, Mohanty P. Endothelial dysfunction, inflammation and diabetes. Rev Endocr Metab Disord 5: 189–197, 2004. [DOI] [PubMed] [Google Scholar]
- 7.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol 130: 963–974, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 52: 1799–1805, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Edwards G, Dora KA, Gardener MJ, Garland C, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396: 269–272, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Félétou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol 26: 1215–1225, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Félétou M, Vanhoutte PM. The alternative: EDHF. J Mol Cell Cardiol 31: 15–22, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald SM, Kemp-Harper BK, Tare M, Parkington HC. Role of endothelium-derived hyperpolarizing factor in endothelial dysfunction during diabetes. Clin Exp Pharmacol Physiol 32: 482–487, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A. Alterations in endothelium-dependent hyperpolarization and relaxation in mesenteric arteries from streptozotocin-induced diabetic rats. Br J Pharmacol 121: 1383–1391, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J 3: 2007–2018, 1989. [PubMed] [Google Scholar]
- 15.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 299: 373–376, 1980. [DOI] [PubMed] [Google Scholar]
- 16.Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in Leprdb mice. Circulation 115: 245–254, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck J, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res 90: 1028–1036, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Gluais P, Edwards G, Weston AH, Falck JR, Vanhoutte PM, Félétou M. Role of SKCa and IKCa in endothelium-dependent hyperpolarizations of the guinea-pig isolated carotid artery. Br J Pharmacol 144: 477–485, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haddy FJ, Vanhoutte PM, Feletou M. Role of potassium in regulating blood flow and blood pressure. Am J Physiol Regul Integr Comp Physiol 290: R546–R552, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Hecker M Endothelium-derived hyperpolarizing factor-fact or fiction? News Physiol Sci 15: 1–5, 2000. [PubMed] [Google Scholar]
- 21.Klover PJ, Clementi AH, Mooney RA. Interleukin-6 depletion selectively improves hepatic insulin action in obesity. Endocrinology 146: 3417–3427, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes 52: 2784–2789, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Makino A, Ohuchi K, Kamata K. Mechanisms underlying the attenuation of endothelium-dependent vasodilatation in the mesenteric arterial bed of the streptozotocin-induced diabetic rat. Br J Pharmacol 130: 549–556, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malmsjo M, Bergdahl A, Zhao XH, Sun XY, Hedner T, Edvinsson L, Erlinge D. Enhanced acetylcholine and P2Y-receptor stimulated vascular EDHF-dilatation in congestive heart failure. Cardiovasc Res 43: 200–209, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Matoba T, Shimokawa H, Morikawa K, Kubota H, Kunihiro I, Urakami-Harasawa L, Mukai Y, Hirakawa Y, Akaike T, Takeshita A. Electron spin resonance detection of hydrogen peroxide as an endothelium-derived hyperpolarizing factor in porcine coronary microvessels. Arterioscler Thromb Vasc Biol 23: 1224–1230, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Miura H, Liu Y, Gutterman DD. Human coronary arteriolar dilation to bradykinin depends on membrane hyperpolarization: contribution of nitric oxide and Ca2+-activated K+ channels. Circulation 99: 3132–3138, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Moncada S, Vane JR. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2 and prostacyclin. Pharmacol Rev 30: 293–331, 1979. [PubMed] [Google Scholar]
- 29.Najibi S, Cowan CL, Palacino JJ, Cohen RA. Enhanced role of potassium channels in relaxations to acetylcholine in hypercholesterolemic rabbit carotid artery. Am J Physiol Heart Circ Physiol 266: H2061–H2067, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Pannirselvam M, Ding H, Anderson TJ, Triggle CR. Pharmacological characteristics of endothelium-derived hyperpolarizing factor-mediated relaxation of small mesenteric arteries from db/db mice. Eur J Pharmacol 551: 98–107, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res 99: 69–77, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286: 327–334, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol 26: 2614–2621, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Shimokawa H, Morikawa K. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J Mol Cell Cardiol 39: 725–732, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol 28: 703–711, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Thengchaisri N, Hein TW, Wang W, Xu X, Li Z, Fossum TW, Kuo L. Upregulation of arginase by H2O2 impairs endothelium-dependent nitric oxide-mediated dilation of coronary arterioles. Arterioscler Thromb Vasc Biol 26: 2035–2042, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Turner NA, Mughal RS, Warburton P, O'regan DJ, Ball SG, Porter KE. Mechanism of TNFalpha-induced IL-1alpha, IL-1beta and IL-6 expression in human cardiac fibroblasts: Effects of statins and thiazolidinediones. Cardiovasc Res 76: 81–90, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Wigg SJ, Tare M, Tonta MA, O'Brien RC, Meredith I, Parkington HC. Comparison of effects of diabetes mellitus on an EDHF-dependent and an EDHF-independent artery. Am J Physiol Heart Circ Physiol 281: H232–H240, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Yada T, Shimokawa H, Hiramatsu O, Kajita T, Shigeto F, Goto M, Ogasawara Y, Kajiya F. Hydrogen peroxide, an endogenous endothelium-derived hyperpolarizing factor, plays an important role in coronary autoregulation in vivo. Circulation 107: 1040–1045, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Zhang C, Hein TW, Wang W, Kuo L. Divergent roles of angiotensin II AT1 and AT2 receptors in modulating coronary microvascular function. Circ Res 92: 322–329, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Zhang C, Xu X, Potter BJ, Wang W, Kuo L, Michael L, Bagby GJ, Chilian WM. TNF-alpha contributes to endothelial dysfunction in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol 26: 475–480, 2006. [DOI] [PubMed] [Google Scholar]