Abstract
FcαRI (CD89) is a human IgA FcR expressed on cells of myeloid lineage such as neutrophils, monocytes, tissue macrophages, eosinophils, and subpopulations of dendritic cells. FcαRI mediates cell activation through Src family kinases and downstream tyrosine-based phosphorylation pathways. However, the role of IgA and the expression and role of its cognate receptor FcαRI (CD89) in platelet activation are undefined. In the current study, we demonstrate that human platelets express FcαRI mRNAs and proteins. Furthermore, we show that the platelet FcαRI is associated with the FcR γ-chain, and cross-linking of FcαRI leads to Syk phosphorylation. Clustering of FcαRI induces pre-mRNA splicing and protein production of tissue factor and IL-1β, suggesting novel roles for human platelet FcαRI and serum IgA in thrombosis and inflammation.
Keywords: CD89, pre-mRNA splicing, IL-1_oblique>β, tissue factor
INTRODUCTION
FcαRI, a type I immune membrane receptor, is expressed on the cells of myeloid lineage including neutrophils, eosinophils, monocytes, tissue macrophages, and subpopulations of dendritic cells [1]. FcαRI binds monomeric and dimeric IgA, and clustering of FcαRI by IgA immune complexes initiates endocytosis, phagocytosis, and other immune responses [2]. The FcR for IgA (FCAR) gene is located in the leukocyte receptor cluster on human chromosome 19q13.4 along with platelet collagen receptor glycoprotein VI (GP-VI), NK cell immune receptors, and leukocyte Ig-like receptors [3,4,5,6,7]. FcαRI shares sequence similarity with platelet collagen receptor GP-VI [5], killer cell inhibitory receptors, and other members of the leukocyte receptor cluster [3, 4]. FcαRI orthologs have been identified in chimpanzees, macaques, cattle, horses, and rats [8,9,10,11]. However, no equivalent gene has been identified in mice [11].
The membrane distal extracellular domain (EC1) of FcαRI is responsible for ligand-binding activity, and FcαRI binds to each IgA CH2–CH3 domain interface by forming a 2:1 complex with a Fcα dimer [12, 13]. FcαRI can associate with the FcR γ-chain through its transmembrane domain. However, FcαRI is often expressed in the absence of γ-chain pairing [14]. FcαRI mRNAs have at least 10 alternatively spliced variants, among which the splicing isoform lacking 22 residues (66 nucleotides) within EC2 is expressed on alveolar macrophages in vivo [1]. The alternatively spliced FcαRI isoforms might have physiologic relevance in IgA-mediated host defense [15].
Human IgA (hIgA) has a central role in host defense on mucosal surfaces. In human serum, IgA is the second-most abundant Ig after IgG, and IgA accounts for one-fifth of the total Ig. hIgA is primarily of the IgA1 subclass and exists mainly in monomeric form (>95%) [16]. In the intestinal lamina propria, the B lineage cells produce polymeric IgA, which exists almost exclusively as dimers. IgA dimers can be joined by a J-chain polypeptide and then linked to the secretory component to form a complex called secretory IgA (SIgA). Through FcαRI, SIgA is able to initiate inflammatory responses, such as respiratory burst of polymorphonuclear leukocytes and degranulation of eosinophils [16, 17].
Human platelets are anucleate cells that have hemostatic and inflammatory functions [18, 19]. As a rich source for cytokines and chemokines, platelets have major roles in the pathogenesis of human inflammatory diseases [20,21,22,23,24,25]. Human platelets possess functional spliceosomes that are able to process pre-mRNAs into mature mRNAs, from which proteins can be translated and processed in platelets [26, 27]. Antiplatelet agents appear to protect renal function in IgA nephropathy patients [28], suggesting that pathogenic IgA immune complexes may be involved in the activation of platelets and in the pathogenesis of IgA nephropathy. In the current study, we demonstrate that human platelets express a functional IgAR (FcαRI). Cross-linking of FcαRI leads to Syk phosphorylation. Furthermore, FcαRI mediates tissue-factor, pre-mRNA splicing and proinflammatory cytokine (IL-1β) production in human platelets, suggesting novel roles for human platelet FcαRI and serum IgA in thrombosis and inflammation.
MATERIALS AND METHODS
Reagents
All mAb used in this study were produced by murine hybridomas. Anti-human FcαRI mAb MIP8a [murine IgG1 (mIgG1)] was purchased from Serotec (Raleigh, NC, USA). Mouse anti-human CD62P (P-selectin) mAb was from eBioscience (San Diego, CA, USA). mAb A59 and A77 are mIgG1 specific for FcαRI as described previously [29]. Anti-FcR γ-chain hybridoma (clone 7D3, mIgG1) was generated and characterized at the Epitope Recognition and Immunoreagent Core Facility of the University of Alabama at Birmingham (UAB; Birmingham, AL, USA). The purified mAb (A59, A77, and 7D3) were produced at the Epitope Recognition and Immunoreagent Core Facility of UAB. The F(ab′)2 preparations of mAb A59 and 7D3 were produced at Rockland Immunochemicals (Gilbertsville, PA, USA), and the purity of these antibody F(ab′)2 preparations was confirmed by SDS gel staining and Western blotting assays. Human serum IgA, FITC-conjugated and nonconjugated goat anti-mouse IgG (H+L), mIgG F(ab′)2, and FITC-conjugated goat anti-hIgA were obtained from Jackson ImmunoResearch (West Grove, PA, USA). Goat anti-hIgA F(ab′)2 was purchased from Southern Biotechnologies (Birmingham, AL, USA). Rabbit anti-CD89 (FcαRI) cytoplasmic domain (CD89cyt) polyclonal antibody (pAb) was produced using GST-CD89cyt fusion protein at Cocalico Biologicals (Reamstown, PA, USA). Anti-Syk rabbit pAb, anti-Syk mAb, and anti-Syk pAb-conjugated agarose beads were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antiphosphotyrosine mAb (clone 4G10), anti-human tissue-factor mAb (clone GMA-320), and rabbit anti-FcR γ-chain pAb were obtained from Upstate Biotech (Waltham, MA, USA). AminoLink kit (cyanogen bromide-activated Sepharose 4B coupling gel and reagents) was purchased from Pierce (Rockford, IL, USA). Human IL-1β cytokine ELISA detection kits were obtained from BD PharMingen (San Jose, CA, USA). Human IgG, fibrinogen, thrombin, and PGE1 were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Preparation of human platelets
The human studies were reviewed and approved by the Institutional Review Board of UAB, and all donors provided written, informed consent. Venous blood (50 ml) was collected from healthy, normal donors in BD Vacutainer tubes containing Na2 EDTA (BD Biosciences, San Jose, CA, USA). Platelets were purified as described by Shiraki el al. [30] with small modification. Briefly, platelet-rich plasma (PRP) was obtained by centrifugation (15 min, 175 g) at room temperature. Packed cells were discarded, and PGE1 (1 μM) was added to the PRP and maintained throughout the purification procedure. PRP was centrifuged again (10 min, 175 g) to remove residual leukocytes. To avoid the contamination of peripheral blood leukocytes, platelets were collected from the upper half to two-thirds of the PRP supernatant for experimental use. Platelets were isolated by centrifugation of the resulting PRP (15 min, 1000 g) at room temperature. Leukocyte contamination was evaluated by flow cytometry with anti-human CD45 and CD14 mAb (Invitrogen, Carlsbad, CA, USA). The contaminated leukocytes were assessed by positive staining for CD45 or CD14 in gating of the entire platelet population. In addition, monocyte contamination was evaluated by amplification of CD14 mRNA by RT-PCR. The purified platelets were resuspended in 10 ml Tyrode-HEPES buffer or platelet activation buffer (HBSS containing 5 mM glucose, 0.1% BSA, and 10 mM HEPES, pH 7.4).
Platelet FcαRI RT-PCR and cDNA sequencing
Total RNA was isolated from 109 platelets by using TRIzol™ total RNA isolation reagent (Gibco-BRL, Grand Island, NY, USA). Total RNA (1 μg) was used to synthesize cDNA with the SuperScript™ preamplification system (Gibco-BRL). RT-PCR was carried out with the forward primer (5′-ATT GAC CAC ATG GAC GCA AAC AAG G-3′ anneals between nt 298 and nt 322) and the reverse primer (5′-CCA AGA GAG CCA CGA GGA CCA GTC-3′ anneals between nt 743 and nt 766) to yield a FcαRI cDNA fragment (469 bp; GenBank Accession Number for FcαRI: X54150). For CD14 RT-PCR, the forward primer (5′-AAA GCA CTT CCA GAG CCT GC-3′) and reverse primer (5′-TCG AGC GTC AGT TCC TTG AGG-3′) were used as described by Panes et al. [31]. The PCR reaction was carried out on a GeneAmp PCR System 9700 (Applied Biosystems Inc., Foster City, CA, USA) using 2 μl cDNA (synthesized with the SuperScript™ preamplification system), 200 nM each primer, 200 μM dNTPs, 1.5 mM MgCl2, and 2.5 U Taq DNA polymerase in a 50-μl reaction vol starting with 95°C for 5 min, 35 cycles of denaturing at 94°C for 30 s, annealing at 56°C for 45 s, and extension at 72°C for 1 min with a final extension at 72°C for 7 min. PCR products were purified with the QIAquick gel extraction kit (Qiagen Inc., Chatsworth, CA, USA). All PCR products were sequenced in both directions on an ABI 3700 sequencer using BigDye terminator cycle sequencing kit (Version 3.1; Applied Biosystems Inc.).
Platelet activation and platelet lysate preparation
For activation experiments, platelets were suspended at the density of 2 × 109 cells/ml in ice-cold platelet activation buffer and divided into 0.5 ml aliquots. mAb A59 F(ab′)2 was added at a final concentration of 10 μg/ml, where required, and all aliquots were incubated on ice for 40 min. Platelets were then centrifuged at 4°C (1 min, 2655 g) in a Beckman GS-15R centrifuge. The supernatants containing unbound antibodies were removed by aspiration, and the platelet pellets were resuspended gently in 0.5 ml ice-cold platelet activation buffer. Platelets were activated at 37°C by thrombin (1 unit/ml) plus fibrinogen (100 μg/ml) or by clustering the bound A59 F(ab′)2 with goat anti-mouse IgG F(ab′)2 (30 μg/ml) for the predetermined time periods. Reactions were terminated by adding an equal volume of 2× Triton X-100 lysis buffer (1% Triton X-100, 10 mM EDTA, 10 mM HEPES, pH 7.4, 2 mM PMSF, 3 mM sodium orthovanadate, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) and incubated on ice for 30 min to ensure the complete lysis of platelets. Platelet lysates were then centrifuged at 4°C (20 min, 12,000 g) to remove cell debris. Alternatively, platelet activations were terminated by the addition of 1 ml ice-cold PBS. The platelets were pelleted with a quick spin in a centrifuge at 4°C (1 min, 2700 g).
Immunoprecipitation and immunoblotting
Nontreated and activated platelet cell lysates were immunoprecipitated at 4°C for 2 h using 2 μg appropriate antibodies conjugated to Sepharose 4B beads. Immunoprecipitates were washed three times with ice-cold 1× Triton X-100 lysis buffer and once with ice-cold PBS. Immunoprecipitated proteins on the beads were dissociated in 30 μl 6× Laemmli sample buffer. For the detection of FcαRI and tissue factor (TF) in human platelets, the platelet whole cell lysates were mixed directly with 6× Laemmli sample buffer. Proteins were denatured by heating (5 min, 100°C) and separated in 12% SDS-PAGE under reducing conditions. The proteins in gels were electrotransferred to nitrocellulose membranes (Sigma Chemical Co.), which were blocked with 5% nonfat milk in PBS containing 0.1% Tween 20 (PBS-T) for 1 h at room temperature and probed overnight at 4°C with appropriate primary antibodies. Blots were washed three times with PBS-T and incubated with the appropriate HRP-conjugated secondary antibody for 60 min at room temperature. Blots were washed three more times and were developed using an ECL detecting system (Pierce). In some experiments, membranes were stripped, and immunoblotting was repeated with another antibody.
Flow cytometry
Aliquots of the washed platelets or cells (106) in 0.1 ml PBS were incubated with fluorescence-labeled or unlabeled, appropriate antibodies (anti-FcαRI mAb at the final concentration of 5 μg/ml) for 45 min on ice and followed by two washes. For indirect immunofluorescence staining, the platelets or cells were then incubated with saturating concentrations of FITC-conjugated goat anti-mouse IgG F(ab′)2 at 4°C for 45 min. After wash, the immunofluorescence intensity of cells was analyzed immediately using a FACScan (BD Biosciences).
For IgA-binding assays, washed platelets (106) in 0.1 ml PBS were pretreated with human IgG (final concentration: 10 μg/ml) for 45 min on ice for the blockade of FcγRs. Then, human serum IgA (final concentration: 100 μg/ml) was added to the platelets, which were incubated on ice for 60 min. After washing with PBS-BSA buffer (PBS containing 1.0% BSA and 0.05% NaN3), platelets were incubated with FITC-conjugated goat anti-hIgA (final concentration: 100 μg/ml) for 45 min on ice. The platelets were washed twice and analyzed by a FACScan (BD Biosciences). To determine whether hIgA binding to platelets was inhibited competitively by anti-FcαRI mAb, platelets (106) were pretreated with anti-FcαRI mAb MIP8a (final concentration: 50 μg/ml) or mIgG1 isotype control (final concentration: 50 μg/ml) for 60 min on ice before incubation with human serum IgA.
To examine IgA-induced platelet activation, hIgA (final concentration: 100 μg/ml) was incubated with platelets. After wash, the surface-bound IgA on platelets was cross-linked with goat anti-hIgA antibody F(ab′)2 (final concentration: 80 μg/ml; Southern Biotechnologies) at 37°C for 30 min. Platelets stimulated by thrombin (1 unit/ml) were used as positive control. The surface levels of platelet CD62P were assayed with flow cytometry using a FACScan.
TF and IL-1β pre-mRNA splicing assays
To examine the TF pre-mRNA splicing, primers that target sequences in TF gene exon four (5′-CTC GGA CAG CCA ACA ATT CAG-3′) and exon five (5′-CGG GCT GTC TGT ACT CTT CC-3′) were used to determine endogenous splicing of TF pre-mRNA in nonstimulated and activated platelets as described by Schwertz et al. [26]. RT-PCR of the mature TF mRNA generates a 297-bp DNA fragment, and the amplification of the unspliced TF pre-mRNA produces a 904-bp DNA fragment. To detect human IL-1β pre-mRNA splicing in platelets, primers that anneal at human IL-1β gene exon one (5′-CGA GGC ACA AGG CAC AAC AG-3′) and exon two (5′-TGT AAT AAG CCA TCA TTT CAC T-3′) were used. These exonic primers allow us to detect the mature IL-1β mRNA (121 bp) and the unspliced IL-1β pre-mRNA (584 bp). The One-Step RT-PCR system (SuperScript III One-Step RT-PCR system with Platinum Taq DNA polymerase) was used to amplify the pre-mRNA and mature mRNA of TF and IL-1β from total RNA purified from the nonstimulated and activated platelets, according to the manufacturer’s instruction (Invitrogen).
Human IL-1β cytokine assays
The purified platelets were resuspended at the density of 5 × 108 cells/ml in RPMI-1640 culture medium without FBS. The platelets were stimulated in 24-well tissue-culture plates (Corning) coated with anti-FcαRI mAb A59 F(ab′)2 (20 μg/ml) or mIgG F(ab′)2 (20 μg/ml), hIgA (20 μg/ml), or thrombin (1 unit/ml) plus fibrinogen (100 μg/ml). The wells were washed twice with PBS and then blocked with 5% BSA prior to the addition of 2.5 × 108 platelets (in 0.5 ml culture medium) for each well. The platelets were incubated at 37°C in 5% CO2 for various periods of time. At each time-point, the platelets in culture media were collected, and platelets were pelleted by centrifugation. The culture supernatants were collected for IL-1β cytokine determination. The levels of human IL-1β in culture medium were quantified by ELISA according to the manufacturer’s instruction (BD PharMingen).
RESULTS
Gene expression of FcαRI in human platelets
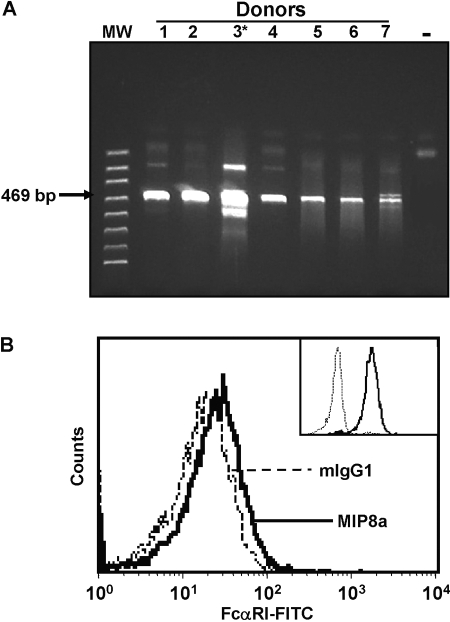
The expression of FcαRI in human myeloid cells is well established, and the functions of FcαRI in human myeloid cells have been characterized extensively [1]. Interestingly, polymorphisms in the FCAR gene are associated with human myocardial infarction, suggesting that FcαRI may be involved in platelet functions [32]. Therefore, we investigated whether human platelets express FcαRI gene products. To rule out the contamination of PBMCs, we carried out flow cytometry assays with the purified platelets. The contamination of PBMCs was less than 1/108, as assayed by flow cytometry with anti-human CD45 (marker for hematopoietic cells), CD14 (marker for monocytes), and CD61 (marker for human platelets; data not shown). The similar degree of purity was reported by others using the same preparation procedure [30]. In addition, we examined the presence of CD14 mRNA (a specific marker of PBMCs) in our platelet preparations by RT-PCR. CD14 mRNA was absent in our platelet preparations (data not shown), indicating that the contamination of leukocytes in platelets was negligible. Total RNA was isolated from the purified platelets, and the platelet mRNAs were transcribed reversely into cDNAs. RT-PCR was carried out to detect FcαRI cDNA. We were able to detect FCAR gene products (cDNAs) in platelets from all normal, healthy donors (Fig. 1A). The identity of FCAR cDNAs was confirmed further by direct sequencing of the cDNA products (data not shown).
Fig. 1.
Expression of FcαRI gene products in human platelets. (A) RT-PCR analysis of FcαRI mRNA in human platelets. Total RNA was isolated from the purified platelets from six individuals (Lanes 1, 2, and 4–7) or from the peripheral blood leukocytes of a healthy donor (Lane 3*, positive control for FcαRI mRNA). Platelet RNA was reversely transcribed, and FcαRI cDNAs were amplified by RT-PCR. The identity of FcαRI cDNA products in human platelets was confirmed further by direct sequencing. (B) Detection of FcαRI on the surface of human platelets. FcαRI was detected using anti-FcαRI mAb MIP8a (bold line) in flow cytometry analysis by comparing with the mIgG1 isotype control (dashed line). The inset shows the staining of FcαRI (bold line) compared with mIgG1 isotype control (thin line) on human neutrophils. This is representative of four independent experiments with at least four normal, healthy donors shown.
Human platelets express FcαRI protein
Next, we carried out flow cytometry assays to examine whether FcαRI is expressed on the surface of human platelets. Anti-FcαRI mAb MIP8a was used to stain the purified human platelets. As shown in Figure 1B, staining of platelets with anti-FcαRI mAb MIP8a caused a fluorescence-intensity shift compared with mIgG1 isotype control, indicating that FcαRI is expressed on the cell surface of human platelets. However, the level of FcαRI expression on human platelets was much lower than that on neutrophils (Fig. 1B, inset). Similar results were obtained with anti-FcαRI mAb A77 and A59 (data not shown), further confirming the expression of FcαRI on platelets. Using Western blot analysis with anti-FcαRI mAb (A77, A59, and MIP8a) and rabbit pAb against the FcαRI cytoplasmic domain, we detected multiple FcαRI protein species in human platelet cell lysates from all healthy donors (Fig. 2), suggesting the human platelet may express multiple splicing forms of FcαRI protein in human platelets.
Fig. 2.
Detection of FcαRI proteins in human platelets, which were purified as described in Materials and Methods. The purified platelets (109) from three normal, healthy donors (Lanes 1–3) were lysed, and cell lysates were separated in 12% SDS-PAGE under reducing conditions. FcαRI proteins were detected on four separate blots with three anti-FcαRI mAb (A77, A59, and MIP8a) and rabbit pAb against the FcαRI cytoplasmic domain in Western blot analysis. FcαRI proteins were shown as multiple protein bands with a molecular weight (Mr) between 50 and 70 kDa. IB, Immunoblot.
hIgA binds to platelet FcαRI
To examine whether platelet FcαRI is able to bind hIgA, we performed flow cytometry analysis. Human platelets were incubated with hIgA, and the platelet-bound hIgA was detected with FITC-conjugated goat anti-hIgA. As shown (see Fig. 3), hIgA was able to bind platelets, and treatment of platelets with anti-FcαRI mAb MIP8a completely blocked the binding of hIgA to platelets. Treatment of platelets with mIgG1 isotype control decreased (but not completely) the binding of hIgA to platelets, indicating that platelets may bind some IgA nonspecifically or may express another IgA-binding receptor (or receptors) that could be blocked by mIgG1. Taken together, our data demonstrate that FcαRI is a platelet IgA receptor capable of binding hIgA. To examine whether clustering of FcαRI leads to platelet activation, we carried out a stimulation experiment. Human platelets were incubated with hIgA, and hIgA on platelets was cross-linked with goat anti-hIgA. As shown in Figure 3B, cross-linking of the IgA receptor significantly increased the platelet CD62P expression, which was comparable with the thrombin stimulation, suggesting that FcαRI can serve as an activation receptor on human platelets.
Fig. 3.
hIgA binds and activates human platelets. (A) Binding of hIgA to platelets, which were preincubated with anti-FcαRI-blocking mAb MIP8a (final concentration: 50 μg/ml; thin line, MIP8a+hIgA), mIgG1 isotype control (final concentration: 50 μg/ml; dashed line), or without any treatment (bold line, hIgA). After washing, human platelets were incubated with hIgA (final concentration: 100 μg/ml) and followed by FITC-conjugated goat anti-hIgA antibody. Platelets stained with FITC-conjugated goat anti-hIgA antibody alone were used as autofluorescence control (shaded histogram). (B) Induction of platelet activation by hIgA. Human platelets were stimulated as described in Materials and Methods. Platelet activation was defined as an increase of CD62P expression. The results were representative of four independent experiments.
Cross-linking of platelet FcαRI leads to Syk kinase phosphorylation
FcαRI is associated with the FcR γ-chain and can mediate activation in human myeloid cells [29, 33]. The FcR γ-chain is present in human platelets, where the FcR γ-chain is physically and stably associated with the platelet collagen receptor GP-VI and serves as a critical signaling adaptor [34,35,36]. To determine whether the platelet FcαRI is associated with the FcR γ-chain, we performed immunoprecipitation with anti-FcαRI mAb. As shown in Figure 4A, the FcR γ-chain was coprecipitated with FcαRI in platelet lysates from all five normal, healthy donors, confirming that FcαRI is constitutively associated with the endogenous FcR γ-chain in human platelets.
Fig. 4.
Induction of Syk phosphorylation by clustering of platelet FcαRI on human platelets. (A) Physical association between the FcαRI and FcR γ-chain in human platelets. Freshly isolated platelets were lysed in 1× Trition X-100 lysis buffer as described in Materials and Methods. Whole platelet cell lysates were immunoprecipitated (IP) with anti-FcαRI mAb A59 F(ab′)2-conjugated agarose beads from five donors (Lanes 1–5) or from a donor with mIgG F(ab′)2-conjugated agarose beads as control (Lane 6). Immunoprecipitated proteins were resolved on SDS-PAGE and immunoblotted with mAb MIP8a (upper panel) or anti-FcR γ-chain pAb (lower panel), followed by HRP-conjugated second antibody. The blots were developed using an ECL-detecting system. The results were representative of three independent experiments. (B) Clustering of FcαRI induced Syk phosphorylation. Purified human platelets (109) were stimulated with anti-FcαRI mAb A59 F(ab′)2 plus goat anti-mouse IgG F(ab′)2 for the various periods of time (in seconds) as indicated. Reactions were terminated by adding an equal volume of 2× lysis buffer, and the FcR γ-chain was immunoprecipitated from platelet lysates with anti-FcR γ-chain mAb 7D3 F(ab′)2-conjugated agarose beads. The immunoprecipitated proteins were resolved in SDS-PAGE and immunoblotted with anti-Syk pAb (top panel), antiphosphotyrosine antibody 4G10 (middle panel), or anti-FcR γ-chain pAb (bottom panel). The results were representative of three independent experiments.
Cross-linking of FcαR on human myeloid cells mediates activation signals via the FcR γ-chain and induces tyrosine phosphorylation of several cellular proteins, including p72Syk, which is a major target of early protein tyrosine kinase activity [1]. Therefore, we analyzed the Syk recruitment after cross-linking of FcαRI on human platelets. The FcR γ-chain was immunoprecipitated with anti-FcR γ-chain mAb 7D3 from lysates of the stimulated or nonstimulated platelets. The FcR γ-chain-associated Syk was detected with anti-Syk by Western blot analysis. No increase of Syk association (with the FcR γ-chain) and phosphorylation was observed in the platelets stimulated with the control mIgG (data not shown). We found that cross-linking of FcαRI significantly increased association of the FcR γ-chain with Syk (Fig. 4B, top panel). In addition, Syk tyrosine phosphorylation was increased significantly after FcαRI clustering (Fig. 4B, middle panel), suggesting platelet FcαRI could mediate activation signals through the FcR γ-chain.
FcαRI-induced platelet TF pre-mRNA splicing and protein production
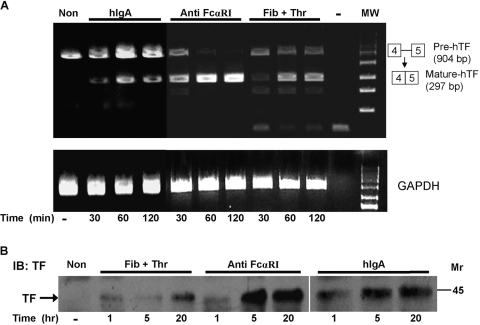
Platelet TF is a 45-kDa transmembrane cell-surface glycoprotein essential for blood clotting. TF also plays an important role in a variety of biological processes such as inflammation, angiogenesis, metastasis, and cell migration [37]. Resting human platelets express TF pre-mRNA that can be processed (or spliced) into mature TF mRNA in response to activation [26]. The process of TF pre-mRNA splicing is required for platelet TF protein production [26]. To investigate whether cross-linking of platelet FcαRI is able to induce TF pre-mRNA splicing, we performed RT-PCR assays. Platelet FcαRI was cross-linked with anti-FcαRI mAb A59 F(ab′)2 plus goat anti-mouse IgG F(ab′)2. As shown in Figure 5A, there was no mature TF mRNA (297 bp) in the nonstimulated platelets. Thirty minutes after cross-linking of FcαRI, mature TF mRNA was produced in the platelets along with the decreased pool of unspliced TF pre-mRNA (904 bp). Sixty minutes after the stimulation of FcαRI, almost all of the TF pre-mRNA was processed into the mature TF mRNA, suggesting that cross-linking of the IgA receptor (FcαRI) on platelets is a potent signal for TF pre-mRNA splicing. Similarly, cross-linking of FcαRI with hIgA plus goat anti-hIgA F(ab′)2 also induced TF pre-mRNA splicing (Fig. 5A).
Fig. 5.
FcαRI-mediated TF pre-mRNA splicing and protein synthesis in platelets. (A) Cross-linking of platelet FcαRI induced mature TF mRNA production. Purified human platelets (5×108) were stimulated with anti-FcαRI mAb A59 F(ab′)2 plus goat anti-mouse IgG F(ab′)2 (Anti FcαRI-marked lanes), with fibrinogen plus thrombin (Fib+Thr-marked lanes), or with hIgA plus goat anti-hIgA F(ab′)2 (hIgA-marked lanes) for various periods of time (in min). Nonstimulated platelets were labeled as Non. Total RNA was isolated from platelet pellets and used for RT-PCR. The lower panel shows RT-PCR results of GAPDH (internal controls for equal amount of total RNA) at each time-point. The results were representative of at least four independent experiments. (B) Induction of TF protein production by clustering of FcαRI. The platelets (5×108) were stimulated in 24-well tissue-culture plates coated with hIgA, anti-FcαRI mAb A59 F(ab′)2 (Anti FcαRI), and fibrinogen plus thrombin, respectively. The platelets were incubated at 37°C in 5% CO2 for various periods of time (hours). Platelets in culture media were collected by centrifugation at various points of time (0, 1, 5, and 20 h) and lysed in 1× radioimmunoprecipitation assay buffer. Proteins in platelet lysates were separated by SDS-PAGE, and the production of TF was analyzed on Western blots. The results were representative of three independent experiments.
Next, we investigated whether stimulation of human platelet FcαRI leads to TF protein production. Platelet FcαRI was cross-linked with plate-bound anti-FcαRI mAb F(ab′)2 or hIgA. As shown in Figure 5B, stimulation of FcαRI for 1 h induced the production of the TF protein in platelets. Stimulation with anti-FcαRI mAb F(ab′)2 (Anti FcαRI) induced more platelet TF protein production as compared with the stimulation with fibrinogen plus thrombin (Fib+Thr) at 5 h and 25 h time-points (Fig. 5B). Similarly, stimulation of platelets with immobilized hIgA also induced TF protein production (Fig. 5B).
FcαRI-mediated IL-1β production in human platelets
IL-1 is a potent, proinflammatory cytokine implicated in inflammation, septic shock, wound healing, and the growth of certain leukemia [38,39,40]. Activated platelets are a rich resource of IL-1β, which constitutes a link between the coagulation and inflammatory cascades [41]. However, platelets contain predominantly IL-1β pre-mRNA that is needed to be spliced into mature IL-1β mRNA in response to platelet activation [27]. To examine whether cross-linking of platelet FcαRI induces IL-1β pre-mRNA splicing, we carried out RT-PCR assay. Purified human platelets were stimulated with anti-FcαRI mAb A59 F(ab′)2 plus goat anti-mouse IgG F(ab′)2 (Anti FcαRI-marked lanes) or without any treatment (nonstimulated platelets). Total RNA was used for quantitative RT-PCR (Fig. 6A). We observed that the mature IL-1β mRNA (121 bp) was increased significantly 30 min and 60 min after clustering of FcαRI along with the decrease of IL-1β pre-mRNA (584 bp) compared with those in the resting platelets. Similarly, cross-linking of FcαRI with hIgA plus goat anti-hIgA F(ab′)2 also induced IL-1β pre-mRNA splicing (Fig. 6A). To learn whether clustering of platelet FcαRI induces IL-1β production, we carried out ELISA assays. The platelets were stimulated with plate-bound mIgG F(ab′)2, thrombin plus fibrinogen, anti-FcαRI mAb A59 F(ab′)2, and hIgA, respectively. As shown in Figure 6B, immobilized anti-FcαRI mAb A59 F(ab′)2 and hIgA induced significantly more platelet IL-1β protein production than the immobilized mIgG F(ab′)2 or fibrinogen plus thrombin (P<0.0001). Taken together, these data demonstrate that human platelet FcαRI is a functional IgA receptor and that hIgA is able to activate platelets.
Fig. 6.
FcαRI-mediated IL-1β pre-mRNA splicing and protein synthesis in human platelets. (A) Cross-linking of platelet FcαRI induced mature IL-1β mRNA production. Purified human platelets (5×108) were stimulated with anti-FcαRI mAb A59 F(ab′)2 plus goat anti-mouse IgG F(ab′)2 (Anti FcαR-marked lanes), with hIgA plus goat anti-hIgA F(ab′)2 (hIgA-marked lanes) or without stimulation (lane labeled as Non). Total RNA was isolated from platelets pellets and used for RT-PCR. The results were representative of at least four independent experiments. (B) Induction of IL-1β release by clustering platelet FcαRI. The platelets (2.5×108) were stimulated with plate-bound mIgG F(ab′)2, thrombin plus fibrinogen, anti-FcαRI mAb A59 F(ab′)2, and hIgA, respectively. IL-1β in culture supernatants was quantified with ELISA at 5 h and 20 h time-points. The data represent the mean ± sem of four independent experiments. *, Significant differences between platelets stimulated with plate-bound A59 F(ab′)2 or hIgA and platelets stimulated with fibrinogen plus thrombin or mIgG F(ab′)2 (P<0.0001).
DISCUSSION
Human platelets are circulating, anucleate cells, which serve as the principal effectors for cellular hemostasis to prevent excessive bleeding caused by endothelial damage. Platelets adhere to the exposed endothelial matrix and aggregate in response to signals for thrombosis, contributing to the formation of platelet-fibrin clots that are critical for sealing vascular disruptions and for ultimate vessel and wound repair. The activation of platelets is tightly controlled, as unrestrained hemostatic responses will result in the formation of a large, occlusive, coronary thrombus, and insufficiency of thrombosis will lead to excessive bleeding. Platelets are implicated in mortality and morbidity in a variety of human diseases including coronary artery disease, diabetes, stroke, renal disease, and autoimmune diseases [21, 42,43,44,45,46,47,48].
The platelets are produced by cytoplasmic budding of the bone marrow megakaryocytes. Although platelets do not contain any genomic DNA, they inherited much of cytoplasmic contents from megakaryocytes and contain large amounts of mRNA with a great capacity for protein synthesis. Platelets contain pre-mRNA for many platelet proteins including TF and IL-1β [26, 27, 41]. Activated platelets are the major source of a number of proinflammatory mediators, and platelets are considered as immune cells that play important roles in the pathogenesis of human inflammatory diseases [20, 23].
Platelets express FcRs for IgG and IgE [49, 50]. As the sole IgG FcR expressed on human platelets, FcγRIIA contributes to the pathophysiology of diseases such as heparin-induced thrombocytopenia and antibody-mediated thrombocytopenia [51,52,53]. In the current study, we demonstrated that human platelets also express the IgA receptor FcαRI, which is capable of mediating platelet activation. To our knowledge, this is the first study to document the existence of a functional IgA receptor on human platelets. Therefore, our findings fill the knowledge gap regarding the ability of hIgA to activate platelets and certainly will extend our understanding of FcαRI functions in humans.
FcαRI, as a multichain immune recognition receptor, can associate with the ITAM-containing FcR γ-chain subunit. The trimeric FcαRI/(FcR γ-chain)2 initiates phosphotyrosine-based signaling cascades. Therefore, FcαRI could serve as a typical activating and proinflammatory receptor [33]. In this study, we observed that FcαRI expressed on human platelets is constitutively associated with the FcR γ-chain and that clustering of FcαRI induced Syk phosphorylation, suggesting that platelet FcαRI may serve as an activation receptor in human platelets. Stimulation of FcαRI induced TF pre-mRNA splicing and production, suggestive of a role for FcαRI in thrombosis. Furthermore, clustering of platelet FcαRI by hIgA and anti-FcαRI mAb induced IL-1β production, signifying that FcαRI is a potent proinflammatory receptor in platelets. Interestingly, cross-linking of platelet FcαRI induced more TF and IL-1β protein production than the established platelet activators (fibrinogen plus thrombin; Figs. 5B and 6B), suggesting that IgA immune complexes may be more effective in the initiation of a proinflammation response than fibrinogen and thrombin.
hIgA plays important roles in the pathogenesis of various human inflammatory diseases. IgA autoantibodies anticardiolipin and anti-β2-glycoprotein I were the most prevalent isotypes in African American systemic lupus erythematosus (SLE) patients [54]. IgA anti-Ro/SSA, anti-La/SSB, anticardiolipin, and anti-β2-glycoprotein-I autoantibodies were found frequently in serum of patients with SLE and Sjogren’s syndrome [55, 56]. IgA anti-β2-glycoprotein-I autoantibodies, as the most important isotype in the antiphospholipid syndrome, were associated strongly with acute myocardial infarction and unexplained, recurrent abortions in women [57,58,59,60,61,62]. Furthermore, a significantly increased IgA rheumatoid factor (IgA-RF; IgA autoantibodies) was found in rheumatoid arthritis (RA) patients, and the appearance of IgA-RF is a good indicator for the development of RA [63]. hIgA also plays important roles in the pathogenesis of other phenotypes, including dermatitis herpetiformis, celiac disease, periodontal disease, and IgA nephropathy. Recently, an association was established between FCAR gene polymorphism and myocardial infarction [32]. The presence of an IgA antibody against β2-glycoprotein I has been associated significantly with strokes, extensive and recurrent venous thrombosis, and coincident arterial and venous thrombosis in cancer patients [64]. Taken together, IgA and the receptors for IgA appear to play critical roles in the pathogenesis of inflammatory and thrombotic diseases.
Antiplatelet agents are widely used in the treatment of IgA nephropathy patients in the Japanese population, and these agents protect renal function in IgA nephropathy patients [28]. It has even been proposed that antiplatelet agents should be used as the standard therapy for IgA nephropathy. However, the mechanism of antiplatelet agents on IgA nephropathy remains unknown [28]. FcαRI-mediated platelet activation may be one of the mechanisms behind the effectiveness of antiplatelet therapy in IgA nephropathy patients. Circulating IgA immune complexes or IgA immune complex deposits in kidneys could directly activate the platelet IgA receptor and induce proinflammatory cytokine (such as IL-1β) production in IgA nephropathy patients. As the absolute number of platelets in the circulation (∼1012) is enormous, the activated platelets may produce more cytokines than other immune cells combined [65]. Therefore, FcαRI-mediated platelet activation may be a reasonable explanation for IgA-mediated human inflammatory and thrombotic diseases. One can envision that antiplatelet agents may inhibit IgA-mediated platelet activation via the FcαRI in IgA nephropathy patients.
Overall, our data demonstrate that human platelets can be activated via FcαRI. Therefore, platelet FcαRI may play critical roles in the process of thrombosis and in the pathogenesis of human inflammatory diseases. Furthermore, our data suggest that human platelets may also express multiple FcαRI isoforms (Fig. 2). A significant change of FcαRI isoform profiles has been observed in neutrophils from pneumonia patients compared with those from healthy individuals, suggesting that the alternatively spliced FcαRI isoform might have physiologic relevance in IgA-mediated host defense [15, 66]. It is possible that the different FcαRI isoforms may have distinct functions in human platelets. The identification of platelet FcαRI isoforms and the detailed signaling pathways of FcαRI in human platelets are currently under the investigation.
Acknowledgments
This work was supported by National Institutes of Health grants R01-AR33062, R01-AR42476, N01-AI40068, M01-RR00032, and P01-AR49084. The FACS Core Facility and Epitope Recognition and Immunoreagent Core Facility of UAB Arthritis and Musculoskeletal Center were supported by the Rheumatic Diseases Core Center (P30-AR48311). K. Q., R. P. K., and J. W. designed research, analyzed data, and co-wrote the manuscript. A. W. G. and J. C. E. contributed intellectual input. K. Q. and F. X. carried out the experiments. All authors reviewed the final version of the manuscript. We also thank Dr. Aijian Qin for his help with our research project. The authors declare that they have no competing financial interest.
References
- Monteiro R C, Van De Winkel J G. IgA Fc receptors. Annu Rev Immunol. 2003;21:177–204. doi: 10.1146/annurev.immunol.21.120601.141011. [DOI] [PubMed] [Google Scholar]
- Fanger M W, Shen L, Pugh J, Bernier G M. Subpopulations of human peripheral granulocytes and monocytes express receptors for IgA. Proc Natl Acad Sci USA. 1980;77:3640–3644. doi: 10.1073/pnas.77.6.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wende H, Colonna M, Ziegler A, Volz A. Organization of the leukocyte receptor cluster (LRC) on human chromosome 19q13.4. Mamm Genome. 1999;10:154–160. doi: 10.1007/s003359900961. [DOI] [PubMed] [Google Scholar]
- Wagtmann N, Rojo S, Eichler E, Mohrenweiser H, Long E O. A new human gene complex encoding the killer cell inhibitory receptors and related monocyte/macrophage receptors. Curr Biol. 1997;7:615–618. doi: 10.1016/s0960-9822(06)00263-6. [DOI] [PubMed] [Google Scholar]
- Clemetson J M, Polgar J, Magnenat E, Wells T N, Clemetson K J. The platelet collagen receptor glycoprotein VI is a member of the immunoglobulin superfamily closely related to FcαR and the natural killer receptors. J Biol Chem. 1999;274:29019–29024. doi: 10.1074/jbc.274.41.29019. [DOI] [PubMed] [Google Scholar]
- Jandrot-Perrus M, Busfield S, Lagrue A H, Xiong X, Debili N, Chickering T, Le Couedic J P, Goodearl A, Dussault B, Fraser C, Vainehenker W, Villeval J L. Cloning, characterization, and functional studies of human and mouse glycoprotein VI: a platelet-specific collagen receptor from the immunoglobulin superfamily. Blood. 2000;96:1798–1807. [PubMed] [Google Scholar]
- Ezumi Y, Uchiyama T, Takayama H. Molecular cloning, genomic structure, chromosomal localization, and alternative splice forms of the platelet collagen receptor glycoprotein VI. Biochem Biophys Res Commun. 2000;277:27–36. doi: 10.1006/bbrc.2000.3624. [DOI] [PubMed] [Google Scholar]
- Morton H C, Pleass R J, Storset A K, Brandtzaeg P, Woof J M. Cloning and characterization of equine CD89 and identification of the CD89 gene in chimpanzees and rhesus macaques. Immunology. 2005;115:74–84. doi: 10.1111/j.1365-2567.2005.02129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton H C, Pleass R J, Storset A K, Dissen E, Williams J L, Brandtzaeg P, Woof J M. Cloning and characterization of an immunoglobulin A Fc receptor from cattle. Immunology. 2004;111:204–211. doi: 10.1111/j.0019-2805.2003.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers K A, Scinicariello F, Attanasio R. Identification and characterization of macaque CD89 (immunoglobulin A Fc receptor) Immunology. 2004;113:178–186. doi: 10.1111/j.1365-2567.2004.01949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruoka T, Nagata T, Kasahara M. Identification of the rat IgA Fc receptor encoded in the leukocyte receptor complex. Immunogenetics. 2004;55:712–716. doi: 10.1007/s00251-003-0626-1. [DOI] [PubMed] [Google Scholar]
- Wines B D, Hulett M D, Jamieson G P, Trist H M, Spratt J M, Hogarth P M. Identification of residues in the first domain of human Fc α receptor essential for interaction with IgA. J Immunol. 1999;162:2146–2153. [PubMed] [Google Scholar]
- Herr A B, Ballister E R, Bjorkman P J. Insights into IgA-mediated immune responses from the crystal structures of human FcαRI and its complex with IgA1-Fc. Nature. 2003;423:614–620. doi: 10.1038/nature01685. [DOI] [PubMed] [Google Scholar]
- Launay P, Patry C, Lehuen A, Pasquier B, Blank U, Monteiro R C. Alternative endocytic pathway for immunoglobulin A Fc receptors (CD89) depends on the lack of FcRγ association and protects against degradation of bound ligand. J Biol Chem. 1999;274:7216–7225. doi: 10.1074/jbc.274.11.7216. [DOI] [PubMed] [Google Scholar]
- Togo S, Shimokawa T, Fukuchi Y, Ra C. Alternative splicing of myeloid IgA Fc receptor (Fc α R, CD89) transcripts in inflammatory responses. FEBS Lett. 2003;535:205–209. doi: 10.1016/s0014-5793(02)03891-7. [DOI] [PubMed] [Google Scholar]
- Van Egmond M, van Garderen E, van Spriel A B, Damen C A, van Amersfoort E S, van Zandbergen G, van Hattum J, Kuiper J, van de Winkel J G. FcαRI-positive liver Kupffer cells: reappraisal of the function of immunoglobulin A in immunity. Nat Med. 2000;6:680–685. doi: 10.1038/76261. [DOI] [PubMed] [Google Scholar]
- Motegi Y, Kita H. Interaction with secretory component stimulates effector functions of human eosinophils but not of neutrophils. J Immunol. 1998;161:4340–4346. [PubMed] [Google Scholar]
- Jurk K, Kehrel B E. Platelets: physiology and biochemistry. Semin Thromb Hemost. 2005;31:381–392. doi: 10.1055/s-2005-916671. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z M. Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- Steinhubl S R. Platelets as mediators of inflammation. Hematol Oncol Clin North Am. 2007;21:115–121. doi: 10.1016/j.hoc.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Huo Y, Ley K F. Role of platelets in the development of atherosclerosis. Trends Cardiovasc Med. 2004;14:18–22. doi: 10.1016/j.tcm.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Weyrich A S, Lindemann S, Zimmerman G A. The evolving role of platelets in inflammation. J Thromb Haemost. 2003;1:1897–1905. doi: 10.1046/j.1538-7836.2003.00304.x. [DOI] [PubMed] [Google Scholar]
- von Hundelshausen P, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res. 2007;100:27–40. doi: 10.1161/01.RES.0000252802.25497.b7. [DOI] [PubMed] [Google Scholar]
- Ferroni P, Santilli F, Guadagni F, Basili S, Davi G. Contribution of platelet-derived CD40 ligand to inflammation, thrombosis and neoangiogenesis. Curr Med Chem. 2007;14:2170–2180. doi: 10.2174/092986707781389664. [DOI] [PubMed] [Google Scholar]
- Ferroni P, Basili S, Davi G. Platelet activation, inflammatory mediators and hypercholesterolemia. Curr Vasc Pharmacol. 2003;1:157–169. doi: 10.2174/1570161033476772. [DOI] [PubMed] [Google Scholar]
- Schwertz H, Tolley N D, Foulks J M, Denis M M, Risenmay B W, Buerke M, Tilley R E, Rondina M T, Harris E M, Kraiss L W, Mackman N, Zimmerman G A, Weyrich A S. Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenicity of human platelets. J Exp Med. 2006;203:2433–2440. doi: 10.1084/jem.20061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M M, Tolley N D, Bunting M, Schwertz H, Jiang H, Lindemann S, Yost C C, Rubner F J, Albertine K H, Swoboda K J, Fratto C M, Tolley E, Kraiss L W, McIntyre T M, Zimmerman G A, Weyrich A S. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taji Y, Kuwahara T, Shikata S, Morimoto T. Meta-analysis of antiplatelet therapy for IgA nephropathy. Clin Exp Nephrol. 2006;10:268–273. doi: 10.1007/s10157-006-0433-8. [DOI] [PubMed] [Google Scholar]
- Wu J, Ji C, Xie F, Langefeld C D, Qian K, Gibson A W, Edberg J C, Kimberly R P. FcαRI (CD89) alleles determine the proinflammatory potential of serum IgA. J Immunol. 2007;178:3973–3982. doi: 10.4049/jimmunol.178.6.3973. [DOI] [PubMed] [Google Scholar]
- Shiraki R, Inoue N, Kawasaki S, Takei A, Kadotani M, Ohnishi Y, Ejiri J, Kobayashi S, Hirata K, Kawashima S, Yokoyama M. Expression of Toll-like receptors on human platelets. Thromb Res. 2004;113:379–385. doi: 10.1016/j.thromres.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Panes O, Matus V, Saez C G, Quiroga T, Pereira J, Mezzano D. Human platelets synthesize and express functional tissue factor. Blood. 2007;109:5242–5250. doi: 10.1182/blood-2006-06-030619. [DOI] [PubMed] [Google Scholar]
- Iakoubova O A, Tong C H, Chokkalingam A P, Rowland C M, Kirchgessner T G, Louie J Z, Ploughman L M, Sabatine M S, Campos H, Catanese J J, Leong D U, Young B A, Lew D, Tsuchihashi Z, Luke M M, Packard C J, Zerba K E, Shaw P M, Shepherd J, Devlin J J, Sacks F M. Asp92Asn polymorphism in the myeloid IgA Fc receptor is associated with myocardial infarction in two disparate populations: CARE and WOSCOPS. Arterioscler Thromb Vasc Biol. 2006;26:2763–2768. doi: 10.1161/01.ATV.0000247248.76409.8b. [DOI] [PubMed] [Google Scholar]
- Morton H C, van den Herik-Oudijk I E, Vossebeld P, Snijders A, Verhoeven A J, Capel P J, van de Winkel J G. Functional association between the human myeloid immunoglobulin A Fc receptor (CD89) and FcR γ chain. Molecular basis for CD89/FcR γ chain association. J Biol Chem. 1995;270:29781–29787. doi: 10.1074/jbc.270.50.29781. [DOI] [PubMed] [Google Scholar]
- Poole A, Gibbins J M, Turner M, van Vugt M J, van de Winkel J G, Saito T, Tybulewicz V L, Watson S P. The Fc receptor γ-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J. 1997;16:2333–2341. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji M, Ezumi Y, Arai M, Takayama H. A novel association of Fc receptor γ-chain with glycoprotein VI and their co-expression as a collagen receptor in human platelets. J Biol Chem. 1997;272:23528–23531. doi: 10.1074/jbc.272.38.23528. [DOI] [PubMed] [Google Scholar]
- Zheng Y M, Liu C, Chen H, Locke D, Ryan J C, Kahn M L. Expression of the platelet receptor GPVI confers signaling via the Fc receptor γ-chain in response to the snake venom convulxin but not to collagen. J Biol Chem. 2001;276:12999–13006. doi: 10.1074/jbc.M009344200. [DOI] [PubMed] [Google Scholar]
- Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24:1015–1022. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- Dower S K, Sims J E, Cerretti D P, Bird T A. The interleukin-1 system: receptors, ligands and signals. Chem Immunol. 1992;51:33–64. [PubMed] [Google Scholar]
- Dinarello C A. The proinflammatory cytokines interleukin-1 and tumor necrosis factor and treatment of the septic shock syndrome. J Infect Dis. 1991;163:1177–1184. doi: 10.1093/infdis/163.6.1177. [DOI] [PubMed] [Google Scholar]
- di Giovine F S, Duff G W. Interleukin 1: the first interleukin. Immunol Today. 1990;11:13–20. doi: 10.1016/0167-5699(90)90005-t. [DOI] [PubMed] [Google Scholar]
- Lindemann S, Tolley N D, Dixon D A, McIntyre T M, Prescott S M, Zimmerman G A, Weyrich A S. Activated platelets mediate inflammatory signaling by regulated interleukin 1β synthesis. J Cell Biol. 2001;154:485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster V, Badimon L, Badimon J J, Chesebro J H. The pathogenesis of coronary artery disease and the acute coronary syndromes (1) N Engl J Med. 1992;326:242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- Strano A, Davi G, Patrono C. In vivo platelet activation in diabetes mellitus. Semin Thromb Hemost. 1991;17:422–425. doi: 10.1055/s-2007-1002648. [DOI] [PubMed] [Google Scholar]
- Bastida E, Ordinas A. Platelet contribution to the formation of metastatic foci: the role of cancer cell-induced platelet activation. Haemostasis. 1988;18:29–36. doi: 10.1159/000215780. [DOI] [PubMed] [Google Scholar]
- del Zoppo G J. The role of platelets in ischemic stroke. Neurology. 1998;51:S9–S14. doi: 10.1212/wnl.51.3_suppl_3.s9. [DOI] [PubMed] [Google Scholar]
- Sagripanti A, Cupisti A, Ferdeghini M, Pinori E, Barsotti G. Molecular markers of hemostasis activation in nephrotic syndrome. Nephron. 1989;51:25–28. doi: 10.1159/000185236. [DOI] [PubMed] [Google Scholar]
- Joseph J E, Harrison P, Mackie I J, Isenberg D A, Machin S J. Increased circulating platelet-leucocyte complexes and platelet activation in patients with antiphospholipid syndrome, systemic lupus erythematosus and rheumatoid arthritis. Br J Haematol. 2001;115:451–459. doi: 10.1046/j.1365-2141.2001.03101.x. [DOI] [PubMed] [Google Scholar]
- Joseph J E, Harrison P, Mackie I J, Machin S J. Platelet activation markers and the primary antiphospholipid syndrome (PAPS) Lupus. 1998;7:S48–S51. doi: 10.1177/096120339800700212. [DOI] [PubMed] [Google Scholar]
- Chacko G W, Duchemin A M, Coggeshall K M, Osborne J M, Brandt J T, Anderson C L. Clustering of the platelet Fc γ receptor induces noncovalent association with the tyrosine kinase p72syk. J Biol Chem. 1994;269:32435–32440. [PubMed] [Google Scholar]
- Hasegawa S, Pawankar R, Suzuki K, Nakahata T, Furukawa S, Okumura K, Ra C. Functional expression of the high affinity receptor for IgE (FcεRI) in human platelets and its intracellular expression in human megakaryocytes. Blood. 1999;93:2543–2551. [PubMed] [Google Scholar]
- Chong B H, Pilgrim R L, Cooley M A, Chesterman C N. Increased expression of platelet IgG Fc receptors in immune heparin-induced thrombocytopenia. Blood. 1993;81:988–993. [PubMed] [Google Scholar]
- McKenzie S E, Taylor S M, Malladi P, Yuhan H, Cassel D L, Chien P, Schwartz E, Schreiber A D, Surrey S, Reilly M P. The role of the human Fc receptor Fc γ RIIA in the immune clearance of platelets: a transgenic mouse model. J Immunol. 1999;162:4311–4318. [PubMed] [Google Scholar]
- Reilly M P, Taylor S M, Hartman N K, Arepally G M, Sachais B S, Cines D B, Poncz M, McKenzie S E. Heparin-induced thrombocytopenia/thrombosis in a transgenic mouse model requires human platelet factor 4 and platelet activation through FcγRIIA. Blood. 2001;98:2442–2447. doi: 10.1182/blood.v98.8.2442. [DOI] [PubMed] [Google Scholar]
- Cucurull E, Gharavi A E, Diri E, Mendez E, Kapoor D, Espinoza L R. IgA anticardiolipin and anti-β2-glycoprotein I are the most prevalent isotypes in African American patients with systemic lupus erythematosus. Am J Med Sci. 1999;318:55–60. doi: 10.1097/00000441-199907000-00009. [DOI] [PubMed] [Google Scholar]
- Pourmand N, Wahren-Herlenius M, Gunnarsson I, Svenungsson E, Lofstrom B, Ioannou Y, Isenberg D A, Magnusson C G. Ro/SSA and La/SSB specific IgA autoantibodies in serum of patients with Sjogren’s syndrome and systemic lupus erythematosus. Ann Rheum Dis. 1999;58:623–629. doi: 10.1136/ard.58.10.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanopoulos D, Teodorescu M R, Varga J, Teodorescu M. High frequency of abnormal levels of IgA anti-β2-glycoprotein I antibodies in patients with systemic lupus erythematosus: relationship with antiphospholipid syndrome. J Rheumatol. 1998;25:675–680. [PubMed] [Google Scholar]
- Greco T P, Amos M D, Conti-Kelly A M, Naranjo J D, Ijdo J W. Testing for the antiphospholipid syndrome: importance of IgA anti-β 2-glycoprotein I. Lupus. 2000;9:33–41. doi: 10.1177/096120330000900107. [DOI] [PubMed] [Google Scholar]
- Diri E, Cucurull E, Gharavi A E, Kapoor D, Mendez E A, Scopelitis E, Wilson W A. Antiphospholipid (Hughes’) syndrome in African-Americans: IgA aCL and aβ2 glycoprotein-I is the most frequent isotype. Lupus. 1999;8:263–268. doi: 10.1191/096120399678847812. [DOI] [PubMed] [Google Scholar]
- Staub H L, Franck M, Ranzolin A, Norman G L, Iverson G M, von Muhlen C A. IgA antibodies to β2-glycoprotein I and atherosclerosis. Autoimmun Rev. 2006;6:104–106. doi: 10.1016/j.autrev.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Staub H L, von Muhlen C A, Norman G L. β2-Glycoprotein I IgA antibodies and ischaemic stroke. Rheumatology (Oxford) 2006;45:645–646. doi: 10.1093/rheumatology/kel033. [DOI] [PubMed] [Google Scholar]
- Lakos G, Kiss E, Regeczy N, Tarjan P, Soltesz P, Zeher M, Bodolay E, Szakony S, Sipka S, Szegedi G. Isotype distribution and clinical relevance of anti-β2-glycoprotein I (β2-GPI) antibodies: importance of IgA isotype. Clin Exp Immunol. 1999;117:574–579. doi: 10.1046/j.1365-2249.1999.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R M, Branch D W, Silver R M. Immunoglobulin A anti-β2-glycoprotein antibodies in women who experience unexplained recurrent spontaneous abortion and unexplained fetal death. Am J Obstet Gynecol. 2001;185:748–753. doi: 10.1067/mob.2001.117659. [DOI] [PubMed] [Google Scholar]
- Rantapaa-Dahlqvist S, de Jong B A, Berglin E, Hallmans G, Wadell G, Stenlund H, Sundin U, van Venrooij W J. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- Yoon K H, Wong A, Shakespeare T, Sivalingam P. High prevalence of antiphospholipid antibodies in Asian cancer patients with thrombosis. Lupus. 2003;12:112–116. doi: 10.1191/0961203303lu328oa. [DOI] [PubMed] [Google Scholar]
- Henn V, Slupsky J R, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek R A. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- Patry C, Sibille Y, Lehuen A, Monteiro R C. Identification of Fc α receptor (CD89) isoforms generated by alternative splicing that are differentially expressed between blood monocytes and alveolar macrophages. J Immunol. 1996;156:4442–4448. [PubMed] [Google Scholar]