Abstract
Hypopituitary Ames dwarf mice have low circulating growth hormone (GH)/IGF-I levels, and they have extended longevity and exhibit many symptoms of delayed aging. To elucidate the vascular consequences of Ames dwarfism we compared endothelial O2•− and H2O2 production, mitochondrial reactive oxygen species (ROS) generation, expression of antioxidant enzymes, and nitric oxide (NO) production in aortas of Ames dwarf and wild-type control mice. In Ames dwarf aortas endothelial O2•− and H2O2 production and ROS generation by mitochondria were enhanced compared with those in vessels of wild-type mice. In Ames dwarf aortas there was a less abundant expression of Mn-SOD, Cu,Zn-SOD, glutathione peroxidase (GPx)-1, and endothelial nitric oxide synthase (eNOS). NO production and acetylcholine-induced relaxation were also decreased in aortas of Ames dwarf mice. In cultured wild-type mouse aortas and in human coronary arterial endothelial cells treatment with GH and IGF significantly reduced cellular O2•− and H2O2 production and ROS generation by mitochondria and upregulated expression of Mn-SOD, Cu,Zn-SOD, GPx-1, and eNOS. Thus GH and IGF-I promote antioxidant phenotypic changes in the endothelial cells, whereas Ames dwarfism leads to vascular oxidative stress.
Keywords: senescence, vascular disease, atherosclerosis, Prop1df/df mice
it is well documented that plasma growth hormone (GH) levels decline with age in humans and in experimental animals, and there are a number of studies linking GH deficiency to age-related pathological conditions, such as cognitive decline, sarcopenia, and frailty [recently reviewed elsewhere (12)].
However, during the last decade important new findings created a controversy regarding the role of GH/IGF pathway in the aging process. Studies in Caenorhabditis elegans provided the first evidence that reduced insulin-like signaling may actually promote longevity in lower organisms by altering oxidative stress resistance and metabolism (reviewed in Ref. 48). The first evidence to support a role of insulin-like signals in regulation of mammalian longevity came from the observation that mice with hereditary dwarfism (Ames dwarf) exhibit a significant extension of life span (>40%) (7). Ames dwarf mice are deficient in GH, prolactin (PRL), and thyroid-stimulating hormone (TSH) because of a mutation in Prop-1, a factor required for differentiation of the pituitary gland during development (45). Since this original observation it has been documented that phenotypically identical Snell dwarf mice (24) and GH receptor/binding protein gene knockout (GHR-KO) mice (1, 25) also live significantly longer than normal, wild-type mice. All of these GH-impaired mutant mice have very low circulating IGF-I levels. A central role for defective IGF signaling in the longevity phenotype is suggested by the finding that female Igf1r+/− mice also live significantly longer than wild-type counterparts. Also, high plasma GH levels in GH-overexpressing mice are associated with a life span one-half that of wild-type control mice (46).
The major cause of death in elderly humans is cardiovascular disease, yet no studies have investigated the cardiovascular consequences of defective GH/IGF signaling in these long-lived animal models. Thus the present study was designed to characterize the effects of Ames dwarfism on endothelial function, nitric oxide (NO) mediation, vascular O2•− and H2O2 production, and expression of antioxidant enzymes in the long-living Ames dwarf mice and wild-type littermates. In addition, we also tested the effects of GH and IGF on endothelial reactive oxygen species (ROS) homeostasis in cultured aortic segments and human coronary arterial endothelial cells (HCAECs).
METHODS
Animals and vessel isolation.
All animal use protocols were approved by the Institutional Animal Care and Use Committee of New York Medical College. The Ames dwarf mice used in these studies were derived from a closed colony (over 20 years) on a heterogeneous background (7), and their endocrine and metabolic status have been characterized previously (3, 7). Ames dwarf (Prop1df/df) mice were produced by mating dwarf (df/df) or carrier (df/+) males to carrier (df/+) females. For each experiment male Ames dwarf mice and their wild-type littermates were used, which approach ensured that the animals were age matched (age: ∼5 mo) and differed only in their Prop1 allele. The male GH transgenic (GH-tg) and wild-type littermates of the same age used in this study were derived from a single male founder [strain (C57BL/6 × SJL)F1] produced by microinjection of the phosphoenolpyruvate carboxykinase (PEPCK) promoter region (300 bp)/bovine GH hybrid gene into the male pronucleus of single-cell embryos (32). The characteristics of each mouse line are given in Table 1.
Table 1.
Body mass and maximum reported life span of mouse lines used in this study
| Common Name | Mean Body Mass, g | MLSP, yr |
|---|---|---|
| Ames dwarf (Prop1df/df) | 11±1 | ∼4.1 |
| Wild type (Prop1+/+) | 23±2 | ∼2.9 |
| GH-tg | 44±3 | ∼2 |
| Wild type (for GH-tg) | 22±2 | ∼3.5 |
Data are means ± SE. MLSP, published maximum life span potential (7); GH, growth hormone; GH-tg, GH transgenic.
All animals were disease free, with no signs of systemic inflammation and/or neoplastic alterations. Animals were killed by CO2. The carotid arteries and the aorta of each animal were carefully exposed and isolated from the surrounding tissues. The vessels were cleaned from the adventitia with an operating microscope and microsurgery instruments. By light microscopic examination we did not detect any gross anatomic abnormalities in the vessels (e.g., atherosclerotic plaques).
Measurement of vascular O2•− production.
Production of O2•− in segments of en face preparations of the aortas was determined. Hydroethidine, an oxidative fluorescent dye, was used to localize superoxide production in situ as we previously reported (16, 18). In brief, vessels were incubated with hydroethidine (3 × 10−6 mol/l at 37°C for 30 min). Then the arteries were washed three times, and optical sections of the endothelial layer were obtained with an inverted confocal laser scanning microscope (Zeiss Pascal LSM 5) at ×40 magnification, with the same settings. Images were analyzed with Zeiss Axionvision imaging software (Carl Zeiss, Gottingen, Germany). Unstained aortas and vessels preincubated with PEG-SOD were used for background correction and negative control, respectively. For quantitative measurements the time course of the buildup of ethidium fluorescence in en face preparations of aortas and in isolated papillary muscle preparations was recorded for 30 min (16). The slope factor was calculated and normalized to Hoechst 33258 fluorescence representing DNA content, which is proportional to the cell number.
Measurement of vascular H2O2 production.
The cell-permeant oxidative fluorescent indicator dye 5(6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate-acetyl ester (C-H2DCFDA or DCF, Invitrogen, Carlsbad, CA) was used to assess H2O2 production in isolated aortas as we reported previously (15, 27). Unstained aortas and vessels preincubated with PEG-catalase were used for background correction and negative control, respectively. In separate experiments the time course of the buildup of C-H2DCFDA fluorescence in en face preparations of the aortas was recorded for 30 min. The slope factor was calculated and normalized to Hoechst 33258 fluorescence representing DNA content (number of cells).
In separate experiments H2O2 production was measured fluorometrically in aortic segments with the Amplex red/horseradish peroxidase (HRP) assay as reported previously (16). H2O2 generation rate was compared by measuring the time course of the buildup of resorufin fluorescence for 60 min.
Measurement of GH- and IGF-I-induced changes in O2•− and H2O2 production in cultured endothelial cells and cardiac myocytes.
HCAECs (Cell Applications, San Diego, CA; after passage 4; age of donors is unknown) and isolated neonatal rat cardiomyocytes [kindly provided by Dr. John G. Edwards (Dept. of Physiology, New York Medical College, Valhalla, NY) (20)] were cultured in 96-well plates as described previously (17). The control medium contained 5% heat-inactivated serum; the cells were cultured in a 21% O2 environment. The cells were treated with GH (Sigma) or recombinant IGF-I (Sigma) for 24 h. Cellular O2•− and H2O2 production were assessed with the dihydroethidine and C-H2DCFDA fluorescence methods, respectively, and a Tecan Infinite M200 plate reader. Hoechst 33258 fluorescence representing DNA content (number of cells) was used for normalization. In other experiments, HCAECs were incubated with C-H2DCFDA and dihydroethidine and buildup of fluorescence was assessed by fluorescent microscopy.
Measurement of mitochondrial ROS production in blood vessels.
MitoSox (Invitrogen), a mitochondrion-specific hydroethidine-derivative fluorescent dye (34), was used to assess mitochondrial O2•− production in situ as reported previously (16, 54). In brief, aortic segments were incubated with MitoSox (5 × 10−6 mol/l at 37°C for 10 min). Hoechst 33258 was used for nuclear staining. The vessels were then washed three times, and confocal images were obtained. Average perinuclear MitoSox fluorescence intensities were assessed. In further experiments, aortic segments (en face) and isolated cardiac papillary muscles were loaded with MitoSox and fluorescent intensities were measured with a Tecan Infinite M200 plate reader. Hoechst 33258 fluorescence representing DNA content (number of cells) was used for normalization.
Measurement of H2O2 production by isolated mitochondria.
Mitochondria were isolated from the hearts by differential centrifugation as described previously (16). Mitochondrial H2O2 generation was determined with previously published methods (16). In brief, mitochondria (final concentration 0.20 mg/ml mitochondrial protein) were incubated with Amplex red (50 μmol/l), HRP (6 U/ml), and SOD (30 U/ml). Succinate (final concentration 5 mmol/l) or glutamate plus malate (5 mmol/l) was added, and changes in fluorescence intensities were followed with an Infinite M200 plate reader. Appropriate corrections for background signals were applied, and standard curves generated with known amounts of H2O2 were used to calculate the rate of H2O2 production.
Measurement of GH- and IGF-I-induced changes in mitochondrial ROS production in HCAECs and cardiac myocytes.
Mitochondrial O2•− production was assessed in GH- and IGF-I-treated HCAECs by flow cytometry (FAScalibur, BD Bioscience, San Jose, CA) with MitoSox Red as previously reported (33, 34). Cell debris (low forward and side scatter), dead cells (Sytox Green and annexin V positive), and apoptotic cells (annexin V positive) were gated out for analysis (33, 34). The data are presented by histogram of mean intensity of MitoSox fluorescence or fold change compared with the untreated control. In separate experiments, HCAECs and cultured neonatal rat cardiomyocytes (20) were grown in 96-well plates and treated with GH and IGF-I (for 24 h). MitoSox fluorescence was assessed with a Tecan Infinite M200 plate reader. Hoechst 33258 fluorescence representing DNA content (number of cells) was used for normalization.
Western blotting.
To compare vascular expression of major antioxidant enzymes in wild-type and Ames dwarf mice Western blotting was performed as described previously (16) with primary antibodies directed against Mn-SOD (no. SOD-110, Stressgen, Ann Arbor, MMI), Cu,Zn-SOD (no. SOD-100, Stressgen), glutathione peroxidase (GPx)-1 (no. 16798, Abcam), catalase (no. 16731, Abcam, Cambridge, MA), gp91phox (no. 07-024, Upstate Biotechnology), Nox-1 (no. sc-25545, Santa Cruz), and endothelial nitric oxide synthase (eNOS) (no. 610298, Transduction Laboratories).
To test the direct effect of GH/IGF-I signaling on vascular antioxidant systems, aortic segments of wild-type mice [maintained in organoid culture as reported previously (17)] or HCAECs were treated with GH or IGF-I. Protein expression of Mn-SOD, Cu,Zn-SOD, GPx-1, and eNOS were assessed. Anti-β-actin (no. 6276, Abcam) was used for normalization purposes.
In separate experiments, aortic segments isolated from wild-type mice were cultured in the presence of serum from Ames dwarf mice or wild-type control mice for 24 h and GPx-1 expression was assessed.
Quantitative real-time PCR.
To complement the protein expression data, mRNA expression of eNOS and GPx-1 was also analyzed in aortic segments of Ames dwarf and wild-type mice as reported previously (17, 18). In brief, total RNA from aortic segments was isolated with the Mini RNA Isolation Kit (Zymo Research, Orange, CA) and was reverse transcribed with Superscript III RT (Invitrogen) as described previously (17, 18). Real-time RT-PCR was used to analyze mRNA expression with the Strategen MX3000, as reported previously (17). Efficiency of the PCR reaction was determined by using dilution series of a standard vascular sample. Quantification was performed with the ΔΔCT method (where CT is threshold cycle). The housekeeping gene HPRT was used for internal normalization. Oligonucleotides used for real-time quantitative RT-PCR (QRT-PCR) are listed in Table 2. Fidelity of the PCR reaction was determined by melting temperature analysis and visualization of product on a 2% agarose gel.
Table 2.
Oligonucleotides for real-time RT-PCR
| mRNA Targets | Sense | Antisense |
|---|---|---|
| Mouse eNOS | ACTGCTAGAGGTGCTGGAG | GCTGGGTGCTGAACTGAC |
| Mouse GPx-1 | TCTGGGACCTCGTGGACTG | CACTTCGCACTTCTCAAACAATG |
| Mouse HPRT | TGCTGCGTCCCCAGACTTTTG | AGATAAGCGACAATCTACCAGAGG |
| Mouse β-actin | AATAAGTGGTTACAGGAAGTC | ATGAAGTATTAAGGCGGAAG |
eNOS, endothelial nitric oxide synthase; GPx, glutathione peroxidase.
Measurement of endothelial NO production.
Basal NO levels in unstimulated vessels from Ames dwarf mice and respective wild-type controls were assessed by using 4,5-diaminofluorescein diacetate (DAF-2DA) as described previously (16). DAF-2DA is nonfluorescent but reacts with NO to form the highly fluorescent compound triazolofluorescein (DAF-2T). En face aortic preparations were loaded with 5 μM DAF-DA for 30 min at 37°C in HEPES buffer (pH 7.4) and washed three times, and fluorescent images of the endothelial layer were obtained. The DAF-2T fluorescence intensities were compared (16).
To assess stimulated NO release, endothelial NO-mediated vasorelaxation was assessed as previously described (14, 16). In brief, carotid arteries of each animal were cut into ring segments 2 mm in length and mounted on 40-μm stainless steel wires in the myograph chambers (Danish Myo Technology) for measurement of isometric tension. The vessels were superfused with Krebs buffer solution (mM: 118 NaCl, 4.7 KCl, 1.5 CaCl2, 25 NaHCO3, 1.1 MgSO4, 1.2 KH2PO4, and 5.6 glucose at 37°C; gassed with 95% air-5% CO2). After an equilibration period of 1 h, during which an optimal passive tension was applied to the rings (as determined from the vascular length-tension relationship), relaxations of precontracted (by 10−6 mol/l phenylephrine) vessels to acetylcholine (ACh; from 10−9 to 10−5 mol/l) and the NO donor S-nitroso-N-acetyl-penicillamine (SNAP, from 10−9 to 10−5 mol/l) were obtained. The effects of the NOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME, 3 × 10−4 mol/l) on ACh-induced responses of the vessels were also tested. In other experiments vascular responses of GH-tg and respective wild-type control animals were also assessed.
Data analysis.
Data were normalized to the respective control mean values and are expressed as means ± SD or SE. Statistical analyses of data were performed by Student's t-test or by two-way ANOVA followed by the Tukey post hoc test, as appropriate. P < 0.05 was considered statistically significant.
RESULTS
Increased vascular O2•− and H2O2 production in Ames dwarf mice.
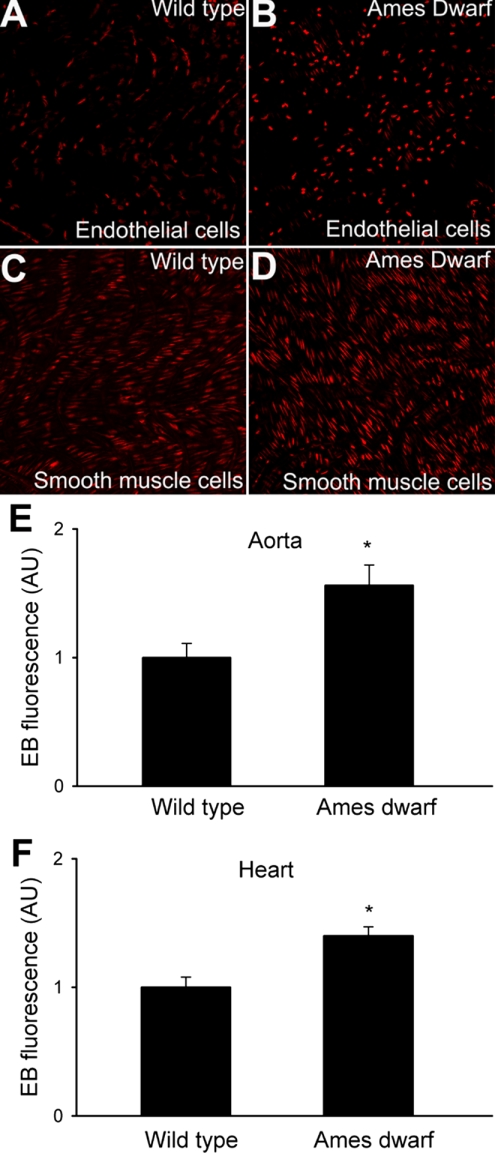
Microscopic analysis of nuclear ethidium staining showed that endothelial and smooth muscle cells in aortas of Ames dwarf mice produced more O2•− than those in vessels of wild-type mice (Fig. 1, A–D). Using a plate reader-based quantitative assay, we found significantly greater buildup of ethidium fluorescence in both vascular and cardiac tissue from Ames dwarf mice (Fig. 1, E and F) than in those of wild-type control animals.
Fig. 1.
Representative confocal images showing ethidium bromide staining (EB, red fluorescence) in endothelial (A and B) and smooth muscle cells (C and D) of aortas from Ames dwarf mice (B and D) and wild-type controls after dihydroethidine (DHE) treatment (A and C; original magnification: ×40). E and F: results from DHE staining experiments. Time course of buildup of EB fluorescence in aortic segments (E) and cardiac muscle preparations (F) of the mice was obtained. Bar graphs are summary data for slope factors normalized to cell number (i.e., nuclear DNA content, assessed by Hoechst fluorescence intensity), representing tissue O2•− production. Data are means ± SE (n = 5 animals in each group). *P < 0.05. Arbitrary units (AU): ΔEB fluorescence/dt/DNA content.
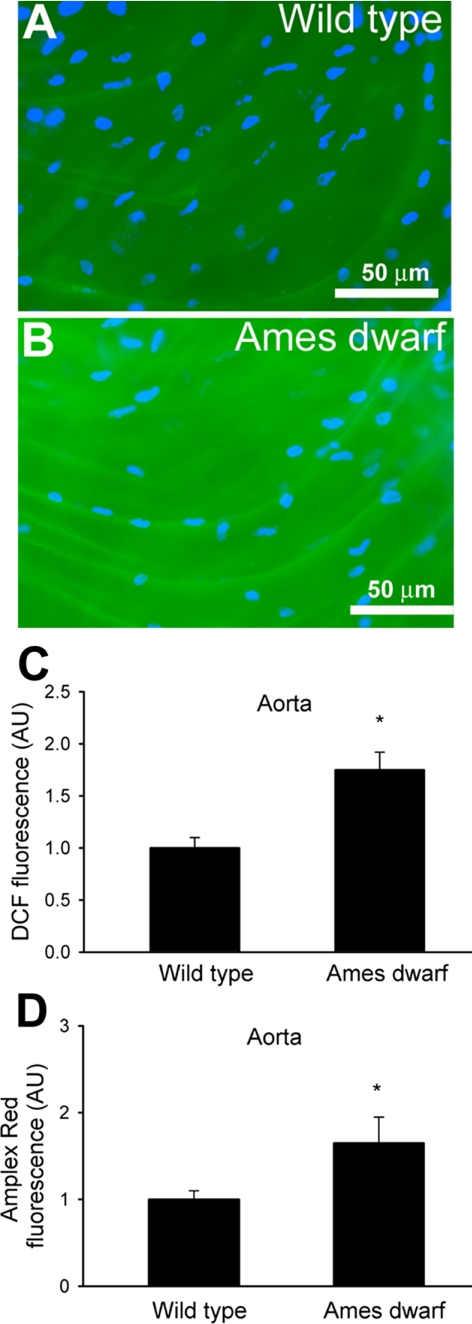
Vascular H2O2 generation was first measured by the dichlorofluorescein (DCF) fluorescence method. We found that endothelial cells in vessels of Ames dwarf mice produced more H2O2 than vessels of wild-type mice (Fig. 2, A–C). Similar results were obtained with the Amplex red/HRP assay (Fig. 2D). The DCF and Amplex red/HRP signals were abolished by pretreatment with PEG-catalase and substantially increased by administration of H2O2, confirming the specificity of the assays for H2O2.
Fig. 2.
A and B: representative fluorescent images showing H2O2 production (measured by the C-H2DCFDA fluorescence method) in endothelial cells of en face preparations of aortas of wild-type (A) and Ames dwarf (B) mice. Green fluorescence, dichlorofluorescein (DCF); blue fluorescence, Hoechst-stained endothelial nuclei (original magnification ×10). C: time course of buildup of DCF fluorescence in aortic segments of the mice was obtained. Bar graphs are summary data for slope factors normalized to cell number (i.e., nuclear DNA content, assessed by Hoechst fluorescence intensity), representing tissue H2O2 production. Bar graphs are summary data of DCF fluorescent intensities in endothelial cells of Ames dwarf mice and wild-type control mice (means ± SE, n = 5 animals for each group; *P < 0.05). AU, ΔDCF fluorescence/dt/DNA content. D: results from Amplex red-horseradish peroxidase assays. Time course of buildup of Amplex red fluorescence by aortic segments of Ames dwarf mice showed a significantly steeper slope than those of wild-type controls. Data are means ± SE. n = 5 animals for each group. *P < 0.05.
GH and IGF-I decrease cellular O2•− and H2O2 production in endothelial cells in vitro.
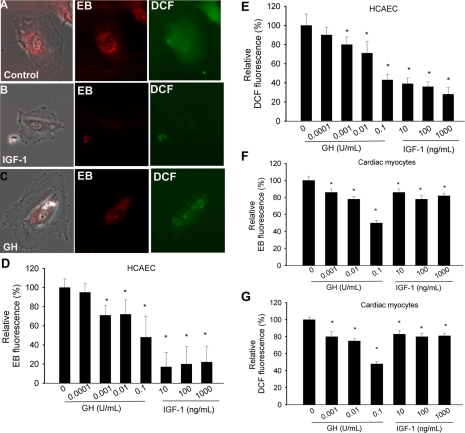
To test the direct effect of GH and IGF-I on endothelial ROS generation, cultured HCAECs were treated with GH (0.0001–0.1 U/ml) and IGF-I (1–1,000 ng/ml). These concentrations overlap with the normal plasma concentration of IGF-I (∼25–500 ng/ml IGF-I, ∼90% of which is protein bound) in mice and humans. In humans GH secretion occurs as several large pulses or peaks each day, and the plasma concentration of GH during these peaks may range up to 35 ng/ml or more (1 mg GH corresponds to 3 international units). GH concentrations used in our studies that exert antioxidant effects thus overlap with the physiological GH peak levels. Imaging of the cells showed that compared with untreated controls pretreatment with GH or IGF-I significantly attenuated ethidium staining and cellular DCF fluorescence in HCAECs (Fig. 3, A and B). Plate reader-based dihydroethidine and DCF assays showed that both GH and IGF-I elicit concentration-dependent decreases in O2•− and H2O2 production in HCAECs (Fig. 3, D and E). In cultured neonatal cardiac myocytes GH or IGF-I also significantly attenuated O2•− and H2O2 generation (as assessed by dihydroethidine and DCF assays; Fig. 3, F and G). We did not assess the effects of the age of the donor and the length of time in culture in the present study.
Fig. 3.
Treatment of human coronary arterial endothelial cells (HCAECs) with growth hormone (GH) and IGF-I (for 24 h) attenuates cellular reactive oxygen species (ROS) production. A–C: representative fluorescent images showing GH- and IGF-I-treated HCAECs [left: phase contrast image; center: red fluorescent EB staining; right: green H2DCFDA (DCF) fluorescence]. D–G: bar graphs are summary data of EB (D and F) and DCF (E and G) fluorescent intensities in GH- and IGF-I treated HCAECs (D and E) and neonatal rat cardiac myocytes (F and G). Data are means ± SE. *P < 0.05.
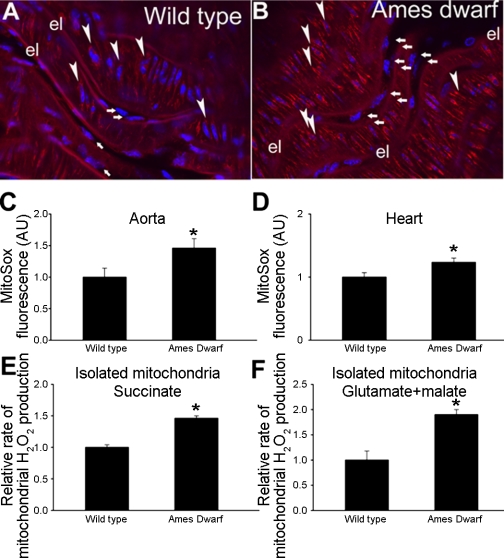
Increased mitochondrial ROS production in Ames dwarf mice.
A series of experiments (Fig. 4) was conducted to examine production of ROS by mitochondria. In Ames dwarf mouse aortas there was a greater perinuclear MitoSox fluorescence than in aortas of wild-type mice (Fig. 4, A and B). Similar results were obtained when endothelial MitoSox fluorescence was measured in en face aorta preparations (Fig. 4C) or when mitochondrial ROS production in cardiac muscle preparations were compared (Fig. 4D). In mitochondria isolated from Ames dwarf mice (with succinate or glutamate + malate as substrate) the rate of ROS production was substantially greater than in mitochondria of wild-type mice (Fig. 4E).
Fig. 4.
A and B: representative confocal images showing stronger perinuclear MitoSox staining (red fluorescence) in Ames dwarf mouse aorta (arrows, endothelial cells; arrowheads, smooth muscle cells; el, internal elastic lamina) compared with wild-type control vessel. Hoechst 33258 (blue fluorescence) was used for nuclear staining (original magnification ×20). C and D: time course of buildup of MitoSox fluorescence in Ames dwarf mouse and wild-type control aortas (C) and cardiac muscles (D) was obtained. Bar graphs are summary data for the slope factor obtained from the time course experiments normalized to tissue mass, representing mitochondrial ROS production in the tissues. Data are means ± SE (n = 5 in each group). *P < 0.05. E and F: relative H2O2 production by heart mitochondria respiring on succinate (E) or glutamate + malate (F) as substrate. For details see methods. Hydrogen peroxide generation rates were determined fluorometrically. Data are means ± SE. *P < 0.05.
GH and IGF-I decrease mitochondrial ROS production in endothelial cells in vitro.
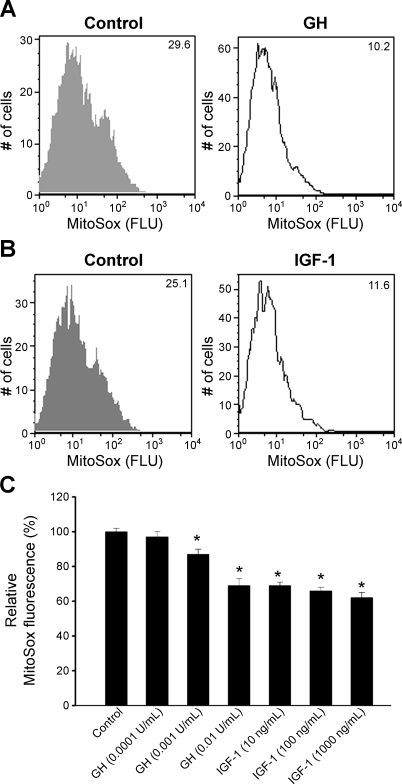
To test the direct effect of GH and IGF-I on mitochondrial ROS generation, cultured HCAECs were treated with GH (0.001–0.1 U/ml) and IGF-I (1 and 1,000 ng/ml). FACS analysis showed that both GH and IGF-I significantly attenuated MitoSox staining in HCAECs (Fig. 5, A and B). A plate reader-based MitoSox assay yielded identical results, showing that IGF-I and GH elicit significant, concentration-dependent decreases in mitochondrial ROS production (Fig. 5C). In cultured neonatal cardiac myocytes GH or IGF-I also significantly attenuated mitochondrial ROS generation (data not shown).
Fig. 5.
A and B: representative histograms of flow cytometry experiments demonstrating that GH (0.001 U/ml, A) and IGF-I (10 ng/ml, B) treatments elicit significant decrease in mean fluorescent intensity of oxidized MitoSox in HCAECs. Experiments were performed in quadruplicates with identical results. FLU, arbitrary fluorescence units. C: time course of buildup of MitoSox fluorescence in GH- and IGF-treated HCAECs was obtained. Bar graphs are summary data for the slope factor obtained from the time course experiments normalized to cell number (i.e., nuclear DNA content, assessed by Hoechst fluorescence intensity), representing mitochondrial O2•− generation. Data are means ± SE (n = 8 in each group). *P < 0.05.
Decreased vascular expression of antioxidant enzymes in Ames dwarf mice.
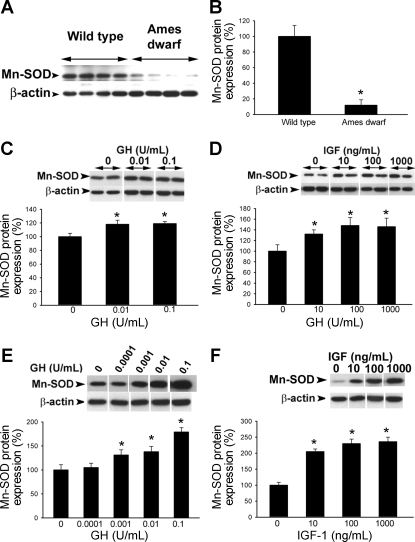
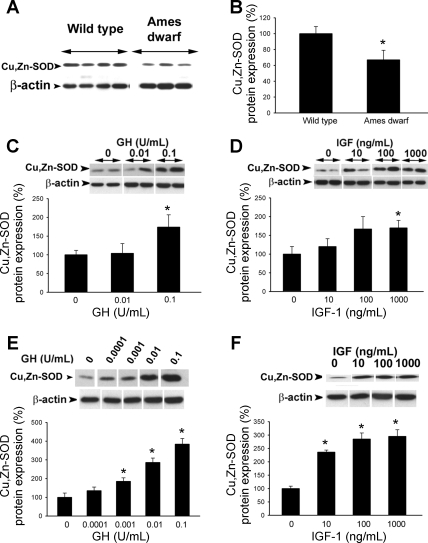
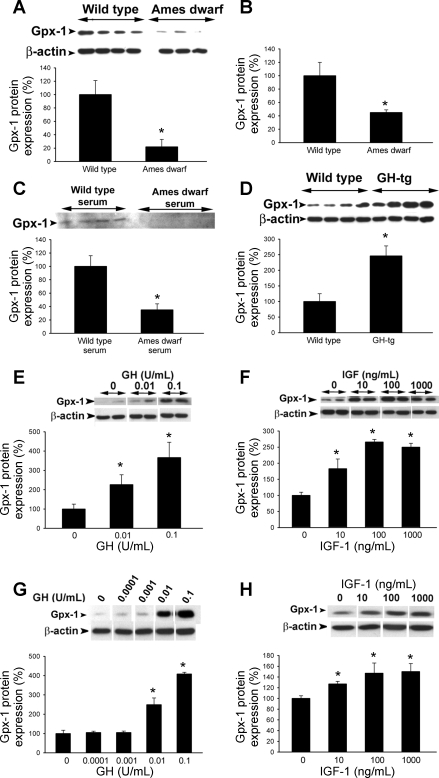
Western blot analysis revealed that in vessels of Ames dwarf mice there is a less abundant expression of Mn-SOD (Fig. 6, A and B), Cu,Zn-SOD (Fig. 7, A and B), and GPx-1 (Fig. 8A), whereas expression of catalase did not differ significantly between the two groups (Fig. 9). We confirmed that GPx-1 expression was downregulated in Ames dwarf mice at the mRNA level as well (Fig. 8B). Also, incubation of control aortic segments with serum from Ames dwarf mice resulted in a significant downregulation of GPx-1 (Fig. 8C). Conversely, aortas of GH-tg mice exhibited an increased GPx-1 expression (Fig. 8D). Expression of the gp91phox subunit of the NAD(P)H oxidase was decreased in Ames dwarf vessels (wild type 100 ± 20%, Ames dwarf 52 ± 11%; P < 0.05), whereas expression of the Nox-1 subunit did not differ significantly between the two groups (wild type 100 ± 22%, Ames dwarf 122 ± 20%; not significant).
Fig. 6.
A: original Western blot showing expression of Mn-SOD in aortas of Ames dwarf mice and wild-type control mice. β-Actin content was used for normalization. B: bar graphs are densitometric data (means ± SE). *P < 0.05. C and D: in cultured aortic segments from wild-type mice, in vitro treatment with GH (C) and IGF (D) elicits dose-dependent upregulation of Mn-SOD (Western blotting). Bar graphs are densitometric data (means ± SE). *P < 0.05 vs. untreated. E and F: in HCAECs in vitro treatment with GH (E) and IGF (F) elicits dose-dependent upregulation of Mn-SOD (Western blotting). Bar graphs are densitometric data (means ± SE). *P < 0.05 vs. untreated.
Fig. 7.
A: original Western blot showing expression of Cu,Zn-SOD in aortas of Ames dwarf mice and wild-type control mice. β-Actin content was used for normalization. B: bar graphs are densitometric data (means ± SE). *P < 0.05. C and D: in cultured aortic segments from wild-type mice in vitro treatment with GH (C) and IGF (D) elicits dose-dependent upregulation of Cu,Zn-SOD (Western blotting). Bar graphs are densitometric data (means ± SE). *P < 0.05 vs. untreated. E and F: in HCAECs in vitro treatment with GH (C) and IGF (D) elicits dose-dependent upregulation of Cu,Zn-SOD (Western blotting). Bar graphs are densitometric data (means ± SE). *P < 0.05 vs. untreated.
Fig. 8.
A: original Western blot showing expression of glutathione peroxidase (GPx)-1 in aortas of Ames dwarf mice and wild-type control mice. β-Actin content was used for normalization. Bar graphs are densitometric data (means ± SE). *P < 0.05. B: in aortas of Ames dwarf mice mRNA expression of GPx-1 was significantly decreased. Analysis of mRNA expression was performed by real-time quantitative RT-PCR (QRT-PCR). β-Actin was used for normalization. Data are means ± SE (n = 5 for each group). *P < 0.05. C: in cultured aortic segments from wild-type mice in vitro treatment with Ames dwarf serum resulted in a marked downregulation of GPx-1 (Western blotting). Bar graphs are densitometric data (means ± SE). *P < 0.05. D: original Western blot showing expression of GPx-1 in aortas of GH-transgenic (GH-tg) mice and wild-type control mice. β-Actin content was used for normalization. Bar graphs are densitometric data (means ± SE). *P < 0.05. E and F: in cultured aortic segments from wild-type mice, in vitro treatment with GH (E) and IGF (F) elicits dose-dependent upregulation of GPx-1 (Western blotting). Bar graphs are densitometric data (means ± SE). *P < 0.05 vs. untreated. G and H: in HCAECs in vitro treatment with GH (G) and IGF (H) elicits dose-dependent upregulation of GPx-1 (Western blotting). Bar graphs are densitometric data (means ± SE). *P < 0.05 vs. untreated.
Fig. 9.
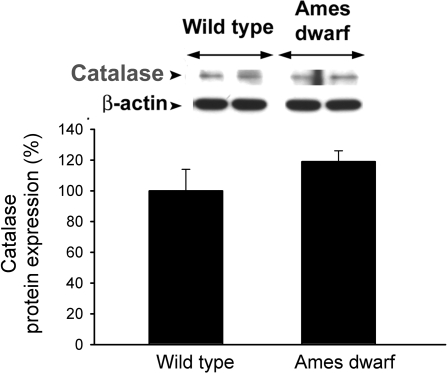
Original Western blot showing expression of catalase in aortas of Ames dwarf mice and wild-type control mice. β-Actin content was used for normalization. Bar graphs are densitometric data (means ± SE).
GH and IGF-I upregulate expression of antioxidant enzymes in cultured vessels and endothelial cells.
To test the direct effect of GH and IGF-I on vascular expression of antioxidant enzymes, cultured aortic segments (isolated from wild-type mice) and HCAECs were treated with GH (0.001–0.1 U/ml) and IGF-I (1 and 10 ng/ml). We found that in both cultured vessels and HCAECs GH elicited significant concentration-dependent increases in the expression of Mn-SOD (Fig. 6, C and E), Cu,Zn-SOD (Fig. 7, C and E), and GPx-1 (Fig. 8, E and G). Similarly, IGF-I upregulated Mn-SOD (Fig. 6, D and F), Cu,Zn-SOD (Fig. 7, D and F, albeit the effect of IGF-I in HCAECs did not reach statistical significance), and GPx-1 (Fig. 8, F and H).
Decreased vascular eNOS expression in Ames dwarf mice.
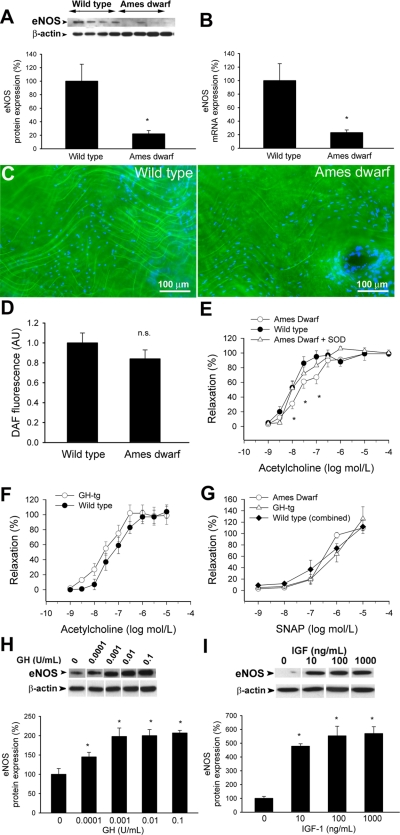
Western blot analysis and QRT-PCR measurements revealed that in vessels of Ames dwarf mice there is a less abundant expression of eNOS (both protein and mRNA; Fig. 10, A and B) than in wild-type mice. DAF fluorescence measurements showed that in the endothelial cells of isolated aortas from Ames dwarf mice basal production of NO tended to be decreased, although the difference did not reach statistical significance (Fig. 10, C and D). There was a rightward shift in the dose-response curve for ACh-induced vasorelaxation (reflecting stimulated NO release) in Ames dwarf mice (Fig. 10E) compared with control animals (maximal relaxation to ACh did not differ between the groups). In the presence of PEG-SOD ACh-induced relaxations of vessels from Ames dwarf mice and wild-type control mice were similar (Fig. 10E). The NOS inhibitor l-NAME abolished ACh-induced responses in both groups. Relaxation to ACh tended to increase in vessels of GH-tg mice (Fig. 10F), although these differences did not reach statistical significance. The NO donor SNAP elicited similar relaxations of vessels from both mouse lines (Fig. 10G). In HCAECs GH and IGF-I treatment elicited significant concentration-dependent increases in the expression of eNOS (Fig. 10, H and I) and NO production (DAF fluorescence assay, not shown).
Fig. 10.
A: original Western blot showing expression of endothelial nitric oxide synthase (eNOS) in aortas of Ames dwarf mice and wild-type control mice. β-Actin content was used for normalization. Bar graphs are densitometric data (means ± SE). *P < 0.05. B: in aortas of Ames dwarf mice, mRNA expression of eNOS was significantly decreased. Analysis of mRNA expression was performed by real-time QRT-PCR. β-Actin was used for normalization. Data are means ± SE (n = 5 for each group). *P < 0.05. C: representative fluorescent images showing that in the endothelial cells of unstimulated Ames dwarf aortas diaminofluorescein fluorescence tended to be weaker than in those of wild-type mice (en face preparations). Hoechst 33258 (blue fluorescence) was used for nuclear staining. D: bar graphs are fluorescent intensity values (means ± SE). n.s., Not significant. E: acetylcholine-induced relaxation in vessels of Ames dwarf mice and wild-type mice (means ± SE). *P < 0.05. vs. wild type. Responses of Ames dwarf vessels in the presence of SOD are also shown (n.s. vs. wild type). F: acetylcholine-induced relaxation in vessels of GH-tg mice and wild-type mice (means ± SE, n.s.). G: vascular relaxations to the nitric oxide donor S-nitroso-N-acetyl-penicillamine (SNAP) (n.s.). H and I: in HCAECs, in vitro treatment with GH (H) and IGF (I) elicits dose-dependent upregulation of eNOS (Western blotting). Bar graphs are densitometric data (means ± SE). *P < 0.05 vs. untreated.
DISCUSSION
There are four interesting findings in this study, namely that in blood vessels of Ames dwarf mice impaired GH/IGF-I signaling is associated with 1) increased endothelial production of O2•− and H2O2, 2) increased mitochondrial ROS production, 3) alterations in the expression of antioxidant enzymes (including downregulation of GPx and SODs), and 4) impaired NO bioavailability.
Vascular aging is characterized by increased ROS production in endothelial cells, which decreases bioavailability of the vasodilator and antiapoptotic NO, increases cardiac oxygen demand, and promotes vascular inflammation by inducing NF-κB (54). On the basis of the oxidative stress theory of aging one would predict that if ROS production is important in determining the rate of aging, then cells of longer-living animals produce less ROS than those from shorter-living animals. Indirect evidence suggests that this may be the case in the brain and liver of long-lived Ames dwarf mice (6, 41). In contrast, vessels of these animals exhibit significantly higher levels of ROS production (Figs. 1 and 2) than arteries of wild-type littermates. Increased ROS generation seems to be present also in the myocardium of Ames dwarfs (Fig. 1F). These findings in cardiovascular tissues from mice deficient in circulating GH/IGF-I are in complete agreement with the observations that in vitro IGF-I and GH treatment also reduces ROS production in cultured endothelial cells (50) (Fig. 3) and cardiac myocytes (Fig. 3). Cardiac oxidative stress was also attenuated in IGF-I transgenic mice (36). Clinical studies also showed that plasma markers of oxidative stress are elevated in GH-deficient subjects and are lowered by GH replacement (22). Thus the idea that insulin-like signaling controls oxidative stress in mammals, similar to the situation in C. elegans, cannot be generalized since both prooxidant and protective effects of GH and insulin have been observed, depending on the tissue or cell type studied (42).
The underlying mechanisms for elevated ROS production in the cardiovascular system of Ames dwarf mice are likely multifaceted. We have found that mitochondrial ROS generation was increased in arteries of Ames dwarf mice (Fig. 4, A–C), which likely contributes to the observed difference between the redox status of Ames dwarf and control arteries. The findings that mitochondrial ROS production was also increased in intact cardiac muscle (Fig. 4D) and isolated cardiac mitochondria (Fig. 4, E and F) from Ames dwarf mice suggest that the GH/IGF pathway acts to reduce mitochondrial oxidative stress in the cardiovascular system. Indeed, treatment of cultured endothelial cells and cardiac myocytes with GH and IGF significantly decreased mitochondrial ROS production (Fig. 5). In this regard, it is interesting to note that cardiac myocyte excitation-contraction coupling is also impaired in Ames dwarf mice (38). Previous results indicated that in Ames dwarf mice oxidative damage to mitochondrial DNA is lower in the brain (41), indicating that the effects of GH/IGF pathway on ROS homeostasis are organ specific (42).
In Ames dwarf aortas Mn-SOD, Cu,Zn-SOD (Fig. 6, A and B, and Fig. 7, A and B), and GPx-1 (Fig. 8, A and B) (but not catalase; Fig. 9) are significantly downregulated compared with the wild-type controls, suggesting that an imbalance in the cellular antioxidant systems may contribute to vascular oxidative stress in these animals. Interestingly, GPx also seems to be lower in the skeletal muscle of Ames dwarf mice (39). In contrast, previous studies have reported an increased expression/activity of antioxidant enzymes in the liver of Ames dwarf mice (8, 10). Organ- and sex-specific alterations in GPx, catalase, and Cu,Zn-SOD expression have been observed also in GHR-KO mice (25). These results suggest that insufficient levels of circulating GH/IGF-I are associated with complex, organ-specific impairment of free radical scavenging systems.
In addition to its endocrine function IGF-I also exerts its biological effects via autocrine/paracrine pathways, and recent studies suggest that circulating IGF-I and tissue IGF-I levels do not always correlate (13, 31, 47). The relative importance of endocrine and autocrine/paracrine IGF-I pathways in various organs likely differs, which may offer another possible explanation for the apparent tissue specificity of the effects of Prop1df/df genotype on cellular antioxidant systems. Our findings that serum of Ames dwarf mice reduced vascular GPx-1 expression in control aortic segments suggest that the circulating GH/IGF-I level is an important regulator of vascular expression of antioxidant enzymes (Fig. 8C). This view is further supported by the findings that GH-tg mice, which have increased circulating GH, IGF-I, and insulin levels (4), exhibit increased vascular GPx-1 expression (Fig. 8D). Yet we cannot rule out that other factors affecting cardiovascular health are predominantly regulated by an autocrine/paracrine IGF-I pathway within the vascular wall, which may or may not be suppressed in Ames dwarf mice.
To further elucidate the endocrine effect of increased GH and IGF-I on vascular antioxidant enzymes, we treated cultured vessel segments and HCAECs with GH and IGF-I. We found that both GH and IGF-I elicited concentration-dependent upregulation of Mn-SOD (Fig. 6, C–F), Cu,Zn-SOD (Fig. 7, C–F), and GPx-1 (Fig. 8, E–H). These in vitro data accord with the ex vivo findings and strongly suggest that the GH/IGF-I axis exerts predominantly antioxidant phenotypic changes in the vascular endothelium. In contrast, in vitro treatment of hepatocytes with high doses of GH and IGF-I downregulated catalase and Mn-SOD (10), further suggesting that the antioxidant effects of the GH/IGF-I pathway are organ specific. Interestingly, we found that expression of NAD(P)H oxidase subunits was decreased (gp91phox) or unaltered (Nox-1) in Ames dwarf vessels, suggesting that induction of vascular NAD(P)H oxidases is not a primary cause of the observed increases in vascular ROS generation. Similarly, a recent microarray study also reported a ∼20% downregulation of the NOX4 subunit of NAD(P)H oxidase in the liver (2).
Another mechanism that is known to counteract the deleterious effects of oxidative stress in the cardiovascular system is the production of endothelium-derived NO. NO is thought to exert vasculoprotective effects, and decreased bioavailability of NO (due to an increased ROS production and/or downregulation of eNOS) has been linked to cardiovascular aging (18, 19). In arteries of Ames dwarf mice eNOS expression (Fig. 10, A and B) and agonist-induced NO-mediated vasorelaxation (Fig. 10E) were impaired and baseline endothelial NO production tended to decrease (Fig. 10, C and D). In contrast, in GH-tg animals endothelial NO-mediated vasorelaxation was intact (Fig. 10F). Sensitivity of the vascular smooth muscle to the vasodilator action of NO was similar in both mouse lines (Fig. 10G). Together, these data suggest that in vivo GH/IGF-I deficiency impairs, rather than improves, endothelial NO synthesis. This view is completely in line with the in vitro data showing that GH and/or IGF-I upregulate eNOS expression (Fig. 10, H and I) and NO production (52) in endothelial cells (49, 50).
The available human data mostly accord with our findings. In GH-deficient subjects flow-mediated dilation of peripheral arteries (which is thought to reflect endothelial NO synthesis) is impaired (22, 23, 44). Treatment of patients with recombinant GH was reported to increase NO production, improve flow-mediated dilation, and decrease peripheral resistance (5, 22, 23, 35, 44). Coronary flow reserve is positively correlated with IGF-I levels (37), and cardiovascular disease risk is elevated among apparently healthy individuals who have serum IGF-I levels in the low-normal range (40). Recent data also suggest a protective role of IGF-I in cardiovascular stem cell physiology (28, 49, 51). In light of these finding, in humans GH and IGF-I deficiency is thought to be associated with premature atherosclerosis and mortality due to cardiovascular disease (21). Interestingly, the most famous person with dwarfism, Charles Sherwood Stratton (who performed with the Barnum Circus in the nineteenth century under the stage name General Tom Thumb), also died of stroke at the age of 45. The findings that cardiac overexpression of IGF-I significantly improves cardiomyocyte contractile function in old mice (29) support the view that loss of IGF-I contributes to cardiac aging. It would be informative to test cardiac and vascular function and cardiac stem cell populations in aged Ames dwarf mice to determine whether aging in these animals results in an exacerbation of the cardiovascular phenotype (i.e., greater dysfunction in aged Ames dwarf mice).
Limitations of study.
We would like to acknowledge that some of the phenotypic changes in Ames dwarf mice may be due, at least in part, to PRL and/or TSH deficiency. In this regard future studies should determine whether treatment of Ames mice with GH or IGF-I would normalize vascular function and ROS generation. In a previous study, Ames dwarf mice were treated with GH for 7 days (9), which increased glutathione levels in brain, muscle, and liver. The same authors also showed that the rate-limiting enzyme of glutathione synthesis is also upregulated by GH treatment in the heart of Ames dwarf mice (11). Yet it is currently unknown how GH treatment affects vascular antioxidant enzymes in Ames dwarf mice. It is also unknown whether there are similar abnormalities present in GHR-KO mice, in which the primary endocrine changes reflect the GH/IGF-I system per se.
The relationship between oxidative stress and life span is still much debated. For example, genetic knockout mice for major cellular antioxidant enzymes often show no major change in maximum life span (30, 43, 55), whereas overexpression of catalase significantly increases longevity (56). It is likely that antioxidant effects of the GH/IGF-I axis are organ specific. Impaired endothelial function and vascular oxidative stress associated with impaired GH/IGF-I signaling per se likely will not affect directly murine life span (through promoting cardiovascular disease), because the primary cause of death in mice is cancer. Indeed, the observed life span extension in Ames dwarf mice is, at least in part, due to the delayed occurrence of fatal neoplastic diseases (26) [additional factors that may also contribute to life span extension in Ames dwarfs include low thyroid activity, reduced body temperature, and/or enhanced insulin sensitivity (7)]. Because endothelial NO plays a central role in tumor neovascularization, we cannot exclude the possibility that alterations in microvascular NO bioavailability contribute to the retardation of tumor growth in Ames dwarf mice. To test this hypothesis, future studies should test whether microvascular function and/or angiogenic processes are blunted in Ames dwarf mice.
Conclusions.
In conclusion, our results support the view that the GH/IGF-I axis exerts primarily vasoprotective functions by upregulating vascular antioxidant defenses, whereas Ames dwarfism elicits endothelial oxidative stress adversely affecting NO bioavailability and endothelium-dependent vasorelaxation. Because plasma GH and IGF-I levels decline with age in healthy humans and in experimental animals, it is possible that GH/IGF-I deficiency may contribute to age-related endothelial oxidative stress and vasodilator dysfunction (53) promoting the development of vascular disease. Our results support the usefulness of Ames dwarf mice as a tool to understand pathophysiological processes associated with human aging.
GRANTS
This work was supported by grants from the American Heart Association (0430108N and 0435140N), the American Diabetes Association (to Z. Ungvari), Philip Morris USA Inc. and Philip Morris International (to Z. Ungvari), the American Federation for Aging Research (to A. Csiszar), the Hungarian Scientific Research Fund (OTKA-K68758), the San Antonio Area Foundation (to A. Podlutsky), and the National Institutes of Health (HL-077256 and HL-43023 to Z. Ungvari; AG-019899 and U-19-AG-023122 to A. Bartke; PO1-HL-74237 to F. A. Recchia; AG-022873 and K07-AG-025063 to S. Austad) and funded by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism (to P. Pacher). F. A. Recchia is an established investigator of the American Heart Association.
Acknowledgments
Some of the experiments took place during the 2007 Molecular Biology of Aging Course at the Marine Biological Laboratory (Woods Hole, MA) organized by S. N. Austad, for which we thank the Ellison Medical Foundation.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor I/insulin signaling and caloric restriction. Endocrinology 146: 851–860, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Amador-Noguez D, Yagi K, Venable S, Darlington G. Gene expression profile of long-lived Ames dwarf mice and Little mice. Aging Cell 3: 423–441, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol 63: 189–225, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Bartke A, Chandrashekar V, Bailey B, Zaczek D, Turyn D. Consequences of growth hormone (GH) overexpression and GH resistance. Neuropeptides 36: 201–208, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Boger RH, Skamira C, Bode-Boger SM, Brabant G, von zur Muhlen A, Frolich JC. Nitric oxide may mediate the hemodynamic effects of recombinant growth hormone in patients with acquired growth hormone deficiency. A double-blind, placebo-controlled study. J Clin Invest 98: 2706–2713, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown-Borg H, Johnson W, Rakoczy S, Romanick M. Mitochondrial oxidant generation and oxidative damage in Ames dwarf and GH transgenic mice. J Am Aging Assoc 24: 85–96, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature 384: 33, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Brown-Borg HM, Rakoczy SG. Catalase expression in delayed and premature aging mouse models. Exp Gerontol 35: 199–212, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Brown-Borg HM, Rakoczy SG. Growth hormone administration to long-living dwarf mice alters multiple components of the antioxidative defense system. Mech Ageing Dev 124: 1013–1024, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Brown-Borg HM, Rakoczy SG, Romanick MA, Kennedy MA. Effects of growth hormone and insulin-like growth factor-1 on hepatocyte antioxidative enzymes. Exp Biol Med (Maywood) 227: 94–104, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Brown-Borg HM, Rakoczy SG, Uthus EO. Growth hormone alters methionine and glutathione metabolism in Ames dwarf mice. Mech Ageing Dev 126: 389–398, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Ceda GP, Dall'Aglio E, Maggio M, Lauretani F, Bandinelli S, Falzoi C, Grimaldi W, Ceresini G, Corradi F, Ferrucci L, Valenti G, Hoffman AR. Clinical implications of the reduced activity of the GH-IGF-I axis in older men. J Endocrinol Invest 28: 96–100, 2005. [PubMed] [Google Scholar]
- 13.Conover CA, Bale LK. Loss of pregnancy-associated plasma protein A extends lifespan in mice. Aging Cell 6: 727–729, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Csiszar A, Ahmad M, Smith KE, Labinskyy N, Gao Q, Kaley G, Edwards JG, Wolin MS, Ungvari Z. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol 168: 629–638, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole rat. Am J Physiol Heart Circ Physiol 293: H919–H927, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy R, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell 6: 783–797, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: role of NF-kappaB inhibition. Am J Physiol Heart Circ Physiol 291: H1694–H1699, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics 17: 21–30, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Edwards JG, Ghaleh B. Divergence of beta-myosin heavy chain (betaMHC) expression in fetal rat cardiomyocytes in vitro and adult rat heart in vivo. Biochem Biophys Res Commun 230: 340–343, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Elhadd TA, Abdu TA, Oxtoby J, Kennedy G, McLaren M, Neary R, Belch JJ, Clayton RN. Biochemical and biophysical markers of endothelial dysfunction in adults with hypopituitarism and severe GH deficiency. J Clin Endocrinol Metab 86: 4223–4232, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Evans LM, Davies JS, Anderson RA, Ellis GR, Jackson SK, Lewis MJ, Frenneaux MP, Rees A, Scanlon MF. The effect of GH replacement therapy on endothelial function and oxidative stress in adult growth hormone deficiency. Eur J Endocrinol 142: 254–262, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Evans LM, Davies JS, Goodfellow J, Rees JA, Scanlon MF. Endothelial dysfunction in hypopituitary adults with growth hormone deficiency. Clin Endocrinol 50: 457–464, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci USA 98: 6736–6741, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauck SJ, Aaron JM, Wright C, Kopchick JJ, Bartke A. Antioxidant enzymes, free-radical damage, and response to paraquat in liver and kidney of long-living growth hormone receptor/binding protein gene-disrupted mice. Horm Metab Res 34: 481–486, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in Ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci 58: 291–296, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Labinskyy N, Csiszar A, Orosz Z, Smith K, Rivera A, Buffenstein R, Ungvari Z. Comparison of endothelial function, O2•− and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am J Physiol Heart Circ Physiol 291: H2698–H2704, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Li B, Wang X, Leri A, Jana KP, Liu Y, Kajstura J, Baserga R, Anversa P. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest 100: 1991–1999, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Q, Wu S, Li SY, Lopez FL, Du M, Kajstura J, Anversa P, Ren J. Cardiac-specific overexpression of insulin-like growth factor 1 attenuates aging-associated cardiac diastolic contractile dysfunction and protein damage. Am J Physiol Heart Circ Physiol 292: H1398–H1403, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Mansouri A, Muller FL, Liu Y, Ng R, Faulkner J, Hamilton M, Richardson A, Huang TT, Epstein CJ, Van Remmen H. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech Ageing Dev 127: 298–306, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, Jimenez-Ortega V, Panici JA, Bonkowski MS, Kopchick JJ, Wang Z, Bartke A Caloric restriction and growth hormone receptor knockout: effects on expression of genes involved in insulin action in the heart. Exp Gerontol 41: 417–429, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGrane MM, de Vente J, Yun J, Bloom J, Park E, Wynshaw-Boris A, Wagner T, Rottman FM, Hanson RW. Tissue-specific expression and dietary regulation of a chimeric phosphoenolpyruvate carboxykinase/bovine growth hormone gene in transgenic mice. J Biol Chem 263: 11443–11451, 1988. [PubMed] [Google Scholar]
- 33.Mukhopadhyay P, Rajesh M, Hasko G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protocols 2: 2295–2301, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukhopadhyay P, Rajesh M, Yoshihiro K, Hasko G, Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun 358: 203–208, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Napoli R, Guardasole V, Matarazzo M, Palmieri EA, Oliviero U, Fazio S, Sacca L. Growth hormone corrects vascular dysfunction in patients with chronic heart failure. J Am Coll Cardiol 39: 90–95, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Norby FL, Aberle NS 2nd, Kajstura J, Anversa P, Ren J. Transgenic overexpression of insulin-like growth factor I prevents streptozotocin-induced cardiac contractile dysfunction and beta-adrenergic response in ventricular myocytes. J Endocrinol 180: 175–182, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Oflaz H, Sen F, Elitok A, Cimen AO, Onur I, Kasikcioglu E, Korkmaz S, Demirturk M, Kutluturk F, Pamukcu B, Ozbey N. Coronary flow reserve is impaired in patients with adult growth hormone (GH) deficiency. Clin Endocrinol 66: 524–529, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Ren J, Brown-Borg HM. Impaired cardiac excitation-contraction coupling in ventricular myocytes from Ames dwarf mice with IGF-I deficiency. Growth Horm IGF Res 12: 99–105, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Romanick MA, Rakoczy SG, Brown-Borg HM. Long-lived Ames dwarf mouse exhibits increased antioxidant defense in skeletal muscle. Mech Ageing Dev 125: 269–281, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Roubenoff R, Parise H, Payette HA, Abad LW, D'Agostino R, Jacques PF, Wilson PW, Dinarello CA, Harris TB. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med 115: 429–435, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Sanz A, Bartke A, Barja G. Long-lived Ames dwarf mice: oxidative damage to mitochondrial DNA in heart and brain. J Am Aging Assoc 25: 119–122, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanz A, Gredilla R, Pamplona R, Portero-Otin M, Vara E, Tresguerres JA, Barja G. Effect of insulin and growth hormone on rat heart and liver oxidative stress in control and caloric restricted animals. Biogerontology 6: 15–26, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Sentman ML, Granstrom M, Jakobson H, Reaume A, Basu S, Marklund SL. Phenotypes of mice lacking extracellular superoxide dismutase and copper- and zinc-containing superoxide dismutase. J Biol Chem 281: 6904–6909, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Smith JC, Evans LM, Wilkinson I, Goodfellow J, Cockcroft JR, Scanlon MF, Davies JS. Effects of GH replacement on endothelial function and large-artery stiffness in GH-deficient adults: a randomized, double-blind, placebo-controlled study. Clin Endocrinol 56: 493–501, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O'Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature 384: 327–333, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Steger RW, Bartke A, Cecim M. Premature ageing in transgenic mice expressing different growth hormone genes. J Reprod Fertil 46: 61–75, 1993. [PubMed] [Google Scholar]
- 47.Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of GH and IGF-I in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging 26: 929–937, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science 299: 1346–1351, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Thum T, Hoeber S, Froese S, Klink I, Stichtenoth DO, Galuppo P, Jakob M, Tsikas D, Anker SD, Poole-Wilson PA, Borlak J, Ertl G, Bauersachs J. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ Res 100: 434–443, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Thum T, Tsikas D, Frolich JC, Borlak J. Growth hormone induces eNOS expression and nitric oxide release in a cultured human endothelial cell line. FEBS Lett 555: 567–571, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, Urbanek K, Nadal-Ginard B, Kajstura J, Anversa P, Leri A. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res 94: 514–524, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Tsukahara H, Gordienko DV, Tonshoff B, Gelato MC, Goligorsky MS. Direct demonstration of insulin-like growth factor-I-induced nitric oxide production by endothelial cells. Kidney Int 45: 598–604, 1994. [DOI] [PubMed] [Google Scholar]
- 53.Ungvari Z, Buffenstein R, Austad SN, Podlutsky A, Kaley G, Csiszar A. Oxidative stress in vascular senescence: lessons from successfully aging species. Front Biosci 13: 5056–5070, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37–H47, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, Nelson J, Strong R, Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics 16: 29–37, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Wu S, Li Q, Du M, Li SY, Ren J. Cardiac-specific overexpression of catalase prolongs lifespan and attenuates ageing-induced cardiomyocyte contractile dysfunction and protein damage. Clin Exp Pharmacol Physiol 34: 81–87, 2007. [DOI] [PubMed] [Google Scholar]