Abstract
The potassium channels IK and IK1, responsible for the action potential repolarization and resting potential respectively, are altered during cardiac hypertrophy. The activation of insulin-like growth factor-I (IGF-I) during hypertrophy may affect channel activity. The aim was to examine the modulatory effects of IGF-I on IK and IK1 through mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathways during hypertrophy. With the use of specific inhibitors for ERK1/2 (PD98059), p38 MAPK (SB203580) and PI3K/Akt (LY294002), Western blot and whole cell patch-clamp were conducted on sham and aorto-caval shunt-induced hypertrophy adult rat myocytes. Basal activation levels of MAPKs and Akt were increased during hypertrophy. Acute IGF-I (10−8 M) enhanced basal activation levels of these kinases in normal hearts but only those of Akt in hypertrophied ones. IK and IK1 activities were lowered by IGF-I. Inhibition of ERK1/2, p38 MAPK, or Akt reduced basal IK activity by 70, 32, or 50%, respectively, in normal cardiomyocytes vs. 53, 34, or 52% in hypertrophied ones. However, basal activity of IK1 was reduced by 45, 48, or 45% in the former vs. 63, 43, or 24% in the latter. The inhibition of either MAPKs or Akt alleviated IGF-I effects on IK and IK1. We conclude that basal IK and IK1 are positively maintained by steady-state Akt and ERK activities. K+ channels seem to be regulated in a dichotomic manner by acutely stimulated MAPKs and Akt. Eccentric cardiac hypertrophy may be associated with a change in the regulation of the steady-state basal activities of K+ channels towards MAPKs, while that of the acute IGF-I-stimulated ones toward Akt.
Keywords: phosphatidylinositol 3-kinase, mitogen activated protein kinase, insulin-like growth factor-I
the adaptive response that the heart undertakes to either pressure or volume overload develops into cardiac hypertrophy. Chronic left ventricular pressure overload leads to ventricular wall thickening, a condition known as concentric cardiac hypertrophy, whereas volume-overload induces ventricular chamber enlargement disproportionate to wall thickening, known as eccentric hypertrophy (17). During the development of eccentric hypertrophy, there is an increase in left ventricular end-diastolic volume (29) and thereof a rise in wall stress, which eventually leads to the addition of sarcomeres mainly in series. This involves the action of specific growth factors that are activated during the development of both types of cardiac hypertrophy, yet with model-related time sequence (40).
One of those growth factors is insulin-like growth factor-1 (IGF-I), which is known for its major role in cellular proliferation and differentiation during heart development (21, 26, 27). Addition of IGF-I to cultured newborn rat cardiocytes stimulates the expression of muscle-specific genes that induce myocyte hypertrophy (28). In addition, there is sufficient evidence showing that IGF-I mRNA and protein increase in parallel with the development of cardiac hypertrophy (9). Furthermore, in the volume-overload model we have shown that cardiac myofibers have an increased receptor affinity to IGF-I, which corroborated with the induction of eccentric hypertrophy (20).
Binding of IGF-I to its receptor stimulates phosphatidylinositol 3-kinase (PI3K), which causes activation of Akt (8, 39). Transgenic overexpression of Akt has been shown to be sufficient in inducing significant cardiac hypertrophy in mice without affecting systolic function (35, 47). Furthermore, it has been demonstrated that IGF-I activates the mitogen-activated protein kinase (MAPK) cascade in addition to the PI3K/Akt one (12). Lavandero et al. (32) showed that short exposure of ventricular cardiocytes to IGF-I leads to the development of cardiac hypertrophy, through activation of the downstream ERK-MAPK pathway via PKC. In addition, Shyu et. al. (48) found that the increased expression of myostatin in hypertrophied cells is mediated by IGF-I, at least in part through p38 MAP kinase.
Thus it seems that IGF-I plays an important role in the development of eccentric cardiac hypertrophy, through the activation of MAPK (namely, ERK1/2 and p38) as well as PI3K pathways. However, their effects on the regulation of the electrical activity of the heart are not yet well studied, specifically in terms of the role of potassium channels (namely, IK and IK1) during eccentric cardiac hypertrophy. Its importance stems from the fact that cardiac hypertrophy is characterized by a prolongation of the action potential duration, an almost universal finding in ventricular cells exposed to chronic workload leading to cardiac hypertrophy (33, 34, 36, 60). Alterations in the activity of the potassium channels have direct effects on the action potential duration and configuration, which are important determinants of myocardial contraction (33, 34). In that regard, physiological volume-overload-induced eccentric cardiac hypertrophy, observed during late pregnancy, causes downregulation of cardiac Kv4.3 channel gene expression by three- to fivefold, both at the mRNA and protein levels. This was paralleled by a reduction in the expression of the transient outward potassium channel (Ito) and a longer action potential duration (11). It has been shown that long-term administration of IGF-I regulates the expression of the K+ channel in cultured neonatal rat ventricular myocytes (18). Most studies regarding the electrophysiological alterations associated with cardiac hypertrophy were performed on the pressure-overload model, which has been associated with reduction in Ito (6, 15, 16, 53) and no change (6, 57) or a decrease in IK (13, 14, 29, 30) and IK1 (5, 15, 37). Interestingly, Brooksby et al. (5) have shown that the electrical remodeling related to cardiac hypertrophy was restricted to a decrease in IK1 current density with no difference in the resting potential level of cardiomyocytes from control and spontaneously hypertensive rats. The fewer electrophysiological studies available in the literature related to volume-overload induced cardiac hypertrophy showed a decrease in IK (50, 56) but an increase in IK1 without any change in Ito (56).
Therefore, there is still a crucial lack of information regarding the intracellular signal transduction events associated with IGF-I regulation of potassium channels activities, especially during the development of eccentric cardiac hypertrophy. Thus the objective of this study was to determine the role of MAPK (ERK1/2 and p38) as well as PI3K in mediating IGF-I effects on the functional expression of potassium channels of adult cardiomyocytes, namely IK and IK1, during the development of eccentric cardiac hypertrophy. Therefore, we hypothesize that during the compensated phase of eccentric cardiac hypertrophy IGF-I can modulate the activity of IK and IK1 through a cross-talk between MAPK (ERK1/2 and p38) and PI3K pathways. In addition, steady-state basal levels of MAPK and PI3K have constitutive positive effects on basal levels of potassium channels. However, acute extrinsic stimulation by IGF-I induces cross-talk between MAPK and PI3K resulting in negative regulation of IK and IK1.
MATERIALS AND METHODS
Animal Preparation
Conformity statement.
As discussed in the following respective sections, all the procedures conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH) publication No. 85-23, revised 1996.
Male Sprague-Dawley rats of 200–250 g body wt were purchased from Charles Rivers (MA). The rats were allowed to recover and acquaint with their new environment upon arrival to the animal house of the College of Medicine for 1 wk. The animals were kept under secure, clean, and controlled room temperature (70–74°F) conditions with a light cycle from 0600 to 1800 and were fed food and water ad libitum.
Eccentric Cardiac Hypertrophy
Induction of volume-overload cardiac hypertrophy.
Adult male Sprague-Dawley rats (200–250 g) were anaesthetized with pentobarbital sodium (30 mg/kg body wt ip). The abdominal aorta was punctured at the union of the segment two-thirds caudal to the renal artery and one-third cephalic to the aortic bifurcation with an 18-G disposable needle. The needle was advanced into the abdominal aorta and vena cava at the point of anastomosis, shunting arterial blood into the venous system. A drop of cyanoacrylate glue was used to seal the aorta-punctured point. The patency of the shunt was verified visually by swelling of the vena cava and the mixing of arterial and venous blood. As a postoperative care, the rat was administered 2.5 mg/kg flunixin. The same procedure was performed on the age-matched sham rats, except for the insertion of the 18-G needle in the abdominal aorta and vena cava. Three weeks were allowed for the cardiac hypertrophy to develop. On the experimentation day, visual inspection of the lungs did not show any sign or symptom of pulmonary edema or pulmonary blood clots in all shunted animal used. This is relevant to the fact that these shunted rats are still in the compensated eccentric cardiac hypertrophy phase and eliminates any decompensatory process to heart failure.
In Vivo Magnetic Resonance Imaging
High-resolution magnetic resonance imaging (MRI) of rat heart were used as a noninvasive method to measure the changes of cardiac muscle during the treatment. All rats were anesthetized during the MRI procedure with 1.5∼2.0 vol % isoflurane mixed with oxygen at a flow rate of 200 cm3/min. The rats were positioned at the center of a Varian 4.7 T horizontal MRI machine. The maximum magnetic field gradient is 60 Gauss/cm. A balloon placed under the abdomen and filled with D2O was used as a respiratory sensor via a plastic tubing connection to a pressure transducer to produce respiratory gating signals. ECG leads made from 25- to 30-G needles were inserted subcutaneously in three of the animal paws. The ECG and respiratory signals were processed to produce respiratory-gated ECG signals for triggering the MRI sequence. A small flip angle gradient echo sequence was used with the following imaging parameters: echo time = 4.0 ms, slice thickness = 1.0 mm, field-of-view = 3.0 × 3.0 cm2, matrix size = 256 × 256, and radio frequency flip angle = 40°. Multiple cross-sectional images of the heart were acquired for each ECG trigger. A time delay of NMR acquisition was inserted immediately after ECG trigger. By varying the delay time, a series of cardiac systole and diastole images was obtained.
Isolated Heart Retrograde Perfusion
Adult hearts were excised after the rat was anesthetized (pentobarbital sodium, 50 mg/kg body wt ip). Immediately the hearts were hung on Langendorff apparatus for retrograde perfusion at 10 ml/min (37°C) for an initial 5-min equilibration with normal Tyrode solution containing the following (mM): 140 NaCl, 5.4 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 20 NaHCO3, 10 d-glucose, 0.1 EGTA, 0.4 NaH2PO4, 20 taurine, and 10 creatine. The experimental protocol consisted of continuing the retrograde perfusion of the hearts for 15 min with Tyrode solutions containing either 1) normal Tyrode as control, 2) IGF-I, or 3) Tyrode containing specific kinase inhibitor followed by IGF-I. At the end of the experiment, hearts were quickly snap frozen in liquid nitrogen for Western blot processing.
Western Blotting
Activation of ERK1/2, p38 MAPK, and Akt was assessed using the Western blot technique. Protein samples were prepared from perfused heart tissue using a lysis buffer containing the following (mM): 20 β-glycerophosphate, 1 EGTA, 0.5 NaVO3, 2 DTT, 10 benzamidine, 0.2 Na3VO4, 2 EDTA, 20 NaF, and 0.6% deoxycholate, 0.1% Triton X-100, and 1 tablet/0.10 ml of Complete protease inhibitors cocktail (Roche; pH 7.5). Cell lysates were incubated on ice for 20 min and centrifuged for 15 min at 14,000 rpm. Samples were matched for protein concentration, separated by SDS-PAGE, and transferred onto nitrocellulose membranes. After blocking in 5% nonfat milk in TBST (10 mM Tris, 150 mM NaCl, and 0.1% Tween 20), the membranes were incubated with dual phospho-specific antibodies to Akt, ERK1/2, or p38 MAPK (Cell Signaling Technology) overnight at 4°C. Afterward, membranes were washed three times in TBST, incubated with appropriate secondary antibody conjugated to horseradish peroxidase (Cell Signaling Technology) for 2 h, and then washed three times with TBST. Bands were visualized by Chemiluminescence (Renaissance, NEN Life Science Products). Films from at least three independent experiments were scanned, and densities of the immunoreactive bands were evaluated using NIH Image software and normalized. Kinase activities were evaluated as the ratio of phosphorylated kinase over total protein kinase per experiment.
Isolation of Adult Rat Cardiomyocytes
All the reagents were purchased from Sigma Chemicals (St. Louis, MO). Double-distilled water from MilliQ system (Millipore, MA) was used to prepare all solutions. Prepared solutions were oxygenated for 20 min before perfusion with 5% CO2-95% O2 and pH at 7.4 with HCl or NaOH.
Animals were injected with sodium heparin (1000 U/kg ip) and anesthetized with pentobarbital sodium (40 mg/kg ip), 30 min before removal of the heart. After excision of the heart, it was quickly transferred to a Langendorff setup for 5-min retrograde coronary perfusion through the aorta (7–10 ml/min at 37°C) with normal Tyrode solution containing the following (mM): 140 NaCl, 5.4 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 20 NaHCO3, 10 d-glucose, 0.1 EGTA, 0.4 NaH2PO4, 20 taurine, and 10 creatine. This was followed by 20-min perfusion with nominal Ca2+-free Tyrode solution to which 0.17 mg/ml liberase Blenzyme 4 (Roche Diagnostics) and 0.18 mg/ml protease type XIV (Sigma) were added for the last 10 min. Then, the heart was perfused for 5 min with high potassium buffer solution containing the following (mM): 120 K-glutamate, 20 KCl, 1 MgCl2, 10 d-glucose, 0.3 K-EGTA, and 20 HEPES. Finally, the ventricles were cut, minced, and filtered. The cells were diluted and kept in a normal Tyrode solution until experimentation. Freshly isolated myocytes showing no signs of blebs or round edges were used for up to 12 h.
Electrophysiological Studies
The whole cell patch-clamp technique was used in the voltage-clamp mode to study the potassium currents in the adult cardiomyocytes. Patch pipettes of 1–2 MΩ resistance were pulled from borosilicate glass capillary tubing with a two-stage puller (David KOPF Instruments). Ventricular myocytes were placed on the stage of an inverted microscope and perfused with an extracellular buffer containing the following (mM): 5 KCl, 1 MgCl2, 140 NaCl, 10 HEPES, 10 d-glucose, 1 CaCl2, and pH 7.4. The pipette solution contained the following (mM): 130 K-glutamate, 20 KCl, 5 EGTA, 5 NaCl, 5 Mg-ATP, 1 MgCl2, 10 HEPES, and pH 7.2.
After the formation of the whole cell patch, the cell capacitance was estimated by integrating the area of the capacitance transient after a −5-mV step from a holding potential of −80 mV. The measured currents were divided by the cell capacitance to normalize for cell size changes between normal and hypertrophied cardiomyocytes.
The cardiomyocytes were stimulated in voltage-clamp mode using pClamp 9.0 software (Molecular Devices) connected to an Axopatch amplifier through a A/D converter (Digidata1320A; Molecular Devices). The resulting ionic currents were displayed on a storage oscilloscope and stored on a computer for analysis with pClamp 9.0. All patch-clamp experiments were performed at room temperature (20–22°C). The osmolarity of all pipette-filling solutions was adjusted to 290 mosM and the extracellular solutions to 310 mosM using sucrose, and the solutions were filtered through a 0.22-μm filter.
Experimental Protocols for Potassium Currents
The voltage dependency of IK and IK1 activation was studied by obtaining data for the respective current-voltage (I-V) relationships. To that end, 400-ms step voltages in 10-mV increments between either −40 and +30 mV for IK, or −80 and −120 mV for IK1, were applied from a holding potential of −40 mV. Steady-state currents, measured at the end of each current response, were plotted as a function of the command potential. The action of IGF-I in the presence and absence of specific blocker were analyzed for their effects on the I-V relationship. Inhibitors for PI3K (LY294002), ERK1/2 MAPK (PD98059), and p38 MAPK (SB203580) were purchased from Cell Signaling Technology.
Statistical Analysis
All statistical analysis was performed using SigmaStat software and verified using both Microsoft Excel and Prism softwares, which all gave the same result. The paired Student's t-test was used to compare data before and after drug treatment of the same animal group. The heteroscedastic two-sample unpaired Student's t-test assuming unequal variances was used when the drug effects were compared between two different animal groups (sham vs. shunt). In addition, we performed one-way ANOVA followed by ad hoc Dunnett's test for comparison of the differences between the effects of inhibitors to a control in each animal model (sham or shunt) and on each potassium channel (IK and IK1). Control data for inhibitors within each animal model and channel type were merged to constitute the control group for the respective Dunnett's test. With the use of the null hypothesis, P ≤ 0.05 was considered significant.
RESULTS
Structural and Functional Parameters
The data on the structural and functional parameters from sham and shunted adult rats confirmed the development of the eccentric cardiac hypertrophy within 3 wk postsurgery as seen in Table 1. The shunted rats did not show a significant difference in their body weight vs. normal sham ones. However, the heart weights (1,737 ± 52 vs. 1,178 ± 21 mg; P < 0.01), as well as the relative heart weight of shunted rats were significantly higher compared with the sham ones (502 ± 18 vs. 349 ± 6 mg/100 g body wt; P < 0.01). In addition, the cellular membrane capacitance was significantly greater in the cardiomyocytes from hypertrophied hearts compared with control ones (211 ± 16 vs. 280 ± 21 pF; P < 0.001). With the assumption of a specific membrane capacity of 1 μF/cm2 (24) for both groups of cardiomyocytes, the surface area of control cells was 21,086 ± 1,593 μm2 and that of hypertrophied ones was 28,049 ± 2,076 μm2 (P < 0.001).
Table 1.
Anatomical parameters for sham and shunt rats
| Body Weight, g | Heart Weight, mg | Relative Heart Weight, mg/100 g body wt | |
|---|---|---|---|
| Sham | 338±2 | 1,178±21 | 349±6 |
| Shunt | 342±5 | 1,737±52* | 502±18* |
Values are means ± SE.
P < 0.01 sham vs. shunt.
Concurrently, in vivo MRI analysis was performed on the same animal before and 3 wk after the surgical creation of the atrioventricular fistula (shunt). In the shunt, the left ventricular wall thickness increased by 39.88 ± 10.03% (P < 0.05) with a concomitant increase in end-diastolic volume (63.15 ± 18.10%; P < 0.05) and in stroke volume (56.38 ± 28.79%). However, there was no change in the ejection fraction (59.93 ± 4.18% for sham and 54.15 ± 3.47% for shunt). These hemodynamic parameters were not altered in the sham rats. This is in support of our model being a compensated form of eccentric hypertrophy.
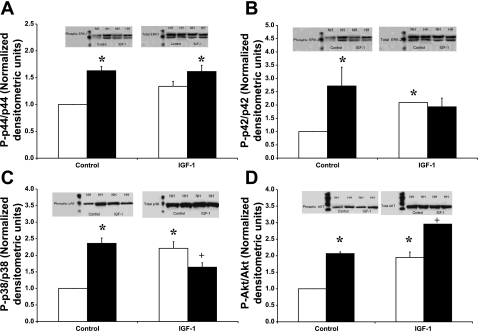
Effects of IGF-I on MAPKs and Akt Activation Levels in Sham and Shunt Hearts
The basal activation levels of the MAPKs and Akt were higher in the hypertrophied hearts compared with sham ones (Fig. 1). Accordingly, hypertrophied hearts showed greater basal activation for Akt (2.06 ± 0.01-fold; P < 0.001), ERK1 (1.63 ± 0.08-fold; P < 0.001), ERK2 (2.72 ± 0.54-fold; P = 0.03), and p38 MAPK (2.36 ± 0.16-fold; P < 0.001). On the other hand, in the normal hearts, acute administration of IGF-I (10−8 M) induced significant activation of Akt (1.95 ± 0.13-fold; P < 0.001), ERK1 (1.34 ± 0.09-fold; P = 0.02), ERK2 (2.09 ± 0.01-fold; P < 0.001), and p38 MAPK (2.21 ± 0.20-fold; P < 0.001) above their respective basal levels. In parallel, acute administration of IGF-I (10−8 M) to the hypertrophied hearts induced significant activation of Akt (2.96 ± 0.01-fold vs. untreated sham; P < 0.001), which is equivalent to a 43.18 ± 3.34% increase compared with untreated shunts (P < 0.001). However, acute IGF-I reduced the basal p38 MAPK activation level by 30.46 ± 9.80% (P = 0.03; with respect to untreated shunts). There was no significant effect of IGF-I on the ERK1/2 basal levels in the hypertrophied hearts.
Fig. 1.
Effects of IGF-I (10−8 M) on the activation level of ERK1 (A), ERK2 (B), p38 MAPK (C), and Akt (D) in normal (open bars) vs. volume-overload-induced hypertrophied (filled bars) hearts. Data are expressed as the ratio of phosphorylated over total protein normalized to control untreated hearts. Insets in each bar graph show representative Western blots for the respective phosphorylated (left) and total kinase (right). Lane 1, normal hearts; lane 2, hypertrophied hearts; lane 3, IGF-I-treated normal hearts; lane 4, IGF-I-treated hypertrophied hearts. Data are average ± SE with n = 3 (from different heart samples). *P < 0.01, compared with normal cardiocytes; +P < 0.01, compared with untreated cardiocytes.
Characteristics of IK and IK1 in Normal and Hypertrophied Cardiomyocytes
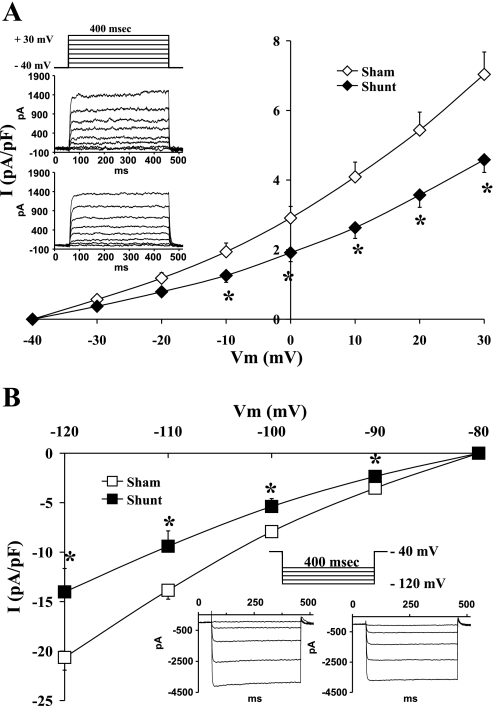
Comparisons of the potassium currents between control and hypertrophied cardiomyocytes showed a significant reduction in the current density of the inward rectifier as well as the outward rectifier potassium channels with eccentric hypertrophy. Figure 2 shows the average current density-voltage relationship of IK (A) and IK1 (B) in normal and hypertrophied cardiomyocytes. When we compared normal to hypertrophied cardiocytes, there were significant differences in the current density of IK at all voltages positive to −10 mV, while IK1 showed significant differences at all membrane potentials between −90 and −120 mV. There was no difference in IK1 between −90 and −60 mV (data not shown).
Fig. 2.
Current density-voltage (I-V) relationship of potassium channels IK (A) and IK1 (B) in normal (open symbols) and volume-overload-induced hypertrophied (closed symbols) cardiocytes. Data are presented as average current density ± SE with n = 10–18. Representative family of currents for IK from sham (top) and shunt (bottom) cardiocytes with the respective stimulation protocol are shown as an inset in A. Representative family of currents for IK1 from sham (left) and shunt (right) cardiocytes with the respective stimulation protocol are shown as an inset in B. *P < 0.05 vs. sham heart value at same voltage.
Hence, the average current density of IK at +30 mV in normal cardiocytes was 7.04 ± 0.64 pA/pF (n = 18) and in the hypertrophied ones it dropped to an average of 4.58 ± 0.37 pA/pF (n = 11; P < 0.001). The slope conductance of IK, calculated between 0 and +30 mV, was lower in the hypertrophied (89.10 ± 5.40 pS/pF) compared with the normal cardiocytes (137.63 ± 11.77 pS/pF; P < 0.001). Furthermore, the average current density of IK1 at −120 mV in normal cardiocytes was −20.64 ± 1.28 pA/pF, while the hypertrophied ones showed a lower average density of −14.02 ± 2.36 pA/pF (P < 0.05). In that regard, IK1 slope conductance was lower in the hypertrophied myocytes (431.55 ± 80.37 pS/pF; n = 10) than in the normal ones (635.78 ± 48.02 pS/pF; P < 0.05; n = 16).
Effects of IGF-I on Potassium Currents
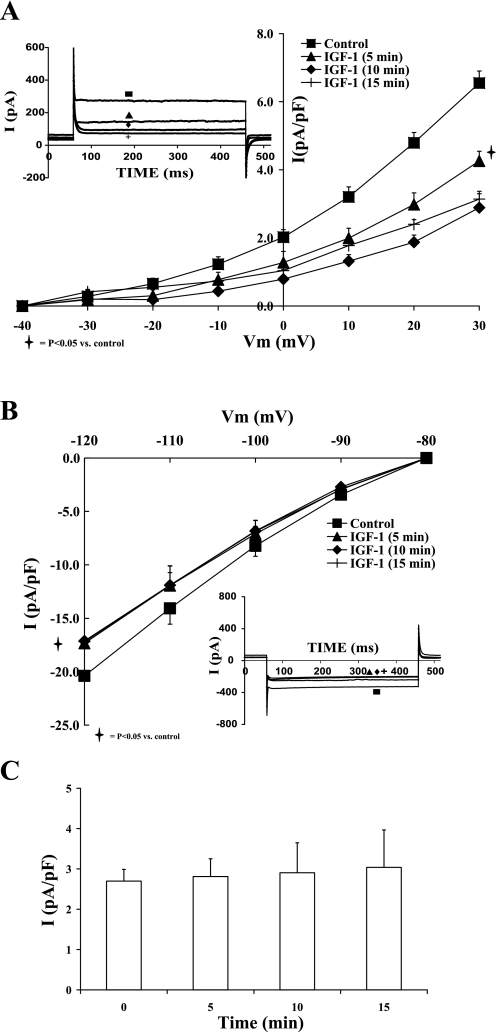
Acute administration of 1 nM IGF-I to adult cardiomyocytes steadily reduced the outward rectifier potassium current by 38.05 ± 8.99% (at +30 mV; P < 0.05). The steady-state effect was reached within 10–15 min of IGF-I exposure. Moreover, the 10 nM IGF-I effect reached steady state within 10 min with greater inhibition of IK (55.96 ± 5.89%; P < 0.01). Figure 3A shows the inhibitory effect of IGF-I on the current-voltage relationship of IK. Higher concentrations of IGF-I (≥0.1 μM) did not yield consistent inhibition levels, due to the variable induction of large nonspecific leaks whereby the currents reversed around 0 mV with concomitant drop in input resistance. Figure 3C shows the steady level of IK at 0 mV in the absence of IGF-I for the same time frame. Similarly, the inward rectifier potassium current was inhibited by 16.02 ± 1.07% (at −120 mV; P < 0.05) with 10 nM IGF-I (Fig. 3B). Such effect reached steady state within 10 min as well. In parallel, acute administration of 10 nM IGF-I showed similar effects on IK and IK1 of shunt cardiomyocytes, with steady-state inhibition reached within 15 min as well. IGF-I inhibition of IK and IK1 was voltage independent with similar percent inhibition levels (as presented above) in the voltage ranges studied.
Fig. 3.
Effects of IGF-I (10−8 M) on the current-voltage relationships of IK (A) and IK1 (B) at 5, 10, and 15 min. C: time control for IK (at 0 mV) in the absence of IGF-I. Data are average current density ± SE with n = 5. Inset in each graph shows the respective representative current responses at +20 mV for IK and −120 mV for IK1 at different time points (control, 5, 10, and 15 min IGF-I).
Effects of MAPK and PI3K on IK
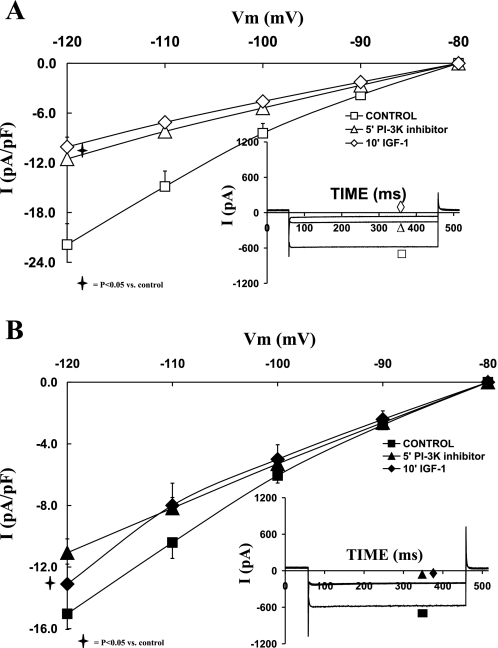
Role of PI3K in the regulation of IK and IGF-I effects on sham and shunt ventricular myocytes.
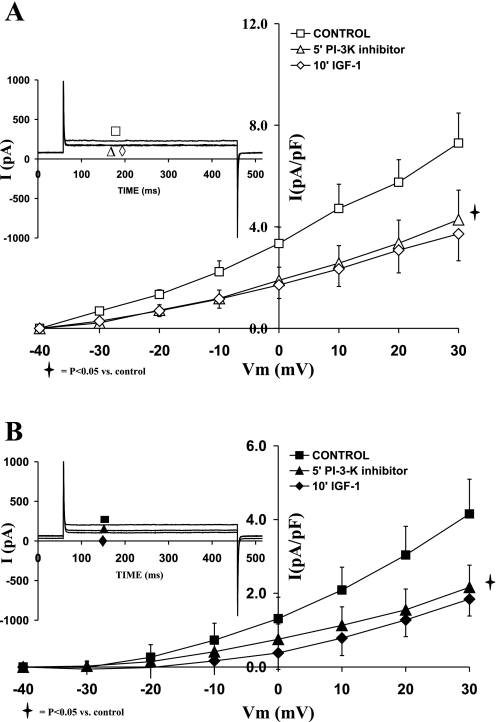
After we established the effects of IGF-I on both IK and IK1, it was important to characterize the intracellular pathways responsible for translating the signal from the IGF-I receptor to IK and IK1 channels. Since PI3K has been also shown to be activated during cardiac hypertrophy, it was interesting to investigate the relationship between PI3K and IGF-I on the activation levels of potassium channels during eccentric hypertrophy compared with normal cardiac myocytes. To that end, the specific membrane permeable PI3K inhibitor LY294002 (1 μM) was used.
The current-voltage relationships of sham and hypertrophied cardiomyocytes (Fig. 4, A and B, respectively) show that LY294002 (1 μM) alone reduced the current density of IK. Thus as shown in Fig. 4A, in the normal cardiomyocytes, IK was reduced by 50.21 ± 11.84% (P = 0.05) with LY294002. Addition of IGF-I did not further reduce IK. In the hypertrophied cardiomyocytes (Fig. 4B), the PI3K inhibitor decreased IK (at +30 mV) by 52.30 ± 6.76 (P < 0.05). Addition of IGF-I in the presence of LY294002 did not affect IK density. The effects of LY294002 on IK current density were not significantly different between sham and shunt cardiomyocytes.
Fig. 4.
Effects of the phosphatidylinositol 3-kinase (PI3K) inhibitor LY 294002 (1 μM), as well as that of IGF-I (10−8 M) in the presence of LY 294002, on the current-voltage relationship of IK in normal (A) and hypertrophied (B) adult cardiomyocytes. Data are average current density ± SE with n = 5. Inset in each graph shows respective representative IK responses at +20 mV.
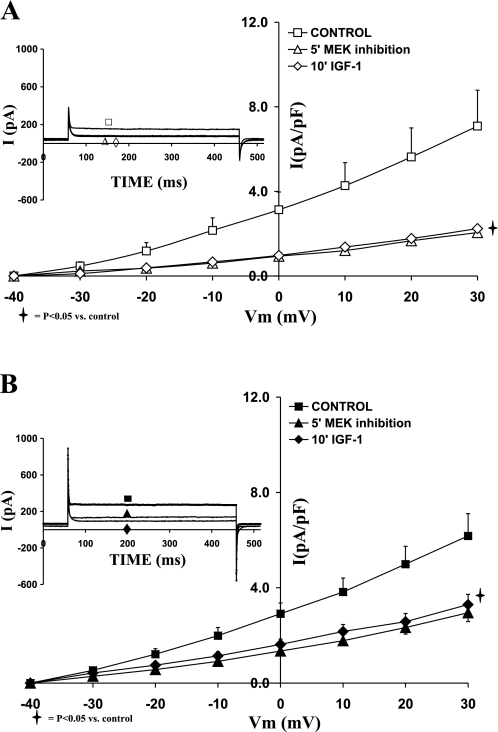
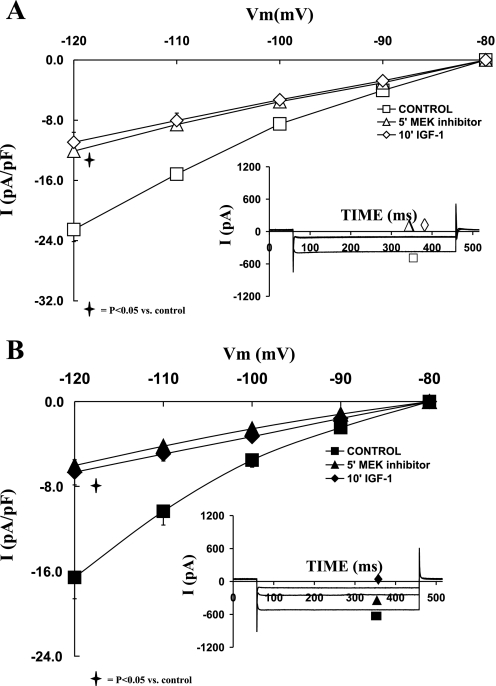
Role of ERK1/2 in the regulation of IK and IGF-I effects on sham and shunt ventricular myocytes.
To evaluate the direct effect of ERK1/2 on potassium channels, and their transduction of the IGF-I signal, we used specific cell permeable inhibitor PD98059 (1 μM). As depicted in the I-V relationships shown in Fig. 5, A and B, for sham and shunt, respectively, inhibition of ERK1/2 reduced the current density of IK in both sham and hypertrophied cardiomyocytes. Accordingly, PD98059 (1 μM) decreased IK density at +30 mV by 69.90 ± 4.77% (P = 0.03) and 53.19 ± 6.05% (P = 0.03) in sham and hypertrophied cardiomyocytes, respectively. Thus ERK1/2 inhibition induced a smaller reduction in the current density of IK in shunt than sham cardiomyocytes (P < 0.05). Addition of IGF-I (10 nM) in the presence of ERK1/2 inhibitor did not affect the I-V relationship for IK in sham or shunt cardiomyocytes.
Fig. 5.
Effects of the MEK1/2 inhibitor PD98059 (1 μM), as well as that of IGF-I (10−8 M) in the presence of PD98059, on the current-voltage relationship of IK in normal (A) and hypertrophied (B) adult cardiomyocytes. Data are average current density ± SE with n = 5. Inset in each graph shows respective representative IK responses at +20 mV.
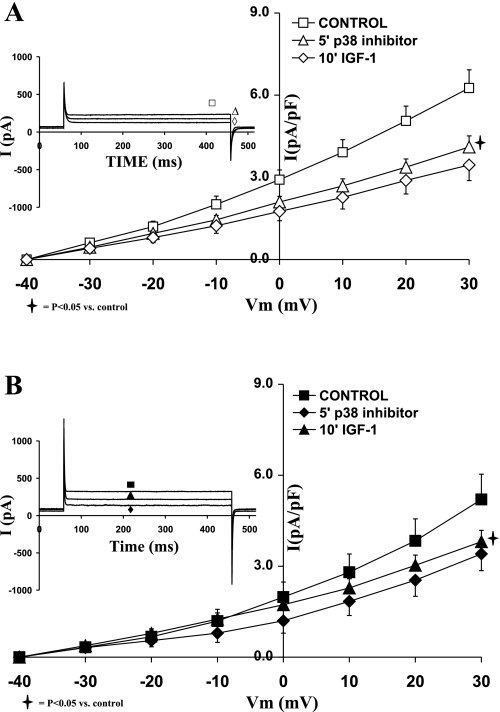
Role of p38 MAPK in the regulation of IK and IGF-I effects on sham and shunt ventricular myocytes.
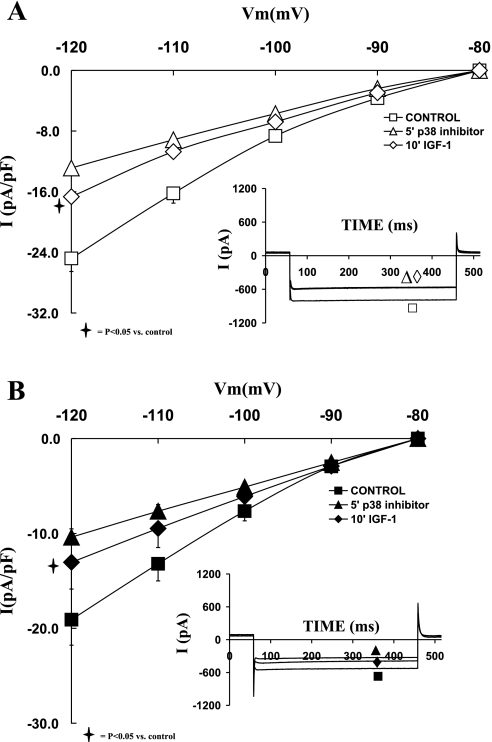
Using a p38 MAPK inhibitor (SB203580; 1 μM), we assessed the role of p38 MAPK in the modulation of IGF-I in relationship to IK1 and IK during eccentric cardiac hypertrophy. Figure 6 shows that inhibition of p38 MAPK reduced the current density of IK in both sham and hypertrophied cardiomyocytes to a similar extent. In that regard, SB203580 decreased IK in sham by 31.51 ± 8.88% (at +30 mV; P = 0.02) and in shunt by 33.96 ± 4.97% (at +30 mV; P = 0.05). Subsequent addition of IGF-I (10 nM) in the presence of the p38 inhibitor did not significantly reduce IK in sham or in the shunt. The effects of SB203580 on IK current density were not significantly different between sham and shunt cardiomyocytes.
Fig. 6.
Effects of the p38 MAPK inhibitor SB203580 (1 μM), as well as that of IGF-I (10−8 M) in the presence of SB203580, on the current-voltage relationship of IK in normal (A) and hypertrophied (B) adult cardiomyocytes. Data are average current density ± SE with n = 5. Inset in each graph shows respective representative IK responses at +20 mV.
Effects of MAPK and PI3K on IK1
Role of PI3K in the regulation of IK1 and IGF-I effects on sham and shunt ventricular myocytes.
The I-V curves in Fig. 7A show that LY294002 (1 μM) alone significantly reduced IK1 of sham cardiomyocytes by 45.08 ± 6.04% at −120 mV (P = 0.007). PI3K inhibition prevented IGF-I-dependent reduction of IK1. In the hypertrophied cells, IK1 was also significantly affected by 24.26 ± 9.33% with LY294002 (P = 0.01), and further addition of IGF-I did not affect the IK1 current density level (Fig. 7B). Thus PI3K inhibition induced a smaller reduction in the current density of IK1 in the hypertrophied cardiomyocytes vs. the normal ones (P < 0.05).
Fig. 7.
Effects of the PI3K inhibitor LY 294002 (1 μM), as well as that of IGF-I (10−8 M) in the presence of LY 294002, on the current-voltage relationship of IK1 in normal (A) and hypertrophied (B) adult cardiomyocytes. Data are average current density ± SE with n = 5. Inset in each graph shows respective representative IK1 responses at −120 mV.
Role of ERK1/2 in the regulation of IK1 and IGF-I effects on sham and shunt ventricular myocytes.
In parallel to IK, inhibition of ERK1/2 by 1 μM PD98059 significantly reduced IK1 current density in both groups as shown in Fig. 8, A and B. Thus PD98059 decreased IK1 current density at −120 mV by 45.25 ± 5.26% (P = 0.02) in sham and by 65.29 ± 9.78% (P < 0.05) in hypertrophied cardiomyocytes. Further addition of IGF-I (10 nM) in the presence of ERK1/2 inhibition did not affect IK1 current density in both groups. The inhibitory effect of PD98059 was significantly greater in the hypertrophied cardiomyocytes compared with sham (P < 0.05).
Fig. 8.
Effects of the MEK1/2 inhibitor PD98059 (1 μM), as well as that of IGF-I (10−8 M) in the presence of PD98059, on the current-voltage relationship of IK1 in normal (A) and hypertrophied (B) adult cardiomyocytes. Data are average current density ± SE with n = 5. Inset in each graph shows respective representative IK1 responses at −120 mV.
Role of p38 MAPK in the regulation of IK1 and IGF-I effects on sham and shunt ventricular myocytes.
Addition of SB203580 also reduced IK1 density in both groups of cardiomyocytes (Fig. 9). Thus IK1 density decreased by 48.36 ± 4.54% in sham (at −120 mV; P < 0.01) and 43.00 ± 5.59% in the hypertrophied cardiomyocytes (at −120 mV; P < 0.05). Thus the inhibitory effect of SB203580 was not significantly different in the hypertrophied myocytes compared with the normal ones. Furthermore, in the presence of SB203580, IGF-I (10−8 M) did not affect IK1 in the presence of p38 MAPK inhibitor in the sham and the hypertrophied cardiomyocytes.
Fig. 9.
Effects of the p38 MAPK inhibitor SB203580 (1 μM), as well as that of IGF-I (10−8 M) in the presence of SB203580, on the current-voltage relationship of IK1 in normal (A) and hypertrophied (B) adult cardiomyocytes. Data are average current density ± SE with n = 5. Inset in each graph shows respective representative IK1 responses at −120 mV.
DISCUSSION
The present work reveals an interesting relationship between the modulation of intracellular kinases by IGF-I and the activity of potassium channels during eccentric cardiac hypertrophy. The data showed that IGF-I has negative regulatory effects on IK1 and IK current densities. Furthermore, these IGF-I effects on both channels are mediated through MAPK and PI3K signaling pathways. Interestingly, this is among the first studies to demonstrate that the basal activation levels of IK and IK1 are regulated by MAPK and PI3K in the adult cardiomyocytes.
Acute Effects of IGF-I on Potassium Currents
Acute IGF-I administration to adult cardiomyocytes showed a significant decrease in the slope conductance of IK and IK1 in response to acute IGF-I. This may indicate a decrease in the opening probability or channel density. However, the effects of IGF-I were evident within 5 min, reaching steady state within 10–15 min. This favors the former interpretation rather than the latter, since it is too short of a time interval to have a decrease in the channel density. This may suggest weaker voltage dependence of potassium channels related to a decrease in their opening probability.
The effect of IGF-I on IK1 is relatively small in the physiological potential range, although most current-density voltage relationships show significance at a potential more negative to −100 mV. This may be related to the fact that the IGF-I effect is voltage independent, and therefore the same percent inhibition (∼15–20%) occurred at all voltages tested. This translated into a smaller effect at −90 mV relative to more polarized potentials showing significant inhibition. Much higher IGF-I concentrations, which are not physiologically relevant, may show inhibitory effects at physiologically relevant potentials. In that regard, the physiological concentration of free IGF-I in the plasma of adult rats was reported to be 30–35 ng/ml, which is equivalent to 4.6 nM (31). Nevertheless, as mentioned earlier, higher IGF-I concentration (10−7 M; i.e., 20 times physiological concentrations) did not yield consistent inhibitory effects.
Basal Regulation of Potassium Channels by MAPK and PI3K
The basal or steady-state integrated activities of MAPKs and PI3K seem to set the fundamental level for IK and IK1 in the absence of acute extrinsic stimulation of the cardiomyocytes. This is due to the fact that inhibition of basal PI3K and/or MAPK significantly reduced the current density of both potassium channels in the adult cardiomyocytes. In the normal cardiomyocytes, it seems that ERK1/2 have greater implications in the activation of basal IK compared with p38 MAPK or PI3K. Nevertheless, such differential influence is not evident for IK1, where all kinases showed a similar positive effect on its basal activity. It is interesting to note that blockade of the basal steady-state activity of MAPKs or PI3K produced partial inhibition of the channels (IK or IK1), favoring a model where basal constitutive (i.e., IGF-I-independent) MAPKs and PI3K may work independently to sustain the fundamental activity of the channel (2). These data are in agreement with recent studies (1, 2, 25, 58, 61) showing direct basal regulation of repolarizing potassium channel activities by MAPK. In that regard, cardiac hypertrophy was associated with MAPK-dependent deregulation of IK and Ito (58). Sun et al. (51) found that LY294002 increases Ca2+ release and myocyte contractility via direct inhibition of cardiac IK, causing action potential prolongation. In addition, a recent study (46) has shown regulation of Kv4.2 channels by ERK through direct phosphorylation of its cytoplasmic carboxy terminus (46).
Due to the changing levels of MAPK and PI3K during cardiac hypertrophy, the extent of basal regulation of the potassium channels by these kinases is likely to be altered. However, it is important to indicate that the kinase activity alone is necessary but not sufficient to determine the ultimate effect on the potassium channels (as discussed later). Nevertheless, the greater influence of ERK1/2 on the nonstimulated basal IK in the normal cardiomyocytes is lost during the development of compensated cardiac hypertrophy, where ERK1/2, p38 MAPK, and PI3K contribute to a similar extent to the regulation of basal IK current density. In comparison, ERK1/2 seems to acquire a greater role than PI3K in the maintenance of basal IK1 current density in the hypertrophied cardiomyocytes. These reduced roles of ERK1/2 and PI3K on IK and IK1, respectively, were concurrent with the decrease in the basal activities of both channels.
Interestingly, there is upregulation in the steady-state activities of Akt and MAPKs in the hypertrophied hearts compared with normal ones. This is in agreement with previous studies showing strong activation of Akt (10, 42), ERK1/2, and p38 MAPK during the development of cardiac hypertrophy (22, 23). We (19) have also recently shown that PI3K/Akt signaling activation participate in the development of cardiac hypertrophy in vivo. Nevertheless, one study (49) has shown a drastic increase in the p38 MAPK activity but not that of ERK1/2; however, their assessment was performed 2 days after the induction of cardiac hypertrophy. Thus their work would only reflect early changes in the signaling pathways involved in the development of cardiac hypertrophy. In contrast, the present study characterizes, for the first time, the long-term condition of the MAPKs and Akt in the compensated volume-overload-induced cardiac hypertrophy. Thus it seems that the transduction efficacy is a limiting factor and a better determinant of the basal channel activity than the level of kinase activation.
Acute IGF-I Regulation of Potassium Channels Through MAPKs and Akt in Normal Cardiomyocytes
IGF-I acute effects on IK and IK1 are shown to be largely mediated through the activation of ERK1/2, p38 MAPK, and PI3K in normal cardiomyocytes. Concurrently, the data demonstrated that inhibition of either MAPKs or PI3K completely blocked the acute effect of IGF-I on IK and IK1 in the normal hearts. This is consistent with the hypothesis that the signaling pathway activated by IGF-I may be transduced through cross-talks between PI3K and MAPKs in series to IK or IK1 channel. A similar mechanism has been proposed in an earlier study (2) on Tandom pore-domain K+ channel current (ITREK) in rat ventricular myocytes, where a dual-MAPK (ERK1/2 and p38 MAPK) activation was necessary for agonist-dependent stimulation of potassium channels.
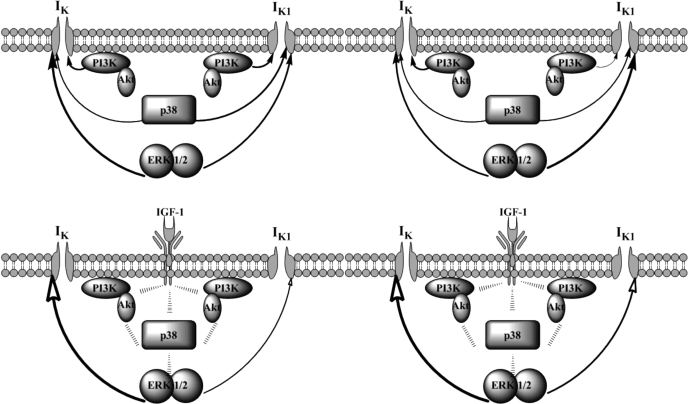
This cross-talk model in series between MAPKs and PI3K should converge on one of these kinases to relay the downstream inhibitory effect on the channel (Fig. 10). In a nonconvergence situation, the inhibition of a single kinase, for example, p38 MAPK, should not lead to the complete alleviation of the IGF-I effect on the channel, since the other kinases conveying IGF-I effect would not be blocked (7). However, the data show a complete impedance of the IGF-I effect on IK or IK1 when one of the related kinases is inhibited. In parallel, the acute IGF-I-induced effect on the inward rectifier potassium channel is also influenced by MAPKs and PI3K, which reinforces the same idea about the convergence of the cross-talk between PI3K and MAPKs to the channel. Analogous signaling convergence and cross-talk between the PI3K and MAPK were recently established for the regulation of sodium channels (54).
Fig. 10.
Diagram representing the steady-state basal (top) and an acute IGF-I-dependent (bottom) regulation of IK and IK1 by MAPKs and PI3K in sham (left) and hypertrophied (right) cardiomyocytes. The thickness of the arrow stem reflects the relative preponderance of the signaling pathway on the channel. Filled arrowheads denote positive effects, whereas empty arrowheads denote negative effects.
Acute IGF-I Regulation of Potassium Channels Through MAPKs and Akt in Cardiomyocytes from Volume-Overloaded Hypertrophied Hearts
Eccentric cardiac hypertrophy brings a changing paradigm in its progression during the compensated phase, where IGF-I levels are significantly higher locally and in the plasma (40). Such chronic exposure of the cardiomyocytes in vivo to elevated amounts of IGF-I increases the level of basal activation of MAPKs and PI3K. This chronic elevation seems to limit further kinase activation by acute IGF-I, except for Akt. This acute IGF-I-dependent elevated activation of Akt was translated into a greater modulatory role on IK channels. Therefore, it is suggested that during eccentric hypertrophy Akt may mediate to a larger extent the acute effect of IGF-I on IK. In parallel, during eccentric cardiac hypertrophy, IGF-I negative regulation on IK1 engages PI3K cross-talk with MAPKs.
Basal Steady-State vs. Acute Stimulation of Potassium Channels
Basal steady-state levels of MAPKs and PI3K show a positive modulatory role on IK and IK1, whereas acute IGF-I exposure induces negative effects on the potassium channels (Fig. 10). This significant dichotomic relationship of the MAPK and PI3K effect on potassium channels is relevant to their hormonally induced stimulation being independent from their constitutive and basal activity. This may insure a reliable hormonal response not affected by changing basal activity levels, which could introduce confounding elements to dilute the signal transduction process. Such dichotomic behavior could be due to different levels of cross-talks that exist between intracellular kinases. A comparable dual effect was recently demonstrated for Atg5-deficient hearts developing cardiac hypertrophy only when extrinsically challenged by pressure or stress (41). The authors concluded that such basal constitutive signaling activity in the heart serves to maintain baseline homeostatic mechanisms, and its up-regulation (by acute extrinsic factors) may activate other adaptive responses for alleviating hemodynamic stress on the cardiomyocyte. The hypertrophic regulatory effects of nuclear factor of activated T cells in cardiac myocytes were also shown to exhibit dichotomic activities dependent on its interaction with p38 (22) and ERK1/2 (44). Furthermore, it was neatly demonstrated in rat ventricular myocytes that an upstream stimulator of p38 MAPK can induce opposing downstream cellular effects depending on the expression profile of the MAPK isoforms and on the isoforms that are dominantly activated (45, 52). This corroborated with recent findings (43, 55) demonstrating contrasting net cellular biological outcome from the same MAPK activation in different cellular kinase contents. In addition, downstream terminal effects of MAPKs are highly dependent on the phosphorylation efficiency of the specific docking domain, which exhibit differential specificity for each MAPK (4). It was concluded that MAPK targeting of a suboptimal domain promotes temporary interactions that are relevant for transient acute effects, whereas phosphorylation of its optimal domain is required for steady-state responses (3, 4).
Concluding Remarks and Clinical Relevance
In conclusion, this work deepens our understanding of the intricate interplay between signaling pathways in the development of eccentric cardiac hypertrophy, namely MAPK and PI3K and their effects on potassium channels. In addition to their trophic effects, this work demonstrates a new dimension in their role towards the regulation of the constitutively basal vs. acutely stimulated potassium channels activities, IK and IK1, in a dichotomic and convergent manner. Thus eccentric cardiac hypertrophy may be associated with a paradigm shift in the regulation of the steady-state basal activities of potassium channels towards MAPKs, while that of the acute IGF-I-stimulated ones towards Akt. This may have an important clinical relevance in the described acute IGF-I-dependent membrane depolarization-mediated cellular survival, through PI3K/Akt downstream activation of transcription factor-myocyte enhancer factor-2 (59). Concurrently, the maintenance of the resting potential is closely tied to the kinase-dependent regulation of the basal potassium channel activities. Most events occurring during the compensated phase of cardiac hypertrophy progressively accentuate leading to the uncompensated phase and failure. Eventual imbalances in kinase regulation of IK would prolong the action potential duration, which may induce arrhythmias, while that of IK1 would directly induce resting potential depolarization and therefore steady-state inactivation of sodium and calcium channels, which would negatively affect cardiac function in terms of arrhythmias, conduction blocks, force and velocity of contraction, as well as ejection fraction, known landmarks of heart failure.
GRANTS
This work was supported in part by National Institute of General Medical Sciences Grant GM-08016-38, National Center for Research Resources Grant 2G12 RR-003048 Research Centers in Minority Institutions, Division of Research Infrastructure, and Specialized Neuroscience Research Program to G. E. Haddad.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem 75: 2277–2287, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Aimond F, Rauzier JM, Bony C, Vassort G. Simultaneous activation of p38 MAPK and p42/44 MAPK by ATP stimulates the K+ current ITREK in cardiomyocytes. J Biol Chem 275: 39110–39116, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Barsyte-Lovejoy D, Galanis A, Clancy A, Sharrocks AD. ERK5 is targeted to myocyte enhancer factor 2A (MEF2A) through a MAPK docking motif. Biochem J 381: 693–699, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barsyte-Lovejoy D, Galanis A, Sharrocks AD. Specificity determinants in MAPK signaling to transcription factors. J Biol Chem 277: 9896–9903, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Brooksby P, Levi AJ, Jones JV. The electrophysiological characteristics of hypertrophied ventricular myocytes from the spontaneously hypertensive rat. J Hypertens 11: 611–622, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Cerbai E, Barbieri M, Li Q, Mugelli A. Ionic basis of action potential prolongation of hypertrophied cardiac myocytes isolated from hypertensive rats of different ages. Cardiovasc Res 28: 1180–1187, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Cobb MH, Ross EM. Principles of cell signaling. In: Cells, edited by Lewin B. Sudbury, MA: Jones and Bartlett publishers, 2007, p. 589–644.
- 8.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation 113: 2097–2104, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Donohue TJ, Dworkin LD, Lango MN, Fliegner K, Lango RP, Benstein JA, Slater WR, Catanese VM. Induction of myocardial insulin-like growth factor-I gene expression in left ventricular hypertrophy. Circulation 89: 799–809, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Dorn GW, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest 115: 527–537, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eghbali M, Deva R, Alioua A, Minosyan TY, Ruan H, Wang Y, Toro L, Stefani E. Molecular and functional signature of heart hypertrophy during pregnancy. Circ Res 96: 1208–1216, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Foncea R, Andersson M, Ketterman A, Blakesley V, Sapag-Hagar M, Sugden PH, LeRoith D, Lavandero S. Insulin-like growth factor-I rapidly activates multiple signal transduction pathways in cultured rat cardiac myocytes. J Biol Chem 272: 19115–19124, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa T, Bassett AL, Furukawa N, Kimura S, Myerburg RJ. The ionic mechanism of reperfusion-induced early afterdepolarizations in feline left ventricular hypertrophy. J Clin Invest 91: 1521–1531, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furukawa T, Myerburg RJ, Furukawa N, Kimura S, Bassett AL. Metabolic inhibition of ICa,L and IK differs in feline left ventricular hypertrophy. Am J Physiol Heart Circ Physiol 266: H1121–H1131, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Gillis AM, Mathison HJ, Kulisz E, Lester WM. Dispersion of ventricular repolarization in left ventricular hypertrophy: influence of afterload and dofetilide. J Cardiovasc Electrophysiol 9: 988–997, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Gomez AM, Benitah JP, Henzel D, Vinet A, Lorente P, Delgado C. Modulation of electrical heterogeneity by compensated hypertrophy in rat left ventricle. Am J Physiol Heart Circ Physiol 272: H1078–H1086, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 56: 56–64, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo W, Kada K, Kamiya K, Toyama J. IGF-I regulates K+-channel expression of cultured neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol 272: H2599–H2606, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Ha T, Li Y, Gao X, McMullen JR, Shioi T, Izumo S, Kelley JL, Zhao A, Haddad GE, Williams DL, Browder IW, Kao RL, Li C. Attenuation of cardiac hypertrophy by inhibiting both mTOR and NFkappaB activation in vivo. Free Radic Biol Med 39: 1570–1580, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Haddad GE, Blackwell K, Bikhazi A. Regulation of insulin-like growth factor-1 by the renin-angiotensin system during regression of cardiac eccentric hypertrophy through angiotensin-converting enzyme inhibitor and AT1 antagonist. Can J Physiol Pharmacol 81: 142–149, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Hanson MC, Fath KA, Alexander RW, Delafontaine P. Induction of cardiac insulin-like growth factor I gene expression in pressure overload hypertrophy. Am J Med Sci 306: 69–74, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Haq S, Choukroun G, Lim H, Tymitz KM, del MF, Gwathmey J, Grazette L, Michael A, Hajjar R, Force T, Molkentin JD. Differential activation of signal transduction pathways in human hearts with hypertrophy vs. advanced heart failure. Circulation 103: 670–677, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7: 589–600, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Hille B Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates, 2001.
- 25.Hu HJ, Glauner KS, Gereau RW. ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. I. Modulation of A-type K+ currents. J Neurophysiol 90: 1671–1679, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Huang CY, Hao LY, Buetow DE. Insulin-like growth factor-II induces hypertrophy of adult cardiomyocytes via two alternative pathways. Cell Biol Int 26: 737–739, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Isgaard J, Wahlander H, Adams MA, Friberg P. Increased expression of growth hormone receptor mRNA and insulin-like growth factor-I mRNA in volume-overloaded hearts. Hypertension 23: 884–888, 1994. [DOI] [PubMed] [Google Scholar]
- 28.Ito H, Hiroe M, Hirata Y, Tsujino M, Adachi S, Shichiri M, Koike A, Nogami A, Marumo F. Insulin-like growth factor-I induces hypertrophy with enhanced expression of muscle specific genes in cultured rat cardiomyocytes. Circulation 87: 1715–1721, 1993. [DOI] [PubMed] [Google Scholar]
- 29.Kleiman RB, Houser SR. Outward currents in normal and hypertrophied feline ventricular myocytes. Am J Physiol Heart Circ Physiol 256: H1450–H1461, 1989. [DOI] [PubMed] [Google Scholar]
- 30.Kleiman RB, Houser SR. Outward currents in hypertrophied feline ventricular myocytes. Prog Clin Biol Res 334: 65–83, 1990. [PubMed] [Google Scholar]
- 31.Lang CH, Vary TC, Frost RA. Acute in vivo elevation of insulin-like growth factor (IGF) binding protein-1 decreases plasma free IGF-I and muscle protein synthesis. Endocrinology 144: 3922–3933, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Lavandero S, Foncea R, Perez V, Sapag-Hagar M. Effect of inhibitors of signal transduction on IGF-I-induced protein synthesis associated with hypertrophy in cultured neonatal rat ventricular myocytes. FEBS Lett 422: 193–196, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Lebeche D, Kaprielian R, del MF, Tomaselli G, Gwathmey JK, Schwartz A, Hajjar RJ. In vivo cardiac gene transfer of Kv4.3 abrogates the hypertrophic response in rats after aortic stenosis. Circulation 110: 3435–3443, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Lebeche D, Kaprielian R, Hajjar R. Modulation of action potential duration on myocyte hypertrophic pathways. J Mol Cell Cardiol 40: 725–735, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem 277: 22896–22901, 2002. [DOI] [PubMed] [Google Scholar]
- 36.McIntosh MA, Cobbe SM, Kane KA, Rankin AC. Action potential prolongation and potassium currents in left-ventricular myocytes isolated from hypertrophied rabbit hearts. J Mol Cell Cardiol 30: 43–53, 1998. [DOI] [PubMed] [Google Scholar]
- 37.McIntosh MA, Hicks MN, Kane KA, Rankin AC, Cobbe SM. A characterized model of left ventricular hypertrophy in the rabbit. Cardioscience 5: 95–100, 1994. [PubMed] [Google Scholar]
- 39.McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, Schinke M, Kong S, Sherwood MC, Brown J, Riggi L, Kang PM, Izumo S. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110alpha) pathway. J Biol Chem 279: 4782–4793, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Modesti PA, Vanni S, Bertolozzi I, Cecioni I, Polidori G, Paniccia R, Bandinelli B, Perna A, Liguori P, Boddi M, Galanti G, Serneri GG. Early sequence of cardiac adaptations and growth factor formation in pressure- and volume-overload hypertrophy. Am J Physiol Heart Circ Physiol 279: H976–H985, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 13: 619–624, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol 37: 449–471, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Pramanik R, Qi X, Borowicz S, Choubey D, Schultz RM, Han J, Chen G. p38 isoforms have opposite effects on AP-1-dependent transcription through regulation of c-Jun. The determinant roles of the isoforms in the p38 MAPK signal specificity. J Biol Chem 278: 4831–4839, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Sanna B, Bueno OF, Dai YS, Wilkins BJ, Molkentin JD. Direct and indirect interactions between calcineurin-NFAT and MEK1-extracellular signal-regulated kinase 1/2 signaling pathways regulate cardiac gene expression and cellular growth. Mol Cell Biol 25: 865–878, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saurin AT, Martin JL, Heads RJ, Foley C, Mockridge JW, Wright MJ, Wang Y, Marber MS. The role of differential activation of p38-mitogen-activated protein kinase in preconditioned ventricular myocytes. FASEB J 14: 2237–2246, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Schrader LA, Birnbaum SG, Nadin BM, Ren Y, Bui D, Anderson AE, Sweatt JD. ERK/MAPK regulates the Kv4.2 potassium channel by direct phosphorylation of the pore-forming subunit. Am J Physiol Cell Physiol 290: C852–C861, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol 22: 2799–2809, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shyu KG, Ko WH, Yang WS, Wang BW, Kuan P. Insulin-like growth factor-1 mediates stretch-induced upregulation of myostatin expression in neonatal rat cardiomyocytes. Cardiovasc Res 68: 405–414, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Sopontammarak S, Aliharoob A, Ocampo C, Arcilla RA, Gupta MP, Gupta M. Mitogen-activated protein kinases (p38 and c-Jun NH2-terminal kinase) are differentially regulated during cardiac volume and pressure overload hypertrophy. Cell Biochem Biophys 43: 61–76, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Stengl M, Ramakers C, Donker DW, Nabar A, Rybin AV, Spatjens RL, van der NT, Wodzig WK, Sipido KR, Antoons G, Moorman AF, Vos MA, Volders PG. Temporal patterns of electrical remodeling in canine ventricular hypertrophy: focus on IKs downregulation and blunted beta-adrenergic activation. Cardiovasc Res 72: 90–100, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Sun H, Oudit GY, Ramirez RJ, Costantini D, Backx PH. The phosphoinositide 3-kinase inhibitor LY294002 enhances cardiac myocyte contractility via a direct inhibition of Ik,slow currents. Cardiovasc Res 62: 509–520, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Tanno M, Bassi R, Gorog DA, Saurin AT, Jiang J, Heads RJ, Martin JL, Davis RJ, Flavell RA, Marber MS. Diverse mechanisms of myocardial p38 mitogen-activated protein kinase activation: evidence for MKK-independent activation by a TAB1-associated mechanism contributing to injury during myocardial ischemia. Circ Res 93: 254–261, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Tomita F, Bassett AL, Myerburg RJ, Kimura S. Diminished transient outward currents in rat hypertrophied ventricular myocytes. Circ Res 75: 296–303, 1994. [DOI] [PubMed] [Google Scholar]
- 54.Tong Q, Booth RE, Worrell RT, Stockand JD. Regulation of Na+ transport by aldosterone: signaling convergence and cross talk between the PI3-K and MAPK1/2 cascades. Am J Physiol Renal Physiol 286: F1232–F1238, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Tourian L, Zhao H, Srikant CB. p38alpha, but not p38beta, inhibits the phosphorylation and presence of c-FLIPS in DISC to potentiate Fas-mediated caspase-8 activation and type I apoptotic signaling. J Cell Sci 117: 6459–6471, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Tsuji Y, Opthof T, Yasui K, Inden Y, Takemura H, Niwa N, Lu Z, Lee JK, Honjo H, Kamiya K, Kodama I. Ionic mechanisms of acquired QT prolongation and torsades de pointes in rabbits with chronic complete atrioventricular block. Circulation 106: 2012–2018, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Volk T, Nguyen TH, Schultz JH, Faulhaber J, Ehmke H. Regional alterations of repolarizing K+ currents among the left ventricular free wall of rats with ascending aortic stenosis. J Physiol 530: 443–455, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walsh KB, Sweet JK, Parks GE, Long KJ. Modulation of outward potassium currents in aligned cultures of neonatal rat ventricular myocytes during phorbol ester-induced hypertrophy. J Mol Cell Cardiol 33: 1233–1247, 2001. [DOI] [PubMed] [Google Scholar]
- 59.Wiedmann M, Wang X, Tang X, Han M, Li M, Mao Z. PI3K/Akt-dependent regulation of the transcription factor myocyte enhancer factor-2 in insulin-like growth factor-1- and membrane depolarization-mediated survival of cerebellar granule neurons. J Neurosci Res 81: 226–234, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Wiedmann M, Wang X, Tang X, Han M, Li M, Mao Z. PI3K/Akt-dependent regulation of the transcription factor myocyte enhancer factor-2 in insulin-like growth factor-1- and membrane depolarization-mediated survival of cerebellar granule neurons. J Neurosci Res 81: 226–234, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci 22: 4860–4868, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]