Abstract
The role of macrophages in the pathogenesis of acetaminophen (APAP)-induced liver injury remains controversial, as it has been demonstrated that these cells display pro-toxicant and hepato-protective functions. This controversy may stem from the heterogeneity and/or plasticity of macrophages and the difficulty in distinguishing and differentially studying subpopulations of macrophages in the liver. In the present study, using flow cytometric analysis and fluorescence-labeled antibodies against specific cell surface macrophage markers, we were able to, for the first time, identify an APAP-induced macrophage (IM) population distinct from resident Kupffer cells. The data demonstrated that the IMs were derived from circulating monocytes that infiltrated the liver following APAP-induced liver injury. The IMs exhibited a phenotype consistent with that of alternatively activated macrophages and demonstrated the ability to phagocytize apoptotic cells and induce apoptosis of neutrophils. Furthermore, in the absence of the IMs, the resolution of hepatic damage following APAP-induced hepatotoxicity was delayed in CCR2−/− mice compared with wild-type mice. These findings likely contribute to the role of the IMs in the processes of tissue repair, including counteracting inflammation and promoting angiogenesis. The present study also demonstrated the ability of separating populations of macrophages and delineating distinct functions of each group in future studies of inflammatory disease in the liver and other tissues.
Keywords: inflammation, monocyte, phagocytosis, neutrophil, apoptosis
INTRODUCTION
Drug-induced liver injury (DILI) attributes to a significant percentage of patient morbidity and mortality [1,2,3,4]. The idiosyncratic nature, severity, and poor diagnosis of DILI make these reactions the most common cause for the withdrawal of drugs from the pharmaceutical market [5]. It is imperative to gain a thorough understanding of the underlying mechanisms of DILI before strategies can be developed to better predict and prevent DILI. An overdose of acetaminophen (APAP), one of the most widely used analgesic and antipyretic drugs, is the leading cause of death from acute liver failure in the United States [3], and the proportion of APAP-induced acute liver failure cases increased dramatically between 1998 (21%) and 2003 (51%) [6]. At present, APAP-induced liver injury in mice is the most widely used animal model to study the mechanisms of DILI. It has been demonstrated that APAP-induced hepatotoxicity is initiated by the formation of a reactive metabolite, N-acetyl-p-benzoquinone imine, which depletes glutathione, covalently binds to cellular proteins [7,8,9], and damages hepatocytes. Recent studies have shown that the initial hepatocyte damage triggers activation of innate immune cells within the liver, such as hepatic macrophages, NK cells, NKT cells, and PMNs, which contribute to the progression of liver injury [10,11,12,13,14,15]. The role of macrophages in the pathogenesis of APAP-induced liver injury remains controversial. It has been demonstrated that hepatic macrophages contribute to APAP-induced hepatotoxicity through the production of proinflammatory cytokines and mediators, including TNF-α, IL-1β, and NO [14, 15]. However, hepatic macrophages also play a hepato-protective role through the production of cytokines and mediators, such as IL-10, IL-6, and IL-18-binding proteins, which counteract inflammatory events and promote liver regeneration [16]. This dichotomy of pro-toxicant and hepato-protective functions of hepatic macrophages may be explained by the heterogeneity and/or plasticity of these cells. The heterogeneity of macrophages is likely emanated from the heterogeneity of circulating monocytes. Recent studies have revealed the presence of at least two distinct subsets of monocytes: a short-lived, inflammatory monocyte population that is preferentially recruited to sites of inflammation and another population that homes constitutively to tissue and serves as precursors to resident cells [17].
It is known that macrophages have diverse activities, and many of them appear to be opposing in nature: proinflammatory versus anti-inflammatory activities, immunogenic versus tolerogenic activities, and tissue-destructive versus tissue-restorative activities [18,19,20]. Two major classes of macrophages have been identified, classically activated macrophages (M1) and alternatively activated macrophages (M2). It has been demonstrated that macrophages can become M1 or M2 depending on their adaptive response to various stimuli [19, 21,22,23,24,25]. In response to microbial infection, LPS, and TH1 cytokines, macrophages differentiate into M1, which generate proinflammatory cytokines and bacterialcidal mediators. In response to TH2 cytokines, such as IL-4, IL-10, and IL-13, as well as corticosteroids and PGs, macrophages differentiate into M2, which play an important role in the down-regulation of inflammation, tissue remodeling, elimination of tissue debris and apoptotic bodies, as well as the induction of angiogenesis [25, 26].
The objective of the present study was to examine whether distinct subpopulations of macrophages are present within the liver of mice after APAP challenge. Using flow cytometric analysis and fluorescence-labeled antibodies against specific cell surface macrophage markers, we were able to differentiate resident Kupffer cells (KCs) from a population of transient, APAP-induced macrophages (IMs). The observed IM-mediated induction of PMN apoptosis as well as the ability to phagocytize apoptotic cells likely contribute to their role in the resolution of inflammation and promotion of tissue repair following APAP-induced liver injury.
MATERIALS AND METHODS
Animal treatment
Eight- to 11-week-old female and male C57Bl/6J wild-type (WT) and CCR2−/− mice (Jackson Laboratories, Bar Harbor, ME, USA) as well as Balb/cJ WT (Jackson Laboratories) and Balb/cJ CCR2−/− mice [provided by Cara L. Mack, M.D., Department of Pediatrics, School of Medicine, University of Colorado Denver (UCD), CO, USA] were used for all experiments. Heterozygous C57Bl/6 CX3CR1GFP/+ mice were obtained from Charles L Edelstein, M.D. (Department of Renal Diseases and Hypertension, School of Medicine, UCD). Animals were maintained according to the Center for Laboratory Animal Care at UCD. Mice were allowed free access to autoclaved food and water until experimental use. Mice were fasted overnight for ∼16 h to deplete glutathione levels [27], prior to i.p. injection of PBS or APAP. For all experiments, female mice were treated with 400 mg APAP/kg, and male mice were treated with 300 mg APAP/kg.
Quantification of hepatic necrosis
Liver samples were obtained from female Balb/cJ WT and CCR2−/− mice at 24, 48, and 72 h after APAP challenge and fixed in 10% formalin. Samples were embedded in paraffin, sectioned, and stained with H&E. Necrosis was examined using low-power (100×) light microscopy, and an image was obtained using a digital camera. The area of necrosis was quantified using Spot Advanced digital software by measuring the total area (mm2) of necrosis within six separate fields per tissue section. The average area of necrosis among the six fields was calculated for each section.
Depletion of IMs by bone marrow irradiation
Bone marrow irradiation was carried out on a Varian 21 CD linear accelerator (6 MeV) within the Radiation Oncology Department at the University of Colorado Hospital. Mice were restrained in 50 mL Falcon tubes without anesthesia and irradiated with a total of 500 radiations (rads) at a dose rate of 600 rads/min. The total irradiation field was symmetric (180×30 cm), and the distance from the source was 100 cm (as calculated to the mid-point of the animal’s torso). Irradiation was performed on Day 0, and all subsequent experiments with the irradiated mice were performed on Day 3.
Isolation of hepatic nonparenchymal cells (NPCs)
Isolation of hepatic macrophages was performed at 24 h after APAP challenge, unless otherwise stated, following a method established previously [28, 29] with slight modifications. In brief, mice were anesthetized, and liver tissues were perfused in situ via the superior vena cava with a perfusion buffer (1× HBSS), followed by a digestion buffer [1× HBSS, supplemented with 0.05% collagenase (Type IV; Sigma Chemical Co., St. Louis, MO, USA), 1.25 mM CaCl2, 4 mM MgSO4, and 10 mM HEPES]. Following digestion, the liver was disrupted in anticoagulant-citrate-dextrose (Acd) solution (1× HBSS, supplemented with 0.5% FBS, 0.6% citrate-dextrose, and 10 mM HEPES), and the cells passed through a 100-μm cell strainer. The cells were centrifuged at 30 g for 3 min to pellet hepatocytes. Cells in the supernatant were then centrifuged at 320 g for 5 min, resuspended in complete RPMI media (RPMI supplemented with 10% FBS, 10 mM HEPES, and 1×penicillin/streptomycin), fractionated using 30% (w/v) Nycodenz (Axis-Shield, Scotland) at 1.155 g/mL to yield liver NPCs free of erythrocytes, and further purified using 30% Percoll (Sigma Chemical Co.) at 1.04 g/mL. At this stage, liver NPCs were resuspended in Acd solution and consisted mainly of hepatic macrophages and liver sinusoidal endothelial cells (LSECs).
Flow cytometry and FACS
To prevent nonspecific binding, liver NPCs were blocked with normal rat serum (Sigma Chemical Co.) and anti-mouse FcγR II/III (clone 93, eBioscience, San Diego, CA, USA). Liver NPCs were subsequently characterized by staining with the following antibodies from eBioscience: FITC-conjugated anti-mouse CD45, PE-conjugated anti-mouse CD11b, PE-conjugated anti-mouse NK-1.1, and allophycocyanin (APC)-conjugated anti-mouse F4/80; and from BD Biosciences (San Jose, CA, USA): PE-conjugated anti-mouse CD3e, CD11c, or CD19. Seven amino-actinomycin D (7-AAD) viability staining solution (eBioscience) was used to determine cellular viability. Cells were analyzed on a FACSCalibur cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA) using FlowJo 6.3.3 software (Tree Star, Inc., Ashland, OR, USA). For flow cytometric analysis, cells were initially gated on forward-scatter (FSC) and side-scatter (SSC) and then gated on alive cells (7-AAD–). CD45 is a marker expressed on cells of hematopoietic origin. Therefore, to exclude LSECs and enrich analysis for macrophages, we gated on CD45+ cells, and from CD45+ cells, we examined the expression of CD11b and F4/80. To purify hepatic macrophages, liver NPCs were stained as described above and sorted using a MoFlo high-performance cell sorter (Cytomation, Fort Collins, CO, USA). The purity of sorted cells was consistently greater than 92%. For morphological analysis, cells were cytospun onto Shandon Cytoslides (Thermo Scientific, Waltham, MA, USA) and stained using the Hema 3 manual staining system (Fisher Scientific, UK).
RT-PCR analysis
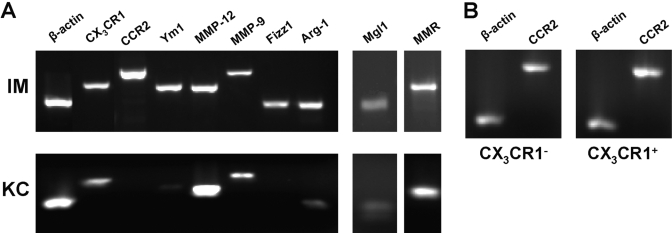
The livers of male Balb/cJ mice at 24 h after APAP challenge were pooled for isolation of IMs and resident KCs via FACS. Total RNA was isolated from the cells using RNeasy micro kits (Qiagen, Valencia, CA, USA), as described by the manufacturer. RNA (1 μg) was reverse-transcribed to cDNA and amplified using JumpStart Taq DNA polymerase (Sigma Chemical Co.) and gene-specific primers (Table 1) for β-actin, CX3CR1, CCR2, Ym1, matrix metalloproteinase 12 (MMP-12), MMP-9, found in inflammatory zone 1 (Fizz1), Arginase 1 (Arg-1), macrophage galactose- and N-acetylgalactosamine-specific C-type lectin 1 (Mgl1), and macrophage mannose receptor (MMR). All PCR products were resolved on 1.5% agarose gels and visualized using ethidium bromide staining.
TABLE 1.
Primer Sequences
| Name | Sequencea | |
|---|---|---|
| β-actin | (F) | 5′-TCT TGG GTA TGG AAT CCT GTG GCA-3′ |
| (R) | 5′-ACT CCT GCT TGC TGA TCC ACA TCT-3′ | |
| CX3CR1 | (F) | 5′-AGC CCA AGA GCA TCA CTG ACA TCT-3′ |
| (R) | 5′-AGA GGA AGA AGG CAA AGA CCA CCA-3′ | |
| CCR2 | (F) | 5′-TGT TAC CTC AGT TCA TCC ACG GCA-3′ |
| (R) | 5′-AGC CCT GTG CCT CTT CTT CTC ATT-3′ | |
| Ym1 | (F) | 5′-AGT TGG GCT AAG GAC AGG CCA ATA-3′ |
| (R) | 5′-TGG AAG TGA GTA GCA GCC TTG GAA-3′ | |
| MMP-12 | (F) | 5′-TGA TGC TGT CAC AAC AGT GGG AGA-3′ |
| (R) | 5′-AGG CTT GAT TCC TGG GAA GTG TGT-3′ | |
| MMP-9 | (F) | 5′-ACT GGG CTT AGA TCA TTC CAG CGT-3′ |
| (R) | 5′-ACA CCC ACA TTT GAC GTC CAG AG-3′ | |
| Fizz1 | (F) | 5′-TCC AGC TGA TGG TCC CAG TGA ATA-3′ |
| (R) | 5′-AGT CAA CGA GTA AGC ACA GGC AGT-3′ | |
| Arginase 1 | (F) | 5′-TTG GCA AGG TGA TGG AAG AGA CCT-3′ |
| (R) | 5′-CGA AGC AAG CCA AGG TTA AAG CCA-3′ | |
| Mgl1 | (F) | 5′-GCA TGA AGG CAG CTG CTA TTG GTT-3′ |
| (R) | 5′-AGC CTT TCT CAA AGT CGG TCC CAT-3′ | |
| MMR | (F) | 5′-AGC TAC CAT GGC ATG AAG CAG AGA-3′ |
| (R) | 5′-ACC CAT TCG AAG GCA TTC CAG AGA-3′ |
(F), Forward primer; (R), reverse primer.
In vivo phagocytosis assay
Male Balb/cJ mice were injected (i.v.) with 250 μL/mouse (1:100 dilution in PBS) Fluoresbrite Polychromatic Red 0.5 μm microspheres (2.62% solids-latex, Polysciences, Inc., Warrington, PA, USA) at 22 h after APAP challenge. Liver NPCs were isolated 2 h after injection of the latex beads and stained with PE-conjugated anti-mouse CD11b and APC-conjugated anti-mouse F4/80. For flow cytometric analysis, we examined the respective red fluorescence of the IMs and resident KCs.
In vitro phagocytosis assay
IMs were isolated via FACS from the pooled livers of male Balb/cJ mice at 24 h following APAP challenge and plated at 5 × 105 cells/well in 24-well cell-culture plates in complete RPMI media. Apoptosis of Jurkat T cells was induced by exposure to ultraviolet irradiation at 254 nm for 10 min, followed by culture for 3 h in complete RPMI media. The percentage of apoptotic cells, as determined by the percentage of Annexin V+ and propidium iodide-negative, was greater than 75%. The apoptotic or viable (control) Jurkat T cells were cocultured (1.5×106 cells/well) with the macrophages for 90 min at a 3:1 ratio (Jurkat T cell:IM). Following coculture, nonphagocytized Jurkat T cells were removed by washing with ice-cold PBS. The adherent macrophages were fixed and stained with Hema 3 manual staining system. The phagocytic index (PI) was calculated as the number of Jurkat T cells ingested divided by the total number of macrophages counted × 100. Apoptotic cells bound to the surface of macrophages, rather than ingested, were not counted. Phagocytosis was scored by visual inspection using light microscopy, with a minimum of 200 macrophages counted per well and three replicate wells per condition.
Apoptosis assay
To exclusively study the ability of IMs to induce apoptosis, we depleted resident KCs using liposome/clodronate (i.v.) 2 days prior to APAP challenge. The virtual elimination of resident KCs from the liver has been demonstrated previously 24 h after liposome/clodronate [30, 31], and this depletion has persisted 3–7 days after treatment [32, 33]. Liver NPCs were isolated from the pooled livers of KC-depleted male Balb/cJ mice at 24 h following APAP challenge. NPCs were stained with biotin-conjugated anti-mouse CD45 (eBioscience), washed, and incubated with anti-biotin MACS microbeads (Miltenyi Biotec, Auburn, CA, USA). The cells were then applied to a LS column (Miltenyi Biotec) in combination with a MACS separator (Miltenyi Biotec). The magnetically retained cells were eluted as the positively selected cell fraction, consisting mainly of IMs. PMNs were isolated via peritoneal washing with ice-cold 1× HBSS at 8 h after inoculation (i.p.) of mice with 3% thioglycolate. A PMN:IM ratio of 1:3 (5×105 PMN:1.5×106 IM) was used in coculture experiments. Cells were plated in 24-well cell-culture plates and incubated for 18 h in complete RPMI media. Experiments were also performed using 1.0 μm cell culture inserts (Becton Dickinson, San Diego, CA, USA). Following coculture, the supernatant within each well, consisting mainly of PMNs, was collected and processed for flow cytometry. Cells were characterized by staining with PE-conjugated anti-mouse Ly-6G (Gr-1, eBioscience) and APC-conjugated anti-mouse F4/80. The total number of PMNs remaining within each well following the coculture was calculated by multiplying the percentage of Gr-1+ cells by the percentage of total cells gated from FSC versus SSC times the total number of cells collected for analysis. Early PMN apoptosis was examined by the expression of membrane phosphatidylserine, detected by Annexin V binding using Annexin V-FITC (BD PharMingen, San Diego, CA, USA) on Gr-1+ cells.
Data analysis
Mean ± sem values were calculated for each experimental group and compared using unpaired Student’s t-test. Probability levels were considered significant when P< 0.05.
RESULTS
Identification of two distinct macrophage populations in the liver of mice following APAP challenge
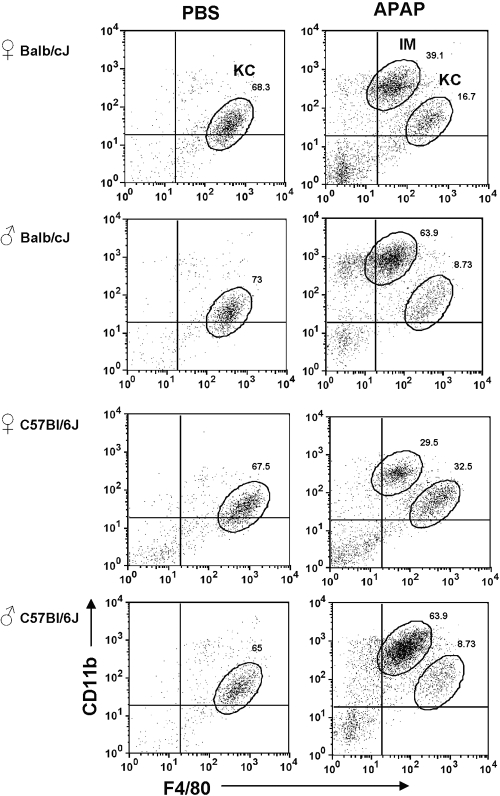
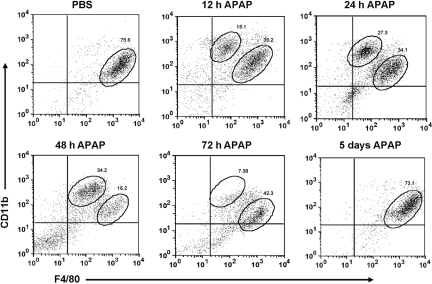
We hypothesized that different populations of macrophages may exist within the liver upon APAP challenge. To examine this hypothesis, we isolated liver NPCs from APAP-treated mice and analyzed these cells using flow cytometry. Hepatic macrophages were identified by using CD11b and F4/80, two commonly used murine macrophage markers [34, 35]. Resident hepatic KCs were observed as a single population, with an expression profile of CD11blow F4/80high in PBS-treated (control) mice (Fig. 1). An additional population of macrophages, with an expression profile of CD11bhigh F4/80low, was observed in APAP-treated mice (Fig. 1). This IM population was similarly induced following APAP challenge in female and male Balb/cJ and C57Bl/6J mice (Fig. 1). Further examination of the IMs demonstrated that they were a transient population, which initially appeared within the liver ∼12 h after APAP challenge and remained present until their eventual disappearance by 5 days after APAP challenge (Fig. 2).
Fig. 1.
Identification of macrophage populations in the liver of mice after APAP challenge. NPCs were isolated from the livers of female and male Balb/cJ and C57Bl/6J mice at 24 h following PBS or APAP challenge. The cells were stained with anti-CD45-FITC, anti-CD11b-PE, and anti-F4/80-APC antibodies, as well as 7-AAD, and analyzed by flow cytometry. 7-AAD– and CD45+ cells were gated, and their expression of CD11b and F4/80 is demonstrated in the dot plots. The percentages of IMs and resident KCs among total 7-AAD– and CD45+ cells are shown. Data shown are representative of six mice per group.
Fig. 2.
Investigation of the time course for the appearance and disappearance of the IMs after APAP challenge. NPCs were isolated from the livers of female C57Bl/6J mice after PBS or at 12 h, 24 h, 48 h, 72 h, and 5 days following APAP challenge. The cells were stained with anti-CD45-FITC, anti-CD11b-PE, and anti-F4/80-APC antibodies, as well as 7-AAD, and analyzed by flow cytometry. 7-AAD– and CD45+ cells were gated, and their expression of CD11b and F4/80 is demonstrated in the dot plots. The percentages of IMs and resident KCs among total 7-AAD– and CD45+ cells are shown. Data shown are representative of three mice per time-point.
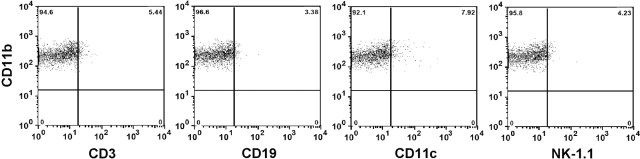
To exclude the possibility of nonmacrophage cell types that also express CD11b, the CD11bhigh F4/80low population was analyzed further for the expression of CD3 (T lymphocyte marker), CD19 (B lymphocyte marker), CD11c (dendritic cell maker), and NK-1.1 (NK cell marker). The expression of CD3, CD19, CD11c, and NK-1.1 was minimal on the IMs (Fig. 3) as well as resident KCs (data not shown), suggesting that both populations were in fact macrophages.
Fig. 3.
Expression of various surface markers by the IMs. NPCs were isolated from livers of male Balb/cJ mice at 24 h following APAP challenge. The cells were stained with anti-CD11b-FITC, anti-F4/80-APC, and anti-CD3-, -CD19-, -CD11c-, or -NK1.1-PE antibodies, as well as 7-AAD, and analyzed by flow cytometry. IMs (CD11bhigh F4/80low) were gated, and their expression of CD3, CD19, CD11c, or NK-1.1 is demonstrated in the dot plots. Data shown are representative of two mice.
Determining the origin of the IMs
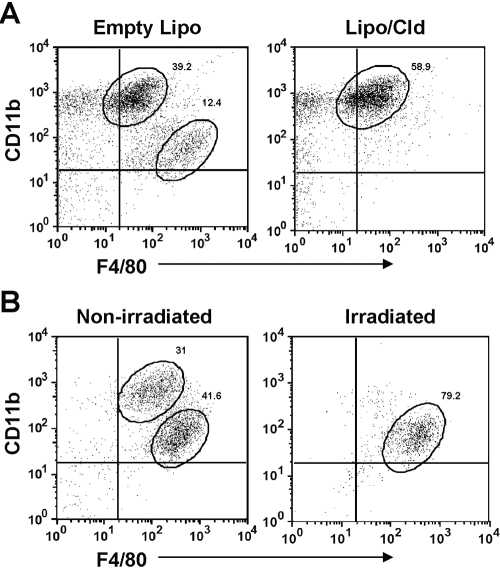
The IMs may represent a subset of activated, resident KCs that have changed their expression profile from CD11blow F4/80high to CD11bhigh F4/80low or may be derived from circulating monocytes that are recruited to the liver upon inflammation. To determine the origin of the IMs, female Balb/cJ mice were injected (i.v.) with liposome/clodronate to deplete resident KCs. Control mice were treated with empty liposomes. After 2 days, mice were challenged with APAP and liver NPCs analyzed. As expected, 24 h following APAP challenge, the resident KCs and IMs were present within the liver of empty liposome-treated, KC-nondepleted mice (Fig. 4A). Interestingly, the IMs were still present in the liver of liposome/clodronate-treated, KC-depleted mice (Fig. 4A). These results suggested that the IMs represented a distinct population from that of resident KCs. To investigate whether the IMs were derived from infiltrating monocytes, female Balb/cJ mice were subjected to bone marrow irradiation to deplete circulating monocytes 3 days prior to APAP challenge. Although resident KCs remained intact in irradiated mice, we observed an almost complete absence of the IMs (Fig. 4B). These results demonstrated that the IMs were derived from circulating monocytes that infiltrated the liver following APAP-induced liver injury.
Fig. 4.
IMs are recruited from the circulation rather than derived from resident KCs. (A) Female Balb/cJ mice were injected (i.v.) with empty liposome (Empty Lipo) or liposome/clodronate (Lipo/Cld) 2 days prior to APAP challenge. Liver NPCs were isolated 24 h after APAP challenge and stained with anti-CD45-FITC, anti-CD11b-PE, and anti-F4/80-APC antibodies, as well as 7-AAD, and analyzed by flow cytometry. 7-AAD– and CD45+ cells were gated, and their expression of CD11b and F4/80 is demonstrated in the dot plots. The percentages of IMs and resident KCs among total 7-AAD– and CD45+ cells are shown. Data shown are representative of four mice per group. (B) Liver NPCs were isolated from female Balb/cJ-nonirradiated (control) and -irradiated mice at 24 h following APAP challenge, stained as described above, and analyzed by flow cytometry. The percentages of IMs and resident KCs among total 7-AAD– and CD45+ cells are shown. Data shown are representative of six mice per group.
Investigation of the phenotype of the IMs
To examine the biological function of the IMs and resident KCs, these cells were purified by FACS at 24 h following APAP challenge, and their expression of a collection of genes associated with macrophage differentiation, activation, and function was examined. Monocytes undergo chemotaxis in response to several chemokines, including fractalkine (CX3CL1) and MCP-1, which bind to the CX3CR1 (also known as the fractalkine receptor) and CCR2, respectively. These two chemokine receptors have been implicated in the migration of monocytes/macrophages to sites of inflammation [36,37,38,39]. Our data demonstrated that the IMs expressed CX3CR1 and CCR2, and the resident KCs appeared to express only CX3CR1 (Fig. 5A). As CX3CL1 and MCP-1 may participate in the recruitment of infiltrating cells to sites of inflammation, we wanted to verify further whether the IMs represented a single population expressing CX3CR1 and CCR2 or two distinct subsets of macrophages each expressing a single chemokine receptor. To clarify this point, we obtained heterozygous C57Bl/6 CX3CR1GFP/+ mice, in which one allele for the gene encoding CX3CR1 has been replaced with the gene encoding GFP. Twenty-four hours following APAP challenge to CX3CR1GFP/+ mice, liver NPCs were analyzed by flow cytometry, and CX3CR1– and CX3CR1+ subpopulations of the IMs were purified by FACS. Gene expression analysis revealed that the CX3CR1– and CX3CR1+ populations expressed comparable levels of CCR2 (Fig. 5B).
Fig. 5.
IMs express genes characteristic of M2. (A) IMs and resident KCs were purified via FACS from the livers of male Balb/cJ mice at 24 h following APAP challenge. RNA was extracted, and the message level of various genes was determined by RT-PCR, including chemokine receptors (CX3CR1, CCR2) and macrophage markers characteristic of an alternative activation (Ym1, MMP-12, MMP-9, Fizz1, Arg-1, Mgl1, and MMR). (B) CX3CR1– and CX3CR1+ subpopulations of the IMs were purified via FACS from the livers of heterozygous C57Bl/6 CX3CR1GFP/+ mice at 24 h following APAP challenge. RNA was extracted, and the message level of CCR2 was determined by RT-PCR.
It is known that compared with M1, M2 express a unique sets of genes, including Ym1, a heparin-binding lectin first identified as transiently expressed and secreted by activated peritoneal macrophages in response to nematode infection [40]; Fizz1, a cysteine-rich, secreted protein, initially found in the bronchoalveolar fluid from mice with experimentally induced allergic pulmonary inflammation [41, 42]; and Arg-1, which competes with NO synthetase for metabolism of l-arginine [43]. Although the IMs expressed significant levels of Ym1, Fizz1, and Arg-1, the resident KCs demonstrated minimal levels of Ym1 and Arg-1 and no apparent expression of Fizz1. M2 have further been observed to express high levels of MMP-12 and MMP-9 [44,45,46], as well as Mgl [47] and MMR [22], all of which have been reported to play important roles in tissue repair processes [48,49,50,51]. Our results revealed that the IMs and resident KCs expressed MMP-12, MMP-9, Mgl1, and MMR (Fig. 5A). Collectively, this expression profile suggested an M2 signature for the IMs, a signature that has been associated with a phenotype involving down-regulation of inflammation, high capacity for phagocytosis, and induction of angiogenesis and tissue repair.
Ability of the IMs to phagocytize apoptotic cells
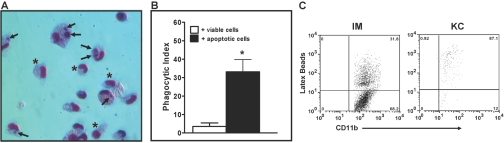
Macrophages are known to be professional phagocytes [52] that play a significant role in the clearance of apoptotic cells, which is critical in the resolution of inflammation. Therefore, we investigated the in vitro ability of the IMs to phagocytize apoptotic cells. IMs were purified by FACS at 24 h following APAP challenge and cocultured with viable or apoptotic Jurkat T cells. Engulfment of apoptotic cells by the IMs is shown in a representative image (Fig. 6A), and phagocytosis was quantified by calculating the PI (Fig. 6B), which for the IMs, was greater than 30%, similar to that of potent phagocytes reported in the literature [53, 54]. The in vivo phagocytic abilities of the IMs and resident KCs were determined at 24 h following APAP challenge after injection of mice with red fluorescent latex beads and detecting the uptake of beads by the two macrophage populations (Fig. 6C). Similar to the results from the in vitro phagocytosis assay, the IMs demonstrated in vivo phagocytic capacity, as ∼31% of the cells had taken up the beads. The data also revealed that resident KCs are more potent phagocytic cells, as ∼88% of the cells could take up the beads.
Fig. 6.
In vitro phagocytosis of apoptotic Jurkat T cells and in vivo phagocytosis of red latex beads by the IMs. Viable or apoptotic (UV-irradiated) Jurkat T cells were cocultured for 90 min with IMs (isolated via FACS from male Balb/cJ mice at 24 h following APAP challenge) at a ratio of 3:1 (Jurkat T cell:IM). (A) Representative image of the IMs following coculture with apoptotic Jurkat T cells. Arrows indicate ingested, apoptotic Jurkat T cells and asterisks indicate IMs that have not phagocytized. (B) PI of the IMs following coculture with viable (open bar) or apoptotic (solid bar) Jurkat T cells. The PI was calculated as the number of Jurkat T cells ingested divided by the total number of macrophages counted × 100. *, P < 0.05, compared with + viable cells. Data shown are representative of three separate experiments with one to two replicates per experiment. (C) Liver NPCs were isolated from male Balb/cJ mice (injected i.v. with red latex beads 2 h prior to sacrifice) at 24 h following APAP challenge. The cells were stained with anti-CD11b-PE and anti-F4/80-APC and analyzed by flow cytometry. The IMs and resident KCs were then gated, and their respective red fluorescence is demonstrated in the dot plots. Data shown are representative of two mice.
Induction of PMN apoptosis by the IMs
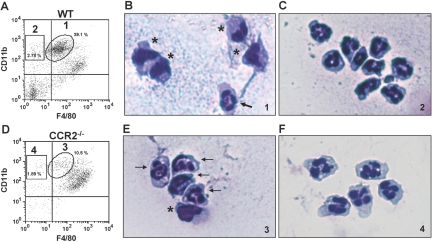
A number of studies have demonstrated that MCP-1 and its receptor CCR2 play a key role in the recruitment of monocytes into inflamed tissue [17, 55, 56]. We examined liver NPCs in CCR2−/− mice at 24 h after APAP challenge and observed a dramatic decrease of the IMs in female Balb/cJ CCR2−/− mice (Fig. 7D) compared with WT mice (Fig. 7A). A similar decrease of the IMs was also observed in male Balb/cJ CCR2−/− mice, as well as female and male C57Bl/6J CCR2−/− mice (data not shown) compared with their respective WT controls. However, the CD11bhigh F4/80low population was still minimally present in CCR2−/− mice (Fig. 7D). A further analysis revealed that these cells in the CCR2−/− mice expressed Gr-1, a granulocyte marker (data not shown), and the CD11bhigh F4/80low population in the WT mice did not (data not shown). To explore differences in hepatic NPC populations between WT and CCR2−/− mice, we examined cellular morphology of various populations after purification by FACS at 24 h following APAP challenge. Although the majority of the CD11bhigh F4/80low population (IMs) in the WT mice had horseshoe- or kidney-shaped nuclei, a morphology consistent with that of monocytes/macrophages (Fig. 7B), the majority of this same population in the CCR2−/− mice, appeared to be PMNs (Fig. 7E), with nuclei consisting of several linked lobes. The small population of CD11bhigh F4/80– cells in WT (Fig. 7C) and CCR2−/− (Fig. 7F) mice also demonstrated a PMN morphology. Furthermore, 24 h following APAP challenge, the number of PMNs in the liver, based on flow cytometric analysis, was increased in the CCR2−/− mice (9.70±2.32×105 cells) compared with WT mice (4.71±1.37×105 cells). A similar increase in the number of PMNs was observed in the peritoneal cavity of thioglycolate-treated CCR2−/− mice compared with WT mice (data not shown). These results suggested a potential relationship between the IMs and PMNs.
Fig. 7.
Morphological analysis of hepatic NPCs in WT and CCR2−/− mice. Liver NPCs were isolated from male Balb/cJ (A) WT and (D) CCR2−/− mice at 24 h following APAP challenge. The cells were stained with anti-CD45-FITC, anti-CD11b-PE, and anti-F4/80-APC antibodies, as well as 7-AAD, and analyzed by flow cytometry. 7-AAD– and CD45+ cells were gated, and their expression of CD11b and F4/80 is demonstrated in the dot plots. The percentages of IMs and resident KCs among total 7-AAD–and CD45+ cells are shown. Data shown are representative of six mice per group. Liver NPC populations were sorted via FACS at 24 h following APAP challenge. The purified populations, labeled 1 (B) and 2 (C) from the WT mice and 3 (E) and 4 (F) from the CCR2−/− mice, were cytospun individually and stained with Hema 3 manual staining system. Each population of cells was examined using light microscopy (original magnification, 400×). Arrows indicate PMNs. *, Macrophages. Data shown are representative of two separate experiments.
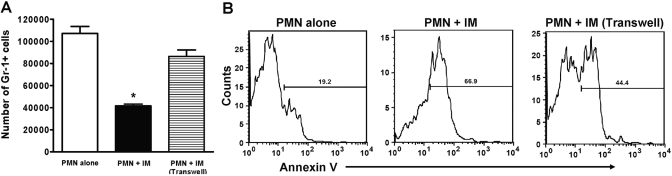
We hypothesized that the IMs are capable of inducing apoptosis of PMNs, and therefore, the lack of IMs may explain the increased number of PMNs present in the liver of CCR2−/− mice following APAP challenge. To investigate the ability of the IMs to induce PMN apoptosis, we prepared cocultures of PMNs (thioglycolate-elicited peritoneal exudate) and IMs (isolated via positive selection at 24 h following APAP challenge). Flow cytometric analysis of the PMNs revealed that their numbers decreased significantly compared with those in cultures of PMNs alone (Fig. 8A). Among the remaining PMNs, the percentage of apoptosis (Annexin V+) increased significantly in the cocultures (67%) compared with that in cultures of PMNs alone (19%; Fig. 8B), suggesting that the IMs are potent inducers of PMN apoptosis. Although macrophage secretory products have been demonstrated to induce apoptosis of PMNs [57,58,59,60,61], it has recently been revealed that this process requires cell-cell contact, in particular, through membrane-bound but not soluble TNF-α [62, 63]. To examine further whether this IM-mediated induction of PMN apoptosis requires direct cell-cell contact, we repeated the cocultures yet separated the PMNs from the IMs using transwell inserts. The data demonstrated that the number of remaining PMNs in the transwell cultures was comparable with those in cultures of PMNs alone (Fig. 8A). The percentage of apoptosis among the remaining PMNs was decreased compared with those cocultured in contact with the IMs (Fig. 8B), suggesting a cell-cell contact-dependent induction of apoptosis. These results indicated an important role for IMs in the clearance of PMNs and protection against prolonged inflammation and tissue damage, as PMNs have been shown to contribute to the development of APAP-induced liver injury [11].
Fig. 8.
The ability of the IMs to induce apoptosis of PMNs. Thioglycolate-elicited peritoneal exudate PMNs were cultured alone and in contact with or separated from IMs (isolated via MACS selection from KC-depleted male Balb/cJ mice at 24 h following APAP challenge) by transwell inserts at a ratio of 1:3 (PMN:IM). Following 18 h coculture, the supernatant was removed and stained with anti-Gr-1-PE antibody. (A) The total number of Gr-1+ cells remaining within each well was calculated by multiplying the percentage of Gr-1+ cells by the percentage of total cells gated from FSC versus SSC times the total number of cells collected in the supernatant for analysis [PMN alone (open bar), PMN+IM in direct contact (solid bar), PMN+IM separated via transwell insert (lined bar)]. *, P < 0.05, compared with PMN alone and PMN + IM (Transwell). (B) PMNs were analyzed for early apoptosis using Annexin V-FITC by flow cytometry. Data shown are representative of two separate experiments with four replicates per experiment.
Resolution of hepatic damage is delayed in the absence of the IMs
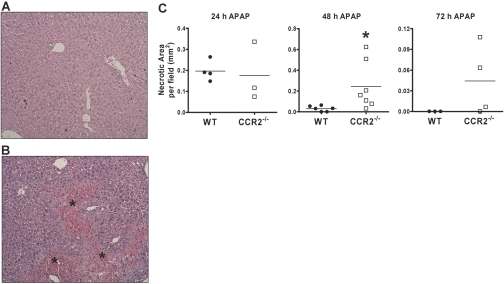
To investigate whether the IMs are involved in APAP-induced liver injury, we challenged female Balb/cJ WT and CCR2−/− mice with APAP. No significant difference was observed in the degree of APAP-induced hepatotoxicity, as measured by alanine aminotransferase (ALT) levels, between WT and CCR2−/− mice at 10 and 24 h following APAP challenge (data not shown). Histological evaluation of liver sections at 24 h after APAP challenge revealed a comparable extent of hepatic damage in WT and CCR2−/− mice, as measured by the average area of necrosis among six separate fields per tissue section (Fig. 9C). Hepatic damage was diminished dramatically and appeared to return to a normal, pathological state in WT mice by 48 (Fig. 9A) and 72 h (data not shown) following APAP challenge. In contrast, extensive necrosis and hemorrhage were still evident and remained elevated in CCR2−/− mice at 48 (Fig. 9B) and 72 h (data not shown) after APAP challenge.
Fig. 9.
Resolution of hepatic damage is delayed in the absence of the IMs. Liver sections were obtained from female Balb/cJ WT and CCR2−/− mice at 24 h, 48 h, and 72 h after APAP challenge and stained with H&E. Representative liver sections from (A) WT and (B) CCR2−/− mice at 48 h following APAP challenge (original magnification, 100×). *, Area of necrosis and hemorrhage. (C) Hepatic necrosis was quantified using light microscopy by measuring the total area (mm2) of necrosis within six separate fields per tissue section. *, P < 0.05, compared with WT. Data are the mean area of necrosis among the six fields from three to seven mice per group per time-point.
DISCUSSION
Studies of APAP-induced liver injury in mice have revealed a dichotomy of pro-toxicant and hepato-protective functions of hepatic macrophages [14,15,16]. This controversy may stem from the heterogeneity and/or plasticity of macrophages and the difficulty in distinguishing subpopulations of macrophages in the liver and studying them separately. The present report described the first study to clearly distinguish a population of infiltrating macrophages from the resident KCs. Our data demonstrated that it is possible to isolate and purify the two macrophage populations separately and examine their functions as distinct cell types. The IMs exhibited a phenotype consistent with that of M2, and furthermore, in the absence of the IMs, the resolution of hepatic necrosis following APAP-induced liver injury was delayed in CCR2−/− mice compared with WT mice.
Hepatic macrophages in APAP-treated mice were examined in a previous study [64] by immunohistochemical analysis using anti-F4/80, which is highly expressed on resident KCs [34, 35, 64] and anti-CD68, recognizing macrosialin that is thought to be expressed on activated macrophages [64,65,66]. The number of F4/80+ cells within the liver decreased by 75% at 24 and 48 h following APAP challenge, which coincided with the accumulation of activated CD68+ macrophages [64]. However, this analysis was unable to distinguish whether the CD68+ macrophages represented an activated subpopulation of resident KCs or a separate macrophage population. We decided to investigate hepatic macrophages by flow cytometric analysis using the macrophage markers CD11b (membrane-activated complex 1) and F4/80. In the liver of APAP-treated mice, we were able to detect resident KCs (CD11blow F4/80high) and a population of IMs exhibiting an expression profile of CD11bhigh F4/80low (Fig. 1). Our data further demonstrated that depletion of resident KCs did not affect the appearance of the IMs (Fig. 4A); however, these cells were absent in bone marrow-irradiated mice (Fig. 4B) and in CCR2−/− mice (Fig. 7D). These results suggested that the IMs represent a bone marrow-derived, circulating monocyte/macrophage population, distinct from resident KCs, which infiltrate the liver in response to APAP challenge.
Investigation into the heterogeneity of peripheral monocytes by Geissmann et al. [17] initially identified two subsets, characterized as CCR2+ CX3CR1int and CCR2– CX3CR1high. Our analysis of the IMs using heterozygous CX3CR1GFP/+ mice revealed that the cells consisted of two subpopulations, characterized as CX3CR1– and CX3CR1+, and that both populations expressed similar message levels of CCR2 (Fig. 5B). The potential discrepancy between the Geissmann et al. [17] study and our data may be explained by the analysis of protein surface expression by Geissmann et al. [17] compared with our gene expression analysis.
A phenotype transition of recruited monocytes during the resolution of inflammation and tissue repair has been demonstrated recently [67]. After injury, skeletal muscle recruited inflammatory monocytes, which switched their phenotype to acquire an anti-inflammatory profile upon phagocytosis of muscle cell debris. It was further demonstrated that in vivo depletion of these circulating monocytes at the time of injury completely prevented muscle regeneration. Our in vitro phagocytosis assay demonstrated that the IMs were competent in taking up apoptotic Jurkat T cells with a relatively high PI value (Fig. 6B). The phagocytic capacity of the IMs was confirmed further by their in vivo engulfment of latex beads (Fig. 6C). The process of phagocytosis of apoptotic cells and cellular debris has been demonstrated to inhibit the production of proinflammatory mediators, such as TNF-α, IL-1β, IL-8, and leukotriene C4 [26, 68], in conjunction with the generation of potent, anti-inflammatory mediators, such as TGF-β and PGE2 [26, 68, 69], which suppress the inflammatory response. It has recently been demonstrated that upon phagocytosis of dead cells, macrophages produce a secretory leukocyte protease inhibitor (SLPI), which contributes to the phenotypic switch of macrophages from a proinflammatory type to an anti-inflammatory, tissue-protective cell type [70]. Impairment in the clearance of apoptotic cells and cellular debris may contribute to a delayed and impaired wound healing, as has been observed in TGF-β null mice [71] and SLPI-deficient mice [72]. Our data and these recent reports suggest that IMs found in the liver following APAP challenge may demonstrate an anti-inflammatory phenotype after engulfment of apoptotic cells or cellular debris and thereby, play an important role in the resolution of APAP-induced inflammation.
APAP-induced liver injury is histopathologically characterized by centrilobular necrosis with a massive infiltration of leukocytes, particularly PMNs [10, 73,74,75]. Recent studies demonstrated a significant decrease in APAP-induced hepatotoxicity as a result of PMN depletion, providing evidence for the involvement of PMNs in the progression of APAP-induced liver injury [11]. Removal of PMNs from inflammatory sites is beneficial, as the recognition and ingestion of apoptotic PMNs protect the surrounding tissue from the potentially proinflammatory and noxious, intracellular contents that inevitably leak from these cells that die by the alternative pathway of necrosis [76]. Furthermore, persistent, PMN-rich inflammatory infiltrates have been associated with the increased tissue destruction associated with unresolved inflammatory reactions, such as those found in adult respiratory distress syndrome, rheumatoid arthritis, and gout [77, 78]. PMN apoptosis, which results in the recognition and uptake of these cells by macrophages, has been proposed as an important mechanism for the removal of PMNs from sites of inflammation [79]. We found that compared with WT mice, in the absence of IMs in CCR2−/− mice, there was a significant increase in the number of PMNs in the liver and peritoneal cavity following APAP challenge and thioglycolate treatment, respectively (data not shown). Furthermore, our data demonstrated that aside from phagocytosis of apoptotic cells, the IMs are capable of actively inducing apoptosis of PMNs through a cell-cell contact-dependent manner (Fig. 8). These results suggest that IM-mediated induction of PMN apoptosis and removal of apoptotic PMNs represent an important hepato-protective mechanism that prevents prolonged inflammation and tissue destruction.
Although no studies have specifically investigated the role of IMs in the pathogenesis of APAP-induced liver injury, one report has demonstrated that CCR2−/− mice exhibited increased sensitivity to APAP compared with WT mice, most notably, at 24 h following APAP challenge [80]. An exaggerated apoptotic and necrotic liver injury as well as significant elevations in the proinflammatory cytokines IFN-γ and TNF-α were observed in the liver of CCR2−/− mice compared with WT mice. The increased toxicity in the CCR2−/− mice was attributed to the lack of hepato-protection provided by CCR2, through its regulation of cytokine expression during APAP challenge. It is important to note that in this above study, APAP failed to induce liver injury in WT mice. Using CCR2−/− mice on a different background strain from this above study, a recent report has shown [64], and our present findings reveal no dramatic difference in the sensitivity of CCR2−/− mice to APAP-induced hepatotoxicity compared with WT mice, as determined by ALT levels (data not shown). We rationalize that these results would be expected, as our data showed that IMs do not begin to appear within the liver until 12 h following APAP challenge, a time-point at which the initiation and propagation of injury are already established. Although the measurement of ALT levels is used routinely as an indicator of liver injury, the clinical gold standard remains histological evaluation. Histological assessment of liver sections during the resolution phase of APAP-induced liver injury revealed a significantly delayed process of tissue recovery in CCR2−/− mice. Extensive necrosis and hemorrhage were still evident in CCR2−/− mice at 48 and 72 h after APAP challenge, whereas the liver returned to an apparent normal, pathological state in WT mice (Fig. 9).
Enhanced expression in M2 compared with M1 has been documented for a number of genes, including Ym1, Fizz1, Arg-1, Mgl1, and MMR [19, 22, 25, 41, 43, 47, 81,82,83,84,85]. Gene expression analysis of the IMs suggested a signature consistent with that of M2 (Fig. 5A). In comparison with IMs, resident KCs expressed similar levels of several M2 genes; however, they exhibited minimal expression levels of Ym1 and Arg-1 and did not express Fizz1. M2 have been observed during the healing phase of acute inflammation [86], in chronic inflammatory diseases such as rheumatoid arthritis [87], and in wound healing [88]. Functionally, M2 are involved in the resolution of inflammation, promotion of angiogenesis and wound healing, and the elimination of apoptotic bodies and tissue debris [25, 89]. Arg-1 and Fizz1 have been demonstrated to promote angiogenesis and wound healing [90, 91]. Proteolytic degradation and remodeling of the extracellular matrix are essential features of tissue repair, of which, MMP-12 and MMP-9 are believed to play an essential role in this process. Furthermore, MMP-9 has been shown to induce promotion of the angiogenesis switch and release of growth factors [92,93,94,95], which may contribute to wound healing and tissue repair. Altogether, the expression of these various genes by the IMs (Fig. 5A) coincides with the phenotype of M2, which play important roles in dampening inflammation and promoting angiogenesis and tissue repair. There is evidence to support that besides hepatocyte damage, APAP overdose also causes microcirulatory dysfunction and LSEC injury [96,97,98], suggesting that angiogenesis represents an important process of liver repair. However, research about the process of tissue repair and more specifically, angiogenesis, following APAP-induced hepatotoxicity, remains scarce. Based on the reports about the tissue repair functions of M2, our data about an M2 signature gene expression by the IMs, and the presence of the IMs at the later stages of hepatotoxicity, we propose that these cells play a key role in the processes of tissue repair following APAP-induced liver injury.
In summary, the present study demonstrated the existence of two subpopulations of hepatic macrophages in mice following APAP-induced liver injury. The IMs represent a circulating monocyte population that infiltrates the liver in response to APAP challenge. Characterization of the IMs suggests that they share a phenotype with M2. In the absence of the IMs, the repair of liver damage following APAP-induced hepatotoxicity was delayed in CCR2−/− mice compared with WT mice. The observed IM-mediated induction of PMN apoptosis as well as the ability to phagocytize apoptotic cells likely contribute to their role in the resolution of inflammation and promotion of tissue repair following APAP-induced liver injury.
Acknowledgments
This work was supported by U.S. National Institutes of Health grant RO1 ES012914 (to C. J.) and Pre- Doctoral Fellowship in Pharmacology/Toxicology from the PhRMA Foundation. The authors thank Drs. Peter Henson and Kathleen McPhillips [Department of Pediatrics, National Jewish Medical and Research Center (NJMRC), Denver, CO, USA] for their assistance with the in vitro phagocytosis assay, as well as Josh Loomis and Shirley Sobus (Cyomtery Core Facility, NJMRC) for their assistance with FACS. The authors also thank Dr. Ross Kedl (Department of Immunology, NJMRC) for his advice and discussions and Dr. Lawrence Lu (Department of Renal Diseases and Hypertension, School of Medicine, UCD) for his assistance with the CX3CR1GFP/+ mice.
References
- Hartleb M, Biernat L, Kochel A. Drug-induced liver damage—a three-year study of patients from one gastroenterological department. Med Sci Monit. 2002;8:CR292–CR296. [PubMed] [Google Scholar]
- Lazarou J, Pomeranz B H, Corey P N. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- Lazerow S K, Abdi M S, Lewis J H. Drug-induced liver disease 2004. Curr Opin Gastroenterol. 2005;21:283–292. doi: 10.1097/01.mog.0000160043.10804.60. [DOI] [PubMed] [Google Scholar]
- Lee W M, Senior J R. Recognizing drug-induced liver injury: current problems, possible solutions. Toxicol Pathol. 2005;33:155–164. doi: 10.1080/01926230590522356. [DOI] [PubMed] [Google Scholar]
- Lee W M. Drug-induced hepatotoxicity. N Engl J Med. 2003;349:474–485. doi: 10.1056/NEJMra021844. [DOI] [PubMed] [Google Scholar]
- Larson A M, Polson J, Fontana R J, Davern T J, Lalani E, Hynan L S, Reisch J S, Schiodt F V, Ostapowicz G, Shakil A O, Lee W M, Acute Liver Failure Study Group Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- Nelson S D. Mechanisms of the formation and disposition of reactive metabolites that can cause acute liver injury. Drug Metab Rev. 1995;27:147–177. doi: 10.3109/03602539509029821. [DOI] [PubMed] [Google Scholar]
- Pumford N R, Halmes N C, Hinson J A. Covalent binding of xenobiotics to specific proteins in the liver. Drug Metab Rev. 1997;29:39–57. doi: 10.3109/03602539709037572. [DOI] [PubMed] [Google Scholar]
- Lee S S, Buters J T, Pineau T, Fernandez-Salguero P, Gonzalez F J. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- Liu Z X, Govindarajan S, Kaplowitz N. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology. 2004;127:1760–1774. doi: 10.1053/j.gastro.2004.08.053. [DOI] [PubMed] [Google Scholar]
- Liu Z X, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology. 2006;43:1220–1230. doi: 10.1002/hep.21175. [DOI] [PubMed] [Google Scholar]
- Liu Z X, Kaplowitz N. Immune-mediated drug-induced liver disease. Clin Liver Dis. 2002;6:755–774. doi: 10.1016/s1089-3261(02)00025-9. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Kondo T, Kimura A, Tsuneyama K, Takayasu T, Mukaida N. Opposite roles of neutrophils and macrophages in the pathogenesis of acetaminophen-induced acute liver injury. Eur J Immunol. 2006;36:1028–1038. doi: 10.1002/eji.200535261. [DOI] [PubMed] [Google Scholar]
- Michael S L, Pumford N R, Mayeux P R, Niesman M R, Hinson J A. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology. 1999;30:186–195. doi: 10.1002/hep.510300104. [DOI] [PubMed] [Google Scholar]
- Laskin D L, Gardner C R, Price V F, Jollow D J. Modulation of macrophage functioning abrogates the acute hepatotoxicity of acetaminophen. Hepatology. 1995;21:1045–1050. [PubMed] [Google Scholar]
- Ju C, Reilly T P, Bourdi M, Radonovich M F, Brady J N, George J W, Pohl L R. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol. 2002;15:1504–1513. doi: 10.1021/tx0255976. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman D R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Stout R D, Suttles J. T cell signaling of macrophage function in inflammatory disease. Front Biosci. 1997;2:d197–d206. doi: 10.2741/a183. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Stout R D, Suttles J. T cell signaling of macrophage activation. Landes R G, editor. TX: Austin; Cell Contact-Dependent and Cytokine Signals. 1995 [Google Scholar]
- Goerdt S, Politz O, Schledzewski K, Birk R, Gratchev A, Guillot P, Hakiy N, Klemke C D, Dippel E, Kodelja V, Orfanos C E. Alternative versus classical activation of macrophages. Pathobiology. 1999;67:222–226. doi: 10.1159/000028096. [DOI] [PubMed] [Google Scholar]
- Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield J S. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci (Lond) 2003;104:27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Erwig L P, Kluth D C, Walsh G M, Rees A J. Initial cytokine exposure determines function of macrophages and renders them unresponsive to other cytokines. J Immunol. 1998;161:1983–1988. [PubMed] [Google Scholar]
- Goerdt S, Orfanos C E. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10:137–142. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- Fadok V A, Bratton D L, Konowal A, Freed P W, Westcott J Y, Henson P M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolone J B, Sparks K, Cohen S D, Khairallah E A. Immunochemical detection of acetaminophen-bound liver proteins. Biochem Pharmacol. 1987;36:1193–1196. doi: 10.1016/0006-2952(87)90069-4. [DOI] [PubMed] [Google Scholar]
- Fennekohl A, Sugimoto Y, Segi E, Maruyama T, Ichikawa A, Puschel G P. Contribution of the two Gs-coupled PGE2-receptors EP2-receptor and EP4-receptor to the inhibition by PGE2 of the LPS-induced TNFα-formation in Kupffer cells from EP2- or EP4-receptor-deficient mice. Pivotal role for the EP4-receptor in wild type Kupffer cells. J Hepatol. 2002;36:328–334. doi: 10.1016/s0168-8278(01)00277-x. [DOI] [PubMed] [Google Scholar]
- Smedsrod B, Pertoft H. Preparation of pure hepatocytes and reticuloendothelial cells in high yield from a single rat liver by means of Percoll centrifugation and selective adherence. J Leukoc Biol. 1985;38:213–230. doi: 10.1002/jlb.38.2.213. [DOI] [PubMed] [Google Scholar]
- Ju C, Pohl L R. Immunohistochemical detection of protein adducts of 2,4-dinitrochlorobenzene in antigen presenting cells and lymphocytes after oral administration to mice: lack of a role of Kupffer cells in oral tolerance. Chem Res Toxicol. 2001;14:1209–1217. doi: 10.1021/tx0100587. [DOI] [PubMed] [Google Scholar]
- Meijer C, Wiezer M J, Diehl A M, Schouten H J, Schouten H J, Meijer S, van Rooijen N, van Lambalgen A A, Dijkstra C D, van Leeuwen P A. Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver. 2000;20:66–77. doi: 10.1034/j.1600-0676.2000.020001066.x. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N, Kors N, vd Ende M, Dijkstra C D. Depletion and repopulation of macrophages in spleen and liver of rat after intravenous treatment with liposome-encapsulated dichloromethylene diphosphonate. Cell Tissue Res. 1990;260:215–222. doi: 10.1007/BF00318625. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Naito M, Moriyama H, Umezu H, Matsuo H, Kiwada H, Arakawa M. Repopulation of murine Kupffer cells after intravenous administration of liposome-encapsulated dichloromethylene diphosphonate. Am J Pathol. 1996;149:1271–1286. [PMC free article] [PubMed] [Google Scholar]
- Lee S H, Starkey P M, Gordon S. Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J Exp Med. 1985;161:475–489. doi: 10.1084/jem.161.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenen P J, de Bruijn M F, Voerman J S, Campbell P A, van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Goda S, Imai T, Yoshie O, Yoneda O, Inoue H, Nagano Y, Okazaki T, Imai H, Bloom E T, Domae N, Umehara H. CX3C-chemokine, fractalkine-enhanced adhesion of THP-1 cells to endothelial cells through integrin-dependent and -independent mechanisms. J Immunol. 2000;164:4313–4320. doi: 10.4049/jimmunol.164.8.4313. [DOI] [PubMed] [Google Scholar]
- Fong A M, Robinson L A, Steeber D A, Tedder T F, Yoshie O, Imai T, Patel D D. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med. 1998;188:1413–1419. doi: 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell C A, Cleary M D, Charo I F. Unique role of the chemokine domain of fractalkine in cell capture. Kinetics of receptor dissociation correlate with cell adhesion. J Biol Chem. 2000;275:34183–34189. doi: 10.1074/jbc.M005731200. [DOI] [PubMed] [Google Scholar]
- Kuziel W A, Morgan S J, Dawson T C, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci USA. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang N C, Hung S I, Hwa K Y, Kato I, Chen J E, Liu C H, Chang A C. A macrophage protein, Ym1, transiently expressed during inflammation is a novel mammalian lectin. J Biol Chem. 2001;276:17497–17506. doi: 10.1074/jbc.M010417200. [DOI] [PubMed] [Google Scholar]
- Raes G, De Baetselier P, Noel W, Beschin A, Brombacher F, Hassanzadeh G G. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597–602. [PubMed] [Google Scholar]
- Holcomb I N, Kabakoff R C, Chan B, Baker T W, Gurney A, Henzel W, Nelson C, Lowman H B, Wright B D, Skelton N J, Frantz G D, Tumas D B, Peale F V, Jr, Shelton D L, Hébert C C. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000;19:4046–4055. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol. 1998;160:5347–5354. [PubMed] [Google Scholar]
- Zheng T, Zhu Z, Wang Z, Homer R J, Ma B, Riese R J, Jr, Chapman H A, Jr, Shapiro S D, Elias J A. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest. 2000;106:1081–1093. doi: 10.1172/JCI10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Noncourt P, Robledo O, Alain T, Kossakowska A E, Urbanski S J, Potworowski E F, St Pierre Y. Leukocyte elastase in murine and human non-Hodgkin lymphomas. J Leukoc Biol. 2001;70:585–591. [PubMed] [Google Scholar]
- Lee C G, Homer R J, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley J M, Gotwals P, Noble P, Chen Q, Senior R M, Elias J A. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β(1) J Exp Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes G, Brys L, Dahal B K, Brandt J, Grooten J, Brombacher F, Vanham G, Noel W, Bogaert P, Boonefaes T, Kindt A, Van den Bergh R, Leenen P J, De Baetselier P, Ghassabeh G H. Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J Leukoc Biol. 2005;77:321–327. doi: 10.1189/jlb.0304212. [DOI] [PubMed] [Google Scholar]
- Gill S E, Parks W C. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol. 2008;40:1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J N, Winter D C, Bouchier-Hayes D. Biological fate and clinical implications of arginine metabolism in tissue healing. Wound Repair Regen. 2006;14:376–386. doi: 10.1111/j.1743-6109.2006.00151.x. [DOI] [PubMed] [Google Scholar]
- Sturge J, Todd S K, Kogianni G, McCarthy A, Isacke C M. Mannose receptor regulation of macrophage cell migration. J Leukoc Biol. 2007;82:585–593. doi: 10.1189/jlb.0107053. [DOI] [PubMed] [Google Scholar]
- Sato K, Imai Y, Higashi N, Kumamoto Y, Onami T M, Hedrick S M, Irimura T. Lack of antigen-specific tissue remodeling in mice deficient in the macrophage galactose-type calcium-type lectin 1/CD301a. Blood. 2005;106:207–215. doi: 10.1182/blood-2004-12-4943. [DOI] [PubMed] [Google Scholar]
- Dini L, Pagliara P, Carla E C. Phagocytosis of apoptotic cells by liver: a morphological study. Microsc Res Tech. 2002;57:530–540. doi: 10.1002/jemt.10107. [DOI] [PubMed] [Google Scholar]
- Bijl M, Reefman E, Horst G, Limburg P C, Kallenberg C G. Reduced uptake of apoptotic cells by macrophages in systemic lupus erythematosus: correlates with decreased serum levels of complement. Ann Rheum Dis. 2006;65:57–63. doi: 10.1136/ard.2005.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardai S J, McPhillips K A, Frasch S C, Janssen W J, Starefeldt A, Murphy-Ullrich J E, Bratton D L, Oldenborg P A, Michalak M, Henson P M. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Serbina N V, Pamer E G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- Xu H, Manivannan A, Dawson R, Crane I J, Mack M, Sharp P, Liversidge J. Differentiation to the CCR2+ inflammatory phenotype in vivo is a constitutive, time-limited property of blood monocytes and is independent of local inflammatory mediators. J Immunol. 2005;175:6915–6923. doi: 10.4049/jimmunol.175.10.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S B, Savill J. Phagocytosis triggers macrophage release of Fas ligand and induces apoptosis of bystander leukocytes. J Immunol. 1999;162:480–485. [PubMed] [Google Scholar]
- Kettritz R, Xu Y X, Kerren T, Quass P, Klein J B, Luft F C, Haller H. Extracellular matrix regulates apoptosis in human neutrophils. Kidney Int. 1999;55:562–571. doi: 10.1046/j.1523-1755.1999.00280.x. [DOI] [PubMed] [Google Scholar]
- Lang R A, Bishop J M. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 1993;74:453–462. doi: 10.1016/0092-8674(93)80047-i. [DOI] [PubMed] [Google Scholar]
- Murray J, Barbara J A, Dunkley S A, Lopez A F, Van Ostade X, Condliffe A M, Dransfield I, Haslett C, Chilvers E R. Regulation of neutrophil apoptosis by tumor necrosis factor-α: requirement for TNFR55 and TNFR75 for induction of apoptosis in vitro. Blood. 1997;90:2772–2783. [PubMed] [Google Scholar]
- Tsuchida H, Takeda Y, Takei H, Shinzawa H, Takahashi T, Sendo F. In vivo regulation of rat neutrophil apoptosis occurring spontaneously or induced with TNF-α or cycloheximide. J Immunol. 1995;154:2403–2412. [PubMed] [Google Scholar]
- Meszaros A J, Reichner J S, Albina J E. Macrophage-induced neutrophil apoptosis. J Immunol. 2000;165:435–441. doi: 10.4049/jimmunol.165.1.435. [DOI] [PubMed] [Google Scholar]
- Allenbach C, Zufferey C, Perez C, Launois P, Mueller C, Tacchini-Cottier F. Macrophages induce neutrophil apoptosis through membrane TNF, a process amplified by Leishmania major. J Immunol. 2006;176:6656–6664. doi: 10.4049/jimmunol.176.11.6656. [DOI] [PubMed] [Google Scholar]
- Dambach D M, Watson L M, Gray K R, Durham S K, Laskin D L. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology. 2002;35:1093–1103. doi: 10.1053/jhep.2002.33162. [DOI] [PubMed] [Google Scholar]
- Kurushima H, Ramprasad M, Kondratenko N, Foster D M, Quehenberger O, Steinberg D. Surface expression and rapid internalization of macrosialin (mouse CD68) on elicited mouse peritoneal macrophages. J Leukoc Biol. 2000;67:104–108. doi: 10.1002/jlb.67.1.104. [DOI] [PubMed] [Google Scholar]
- Rabinowitz S S, Gordon S. Macrosialin, a macrophage-restricted membrane sialoprotein differentially glycosylated in response to inflammatory stimuli. J Exp Med. 1991;174:827–836. doi: 10.1084/jem.174.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold L, Henry A, Poron F, Baba-Am Y, van Rooijen N, Plonquet A, Gherardi R K, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald P P, Fadok V A, Bratton D, Henson P M. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-β in macrophages that have ingested apoptotic cells. J Immunol. 1999;163:6164–6172. [PubMed] [Google Scholar]
- Huynh M L, Fadok V A, Henson P M. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odaka C, Mizuochi T, Yang J, Ding A. Murine macrophages produce secretory leukocyte protease inhibitor during clearance of apoptotic cells: implications for resolution of the inflammatory response. J Immunol. 2003;171:1507–1514. doi: 10.4049/jimmunol.171.3.1507. [DOI] [PubMed] [Google Scholar]
- Crowe M J, Doetschman T, Greenhalgh D G. Delayed wound healing in immunodeficient TGF-β 1 knockout mice. J Invest Dermatol. 2000;115:3–11. doi: 10.1046/j.1523-1747.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- Ashcroft G S, Lei K, Jin W, Longenecker G, Kulkarni A B, Greenwell-Wild T, Hale-Donze H, McGrady G, Song X Y, Wahl S M. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat Med. 2000;6:1147–1153. doi: 10.1038/80489. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Kondo T, Ohshima T, Fujiwara H, Iwakura Y, Mukaida N. A pivotal involvement of IFN-γ in the pathogenesis of acetaminophen-induced acute liver injury. FASEB J. 2002;16:1227–1236. doi: 10.1096/fj.02-0046com. [DOI] [PubMed] [Google Scholar]
- Lawson J A, Farhood A, Hopper R D, Bajt M L, Jaeschke H. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol Sci. 2000;54:509–516. doi: 10.1093/toxsci/54.2.509. [DOI] [PubMed] [Google Scholar]
- Smith G S, Nadig D E, Kokoska E R, Solomon H, Tiniakos D G, Miller T A. Role of neutrophils in hepatotoxicity induced by oral acetaminophen administration in rats. J Surg Res. 1998;80:252–258. doi: 10.1006/jsre.1998.5441. [DOI] [PubMed] [Google Scholar]
- Newman S L, Henson J E, Henson P M. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982;156:430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malech H L. Phagocytic cells: egress from marrow and diapedesis. Gallin J I, Goldstein M, Snyderman R, editors. New York, NY, USA: Raven; InflammationBasic Principles and Clinical Correlates. 2008 [Google Scholar]
- Weiss S J. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Savill J. Apoptosis in resolution of inflammation. J Leukoc Biol. 1997;61:375–380. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- Hogaboam C M, Bone-Larson C L, Steinhauser M L, Matsukawa A, Gosling J, Boring L, Charo I F, Simpson K J, Lukacs N W, Kunkel S L. Exaggerated hepatic injury due to acetaminophen challenge in mice lacking C-C chemokine receptor 2. Am J Pathol. 2000;156:1245–1252. doi: 10.1016/S0002-9440(10)64995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan S A, Martinez-Pomares L, Stahl P D, Gordon S. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: in situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J Exp Med. 1999;189:1961–1972. doi: 10.1084/jem.189.12.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuochi S, Akimoto Y, Imai Y, Hirano H, Irimura T. Unique tissue distribution of a mouse macrophage C-type lectin. Glycobiology. 1997;7:137–146. doi: 10.1093/glycob/7.1.137. [DOI] [PubMed] [Google Scholar]
- Nair M G, Cochrane D W, Allen J E. Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunol Lett. 2003;85:173–180. doi: 10.1016/s0165-2478(02)00225-0. [DOI] [PubMed] [Google Scholar]
- Nair M G, Gallagher I J, Taylor M D, Loke P, Coulson P S, Wilson R A, Maizels R M, Allen J E. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect Immun. 2005;73:385–394. doi: 10.1128/IAI.73.1.385-394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J M, Yoon M, Anver M R, Haines D C, Kudo G, Gonzalez F J, Kimura S. Hyalinosis and Ym1/Ym2 gene expression in the stomach and respiratory tract of 129S4/SvJae and wild-type and CYP1A2-null B6, 129 mice. Am J Pathol. 2001;158:323–332. doi: 10.1016/S0002-9440(10)63972-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S K, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F, Vago L, Nebuloni M, Mantovani A, Sica A. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-κB and enhanced IRF-3/STAT1 activation) Blood. 2006;107:2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- Ito Y, Bethea N W, Abril E R, McCuskey R S. Early hepatic microvascular injury in response to acetaminophen toxicity. Microcirculation. 2003;10:391–400. doi: 10.1038/sj.mn.7800204. [DOI] [PubMed] [Google Scholar]
- Ito Y, Machen N W, Abril E R, McCuskey R S. Effects of acetaminophen on hepatic microcirculation in mice. Comp Hepatol. 2004;3:S33. doi: 10.1186/1476-5926-2-S1-S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Kampfer H, Pfeilschifter J, Frank S. Expression and activity of arginase isoenzymes during normal and diabetes-impaired skin repair. J Invest Dermatol. 2003;121:1544–1551. doi: 10.1046/j.1523-1747.2003.12610.x. [DOI] [PubMed] [Google Scholar]
- Teng X, Li D, Champion H C, Johns R A. FIZZ1/RELMα, a novel hypoxia-induced mitogenic factor in lung with vasoconstrictive and angiogenic properties. Circ Res. 2003;92:1065–1067. doi: 10.1161/01.RES.0000073999.07698.33. [DOI] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu T H, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L M, Tinkle C L, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson V, de la Ballina L R, Munaut C, Wielockx B, Jost M, Maillard C, Blacher S, Bajou K, Itoh T, Itohara S, Werb Z, Libert C, Foidart J M, Noël A. Contribution of host MMP-2 and MMP-9 to promote tumor vascularization and invasion of malignant keratinocytes. FASEB J. 2005;19:234–236. doi: 10.1096/fj.04-2140fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, DeBusk L M, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian L M, Carbone D P, Lin P C. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Ito Y, Bethea N W, Abril E R, McCuskey R S. Early hepatic microvascular injury in response to acetaminophen toxicity. Microcirculation. 2003;10:391–400. doi: 10.1038/sj.mn.7800204. [DOI] [PubMed] [Google Scholar]
- Williams A M, Langley P G, Osei-Hwediah J, Wendon J A, Hughes R D. Hyaluronic acid and endothelial damage due to paracetamol-induced hepatotoxicity. Liver Int. 2003;23:110–115. doi: 10.1034/j.1600-0676.2003.00808.x. [DOI] [PubMed] [Google Scholar]
- McCuskey R S, Bethea N W, Wong J, McCuskey M K, Abril E R, Wang X, Ito Y, DeLeve L D. Ethanol binging exacerbates sinusoidal endothelial and parenchymal injury elicited by acetaminophen. J Hepatol. 2005;42:371–377. doi: 10.1016/j.jhep.2004.11.033. [DOI] [PubMed] [Google Scholar]