Abstract
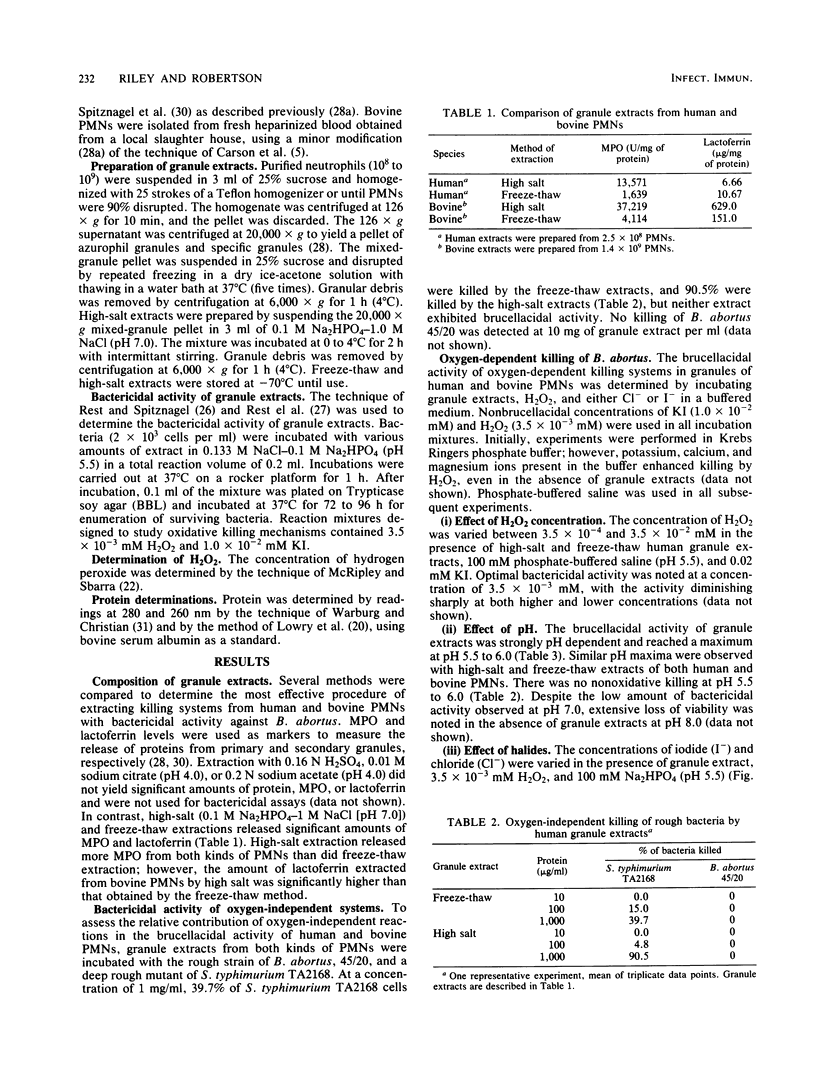
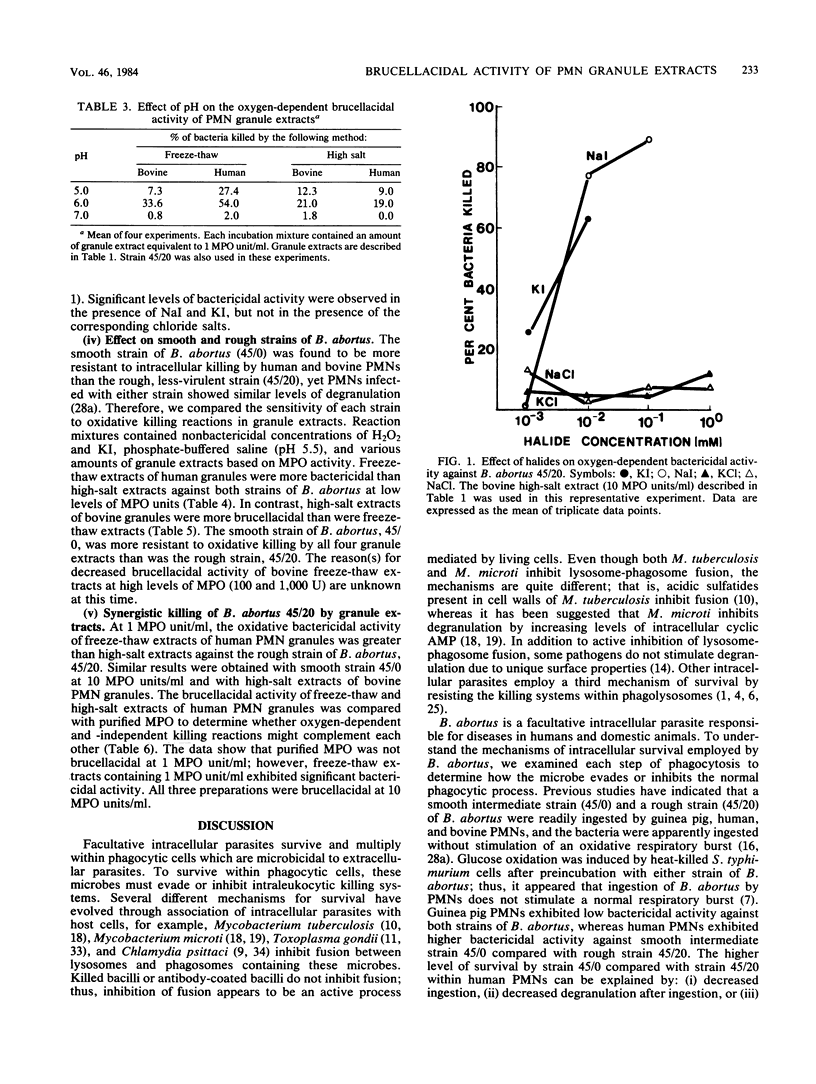
The microbicidal activities of freeze-thaw and high-salt extracts of human and bovine polymorphonuclear leukocyte (PMN) granules were tested against a smooth intermediate strain (45/0) and a rough strain (45/20) of Brucella abortus which differ in virulence and survival within PMNs. Freeze-thaw extracts of human PMN granules were more brucellacidal than high-salt extracts when supplemented with hydrogen peroxide (H2O2) and potassium iodide (KI), whereas the opposite was found with freeze-thaw and high-salt extracts of bovine PMN granules. There was no oxygen-independent killing of either the smooth or rough strain of B. abortus by amounts of granule extracts which caused 100% killing of a deep rough mutant (Re) of Salmonella typhimurium. The oxygen-dependent brucellacidal activity of granule extracts was dependent on concentrations of myeloperoxidase (MPO) units, H2O2, and KI. Maximal brucellacidal activity was observed at pH 5.5 to 6.0. The smooth strain, 45/0, was more resistant to oxygen-dependent killing by granule extracts than was the rough strain, 45/20. Granule extracts were more brucellacidal than purified MPO at equivalent levels of MPO enzyme units, suggesting that at least one other reaction enhances killing by the MPO-H2O2-I- system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J., Vickerman K. Fusion of host cell secondary lysosomes with the parasitophorous vacuoles of Leishmania mexicana-infected macrophages. J Protozool. 1975 Nov;22(4):502–508. doi: 10.1111/j.1550-7408.1975.tb05219.x. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Carrol M. E., Jackett P. S., Aber V. R., Lowrie D. B. Phagolysosome formation, cyclic adenosine 3':5'-monophosphate and the fate of Salmonella typhimurium within mouse peritoneal macrophages. J Gen Microbiol. 1979 Feb;110(2):421–429. doi: 10.1099/00221287-110-2-421. [DOI] [PubMed] [Google Scholar]
- Carson C. A., Sells D. M., Ristic M. A method for separation of bovine blood leukocytes for in vitro studies. Am J Vet Res. 1975 Aug;36(08):1091–1094. [PubMed] [Google Scholar]
- Chang K. P., Dwyer D. M. Multiplication of a human parasite (Leishmania donovani) in phagolysosomes of hamster macrophages in vitro. Science. 1976 Aug 20;193(4254):678–680. doi: 10.1126/science.948742. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R. Initiation of the respiratory burst in human polymorphonuclear neutrophils: a critical review. J Reticuloendothel Soc. 1978 Jul;24(1):73–91. [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Phagocyte strategy vs. microbial tactics. Rev Infect Dis. 1980 Sep-Oct;2(5):817–838. doi: 10.1093/clinids/2.5.817. [DOI] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B., D'Arcy Hart P., Young M. R., Armstrong J. A. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2510–2514. doi: 10.1073/pnas.73.7.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Kossack R. E., Guerrant R. L., Densen P., Schadelin J., Mandell G. L. Diminished neutrophil oxidative metabolism after phagocytosis of virulent Salmonella typhi. Infect Immun. 1981 Feb;31(2):674–678. doi: 10.1128/iai.31.2.674-678.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer D. L., Buller C. S., Robertson D. C. Chemical characterization and biological properties of lipopolysaccharides isolated from smooth and rough strains of Brucella abortus. Infect Immun. 1979 Mar;23(3):811–818. doi: 10.1128/iai.23.3.811-818.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer D. L., Dreyfus L. A., Robertson D. C. Interaction of polymorphonuclear leukocytes with smooth and rough strains of Brucella abortus. Infect Immun. 1979 Mar;23(3):737–742. doi: 10.1128/iai.23.3.737-742.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer D. L., Robertson D. C. Surface macromolecules and virulence in intracellular parasitism: comparison of cell envelope components of smooth and rough strains of Brucella abortus. Infect Immun. 1979 Mar;23(3):819–828. doi: 10.1128/iai.23.3.819-828.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowrie D. B., Aber V. R., Jackett P. S. Phagosome-lysosome fusion and cyclic adenosine 3':5'-monophosphate in macrophages infected with Mycobacterium microti, Mycobacterium bovis BCG or Mycobacterium lepraemurium. J Gen Microbiol. 1979 Feb;110(2):431–441. doi: 10.1099/00221287-110-2-431. [DOI] [PubMed] [Google Scholar]
- Lowrie D. B., Jackett P. S., Ratcliffe N. A. Mycobacterium microti may protect itself from intracellular destruction by releasing cyclic AMP into phagosomes. Nature. 1975 Apr 17;254(5501):600–602. doi: 10.1038/254600a0. [DOI] [PubMed] [Google Scholar]
- Mandell G. L. Bactericidal activity of aerobic and anaerobic polymorphonuclear neutrophils. Infect Immun. 1974 Feb;9(2):337–341. doi: 10.1128/iai.9.2.337-341.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XII. Hydrogen peroxide-myeloperoxidase bactericidal system in the phagocyte. J Bacteriol. 1967 Nov;94(5):1425–1430. doi: 10.1128/jb.94.5.1425-1430.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Mechanisms for the microbicidal activity of cationic proteins of human granulocytes. Infect Immun. 1976 Dec;14(6):1269–1275. doi: 10.1128/iai.14.6.1269-1275.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Microbicidal mechanisms of human granulocytes: synergistic effects of granulocyte elastase and myeloperoxidase or chymotrypsin-like cationic protein. Infect Immun. 1976 Dec;14(6):1276–1283. doi: 10.1128/iai.14.6.1276-1283.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quie P. G. Perturbation of the normal mechanisms of intraleukocytic killing of bacteria. J Infect Dis. 1983 Aug;148(2):189–193. doi: 10.1093/infdis/148.2.189. [DOI] [PubMed] [Google Scholar]
- Rest R. F., Cooney M. H., Spitznagel J. K. Bactericidal activity of specific and azurophil granules from human neutrophils: studies with outer-membrane mutants of Salmonella typhimurium LT-2. Infect Immun. 1978 Jan;19(1):131–137. doi: 10.1128/iai.19.1.131-137.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Cooney M. H., Spitznagel J. K. Susceptibility of lipopolysaccharide mutants to the bactericidal action of human neutrophil lysosomal fractions. Infect Immun. 1977 Apr;16(1):145–151. doi: 10.1128/iai.16.1.145-151.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Spitznagel J. K. Subcellular distribution of superoxide dismutases in human neutrophils. Influence of myeloperoxidase on the measurement of superoxide dismutase activity. Biochem J. 1977 Aug 15;166(2):145–153. doi: 10.1042/bj1660145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley L. K., Robertson D. C. Ingestion and intracellular survival of Brucella abortus in human and bovine polymorphonuclear leukocytes. Infect Immun. 1984 Oct;46(1):224–230. doi: 10.1128/iai.46.1.224-230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Dalldorf F. G., Leffell M. S., Folds J. D., Welsh I. R., Cooney M. H., Martin L. E. Character of azurophil and specific granules purified from human polymorphonuclear leukocytes. Lab Invest. 1974 Jun;30(6):774–785. [PubMed] [Google Scholar]
- Weiss J., Elsbach P., Olsson I., Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem. 1978 Apr 25;253(8):2664–2672. [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrick P. B., Brownridge E. A. Growth of Chlamydia psittaci in macrophages. Infect Immun. 1978 Mar;19(3):1054–1060. doi: 10.1128/iai.19.3.1054-1060.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


