Abstract
The role of cannabinoid receptors, CB1 and CB2, in immune competence and modulation by Δ9-tetrahydrocannabinol (Δ9-THC) was investigated in CB1−/−/CB2−/− mice. Immunofluorescence analysis of splenic leukocytes showed no significant differences in the percentage of T cell subsets, B cells, or macrophages between wild-type and CB1−/−/CB2−/− mice. Lymphoproliferative control responses to PHA, phorbol ester plus ionomycin, or LPS and sensitivity to suppression by Δ9-THC showed no profound differences between the two genotypes, although some differences were observed in control baseline responses. Likewise, similar control responses and sensitivity to Δ9-THC were observed in mixed lymphocyte responses (MLR) and in IL-2 and IFN-γ production in both genotypes. Conversely, humoral immune responses showed a markedly different profile of activity. Δ9-THC suppressed the in vivo T cell-dependent, anti-sheep RBC (anti-sRBC) IgM antibody-forming cell (AFC) response in wild-type but not in CB1−/−/CB2−/− mice, and the in vitro anti-sRBC IgM response in CB1−/−/CB2−/− splenocytes was too low to rigorously assess CB1/CB2 involvement in modulation by Δ9-THC. Conversely, comparable in vitro IgM AFC control responses to LPS and CD40 ligand (CD40L) activation were observed in the two genotypes. Interestingly, LPS-induced IgM responses were refractory to suppression by Δ9-THC, regardless of genotype, and CD40L-induced IgM responses were only suppressed by Δ9-THC in wild-type but not in CB1−/−/CB2−/− B cells. Collectively, we demonstrate differential involvement of CB1 and/or CB2 in immune modulation by Δ9-THC and in some control responses. Moreover, CB1/CB2 involvement was observed in humoral responses requiring CD40-initiated signaling for suppression by Δ9-THC.
Keywords: immunopharmacology, CD40 ligand, humoral immunity, cell-mediated immunity
INTRODUCTION
Cannabinoid receptors 1 and 2 (CB1 and CB2) are Gi protein-coupled receptors that mediate several of the pharmacological effects of synthetic, plant-derived, and endogenous cannabinoid compounds. For example, much evidence exists that the psychotropic effects of Δ9-tetrahydrocannabinol (Δ9-THC), the main psychoactive congener in marijuana [1], occur via CB1 receptors in the CNS [2, 3]. On the other hand, there is a growing body of evidence demonstrating that Δ9-THC and other cannabinoid compounds do not exert all of their effects via CB1 or CB2 [4,5,6,7,8,9]. Thus, the role of CB1 and CB2, in the absence and presence of exogenous cannabinoid compounds, remains to be elucidated.
In addition to other organ systems, the role of CB1 and CB2 has not been established definitively in the immune system. Initially, the discovery of CB2, in particular, with its predominance in the periphery, including blood mononuclear cells [10], prompted many to hypothesize that cannabinoid-induced immune modulation must be mediated through CB1 and/or CB2. Yet, the mechanism by which cannabinoid compounds modulate immune function cannot be explained fully, based solely on the involvement of CB1 and CB2 receptors, as evidenced by an absence of structure–activity relationships and lack of antagonism by cannabinoid receptor antagonists, SR141716A and SR144528, for a growing list of immune responses [4,5,6]. Further confusion surrounding the role of CB1 and CB2 in cannabinoid-mediated actions comes from observations that cannabinoid receptor antagonists under various experimental conditions exhibit inverse agonism [11,12,13,14], partial agonism [15], or off-target (i.e., non- CB1-, non-CB2-mediated) effects [5, 16].
As opposed to antagonists, CB1−/−/CB2−/− mice provide a more rigorous model for characterizing the role of CB1 and CB2 in cannabinoid-mediated actions. This is particularly crucial in cells of the immune system, in which both CB1 and CB2 are expressed. CB1−/−/CB2−/−mice have no gross observable defects, with the exception of being prone to the development of conjunctivitis and two reports of exaggerated T cell-associated immune responses. Specifically, CB1−/−/CB2−/− mice developed chronic ear ulceration in response to nickel-containing ear clips [17]. In the same study, the contact sensitizer 2,4-dinitrofluorobenzene induced more pronounced ear swelling in CB1−/−/CB2−/− mice than in the wild-type controls [17]. Finally, in CB1−/−/CB2−/− mice challenged with influenza, there was a markedly decreased viral load in the absence of Δ9-THC and increased inflammation and pulmonary injury as assessed by histopathology in the presence of Δ9-THC when compared with wild-type mice, indicating a more vigorous immune response to influenza virus [18].
With the demonstration that CB1 and/or CB2 likely play critical roles in immune homeostasis, the objectives of these studies were to characterize the role of CB1 and CB2 in immune functionality and determine whether CB1 and/or CB2 contribute to the mechanism by which Δ9-THC modulates immune responses. These studies provide a comprehensive characterization of immune function using assessment of AFC development, cytokine production, MLR, proliferation, and phenotypic characterization of splenocytes.
MATERIALS AND METHODS
Animals
Virus-free female C57BL/6 mice (6–7 weeks of age) were purchased from Charles River (Portage, MI, USA). Upon arrival, mice were randomized five/cage and quarantined for 1 week prior to use. CB1−/−/CB2−/− mice were generated as described previously [19] and were a generous gift from Dr. Andreas Zimmer (University of Bonn, Germany). Homozygous CB1−/−/CB2−/− mice, which were bred at Michigan State University (East Lansing, MI, USA), were subjected to an extensive battery of serological screens prior to breeding, as well as designated offspring sentinels after breeding. Tests for all pathogens assayed were negative. Mice were housed together at no more than five/cage in polycarbonate boxes on Cell-Sorb Plus bedding (A&W Products, Cincinnati, OH, USA) covered with filtered lids. All mice were given food (Purina Certified Laboratory Chow) and water ad libitum. Room lights were set on a 12-h light/dark cycle beginning at 6:00 am, and the temperature and relative humidity were maintained between 21°C and 24°C and 40–55% humidity, respectively. Mice were matched for sex and as closely as breeding would allow for age in all studies. Mice were used in accordance with guidelines set forth by the Michigan State University Institutional Animal Care and Use Committee.
Reagents
Δ9-THC was provided by the National Institute on Drug Abuse (Bethesda, MD, USA). All reagents were obtained from Sigma Chemical Co. (St. Louis, MO, USA) unless noted otherwise.
Preparation of lymphocyte culture
Mice were killed, and spleens were removed aseptically. Single-cell suspensions were prepared and cultured in RPMI-1640 media supplemented with bovine calf serum (BCS; percent BCS dependent on length of culture and assay; Hyclone, Logan, UT, USA), 100 units penicillin/ml, 100 μg streptomycin/ml, and 50 μM 2-ME. Splenocytes were cultured in a humidified atmosphere at 37°C and 5% CO2.
Immunofluorescence analysis
Single spleen cell suspensions were prepared and erythrocytes lysed using ammonium phosphate lysis solution (150 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA; 1 ml/spleen). One million cells were added to each reaction tube and subjected to Fcγ blocking using anti-CD16/32 (clone 2.4G2; BD Biosciences, San Jose, CA, USA). Spleen cells were stained with fluorescently conjugated antibodies purchased from BD Biosciences, except where noted: B cells, anti-CD19 (clone 1D3); total T cells, anti-CD3 (clone 145-2C11); Th cells, anti-CD4 (clone RM4-5); T cytotoxic cells, anti-CD8 (clone 53-6.7), and macrophages, anti-F4/80 (clone BM8 from eBioscience, San Diego, CA, USA). In addition, splenocytes (5×106 cells) were stained following activation in complete medium containing 10% heat-inactivated BCS in 48-well culture plates for 5 days with 6.5 × 106 sRBC (Colorado Serum, Denver, CO, USA) in a manner consistent with the in vitro anti-sRBC IgM AFC assay. Cells were harvested and stained with antibodies directed against CD4 and CD25 (clone 2A3). Expression of CD40 ligand (CD40L) on the cell surface of CD40L–L cells (generously provided by Dr. David Sherr, Boston University, Boston, MA, USA) was verified prior to each experiment using anti-CD40L (clone TNF-related activation protein 1). Leukocytes in all of the above instances were analyzed using a FACSCalibur (BD Biosciences). Cells were gated on forward- and side-scatter, and data were analyzed using CellQuest software (BD Biosciences). Appropriate isotype controls were included in the analysis for each sample set.
In vitro lymphoproliferation
Wild-type or CB1−/−/CB2−/− splenocytes were cultured in complete RPMI supplemented with 5% BCS, aliquoted at 2 × 105 cells/well in quadruplicate into 96-well culture plates, treated with Δ9-THC or vehicle (VH; 0.1% ethanol) for 30 min, and then stimulated with mitogen for 72 h [except phorbol ester and ionomycin (PMA/Io), which was 48 h]. Three different stimuli were used to induce proliferation: PHA (10 μg/ml), LPS (10 or 100 μg/ml), or PMA/Io (40 nM/0.5 μM). During the last 16 h, the cultures were pulsed with 1 μCi [3H]-thymidine (6.7 Ci/mmol; Perkin Elmer, Waltham, MA). Cells were harvested, and [3H]-thymidine incorporation was measured by a scintillation counter, model LS1701 (Beckman Coulter, Fullerton, CA, USA). Results are expressed as mean CPM ± se of quadruplicate cultures.
MLR
The MLR was performed using a method described previously [20] with minor modifications. Briefly, DBA/2 stimulator splenocytes (5×106 cells/ml) were inactivated with mitomycin C (40 μg/ml) for 60 min at 37°C and washed four times in RPMI. Wild-type or CB1−/−/CB2−/− splenocytes (1×105/well) were cocultured with 4 × 105 DBA/2 stimulator cells in the presence of Δ9-THC or vehicle (0.1% ethanol) for 3, 4, or 5 days. During the last 20 h of culture, each well was pulsed with 1 μCi [3H]-thymidine (6.7 Ci/mmol; Perkin Elmer). Cells were harvested, and [3H]-thymidine incorporation was measured by a scintillation counter, model LS1701 (Beckman Coulter). Results are expressed as CPM ± se of quadruplicate cultures.
IL-2 and IFN-g ELISAs
Splenocytes (8×105 cells) were treated with D9-THC (0.5–15 μM) for 30 min at 37°C, followed by cellular activation with 40 nM/0.5 μM PMA/Io for 18–24 h in complete medium containing 2% BCS in 48-well culture plates at 0.8 ml/well. Cells were harvested and supernatants were collected and assayed for murine IL-2 or IFN-γ production by ELISA. Recombinant IL-2 and IFN-γ were used as standards. Purified and biotinylated anti-mouse IL-2 and IFN-γ antibodies were purchased from BD Biosciences (San Jose, CA, USA). Color development was performed using streptavidin peroxidase followed by tetramethylbenzidine (Fluka/Sigma, St. Louis, MO, USA). Reactions were stopped with 6N H2SO4, after which samples were read at 450 nm.
In vivo AFC assays
Wild-type or CB1−/−/CB2−/− mice (five/treatment group) received Δ9-THC or corn oil vehicle by oral gavage for 3 consecutive days. Mice were sensitized with sRBC (Colorado Serum) on Day 2 of treatment by i.p. injection (5×108 sRBC/mouse). By sensitizing with antigen on Day 2, we insured that Δ9-THC was present during the critical stages of the T cell-dependent antibody response including antigen recognition, processing, and presentation as well as during T cell accessory cell function (i.e., activation, T cell–B cell interaction, proliferation, and cytokine production) and during B cell activation and proliferation. Four days postsensitization with sRBC, the anti-sRBC IgM AFC response was enumerated as described previously [21]. Briefly, spleens were isolated, and a single-cell suspension was prepared from each mouse individually. Splenocytes were diluted 1:30 in HBSS, and 100 μl diluted cell suspension was added to a glass 12 × 75 mm heated culture tube containing 350 μl 0.5% dissolved agar (Difco, Detroit, MI, USA) and 25 μl indicator sRBC. Upon removal from the heated water bath, 25 μl guinea pig complement was added, the tube vortex mixed, and the cell mixture poured onto a 100 × 15-mm petri dish and covered immediately with a 45 × 50-mm glass coverslip. Upon solidification of the agar, the petri dishes were placed in a humidified 37°C incubator overnight to allow for plaque formation. The number of splenocytes from each spleen was determined using a Coulter counter (Beckman Coulter). Results were expressed as mean AFC/106 recovered splenocytes ± se.
In vitro T cell-dependent and polyclonal IgM AFC response
Single-cell suspensions of splenocytes from naïve wild-type or CB1−/−/CB2−/− mice were adjusted to 1 × 107 cells/ml for the T cell-dependent IgM AFC response or 5 × 106 cells/ml for the polyclonal IgM AFC response in RPMI supplemented with 10% BCS. Spleen cells were transferred to 48-well culture plates in 500 μl aliquots with four wells/treatment group. Δ9-THC and/or vehicle (0.1% ethanol) was added directly to each well in 5 μl aliquots. For the anti-sRBC IgM AFC response, each well was sensitized with 6.5 × 106 sRBC and cultured for 5 days. For the polyclonal IgM AFC response to LPS, splenocytes were sensitized with 10 or 100 μg/ml LPS and cultured for 3 days. The cell cultures were placed in a pressurized chamber at 5.0 pounds per square inch containing 10% O2, 7% CO2, and 83% N2 gas mixture and maintained with continuous rocking at 37°C. Enumeration of the AFC response was performed as described above. For enumeration of the polyclonal AFC, indicator sRBC were haptenated with trinitrophenol (TNP) as described previously [22]. Results are expressed as AFC/106 viable splenocytes ± se. Splenocyte viability was measured using pronase (EMD Biosciences, San Diego, CA, USA) as described previously [20]. Briefly, following 3 or 5 days of culture, splenocytes were resuspended, and 100 μl cell suspension was incubated with an equal volume of pronase for 10 min at 37°C. Following the incubation, the splenocyte solution was diluted with 10 ml Isoton (Beckman Coulter) and the cells counted on a Coulter counter. The percent of nonviable cells was determined by comparing the cell counts for each sample with and without pronase: (cell counts without pronase–cell counts with pronase)/(cell counts without pronase) × 100%.
Purification of splenic B cells
Spleens were isolated aseptically from wild-type or CB1−/−/CB2−/− mice and made into a single-cell suspension. Splenic B cells were purified by negative selection from the spleen cell preparation using a B cell isolation kit (Miltenyi Biotec, Auburn, CA, USA). Briefly, 40 μL MACS buffer (PBS solution or PBS, pH 7.2, supplemented with 0.5% BSA and 2 mM EDTA)/1 × 107 cells was used to suspend the cells, and 10 μl biotin-antibody cocktail (Miltenyi Biotec)/107 cells was added to label non-B cells. After incubation at 4°C for 10 min, 30 μl buffer and 20 μl anti-biotin microbeads (Miltenyi Biotec)/107 cells were added. The cell suspension was incubated for 15 min at 4°C, washed with 10–20× labeling volume, and then centrifuged at 300 g for 5 min. The cell pellet was finally resuspended in 500 μL buffer/108 cells and passed through the prerinsed LS column (Miltenyi Biotec), followed by four washes with 3 ml MACS buffer. The entire effluent was collected and used for total B cell quantification. The isolated B cells were evaluated for purity as determined by immunofluorescence analysis using an anti-CD19 antibody (BD Biosciences) and routinely yielded >95% pure CD19+ cells.
CD40L-induced IgM antibody responses
Purified, splenic B cells were suspended in RPMI, supplemented with 10% heat-inactivated BCS to a final density of 2.5 × 106 cell/ml. B cells were then transferred to 24-well microtiter plates preseeded with irradiated CD40L–L cells and treated with Δ9-THC and/or vehicle (0.1% ethanol). A control group with no CD40L–L cells was included to assess background supernatant IgM and IgM AFC. The cultures were also supplemented with IL-2 (10 units/ml) and IL-6 (100 units/ml) at the initiation of the cultures. After 3 days of culture, B cells were transferred to a 96-well microtiter plate without CD40L–L cells for 3 additional days of culture. On Day 6, B cells were harvested for IgM AFC using TNP-haptenated sRBC as described above, and supernatants were assayed for IgM by ELISA.
Anti-IgM ELISA
Supernatants were collected from the same experimental cultures from which CD40L-induced IgM antibody responses were assessed. Supernatant IgM was detected by sandwich ELISA. Briefly, 100 μl 5 μg/ml anti-mouse IgM capture antibody (Boehringer Mannheim, Indianapolis, IN, USA) was added to wells of a 96-well microtiter plate and stored at 4°C overnight. After the precoating step, the plate was washed twice with 0.05% Tween-20 in PBS and three times with dH2O. Then 200 μl 3% BSA–PBS buffer was added to the wells and incubated at room temperature for 1.5 h to block nonspecific binding, followed by the same washing steps described above. Standard (mouse IgM) or supernatant samples (100 μl) were added to the coated plate, respectively, and then incubated at 37°C for 1.5 h. After the incubation, the plate was washed again, followed by addition of 100 μl HRP-conjugated goat anti-mouse IgM detection antibody. After 1.5 h at 37°C, an unbound detection antibody was washed away from the plate, and 100 μl ABTS (1 mg/ml; Boehringer Mannheim) was added. The detection of the HRP substrate reaction was conducted over a 1-h period using a Synergy HT automated Microplate reader with a 405-nm filter (Bio-Tek, Winooski, VT, USA). The KC4 computer analysis program (Bio-Tek) calculated the concentration of IgM in each sample based on a standard curve generated from the absorbance readings of known IgM concentrations.
Statistical analysis
The mean ± se was determined for each treatment group in the individual experiments. Homogeneous data were evaluated by parametric ANOVA. When significant differences occurred, treatment groups were compared using Dunnett’s two-tailed t-test [23]. Percent data were transformed prior to statistical analysis.
RESULTS
Phenotypic analysis of leukocyte subpopulations in spleen
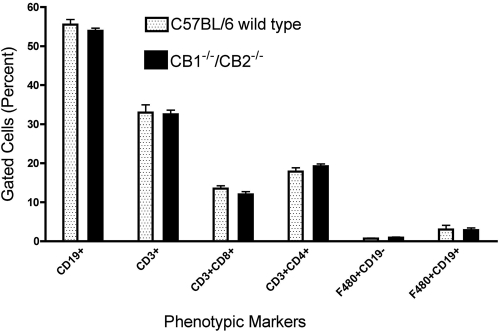
Flow cytometric analysis of spleen cell subpopulations was performed comparing C57BL/6 wild-type and CB1−/−/CB2−/− mice to determine whether targeted deletion of CB1 and CB2 significantly altered the cellular composition of leukocytes in the spleen. No significant differences were observed between wild-type and CB1−/−/CB2−/− mice with respect to any of the subpopulations analyzed based on the relative number of cells or in the mean fluorescence intensity of the specific epitopes used to identify T cells (CD3+), Th cells (CD3+, CD4+), cytotoxic T cells (CD3+, CD8+), B cells (CD19+), and macrophages (F4/80+; Fig. 1). In addition, no significant differences have been observed in the total number of splenocytes recovered between wild-type and CB1−/−/CB2−/− mice.
Fig. 1.
Splenic cellular subpopulation profiles in naïve C57BL/6 wild-type and CB1−/−/CB2−/− mice. Splenocytes were labeled using the appropriate fluorochrome-conjugated mAb and analyzed using flow cytometry by gating on the leukocyte population. Data are expressed as mean ± sem; n = 7.
The effect of Δ9-THC on PMA/Io or PHA-induced proliferation
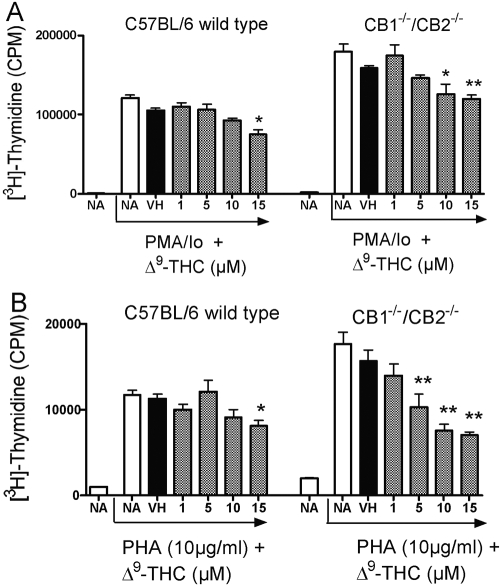
Based on the absence of phenotypic differences in the major spleen cell subpopulations between wild-type and CB1−/−/CB2−/− mice, the sensitivity of splenocytes to mitogenic stimuli, including PMA/Io and PHA, in the absence and presence of Δ9-THC, was evaluated. Interestingly, in the absence of Δ9-THC, there was a modest trend for a greater magnitude of proliferation by splenocytes derived from CB1−/−/CB2−/− mice as compared with wild-type with PMA/Io and PHA activation (Fig. 2). In the presence of Δ9-THC, a concentration-related suppression of proliferation was observed after PMA/Io and PHA stimulation in wild-type and CB1−/−/CB2−/− splenocytes, and CB1−/−/CB2−/− mice exhibited modestly greater sensitivity to Δ9-THC (Fig. 2).
Fig. 2.
The effect of Δ9-THC on C57/BL6 wild-type and CB1−/−/CB2−/− splenocyte proliferation. Splenocytes were pretreated with Δ9-THC for 30 min followed by stimulation with mitogens: PMA/Io (40 nM/0.5 μM; A) or PHA (10 μg/ml; B). Pretreated, stimulated cells were cultured for 48 (PMA/Io) or 72 h. [3H]-Thymidine incorporation is expressed as mean ± se of quadruplicate samples. * or **, Values that are significantly different from vehicle (VH) at P < 0.05 or P < 0.01, respectively, within each genotype. NA, Naive.
Suppression by Δ9-THC of the MLR and supernatant IL-2 and IFN-γ levels
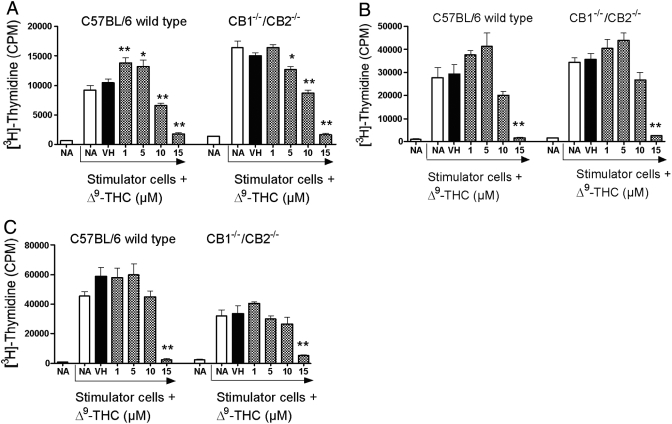
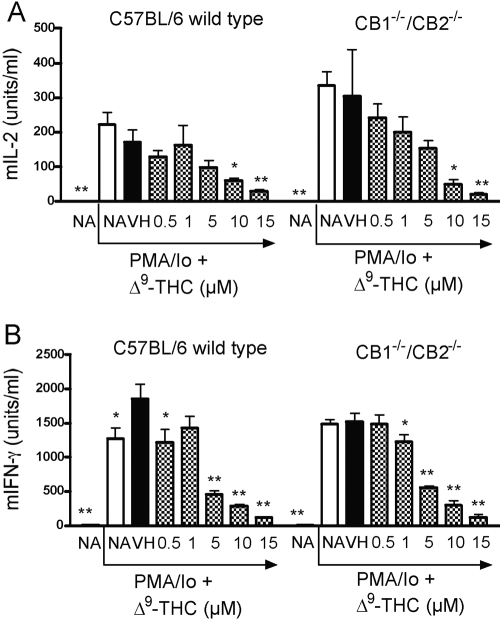
With the demonstration that Δ9-THC suppressed spleen cell-proliferative responses in response to various stimuli regardless of genotype, the role of CB1/CB2 on the one-way MLR was evaluated. The MLR was assayed on Days 3, 4, and 5 in the event that the deletion of CB1 and/or CB2 altered the kinetics for the peak time of the response in the absence or presence of Δ9-THC (Fig. 3). No significant differences were observed between CB1−/−/CB2−/− and wild-type splenocytes in the absence or presence of Δ9-THC on any of the days examined. Δ9-THC produced a concentration-related suppression of the MLR in wild-type and CB1−/−/CB2−/− splenocytes on Days 3–5. Interestingly, on Day 4, lower treatment concentrations of Δ9-THC (1 and 5 μM) produced a modest enhancement of the MLR in wild-type and CB1−/−/CB2−/− splenocytes. Based on the suppression observed in T cell proliferation in the MLR, the effects of Δ9-THC on IL-2 production were assessed. Δ9-THC suppressed IL-2 secretion by splenocytes from wild-type and CB1−/−/CB2−/− mice in response to PMA/Io activation (Fig. 4). Supernatants were also assayed for IFN-γ, which exhibited a similar concentration-related suppression in the presence of Δ9-THC by splenocytes derived from wild-type and CB1−/−/CB2−/− mice.
Fig. 3.
Alteration of the mixed lymphocyte response by Δ9-THC at Day 3 (A), Day 4 (B), or Day 5 (C) in C57BL/6 wild-type and CB1−/−/CB2−/− splenocytes. Stimulator cells from DBA/2 mice were isolated and inactivated with 40 μg/ml mitomycin C. Responder cells from each C57BL/6 wild-type or CB1−/−/CB2−/− mice were isolated. Responder cells (1×105) and stimulator cells (4×105) were cultured with VH or the indicated concentration of Δ9-THC for 3, 4, or 5 days. [3H]-Thymidine incorporation is expressed as mean ± se of quadruplicate samples. * or **, Values that are significantly different from VH at P < 0.05 or P < 0.01, respectively, within each genotype.
Fig. 4.
The effect of Δ9-THC on PMA/Io-induced cytokine production in C57BL/6 wild-type and CB1−/−/CB2−/− splenocytes, which were treated with indicated concentrations of Δ9-THC and stimulated with PMA/Io (40 nM/0.5 μM). Cultures were incubated for 18–24 h, after which supernatants were analyzed for IL-2 [murine IL-2 (mIL-2; A)] or IFN-γ (mIFN- γ; B) by ELISA. * or **, Values that are significantly different from VH at P < 0.05 or P < 0.01, respectively, within each genotype. Results are representative of at least three separate experiments.
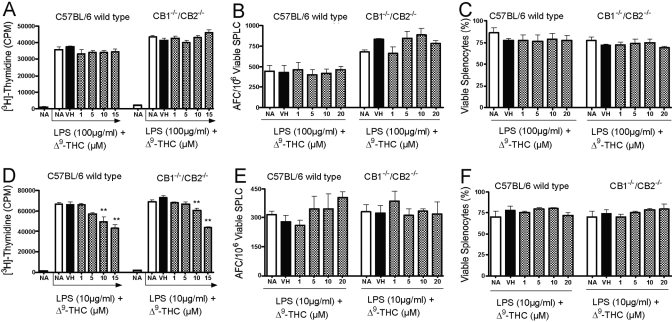
The effect of Δ9-THC on LPS-induced proliferation and the in vitro IgM AFC response
To assess the functional responsiveness of B cells from CB1−/−/CB2−/− mice in vitro, splenocytes were activated with the polyclonal B cell activator, LPS, followed by assessment of B cell proliferation and the IgM AFC response in the absence and presence of Δ9-THC. In the absence of Δ9-THC, the magnitude of LPS-induced proliferation was similar in splenocytes from CB1−/−/CB2−/− and wild-type mice. At lower concentrations of LPS (10 μg/ml), Δ9-THC produced a modest and comparable suppression of LPS-induced proliferation in wild-type and CB1−/−/CB2−/− splenocytes, which was not observed at higher concentrations of LPS (100 μg/ml; Fig. 5, A and D). In contrast, Δ9-THC exhibited no effect on the in vitro IgM AFC response following activation with 10 μg/ml or 100 μg/ml LPS in wild-type or CB1−/−/CB2−/− splenocytes (Fig. 5, B and E). Likewise, Δ9-THC had no effect on spleen cell viability at any of the concentrations used when compared with the vehicle control in the wild-type or CB1−/−/CB2−/− splenocytes as assessed on Day 3, the peak day of the IgM AFC response (Fig. 5, C and F).
Fig. 5.
The effect of Δ9-THC on proliferation, polyclonal IgM AFC response, and viability following LPS stimulation in C57BL/6 wild-type and CB1−/−/CB2−/− splenocytes (SPLC), which were treated with indicated concentrations of Δ9-THC and stimulated with LPS at 100 mg/ml (A–C) or 10 mg/ml (D–F). Cultures were analyzed subsequently for proliferation (A and D), enumerated for an AFC response (B and E), or analyzed for spleen cell viability (C and F). Results are expressed as mean ± sem from quadruplicate samples. **, Values that are significantly different from the VH at P < 0.01 within each genotype.
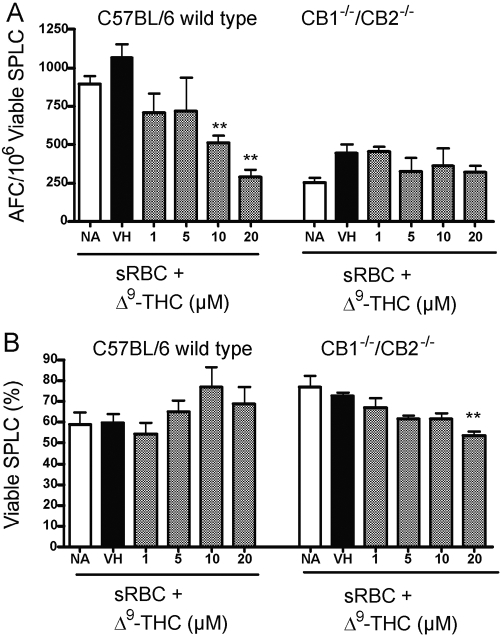
Effects of Δ9-THC on the in vitro T cell-dependent IgM AFC response
This laboratory has previously reported marked suppression of the in vitro IgM AFC response to the T cell-dependent antigen, sRBC, after direct addition of Δ9-THC to naïve, cultured splenocytes from B6C3F1 mice [22]. Direct addition of Δ9-THC to naïve splenocytes from wild-type C57BL/6 mice produced a similar concentration-related suppression of the anti-sRBC AFC IgM response. No effect on spleen cell viability was observed at any of the concentrations of Δ9-THC used in the experiment (Fig. 6). In contrast and following repeated attempts, two significant phenomena were observed in splenocytes derived from CB1−/−/CB2−/− splenocytes. The first was that only a modest anti-sRBC IgM AFC response was inducible in vitro with spleen cells from CB1−/−/CB2−/− mice. Typically, the control response following sRBC sensitization was approximately one-fifth or less of that observed with wild-type splenocytes. The second observation was that Δ9-THC did not significantly suppress the anti-sRBC IgM AFC response in splenocytes from CB1−/−/CB2−/− mice.
Fig. 6.
The effect of Δ9-THC on a C57BL/6 wild-type and CB1−/−/CB2−/− T cell-dependent, AFC response in vitro. Splenocytes were treated with Δ9-THC, stimulated with sRBC, and incubated for 5 days. Cultures were subsequently enumerated for the AFC response (A) and viability (B). Results are expressed as mean ± sem from quadruplicate samples. **, Values that are significantly different from VH at P < 0.01 within each genotype.
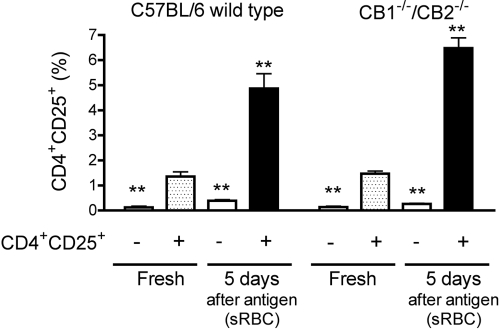
Based on the observation that the magnitude of the in vitro anti-sRBC IgM AFC response in CB1−/−/CB2−/− mice was approximately one-fifth of that induced in C57BL/6 wild-type mice, the percentage of putative T regulatory cells (Tregs) was assessed. Tregs have been demonstrated to attenuate the magnitude of humoral responses [24, 25] and Tregs have been shown to expand preferentially under common culture conditions in vitro [26]. Although the mRNA expression of forkhead box p3 (foxp3), a gene associated with Tregs, was higher in T cells than total splenocytes derived from C57BL/6 wild-type or CB1−/−/CB2−/− mice, there was no difference in the expression level of foxp3 between genotypes (data not shown). Using the cell-surface markers CD4 and CD25, the percentage of CD4+CD25+ T cells was low in splenocytes from C57BL/6 wild-type or CB1−/−/CB2−/− mice (Fig. 7). Following the 5-day culture period in the presence of sRBC, the percentage of CD4+CD25+ T cells increased in genotypes, and there was a trend toward an increased percentage of CD4+CD25+ cells in the cultures derived from CB1−/−/CB2−/− splenocytes.
Fig. 7.
The effect of in vitro culture on expression of CD4 and CD25. Splenocytes (5×106 cells) were stained fresh (i.e., upon generation of the single-cell suspension) or following activation. Splenocytes were activated with sRBC for 5 days in culture. Cells were harvested and stained with antibodies directed against CD4 and CD25. **, Values significantly different from fresh CD4+CD25− group at P < 0.01 within each genotype.
Effects of Δ9-THC administration on the in vivo anti-sRBC IgM AFC response
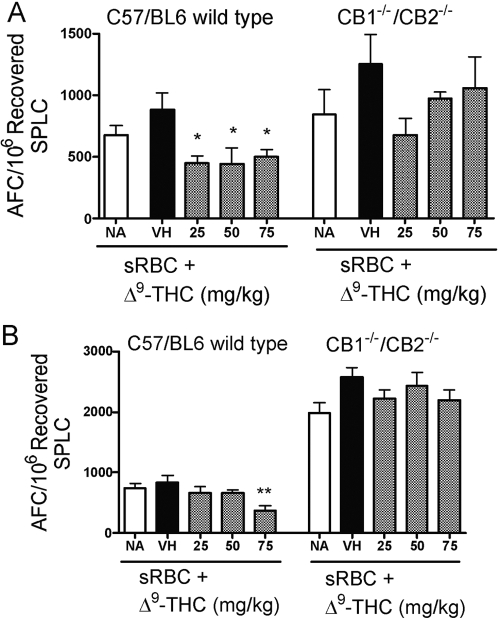
In light of the remarkably modest in vitro anti-sRBC IgM AFC response observed by naïve splenocytes from CB1−/−/CB2−/− mice, similar studies were conducted using administration of Δ9-THC (25–200 mg/kg) by oral gavage, followed by in vivo sensitization with sRBC and assessment of the anti-sRBC IgM AFC response. Interestingly, the magnitude of the anti-sRBC IgM AFC response following in vivo antigen sensitization was similar in wild-type and CB1−/−/CB2−/− mice. In fact, there was a trend in multiple experiments in which the control anti-sRBC IgM AFC response was modestly greater in CB1−/−/CB2−/− mice than in wild-type mice. Δ9-THC, at doses as high as 75 mg/kg, administered over 3 consecutive days surrounding the day of antigen sensitization, did not suppress the in vivo anti-sRBC IgM AFC response in CB1−/−/CB2−/− mice. In contrast, Δ9-THC administration suppressed the anti-sRBC IgM AFC response in wild-type C57BL6 mice, although the magnitude of suppression observed with Δ9-THC administration in wild-type C57BL6 mice was not as marked as we have observed previously in B6C3F1 mice [22]. No treatment-related effects were observed on body weight in wild-type or CB1−/−/CB2−/− mice. At 75 mg/kg, wild-type mice exhibited a significant decrease in spleen weight as compared with the vehicle control group, which was not observed in CB1−/−/CB2−/− mice (Fig. 8). There was also no consistent alteration of thymus weight as a result of Δ9-THC treatment in wild-type or CB1−/−/CB2−/− mice, as compared with their respective vehicle control group (data not shown).
Fig. 8.
The effect of Δ9-THC on the T cell-dependent AFC response in vivo in C57BL/6 wild-type and CB1−/−/CB2−/− mice (five/group), which were treated with Δ9-THC or corn oil vehicle for 3 days. On Day 2, mice were injected with sRBC. On Day 6, mice were killed, and the AFC response was assayed. Two separate experiments are shown (A and B). Results are expressed as mean ± sem. * or **, Values that are significantly different from VH at P < 0.05 or P < 0.01, respectively, within each genotype.
Splenocytes from sRBC-sensitized, wild-type and CB1−/−/CB2−/− mice were also characterized by flow cytometry on the day of sacrifice. No significant differences in the percentages of the spleen cell subpopulation were observed as a result of Δ9-THC treatment. A modest trend toward a greater percentage of macrophages (F4/80+ cells) in CB1−/−/CB2−/− spleens, albeit not statistically different from wild-type, was observed in both experiments (data not shown).
CD40L-induced humoral immune responses
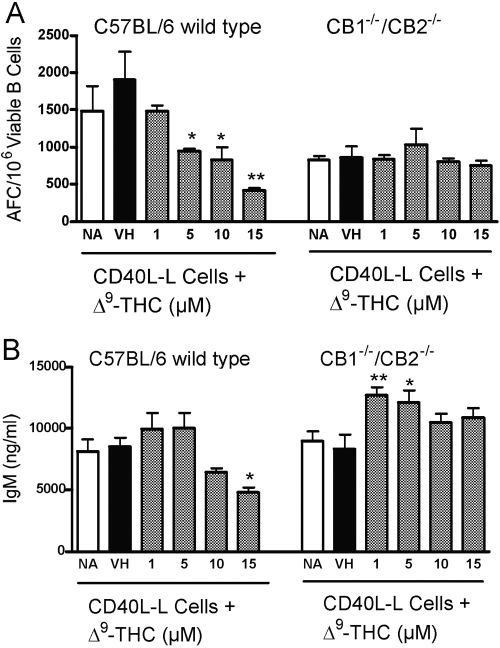
To further investigate the putative mechanism responsible for the strikingly modest in vitro anti-sRBC IgM AFC response by CB1−/−/CB2−/− splenocytes, CD40L-mediated activation was used. By using CD40L-expressing L cells (plus IL-2 and IL-6) to activate purified B cells from CB1−/−/CB2−/− and wild-type mice, it was possible to characterize B cell function directly in the absence of cognate accessory cell participation in a manner somewhat analogous to the polyclonal B cell activator LPS. B cells from CB1−/−/CB2−/− and wild-type mice activated by CD40L exhibited comparable IgM AFC responses (Fig. 9). In fact, in several experiments of B cells from CB1−/−/CB2−/− mice showed a modestly greater in vitro IgM AFC response than B cells from wild-type mice. Studies were also performed to assess the sensitivity of B cells to Δ9-THC-mediated suppression of the IgM response after CD40L activation. Direct addition of Δ9-THC produced a concentration-related suppression of the IgM AFC response in wild-type but not in CB1−/−/CB2−/− B cells. The corresponding culture supernatants were also assayed for IgM and revealed almost identical trends, as observed in the AFC response (Fig. 9). Control IgM levels between wild-type and CB1−/−/CB2−/− B cells were comparable in magnitude, and direct addition of Δ9-THC to culture produced a concentration-related suppression of supernatant IgM in wild-type but not in CB1−/−/CB2−/− B cells.
Fig. 9.
The effect of Δ9-THC on the B cell AFC response in vitro in C57BL/6 wild-type and CB1−/−/CB2−/− mice. Purified, splenic B cells were cocultured with CD40L-expressing L cells and treated with Δ9-THC on Day 1. After 3 days of culture, Δ9-THC-treated B cells were transferred to plates without L cells for 3 additional days. Cultures were subsequently enumerated for the AFC response (A), or supernatants were assayed for IgM (B). Results are expressed as mean ± sem from quadruplicate samples. **, Values that are significantly different from VH at P 0.05 or P < 0.01, respectively, within each genotype.
DISCUSSION
The physiological role of CB1 and CB2 expression within the immune system has remained elusive in spite of the fact that almost two decades have passed since both receptors were identified and cloned and their expression detected in leukocytes. Recent findings in mice null for CB1 and CB2 have provided interesting, new clues, suggesting that a principle role of one or both of the cannabinoid receptors may be in the maintenance of immune homeostasis by transducing inhibitory signals to down-regulate immunocompetent cells. Concordant with this premise, CB1−/−/CB2−/− mice were reported to be prone toward developing hypersensitivity to nickel ear clips and to exhibit an enhanced response to the contact sensitizer 2,4-dinitrofluorobenzene when compared with wild-type mice [17]. Likewise, we have reported recently that CB1−/−/CB2−/− mice infected with a sublethal dose of influenza more effectively clear virus while developing more severe pulmonary damage as a result of mounting a more robust, antiviral immune response than wild-type mice [27]. In both cases, cell-mediated immunity was augmented and resulted in detrimental consequences to the host. Based on these intriguing observations and wanting to gain further insight into the role of CB1 and CB2 on immune competence, a comprehensive, immunologic evaluation of CB1−/−/CB2−/− mice was conducted that included a flow cytometric characterization of leukocyte subpopulations, lymphoproliferative responses to polyclonal stimuli, and measurements of various aspects of cell-mediated and humoral immunity in the presence and absence of cannabinoid treatment.
The effect of CB1/CB2 deletion on the phenotypic composition of leukocyte populations within the spleen was characterized by flow cytometry, and the flow cytometric analysis of the spleen revealed no significant differences in the major splenic leukocyte subpopulations including total B cells (CD19+), total T cells (CD3+), Th cells (CD3+CD4+), cytotoxic T cells (CD3+/CD8+), and macrophages (F4/80+) and in spleen cellularity between CB1−/−/CB2−/− and wild-type mice. Our results parallel similar studies conducted in CB2−/− and CB2+/− mice, in that no significant differences between the two genotypes were found with respect to the total number of splenic CD19+ B cells and CD3+ T cells [28]. However, further flow cytometric analysis of splenic B and T cell subpopulations by Ziring and coworkers [28] did reveal subtle differences between CB2−/− and CB2+/− mice. Fractionation of splenic regions revealed a three-fold reduction in the midzonal B cell subpopulation in CB2−/− mice, which was identified as a CD19+CD21hiCD23− splenic population. No significant differences were observed in the B cell subpopulation in other regions of the spleen between CB2−/− and CB2+/− mice. Likewise, Ziring and coworkers [28] observed no differences in the total number of CD3+ T cells between CB2−/− and wild-type mice but again, observed subtle differences this time in the ratio of naïve:memory T cell subsets between CB2−/− and CB2+/− mice. In particular, CB2−/− mice exhibited a reduced number of memory T cells as compared with heterozygous littermates. Although the modest, potentially important differences in the percentages of leukocyte subsets reported by Ziring et al. [28] when comparing CB2−/− null, heterozygous, and wild-type mice are interesting, in the absence of a more comprehensive characterization to identify specific differences in immune competence between CB1−/−/CB2−/− and wild-type mice, we were discouraged from pursuing more refined leukocyte phenotyping at this time.
Based on the above, the major goal of this investigation was to characterize functional immune responses in CB1−/−/CB2−/− and wild-type mice and compare their respective sensitivity to Δ9-THC treatment in vivo and in vitro. Proliferative responses to a variety of activation stimuli, including PHA, LPS, PMA/Io, and allogeneic MHC (as assessed in the MLR), were all strikingly similar with the exception that there was a modest trend toward a greater proliferative response to PHA and PMA/Io activation in the CB1−/−/CB2−/− compared with wild-type mice. Likewise, we observed that these proliferative responses were suppressed in a concentration-related manner by Δ9-THC treatment in CB1−/−/CB2−/− and wild-type splenocytes, demonstrating that the Δ9-THC-mediated, antiproliferative effects occur through mechanisms independent of CB1 and CB2. Interestingly, at 10 μg/ml LPS, a modest suppression of the proliferative response was produced by Δ9-THC, which was not observed at 100 μg/ml LPS. Somewhat analogous to what was observed with LPS here, we have previously reported biphasic effects with Δ9-THC on the IL-2 response, which was closely linked to the magnitude of the activation stimulus. Specifically, we observed that with optimum or supraoptimum activation, using a variety of stimuli, including anti-CD3/anti-CD28 and phorbol ester plus calcium ionophore, T cell-derived IL-2 production was suppressed by Δ9-THC, and the same concentration of Δ9-THC in the presence of suboptimal T cell activation enhanced IL-2 production [29]. In light of the suppressed proliferative responses by Δ9-THC in the presence of optimum activation stimuli, we also examined control responses of two important cytokines reported previously to be modulated by Δ9-THC and critical to T cell clonal expansion and/or immunity to viral infection. Measurements of IL-2 and IFN-γ secretion after activation with PMA/Io revealed strong expression of cytokines in CB1−/−/CB2−/− and wild-type splenocytes as well as sensitivity to suppression by Δ9-THC. These results once again clearly demonstrate the absence of CB1 and/or CB2 involvement in Δ9-THC-mediated modulation of IL-2 and IFN-γ.
The most significant differences observed between CB1−/−/CB2−/− and wild-type mice were in humoral immune responses, in particular, with respect to the overall magnitude of the control immune responses and their respective sensitivity to Δ9-THC treatment. Splenocytes from CB1−/−/CB2−/− mice showed a strikingly modest in vitro T cell-dependent anti-sRBC IgM AFC response. This observation was in contrast with the in vivo anti-sRBC IgM AFC response, in which CB1−/−/CB2−/− and wild-type mice demonstrated a strong response. In fact, in one study, CB1−/−/CB2−/− mice exhibited a markedly more pronounced in vivo anti-sRBC IgM AFC response than wild-type. The in vivo anti-sRBC IgM AFC response studies also demonstrated differential sensitivity between the two genotypes to Δ9-THC treatment as evidenced by suppression of the humoral immune response in wild-type but not in CB1−/−/CB2−/− mice, suggesting involvement of CB1 and/or CB2. Unfortunately, as a result of the extremely low in vitro anti-sRBC IgM AFC response by splenocytes from CB1−/−/CB2−/− mice, it was not possible to rigorously assess whether the same differential sensitivity to Δ9-THC treatment between the two genotypes would be observed under in vitro conditions. However, based on a recent report showing that common culture conditions can lead to the preferential expansion of Tregs [26], which are known to suppress humoral immune responses [24, 25], the involvement of Tregs was explored in an attempt to explain the modest in vitro control antibody response by CB1−/−/CB2−/− B cells. Although there appeared to be a slight trend toward an increased number of CD4+/CD25+ T cells after 5 days of culture and sRBC activation in CB1−/−/CB2−/− splenocytes when compared with wild-type, splenic cultures, the differences were modest and not statistically significant. Likewise, no significant differences were observed in the percentage of CD4+/CD25+ T cells between CB1−/−/CB2−/− and wild-type mice immediately upon removal of the spleens, as well as no differences between the two genotypes in the expression of foxp3, a gene associated with Tregs. Collectively, these results suggest that Tregs are unlikely to account for the differences observed in the magnitude of the control in in vitro IgM AFC responses between wild-type and CB1−/−/CB2−/− mice.
Another significant finding and in striking contrast to the in vitro anti-sRBC IgM AFC responses was the observation that in vitro activation of purified B cells with CD40L plus cytokines produced a comparable control-immune response in both genotypes. Clearly polyclonal activation of B cells by CD40L or LPS was capable of strongly driving B cell differentiation and IgM secretion in vitro, regardless of CB1/CB2 expression, which also suggested that class-switching was unlikely responsible for the modest in vitro anti-sRBC IgM AFC responses. However, significant differences were observed with respect to Δ9-THC treatment. Wild-type but not CB1−/−/CB2−/− B cells exhibited suppression of the IgM AFC response and IgM secretion induced by CD40L. As irradiated, CD40L-expressing L cells were used as the source of CD40L, the Δ9-THC-induced, suppressive effects on plasma cell formation and IgM secretion were mediated through a direct effect on B cells and dependent on CB1 and/or CB2 expression under the current experimental conditions. It is important to emphasize that the findings should be interpreted carefully, as they do not rule out the possibility that Δ9-THC treatment impairs accessory cell functions including antigen processing and presentation, as well as Th cell-derived cytokine production, as neither are required in the CD40L activation protocol used here. In fact, impairment of antigen processing by macrophages and cytokine production by T cells has been described previously [30,31,32,33]. In addition, previous findings from our laboratory using B6C3F1 mice showed that splenocytes are refractory to Δ9-THC-mediated suppression of T cell-independent IgM AFC responses to DNP-Ficoll and LPS but not to the T cell-dependent antigen, sRBC [22], which is dependent on CD40/CD40L interactions between B and Th cells. These new findings clearly show that B cells are not refractory to Δ9-THC-mediated suppression and strongly implicate the impairment of a critical, regulatory event linked with signal transduction initiated through CD40.
With the exception of the in vivo anti-sRBC IgM response, the majority of the Δ9-THC-mediated effects studied in this investigation appeared to occur in a CB1/CB2-independent manner, as evidenced by the ability of Δ9-THC to induce similar responses in CB1−/−/CB2−/− and wild-type mice. Again, these results also need to be interpreted cautiously, in that they only rule out the involvement of known cannabinoid receptors. There is increasing evidence suggesting additional cannabinoid molecular targets, whether discrete bona fide receptors or perhaps signaling intermediates such as kinases or phosphatases. For example, recent findings from our laboratory revealed that the cannabinoid-induced (Δ9-THC, cannabinol, and HU-210) rise in intracellular calcium, which was observed in resting splenic T cells from wild-type and CB1−/−/CB2−/− mice, was attenuated significantly in both genotypes by the cannabinoid receptor antagonists SR141716A (CB1) and SR144528 (CB2) [5]. The ability of both CB1/CB2 receptor antagonists to block a biological response induced by three different but structurally related cannabinoids strongly implicates the existence of additional common cellular targets. The CB1−/−/CB2−/− mouse represents a valuable tool to identify these non-CB1/CB2 receptor cannabinoid molecular targets.
The findings reported here have a number of important implications about the role of CB1 and/or CB2 in immune competence and about the sensitivity of the immune system to modulation by the prototypic cannabinoid Δ9-THC. In spite of recent reports by several laboratories, including our own, about enhanced T cell-mediated immune responses in CB1−/−/CB2−/− mice, including delayed-type hypersensitivity and antiviral responses to influenza, targeted disruption of CB1 and CB2 did not produce profound effects on immune competence, as assessed by well-established and widely used standard immune function assays. Likewise, no profound differences between CB1−/−/CB2−/− and wild-type mice were observed in the percentages of major leukocyte subpopulations, as well as in control responses to a variety of mitogenic stimuli, the mixed lymphocyte response, the in vivo anti-sRBC IgM response, and the production of several major cytokines (i.e., IL-2 and IFN-γ). Clearly, differences were observed within the context of baseline immune responses between CB1−/−/CB2−/− and wild-type mice, but with the exception of the in vitro anti-sRBC IgM AFC response, at this point, it is difficult to discern whether these differences are mechanistically meaningful or simply within the realm of biological variability. As the CB1−/−/CB2−/− mice are characterized further, the basis of these rather subtle differences within the two genotypes will become better understood. In addition, with the exception of humoral immune responses requiring CD40/CD40L interactions (i.e., sRBC- or CD40L plus cytokine-induced IgM AFC responses), suppression of the respective responses occurred in a CB1/CB2-independent manner but does not rule out the possibility of other yet-to-be-identified molecular targets. Conversely, suppression of humoral immune responses involving CD40 appeared to be suppressed significantly by Δ9-THC and dependent on expression of CB1 and/or CB2. The CB1/CB2-dependent effects on humoral immune responses by Δ9-THC at low micromolar concentrations also, once again, raise intriguing questions about why relatively high concentrations of cannabinoids are required to suppress in vitro immune responses, even when acting through cannabinoid receptors. This phenomenon is likely related, at least in part, to the lipophilic properties of cannabinoids, which promote nonspecific binding with serum lipids and proteins. Collectively, this investigation provides a comprehensive, initial characterization of the effects produced by targeted disruption of CB1 and CB2 on immune competence as well as sensitivity to Δ9-THC treatment, which we believe will be critically important to the interpretation of future studies using CB1−/−/CB2−/− mice and in the search of other cannabinoid molecular targets. Likewise, using Δ9-THC as well as other plant-derived cannabinoids as a pharmacological probe in combination with CB1−/−/CB2−/− mice may provide important mechanistic insights into further understanding the potential effects on immune competence associated with cannabis use, especially within the context of host resistance to viral and bacterial pathogens.
Acknowledgments
This work was supported in part by National Institutes of Health grants DA12740, DA07908, and T32 ES07255. We acknowledge Dr. David Sherr for generously providing the CD40L–L cells and Dr. Andreas Zimmer for CB1−/−/CB2−/− mice. We thank Mrs. Kimberly Hambleton for administrative assistance with the preparation of the manuscript.
References
- Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647. [Google Scholar]
- Huestis M A, Gorelick D A, Heishman S J, Preston K L, Nelson R A, Moolchan E T, Frank R A. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Varvel S A, Bridgen D T, Tao Q, Thomas B F, Martin B R, Lichtman A H. Δ9-Tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. J Pharmacol Exp Ther. 2005;314:329–337. doi: 10.1124/jpet.104.080739. [DOI] [PubMed] [Google Scholar]
- Kaplan B L, Rockwell C E, Kaminski N E. Evidence for cannabinoid receptor-dependent and -independent mechanisms of action in leukocytes. J Pharmacol Exp Ther. 2003;306:1077–1085. doi: 10.1124/jpet.103.051961. [DOI] [PubMed] [Google Scholar]
- Rao GK, Kaminski NE. Cannabinoid-mediated elevation of intracellular calcium: a structure-activity relationship. J Pharmacol Exp Ther. 2006;317:820–829. doi: 10.1124/jpet.105.100503. [DOI] [PubMed] [Google Scholar]
- Rockwell C E, Snider N T, Thompson J T, Vanden Heuvel J P, Kaminski N E. Interleukin-2 suppression by 2-arachidonyl glycerol is mediated through peroxisome proliferator-activated receptor γ independently of cannabinoid receptors 1 and 2. Mol Pharmacol. 2006;70:101–111. doi: 10.1124/mol.105.019117. [DOI] [PubMed] [Google Scholar]
- Curran N M, Griffin B D, O'Toole D, Brady K J, Fitzgerald S N, Moynagh P N. The synthetic cannabinoid R(+)WIN 55,212-2 inhibits the interleukin-1 signaling pathway in human astrocytes in a cannabinoid receptor-independent manner. J Biol Chem. 2005;280:35797–35806. doi: 10.1074/jbc.M507959200. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Andersson DA, Hogestatt ED. (9-Tetrahydrocannabinol and cannabinol activate capsaicin-sensitive sensory nerves via a CB1 and CB2 cannabinoid receptor-independent mechanism. J Neurosci. 2002;22:4720–4727. doi: 10.1523/JNEUROSCI.22-11-04720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D A, Owens W A, Gould G G, Frazer A, Roberts J L, Daws L C, Giuffrida A. CB1-independent inhibition of dopamine transporter activity by cannabinoids in mouse dorsal striatum. J Neurochem. 2007;101:389–396. doi: 10.1111/j.1471-4159.2006.04383.x. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Shire D, Calandra B, Bouaboula M, Barth F, Rinaldi-Carmona M, Casellas P, Ferrara P. Cannabinoid receptor interactions with the antagonists SR 141716A and SR 144528. Life Sci. 1999;65:627–635. doi: 10.1016/s0024-3205(99)00285-4. [DOI] [PubMed] [Google Scholar]
- Landsman R S, Burkey T H, Consroe P, Roeske W R, Yamamura H I. SR141716A is an inverse agonist at the human cannabinoid CB1 receptor. Eur J Pharmacol. 1997;334:R1–R2. doi: 10.1016/s0014-2999(97)01160-6. [DOI] [PubMed] [Google Scholar]
- Rhee M H, Kim S K. SR144528 as inverse agonist of CB2 cannabinoid receptor. J Vet Sci. 2002;3:179–184. [PubMed] [Google Scholar]
- Portier M, Rinaldi-Carmona M, Pecceu F, Combes T, Poinot-Chazel C, Calandra B, Barth F, le Fur G, Casellas P. SR 144528, an antagonist for the peripheral cannabinoid receptor that behaves as an inverse agonist. J Pharmacol Exp Ther. 1999;288:582–589. [PubMed] [Google Scholar]
- Krylatov A V, Maslov L N, Lasukova O V, Pertwee R G. Cannabinoid receptor antagonists SR141716 and SR144528 exhibit properties of partial agonists in experiments on isolated perfused rat heart. Bull Exp Biol Med. 2005;139:558–561. doi: 10.1007/s10517-005-0344-9. [DOI] [PubMed] [Google Scholar]
- Batkai S, Pacher P, Jarai Z, Wagner J A, Kunos G. Cannabinoid antagonist SR-141716 inhibits endotoxic hypotension by a cardiac mechanism not involving CB1 or CB2 receptors. Am J Physiol Heart Circ Physiol. 2004;287:H595–H600. doi: 10.1152/ajpheart.00184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsak M, Gaffal E, Date R, Wang-Eckhardt L, Rehnelt J, Petrosino S, Starowicz K, Steuder R, Schlicker E, Cravatt B, Mechoulam R, Buettner R, Werner S, Di Marzo V, Tuting T, Zimmer A. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science. 2007;316:1494–1497. doi: 10.1126/science.1142265. [DOI] [PubMed] [Google Scholar]
- Buchweitz J P, Karmaus P W, Williams K J, Harkema J R, Kaminski N E. Targeted deletion of cannabinoid receptors CB1 and CB2 produced enhanced inflammatory responses to influenza A/PR/8/34 in the absence and presence of Δ9-tetrahydrocannabinol. J Leukoc Biol. 2008;83:785–796. doi: 10.1189/jlb.0907618. [DOI] [PubMed] [Google Scholar]
- Jarai Z, Wagner J A, Varga K, Lake K D, Compton D R, Martin B R, Zimmer A M, Bonner T I, Buckley N E, Mezey E, Razdan R K, Zimmer A, Kunos G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Yang K H, Kaminski N E. Effect of putative endogenous cannabinoid receptor ligands, anandamide and 2-arachidonyl-glycerol, on immune function in B6C3F1 mouse splenocytes. J Pharmacol Exp Ther. 1995;275:529–536. [PubMed] [Google Scholar]
- Kaminski N E, Holsapple M P. Inhibition of macrophage accessory cell function in casein-treated B6C3F1 mice. J Immunol. 1987;139:1804–1810. [PubMed] [Google Scholar]
- Schatz A R, Koh W S, Kaminski N E. Δ9-Tetrahydrocanabinol selectively inhibits T-cell dependent humoral immune responses through direct inhibition of accessory T-cell function. Immunopharmacology. 1993;26:129–137. doi: 10.1016/0162-3109(93)90005-b. [DOI] [PubMed] [Google Scholar]
- Dunnett C W. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–1121. [Google Scholar]
- Bystry R S, Aluvihare V, Welch K A, Kallikourdis M, Betz A G. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–1132. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- Eddahri F, Oldenhove G, Denanglaire S, Urbain J, Leo O, Andris F. CD4+ CD25+ regulatory T cells control the magnitude of T-dependent humoral immune responses to exogenous antigens. Eur J Immunol. 2006;36:855–863. doi: 10.1002/eji.200535500. [DOI] [PubMed] [Google Scholar]
- Mesel-Lemoine M, Cherai M, Le Gouvello S, Guillot M, Leclercq V, Klatzmann D, Thomas-Vaslin V, Lemoine F M. Initial depletion of regulatory T cells: the missing solution to preserve the immune functions of T lymphocytes designed for cell therapy. Blood. 2006;107:381–388. doi: 10.1182/blood-2005-07-2658. [DOI] [PubMed] [Google Scholar]
- Buchweitz J P, Harkema J R, Kaminski N E. Time-dependent airway epithelial and inflammatory cell responses induced by influenza virus A/PR/8/34 in C57BL/6 mice. Toxicol Pathol. 2007;35:424–435. doi: 10.1080/01926230701302558. [DOI] [PubMed] [Google Scholar]
- Ziring D, Wei B, Velazquez P, Schrage M, Buckley N E, Braun J. Formation of B and T cell subsets require the cannabinoid receptor CB2. Immunogenetics. 2006;58:714–725. doi: 10.1007/s00251-006-0138-x. [DOI] [PubMed] [Google Scholar]
- Jan T R, Rao G K, Kaminski N E. Cannabinol enhancement of interleukin-2 (IL-2) expression by T cells is associated with an increase in IL-2 distal nuclear factor of activated T cell activity. Mol Pharmacol. 2002;61:446–454. doi: 10.1124/mol.61.2.446. [DOI] [PubMed] [Google Scholar]
- Matveyeva M, Hartmann C B, Harrison M T, Cabral G A, McCoy K L. Δ(9)-Tetrahydrocannabinol selectively increases aspartyl cathepsin D proteolytic activity and impairs lysozyme processing by macrophages. Int J Immunopharmacol. 2000;22:373–381. doi: 10.1016/s0192-0561(99)00092-2. [DOI] [PubMed] [Google Scholar]
- McCoy K L, Matveyeva M, Carlisle S J, Cabral G A. Cannabinoid inhibition of the processing of intact lysozyme by macrophages: evidence for CB2 receptor participation. J Pharmacol Exp Ther. 1999;289:1620–1625. [PubMed] [Google Scholar]
- Condie R, Herring A, Koh W S, Lee M, Kaminski N E. Cannabinoid inhibition of adenylate cyclase-mediated signal transduction and IL-2 expression in the murine T-cell line, EL4.IL-2. J Biol Chem. 1996;271:13175–13183. doi: 10.1074/jbc.271.22.13175. [DOI] [PubMed] [Google Scholar]
- Jan T R, Farraj A K, Harkema J R, Kaminski N E. Attenuation of the ovalbumin-induced allergic airway response by cannabinoid treatment in A/J mice. Toxicol Appl Pharmacol. 2003;188:24–35. doi: 10.1016/s0041-008x(03)00010-3. [DOI] [PubMed] [Google Scholar]