Abstract
The ubiquitin-proteasome system plays an important role in many cellular processes through degradation of specific proteins. Low molecular mass polypeptide 2 (LMP-2 or β1i) is one important subunit of the immunoproteasome. Ischemic preconditioning (IPC) activates cell signaling pathways and generates cardioprotection but has not been linked to LMP-2 function previously. LMP-2 knockout mice (C57BL6 background) and wild-type C57BL6 mice were subjected to 30 min of ischemia (I-30) and 120 min of reperfusion (R-120) with or without preceding IPC (10 min of infusion and 5 min of reperfusion). IPC significantly increased left ventricular developed pressure and decreased infarct size in wild-type mice, but this protective effect of IPC was lost in LMP-2 knockout mice. IPC-mediated degradation of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) and activation of the downstream protein kinase Akt were impaired in LMP-2 knockout hearts. The impairment of PTEN degradation was associated with defective immunoproteasomes and decreased proteolytic activities. When LMP-2 knockout mice were pretreated with the PTEN inhibitor bpV(HOpic), cardiac function was significantly improved, and myocardial infarct size was significantly reduced after I-30/R-120. In conclusion, LMP-2 is required for normal proteasomal function and IPC induction in the heart. Its action may be related to PTEN protein degradation.—Cai, Z. P., Shen, Z., Van Kaer, L., Becker, L. C. Ischemic preconditioning-induced cardioprotection is lost in mice with immunoproteasome subunit low molecular mass polypeptide-2 deficiency.
Keywords: ubiquitin-proteasome system, reperfusion injury, ubiquitin, phosphatase and tensin homologue deleted on chromosome 10, PTEN, Akt
Ischemic preconditioning (IPC), consisting of one or multiple brief periods of ischemia and reperfusion, is capable of generating powerful protection against cardiac injury (1, 2). IPC activates several signaling pathways, including protein kinase C (PKC), mitogen-activated protein kinase (MAPK), and phosphatidylinositol 3-kinase (PI3K) (2,3,4,5,6,7). Moreover, IPC inactivates the phosphatase and tensin homologue deleted on chromosome 10 (PTEN) (8). There is also significant cross-talk between the phosphatase PTEN and the survival protein kinases (8,9,10). PTEN antagonizes PI3K by dephosphorylating its product phosphatidylinositol-3,4,5-triphosphate (PIP3), which activates Akt directly or through PIP3-dependent protein kinase 1 (PDK1) (10). PDK1 is a protein that functions upstream of PKC (11). Therefore, PTEN may play a pivotal role in controlling cell fate (4). IPC also activates the ubiquitin-proteasome system (UPS) in the brain (12), and proteasome inhibition leads to blockade of IPC protection in neurons (13, 14). However, whether proteasome activity is necessary for IPC induction in the heart has not been investigated previously.
Cellular proteins are predominantly degraded through the UPS. The process of protein degradation can be divided into two distinct steps, polyubiquitination and proteasome digestion (15,16,17,18,19). Ubiquitins are first targeted to substrates through specific ligases. Polyubiquitinated substrates are rapidly degraded by the 26S proteasome, consisting of two regulatory 19S complexes and one catalytic 20S proteasome core (19). The 20S proteasome contains four stacked rings. The inner two rings are composed of two sets of seven β-subunits and two sets of seven α-subunits are aligned over their corresponding β-subunits as outer rings. The β-subunits β1, β2, and β5 are linked to caspase-like (Cas-L), tryptic-like (Tryp-L), and chymotryptic-like (Chym-L) activities, respectively. These constitutive subunits can be replaced by the inducible immunoproteasome subunits low molecular mass polypeptide (LMP) -2/β1i, multicatalytic endopeptidase complex-like (MECL) -1/β2i, and LMP-7/β5i (20, 21). After these subunits are incorporated and processed, the proteolytic activity and specificity of the proteasome are changed (22,23,24).
LMP-2 is induced by inflammatory stimuli, especially interferon-γ; however, it is also constitutively expressed in several tissues (25,26,27), and its function is not limited to antigen presentation. LMP-2 has also been implicated in controlling the accumulation of damaged proteins. LMP-2 deficiency results in a decrease in 20S proteasome activity and accumulation of oxidized proteins in the brain and liver of mice (28). Moreover, LMP-2 regulates estrogen receptor-mediated gene transcription through the UPS (29). Finally, in addition to interferon-γ, other regulators of LMP-2 expression such as nitric oxide and heart shock have been reported (30, 31). Therefore, LMP-2 may act as a modulator of proteasome activity in the cell. In this study, we have tested the hypothesis that LMP-2 plays a critical role in cardioprotection in response to IPC.
MATERIALS AND METHODS
Materials
A 20S proteasome activity kit was purchased from Chemicon International (Billerica, MA, USA). Antibodies against PTEN (rabbit), phospho (p)-Akt (S-473), and total Akt were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies against ubiquitin, 20S β1subunit, PTEN (mouse), and β-actin were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Antibodies against LMP-2 and LMP-7 were from Abcam Inc. (Cambridge, MA, USA). Anti-MECL-1 antibody and all fluorogenic substrates were purchased from Biomol International L.P. (Plymouth Meeting, PA, USA). bpV(HOpic) (referred to as HOpic hereafter) was from EMD Chemicals Inc. (La Jolla, CA, USA).
Animals
All experiments were performed with age-matched male mice that were either wild-type (WT) (C57BL6) or LMP-2 knockout (KO) (LMP-2−/−) (backcrossed 10 times to C57BL6). LMP-2−/− mice were generated by deletion of the corresponding gene using embryonic stem cell technology (27). At the time of the experiment, mice were 2–3 months old and weighed 22–32 g. All procedures were approved by The Johns Hopkins University Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996).
In situ ischemia and reperfusion mouse model
Mice were anesthetized by intraperitoneal injection of pentobarbital (70 mg/kg) and were mechanically ventilated (32). A left thoracotomy was performed, and the mouse was then turned slightly to the right. The left coronary artery was identified visually, and a 7-0 suture was placed around the artery ∼1 mm below the left auricle. The electrocardiogram (ECG) was recorded continuously. The mice were warmed to 37°C with heating lamps and kept continuously at this temperature, monitored with a rectal probe. A loop was made in the suture around the coronary artery and tightened over a small piece of polyethylene tubing. Occlusion of the left coronary artery resulted in changes in heart color (pallor) and ECG (ST-segment elevation and widening of the QRS complex). Reperfusion was accomplished by loosening the loop and confirmed by return of red color to the region and improved cardiac contraction. All mice were subjected to 30 min of ischemia (I-30) and 120 min of reperfusion (R-120) with or without preceding IPC, which consisted of a single episode of 10 min of ischemia (I-10) and 5 min of reperfusion (R-5). The PTEN inhibitor HOpic was administrated by intraperitoneal injection at a dose of 50 μg/kg (body weight) 15 min before ischemia-reperfusion. Saline was used as a control. At the end of the experiment, the animals were euthanized by transecting the aorta and removing the heart for infarct size determination.
Measurement of left ventricular pressure
After exposure of the heart, a 26-gauge needle was used to make a stab wound near the left ventricular apex. A Mikro-tip catheter (Millar Instruments, Houston, TX, USA) containing a tiny pressure sensor at the tip was inserted through the stab wound into the left ventricle. Left ventricular pressure was directly measured with the Powerlab Data Acquisition System and displayed on a computer. Left ventricular developed pressure (LVDP=systolic pressure−end-diastolic pressure), heart rate, positive maximal left ventricular pressure (LVP) derivative (+dp/dtm), and negative maximal LVP derivative (−dp/dtm) were automatically calculated using Chart 5 software.
Assessment of area at risk and infarct size
The loop around the coronary artery was retightened just before euthanasia. Then 1% Evans Blue solution was injected into the aorta, and the dye was passed into the coronary arteries and distributed throughout the nonrisk ventricular wall proximal to the coronary artery ligature. The heart was frozen and then cut transversely into five sections, each of which was weighed and incubated in 1.5% triphenyltetrazolium chloride (TTC) for 15 min at 37°C. The area not at risk appeared blue. Viable myocardium in the area at risk stained brick red and infarcted tissue was pale white. Two sides (A and B) of each section were photographed. The areas of blue, red, or white color were measured by computerized planimetry (Image J; National Institutes of Health, Bethesda, MD, USA). The following indices were calculated: infarct size as a percentage of left ventricle (IS/LV) = total weight of white tissue/weight of left ventricle (LV) × 100%; area at risk (AAR) as a percentage of LV (AAR/LV): total weight of white tissue + red tissue/weight of LV × 100%; and infarct size as a percentage of the area at risk (IS/AAR) = total weight of white tissue/total weight of white tissue + red tissue × 100%.
Mouse Langendorff preparation
Mice were anesthetized by intraperitoneal injection of pentobarbital (70 mg/kg). After the chest was opened, the heart was excised, and the ascending aorta was cannulated with a blunt needle. The heart was perfused at a constant pressure of 100 cm H2O with Krebs-Henseleit buffer (17 mM glucose, 120 mM NaCl, 25 mM NaHCO3, 2.5 mM CaCl2, 5.9 mM KCl, 1.2 mM MgSO4, and 0.5 mM EDTA), which was maintained at 37°C and bubbled continuously with a mixture of 95% O2 and 5% CO2. Global ischemia was induced by cessation of perfusion, followed by reperfusion. IPC was induced by a single episode of 10 min of ischemia and 5 min of reperfusion. HOpic (final concentration 100 nM) was injected into the perfusion line by a syringe pump according to the coronary flow rate.
Measurement of proteolytic activities
Hearts were homogenized on ice in the assay buffer (25 mM HEPES, pH 7.5; 0.5 mM EDTA; and 0.03% SDS) as described previously (33). Chymotryptic-like activity and caspase-like activity were measured using the fluorogenic substrates Suc-LLVY-aminomethycoumarin (AMC) and Z-LLE-AMC. Equal amounts (0.65 g) of protein were reacted with 50 μM concentrations of the substrates in 100 μl of assay buffer for 24 h at room temperature. The cleavage product AMC was analyzed in a fluorometer (excitation/emission: 355/460 nm). Background activity (caused by nonproteasomal degradation) was determined by addition of the proteasome inhibitor lactacystin at a final concentration of 50 μM. Proteolytic activities are presented as relative activity (RA) to wild-type control. A similar protocol was used to measure tryptic-like activity. In this assay, Boc-LLR-AMC was reacted with 6.5 g of protein in a separate buffer (25 mM HEPES, pH 7.5; 0.5 mM EDTA; 0.05% Nonidet P-40; and 0.001% SDS).
Immunoprecipitation and Western blot analysis
Hearts were homogenized in lysate buffer (20 mM Tris, pH 7.5; 150 mM NaCl; 1 mM EDTA; 1mM EGTA; 1 mM phenylmethylsulfonyl fluoride; 1 mM Na3VO4; and 1% Triton). PTEN was immunoprecipitated from 300 μg of heart lysates with 20 μl of anti-PTEN antibody overnight, followed by incubation with protein G-agarose beads (Santa Cruz Biotechnology, Inc.) for 2 h at 4°C. The precipitates were washed 3 times with lysate buffer and then dissolved in sample buffer. Proteins were separated on a precast NuPAGE Bis-Tris gel (Invitrogen, Carlsbad, CA, USA) and transferred to a nitrocellulose membrane. Proteins were detected using primary antibodies, followed by horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescence.
Statistical analysis
Data are presented as mean ± se values. The difference among groups was analyzed by ANOVA or Student’s t test. Differences were considered significant if P < 0.05.
RESULTS
General characteristics of LMP-2−/− mice
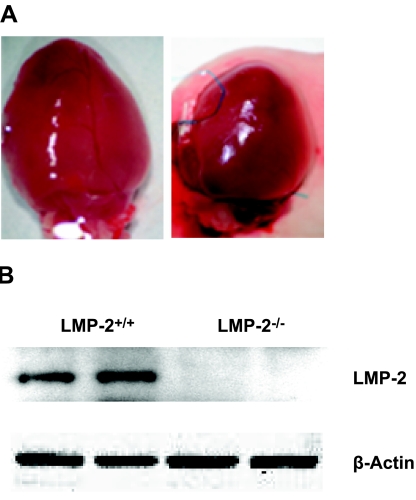
To determine the effects of LMP-2 deficiency on the heart, we determined total body and heart mass and measured LVP and heart rate after anesthesia. LMP-2−/− mice were larger than LMP-2+/+ mice (27±1.3 vs. 24±0.7 g, P<0.05) (Table 1), but their hearts were significantly smaller (147±7.1 vs. 178±10.5 mg, P<0.05) (Fig. 1A). However, within each genotype (LMP-2+/+ or LMP-2−/−), control (CON) and IPC groups did not differ with respect to body weight or heart weight (Table 1). LMP-2 protein was undetectable in LMP-2−/− hearts (Fig. 1B). LMP-2 deficiency did not significantly alter LVDP or cardiac contractility (+dp/dtm and −dp/dtm) but did reduce heart rate (346±16 vs. 412±24 beats/min, P<0.05) (Table 1). These data indicate that LMP-2 deficiency may result in anatomic and functional changes in the heart.
TABLE 1.
General cardiac characteristics of LMP-2−/− mice
| Characteristic | LMP-2+/+
|
LMP-2−/−
|
||
|---|---|---|---|---|
| CONWT | IPCWT | CONKO | IPCKO | |
| Body weight (g) | 24.6 ± 1.1 | 23.4 ± 0.9 | 28 ± 2.1 | 26.0 ± 1.5 |
| Heart weight (g) | 0.166 ± 0.016 | 0.191 ± 0.013 | 0.153 ± 0.011 | 0.141 ± 0.01 |
| Left ventricular systolic pressure (mmHg) | 72.4 ± 3.9 | 66.2 ± 4.6 | 69.2 ± 7.3 | 75.4 ± 1.6 |
| Left ventricular end-diastolic pressure (mmHg) | –2.6 ± 3.4 | –4.6 ± 1.6 | –5.0 ± 1.3 | –6.0 ± 0.9 |
| Heart rate (beats/min) | 430 ± 44 | 422 ± 23 | 339 ± 15 | 354 ± 24 |
| +dp/dtm (mmHg/s) | 4528 ± 359 | 4166 ± 412 | 3928 ± 412 | 4578 ± 277 |
| –dp/dtm (mmHg/s) | 3639 ± 369 | 3708 ± 353 | 3336 ± 440 | 4012 ± 175 |
n = 5/group.
Figure 1.
Heart size and LMP-2 levels in mouse hearts. A) Representative hearts from two age-matched LMP-2+/+ and LMP-2−/− mice. B) LMP-2 protein levels in two separate hearts from LMP-2+/+ and LMP-2−/− mice. The data are representative of 10 LMP-2+/+ mice and 10 LMP-2−/− mice.
Ischemic preconditioning is impaired in LMP-2−/− mice
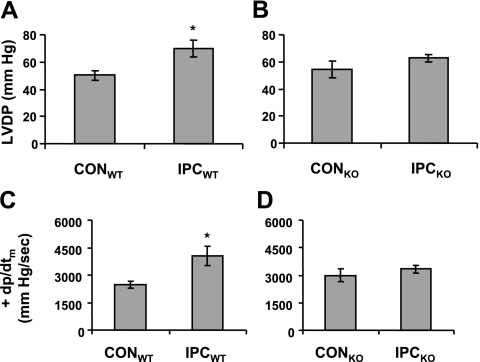
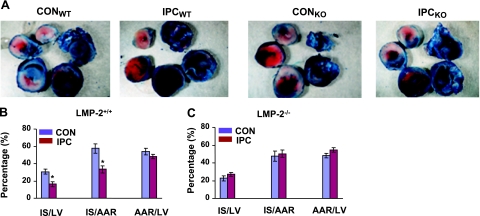
To determine whether LMP-2 is involved in IPC, we subjected the LMP-2+/+ and LMP-2−/− mice to IPC, one cycle of I-10/R-5, followed by I-30/R-120. In LMP-2+/+ mice, IPC resulted in significantly higher LVDP at the end of reperfusion compared with mice without IPC (CON) (70±6 vs. 50±4 mmHg, P<0.05) (Fig. 2A). However, in LMP-2−/− mice, IPC did not significantly improve LVDP compared with that in littermate controls (63±3 vs. 54±6 mmHg, P>0.05) (Fig. 2B). Similarly, IPC increased cardiac contractility +dp/dtm in LMP-2+/+ mice (4060±529 vs. 2477±194 mmHg/s, P<0.05) (Fig. 2C) but not in LMP-2−/− mice (3328±208 vs. 2997±360 mmHg/s, P>0.05) (Fig. 2D). Consistent with the changes in cardiac function, IPC decreased myocardial infarct size in LMP-2+/+ mice compared with control mice (IS/AAR: 33±4 vs. 58±6%, P<0.01) (Fig. 3A, B) but not in LMP-2−/− mice (IS/AAR: 50±4 vs. 47±6%, P>0.05) (Fig. 3A, C). Similar results were obtained using IS/LV as an index of infarct size (LMP-2+/+ mice: 16±3 vs. 31±3%, P<0.01; LMP-2−/− mice: 27±2 vs. 22±3%, P>0.05) (Fig. 3B, C). There was no significant difference in AAR/LV between CON and IPC in LMP-2+/+ or LMP-2−/− mice (Fig. 3A–C). These data show that LMP-2 deficiency leads to impairment of IPC cardioprotection.
Figure 2.
Cardiac functional recovery after ischemia-reperfusion. Mice were subjected to I-30/R-120 with or without preceding IPC, I-10/R-5. A, B) IPC resulted in increased recovery of LVDP in LMP-2+/+ (WT) mice, but not in LMP-2−/− (KO) mice. C, D) IPC increased +dp/dtm after I-30/R-120 in WT mice, but not in KO mice. *P < 0.05 vs. CON; n = 5/group.
Figure 3.
Myocardial infarct size after ischemia-reperfusion. Hearts were cut into five sections, incubated with TTC and photographed. A) Representative pictures of TTC staining from each group. B) IPC decreased myocardial infarct size (in IS/AAR or IS/LV) after ischemia-reperfusion in LMP-2+/+ (WT) mice. *P < 0.01 vs. CON; n=5/group. C) The differences in infarct size between IPC and CON were not significant in LMP-2−/− (KO) mice.
PTEN degradation and Akt phosphorylation are decreased in preconditioned LMP-2−/− hearts
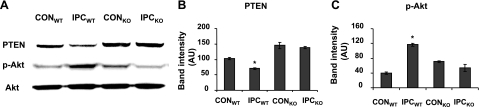
PTEN is down-regulated during ischemia and reperfusion by a proteasome-dependent mechanism (8). We therefore determined whether LMP-2 deficiency affects IPC-mediated PTEN inactivation. Isolated perfused hearts from LMP-2+/+ and LMP-2−/− mice were exposed to one episode of IPC (I-10/R-5) or CON (nonischemia), and then they were assayed for PTEN, p-Akt, and total Akt by Western blot. Under basal conditions, PTEN levels were significantly increased in LMP-2−/− hearts compared with those in LMP-2+/+ hearts [145±10 vs. 103±4 arbitrary units (AU), P<0.05] (Fig. 4A, B). In LMP-2+/+ mice, IPC significantly decreased PTEN levels (70±3 vs. 102±4 AU, P<0.01) (Fig. 4B) and increased Akt phosphorylation (117±4 vs. 40±2 AU, P<0.01) (Fig. 4C). However, in LMP-2−/− mice, IPC did not significantly change PTEN or p-Akt levels (PTEN: 138±5 vs. 145±10 AU, P>0.05; p-Akt: 53±10 vs. 71±2 AU, P>0.05) (Fig. 4A–C).
Figure 4.
Impairment of PTEN degradation in LMP-2−/− hearts after IPC. Isolated hearts from LMP-2+/+ and LMP-2−/− mice were subjected to control (nonischemia) or IPC (I-10/R-5). A) Representative Western blots of PTEN, p-Akt, and Akt. B) IPC significantly decreased PTEN protein levels in LMP-2+/+ hearts, but not in LMP-2−/− hearts. C) Phosphorylated downstream Akt was significantly increased in LMP-2+/+ hearts, but not in LMP-2−/− hearts after IPC. *P < 0.01 vs. CONWT; n = 3/group.
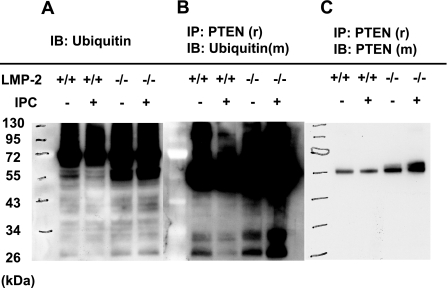
Degradation of ubiquitinated proteins is impaired in LMP-2−/− hearts
To determine whether the impairment of PTEN down-regulation by IPC is due to deficient UPS function in LMP-2−/− hearts, we first detected ubiquitinated protein levels in isolated LMP-2+/+ and LMP-2−/− hearts with or without IPC. IPC decreased the levels of ubiquitinated proteins in LMP-2+/+ hearts, suggesting an increase in UPS function. In contrast, in LMP-2−/− hearts, the basal level of ubiquitinated proteins was increased compared with that in LMP-2+/+ hearts, but there was no change after IPC (Fig. 5A). Next, to determine whether UPS-mediated PTEN degradation is impaired in LMP-2−/− hearts after IPC, we first precipitated PTEN with anti-rabbit PTEN antibody and then transferred it to a membrane, which was blotted with anti-mouse ubiquitin antibody. IPC decreased PTEN ubiquitination in LMP-2+/+ hearts, but in LMP-2−/− hearts, IPC increased PTEN ubiquitination (Fig. 5B). Last, to determine whether the same amounts of PTEN were pulled down from the hearts, we stripped this Western blot. After no signal was detected from the membrane, we reblotted it with anti-mouse PTEN antibody. The PTEN bands (with molecular mass of 55 kDa) had a similar density in the lysates from CON and IPC hearts (Fig. 5C). However, there was a second band with slightly higher molecular mass in the samples from LMP-2−/− hearts. This might be due to PTEN monoubiquitination. These results suggest that proteasome function is impaired in LMP-2−/− hearts.
Figure 5.
Accumulation of ubiquitinated proteins in LMP-2−/− hearts. Isolated mouse hearts were subjected to CON or IPC. A) Increased accumulation of ubiquitinated proteins in LMP-2−/− hearts. IPC decreased levels of ubiquitinated proteins in LMP-2+/+ hearts, but not in LMP-2−/− hearts. B) Accumulation of ubiquitinated PTEN in preconditioned LMP-2−/− hearts. PTEN was immunoprecipitated by anti-rabbit (r) PTEN antibody and then immunoblotted by anti-mouse (m) ubiquitin antibody. The levels of ubiquitinated PTEN were decreased in LMP-2+/+ hearts, but increased in LMP-2−/− hearts after IPC. C) The same amounts of PTEN (55 kDa) were pulled down from CON and IPC hearts. After stripped with the buffer, the membrane was blotted by anti-mouse PTEN antibody. Each blot is representative of 3 independent experimentals.
Proteolytic activities are decreased in LMP-2−/− hearts after IPC
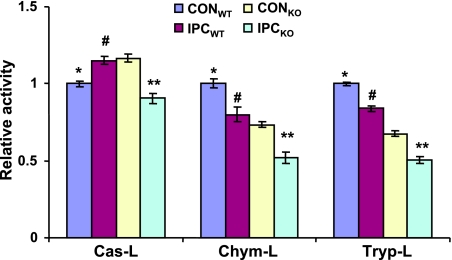
To determine whether proteolytic activities are decreased in LMP-2−/− hearts, we exposed isolated LMP-2+/+ and LMP-2−/− hearts to IPC or CON and then measured Cas-L, Chym-L, and Tryp-L activities. IPC increased Cas-L activity in LMP-2+/+ hearts (1.15±0.02 vs. 1.00±0.02 RA, P<0.01) (Fig. 6) compared with CON. However, in LMP-2−/− hearts, IPC significantly decreased this activity (0.90±0.03 vs. 1.17±0.02 RA, P<0.001) (Fig. 6). Under basal conditions, Chym-L activity and Tryp-L activity were decreased in LMP-2−/− hearts compared with LMP-2+/+ hearts (Chym-L activity: 0.73±0.02 vs. 1.00±0.03 RA, P<0.01; Tryp-L activity: 0.67±0.02 vs. 1.00±0.01 RA, P<0.01) (Fig. 6). IPC caused a decrease in Chym-L and Tryp-L activities in LMP-2+/+ hearts (Chym-L: 0.80±0.05 vs. 1.00±0.03 RA, P<0.01; Tryp-L: 0.83±0.02 vs. 1.00±0.01 RA, P<0.01) (Fig. 6) and LMP-2−/− hearts (Chym-L: 0.52±0.04 vs. 0.73±0.02 RA, P<0.01; Tryp-L: 0.50±0.02 vs. 0.67±0.02 RA, P<0.01) (Fig. 6). Therefore, in LMP-2−/− hearts, proteolytic activities are dysregulated under basal conditions and worsened by IPC.
Figure 6.
Decreased proteolytic activities in LMP-2−/− hearts after IPC. Homogenates were generated from isolated LMP-2+/+ and LMP-2−/− hearts with or without IPC. Cas-L, Chym-L, and Tryp-L were measured using the fluorogenic substrates Z-LLE-AMC, Suc-LLVY-AMC, and Boc-LRR-AMC, respectively. LMP-2−/− hearts have lower Chym-L and Tryp-L activities but higher Cas-L activity under basal conditions (*P<0.01 vs. CONKO each activity). IPC increased Cas-L activity in LMP-2+/+ hearts, but decreased Cas-L activity in LMP-2−/− hearts (#P<0.01 vs. CONWT). IPC decreased Chym-L and Tryp-L activities in both LMP-2+/+ and LMP-2−/− hearts (**P<0.01 vs. CONKO, #P<0.01 vs. CONWT each activity). n = 6/group.
The immunoproteasome is defective in LMP-2−/− hearts
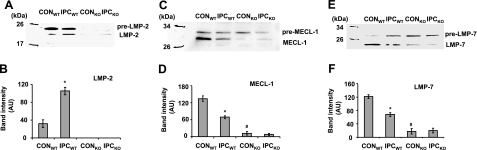
Because proteolytic activities are executed in the 20S proteasome, we wondered whether the immunoproteasome is defective in LMP-2−/− hearts and whether IPC regulates the immunoproteasome in LMP-2+/+ hearts. We determined protein levels of LMP-2, LMP-7, MECL-1, and their precursors in LMP-2+/+ and LMP-2−/− hearts with or without preceding IPC. IPC significantly increased LMP-2 levels in LMP-2+/+ hearts (105±8 vs. 31±9 AU, P<0.01) (Fig. 7A, B), but it decreased MECL-1 (68±5 vs. 134±10 AU, P<0.001) (Fig. 7C, D) and LMP-7 (43±10 vs. 129±4 AU, P<0.001) (Fig. 7E, F). Under basal conditions, LMP-2 was undetectable in LMP-2−/− hearts. Interestingly, MECL-1 and LMP-7 were also markedly decreased in these hearts compared with LMP-2+/+ hearts [MECL-1: 11±6 vs.134±10 AU, P<0.001 (Fig. 7C, D); LMP-7: 8±6 vs. 129±4 AU, P<0.001 (Fig. 7E, F)]. IPC did not significantly affect these protein levels in LMP-2−/− hearts. Together, the results show that the immunoproteasome is defective in LMP-2−/− hearts during CON and IPC and that IPC regulates immunoproteasome subunits in LMP-2+/+ hearts.
Figure 7.
Regulation of the immunoproteasome by IPC. Lysates were generated from isolated LMP-2+/+ and LMP-2−/− hearts with or without IPC. A, B): LMP-2 was increased in LMP-2+/+ hearts after IPC but was undetectable in LMP-2−/− hearts. *P < 0.001 vs. CONWT. C, D) MECL-1 was decreased in LMP-2+/+ hearts after IPC. Under basal conditions, MECL-1 was less in LMP-2−/− hearts. *P < 0.01, #P < 0.001 vs. CONWT. E, F) IPC decreased LMP-7 protein levels in LMP-2+/+ heart, and there was less LMP-7 in LMP-2−/− hearts. *P < 0.01, #P < 0.01 vs. CONWT. n = 4/group.
Preconditioning with the PTEN inhibitor HOpic induces cardioprotection in LMP-2−/− mice
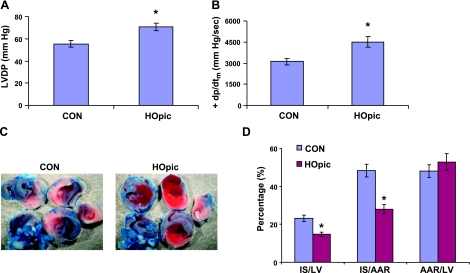
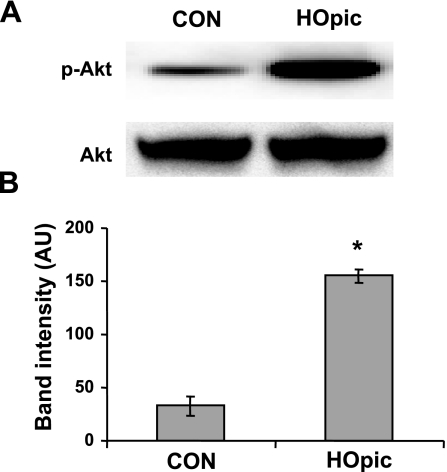
To determine whether PTEN inhibition is cardioprotective, we used the PTEN inhibitor HOpic to precondition LMP-2−/− hearts for 15 min, followed by I-30/R-120. HOpic significantly increased LVDP (71±3 vs. 55±2 mmHg, P<0.01) (Fig. 8A) and maximal dp/dt (4499±371 vs. 3112±237 mmHg/s, P<0.01) (Fig. 8B). Moreover, HOpic significantly reduced myocardial infarct size after I-30/R-120 compared with the saline control (IS/AAR: 28±2 vs. 48±3%, P<0.001) (Fig. 8C, D), with no significant difference in the area at risk (AAR/LV: 53±4 vs. 48±3%, P>0.05) (Fig. 8D). To confirm that PTEN is inactivated by HOpic in the heart, we measured p-Akt levels after isolated hearts were treated with 100 nM HOpic for 15 min. HOpic significantly increased Akt phosphorylation compared with saline (155±6 vs. 32±9 AU, P<0.01) (Fig. 9A, B). These results indicate that PTEN inactivation induces cardioprotection.
Figure 8.
Induction of cardioprotection by the PTEN inhibitor HOpic. Mice were pretreated with HOpic or saline (CON) for 15 min and then exposed to I-30/R-120 by occluding and reopening the left coronary artery. A, B) HOpic increased LVDP and +dp/dtm after ischemia and reperfusion. C, D) HOpic reduced myocardial infarct size. *P < 0.01 vs. CON; n = 8/group).
Figure 9.
Effect of HOpic on Akt phosphorylation. LMP-2−/− hearts were isolated and exposed to CON or HOpic for 15 min. A) Representative Western blots of p-Akt and total Akt. B) HOpic increased Akt phosphorylation in LMP-2−/− hearts. *P < 0.01 vs. CON; n = 4/group.
DISCUSSION
We have made three important observations. First, we found that LMP-2 deficiency contributes to the dysfunction of the UPS in the heart under basal conditions and in response to IPC. Second, LMP-2 deficiency leads to lower cardiac mass and heart rate, indicating that LMP-2 expression may be necessary for normal cardiac development. Third, and most important, LMP-2−/− mice failed to generate cardioprotection in response to IPC. Further studies revealed that PTEN degradation and Akt activation are impaired in preconditioned LMP-2−/− hearts and that PTEN inhibition induces cardioprotection in LMP-2−/− mice. Therefore, we report for the first time that LMP-2 as a key subunit of the immunoproteasome is involved in the physiological phenomenon IPC, probably through PTEN degradation.
LMP-2 deficiency prevents immunoproteasome formation
The UPS plays a predominant role in the control of protein quality and quantity (15). Its dysfunction has been implicated in various cardiovascular diseases including ischemia and reperfusion injury, cardiac hypertrophy, and heart failure (16,17,18). However, little is known about the regulation of the UPS. The 20S proteasome executes protein digestion. Its proteolytic activities are generated from the constitutive β1, β2, and β5 subunits. In response to stimulation of interferon-γ, the 20S proteasome is modulated by incorporation of LMP-2, MECL-1, and LMP-7. The process includes assembly of their precursors and subsequent cleavage of prosequences (34). These inducible subunits assemble the immunoproteasome in a cooperative manner. LMP-2 plays an important role in this process. It is essential for incorporation and processing of MECL-1 (21, 35). Consistent with prior observations, the present study showed that MECL-1 and LMP-7 are markedly reduced in LMP-2−/− hearts despite the presence of their precursors, suggesting that the immunoproteasome is defective in these mice. The decrease in MECL-1 and LMP-7 protein levels may explain the decreased Chym-L and Tryp-L activities in LMP-2−/− hearts. Decreased proteolytic activities are also found in the brain and liver of LMP-2−/− mice (27, 28). LMP-2 may suppress Cas-L activity in the heart under basal conditions. Overexpression of LMP-2 causes decreased Cas-L activity in cells (23, 24), and LMP-2 deficiency leads to increased Cas-L activity in certain tissues (27). We showed that Cas-L activity is increased in LMP-2−/− hearts. Therefore, LMP-2 is essential for immunoproteasome formation and normal proteolytic activities in the heart.
The immunoproteasome is necessary for normal UPS function in the heart
The immunoproteasome has been implicated in protein degradation by the UPS. It has been reported that decreased Chym-L and Tryp-L activities are associated with accumulation of oxidized proteins in LMP-2−/− mice (28). Moreover, LMP-2 inhibition blocks inhibitory NF-κBα degradation and p50 generation during NF-κB activation in a human invasive extravillous trophoblast cell line (36). In the present study, ubiquitinated proteins were increased in LMP-2−/− mice after IPC. Notably, the degradation of the proapoptotic protein PTEN was impaired after ubiquitination. IPC promotes degradation of ubiquinated proteins in LMP-2+/+ hearts. However, with the defective immunoproteasome induced by LMP-2 deficiency, the heart is unable to remove ubiquitinated proteins, consistent with an important role for the immunoproteasome in UPS function in the heart. IPC modulates proteolytic activities, probably by regulating immunoproteasome subunits. Decreased Chym-L and Tryp-L activities were associated with decreased LMP-7 and MECL-1 in LMP-2+/+ hearts after IPC (I-10/R-5) in our study. The result is consistent with results of a previous study, which showed that a longer period of ischemia and reperfusion (I-30/R-60) decreased Chym-L activity in the heart (44). The explanation for why we found decreased Chym-L activity after such a brief period of ischemia, unlike the early study, is uncertain but could be related to the more sensitive assay used in our study.
Interestingly, IPC increases endogenous LMP-2 levels and Cas-L activity in LMP-2+/+ hearts. The increased Cas-L activity may be necessary to degrade ubiquitinated proteins as part of IPC-induced cardioprotection. An increase in endogenous LMP-2 appears to have a different effect on Cas-L activity than exogenous overexpression of LMP-2 (23, 24). The underlying mechanism for this is not known. LMP-2 deficiency also affects the regulation of the other inducible subunits mediated by IPC. There is no significant difference in LMP-7 and MECL-1 between CON and IPC in LMP-2−/− hearts. However, all three proteolytic activities are decreased in these samples after IPC. Because IPC produces reactive oxygen species (ROS), the decreased proteolytic activities in LMP-2−/− hearts may result from further damage of the 20S proteasome by ROS (37). Notably, the degradation of ubiquitinated proteins is increased in LMP-2+/+ hearts but impaired in LMP-2−/− hearts after IPC.
LMP-2 deficiency contributes to cardiac underdevelopment
The UPS is involved in cellular growth, differentiation, and survival. Proteasome inhibition attenuates cardiac hypertrophy induced by pressure overload and isoproterenol injection (38, 39). In this study, we showed that PTEN levels are elevated in LMP-2−/− hearts. It has been reported that overexpression of wild-type PTEN promotes apoptosis in cardiomyocytes (40). Therefore, increased PTEN protein levels could be an explanation for impaired cardiac development in LMP-2−/− mice. Interestingly, despite a smaller heart size, these mice have a larger body size than LMP-2+/+ mice, as described previously (41). Although the underlying mechanism is not clear, the abnormality is associated with increased motor function (41). Heart rate is also lower in LMP-2−/− mice than in LMP-2+/+ mice. This finding is consistent with a recent report, in which proteasome inhibition decreased ventricular tachyarrhythmias caused by ischemia and reperfusion injury (42).
Impairment of PTEN degradation contributes to loss of IPC-induced cardioprotection
IPC generates profound cardioprotection in various models (2,3,4,5,6,7). In the IPC model used here, a single cycle of 10 min of ischemia followed by 5 min of reperfusion significantly decreased infarct size (in IS/AAR) by 42% and increased cardiac functional recovery (in LVDP) by 39%. However, IPC cardioprotection was lost in LMP-2−/− mice. Although several survival protein kinases have been implicated in IPC, including PI3K, PKC, and MAPK, new signaling molecules continue to emerge. Recently, we reported that PTEN inactivation by IPC is associated with its cardioprotection (8). PTEN protein is subjected to proteasomal degradation (8, 43). In the present study, we demonstrated that PTEN degradation and Akt activation are impaired in LMP-2−/− hearts after IPC and that PTEN inhibition induces IPC-like cardioprotection in these hearts. These results provide additional evidence that PTEN plays an important role in IPC. Moreover, our study suggests that LMP-2 expression is necessary for proteasome-mediated PTEN degradation and IPC protection. Indeed, the down-regulation of proapoptotic proteins in IPC may be crucial for tissues to tolerate later lethal injury. In the brain, the proteasome mediates IPC protection through degradation of the proapoptotic protein Bim (14). Although the UPS can potentially regulate G protein-coupled receptors and receptor tyrosine kinases, it remains unclear whether the UPS is actually involved in the activation of the survival protein kinases through these pathways during IPC.
In conclusion, inducible LMP-2 is essential for immunoproteasome formation and function in the heart. IPC induces cardioprotection through UPS-mediated signaling, probably PTEN protein degradation and Akt activation. Our study also suggests that LMP-2 plays an important role in cardiac development. Therefore, by regulating LMP-2 activity, it might be possible to generate novel therapeutic treatments for various cardiovascular diseases, including ischemia-reperfusion injury, cardiac hypertrophy, and heart failure.
Acknowledgments
This work was supported by U.S. Public Health Service grant P01-HL65608 from the National Heart, Lung, and Blood Institute, National Institutes of Health.
References
- Murry C E, Jennings R B, Reimer K A. Preconditioning with ischemia: a delay of lethal injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Yellon D M, Downey J M. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- Murphy E. Primary and secondary signaling pathways in early preconditioning that converge on the mitochondria to produce cardioprotection. Circ Res. 2003;94:7–16. doi: 10.1161/01.RES.0000108082.76667.F4. [DOI] [PubMed] [Google Scholar]
- Mocanu M M, Yellon D M. PTEN, the Achilles’ heel of myocardial ischaemia/reperfusion injury? Br J Pharmacol. 2007;150:833–838. doi: 10.1038/sj.bjp.0707155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohm C, Barancik T, Bruhl M L, Kilian S A, Schaper W. Inhibition of the ER-kinase cascade by PD98059 and UO126 counteracts ischemic preconditioning in pig myocardium. J Cardiovasc Pharmacol. 2000;36:218–229. doi: 10.1097/00005344-200008000-00012. [DOI] [PubMed] [Google Scholar]
- Tong H, Chen W, Steenbergen C, Murphy E. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ Res. 2000;87:309–315. doi: 10.1161/01.res.87.4.309. [DOI] [PubMed] [Google Scholar]
- Ping P, Zhang J, Qiu Y, Tang X L, Manchikalapudi S, Cao X, Bolli R. Ischemic preconditioning induces selective translocation of protein kinase C isoforms ε and η in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res. 1997;81:404–414. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- Cai Z, Semenza G L. PTEN activity is modulated during ischemia and reperfusion: involvement in the induction and decay of preconditioning. Circ Res. 2005;97:1351–1359. doi: 10.1161/01.RES.0000195656.52760.30. [DOI] [PubMed] [Google Scholar]
- Mahimainathan L, Choudhury G G. Inactivation of platelet-derived growth factor receptor by the tumor suppressor PTEN provides a novel mechanism of action of the phosphatase. J Biol Chem. 2004;279:15258–15268. doi: 10.1074/jbc.M314328200. [DOI] [PubMed] [Google Scholar]
- Oudit G Y, Sun H, Kerfant B G, Crackower M A, Penninger J M, Backx P H. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Balendran A, Hare G R, Kieloch A, Williams M R, Alessi D R. Further evidence that 3-phosphoinositide-dependent protein kinase-1 (PDK1) is required for the stability and phosphorylation of protein kinase C (PKC) isoforms. FEBS Lett. 2000;484:217–223. doi: 10.1016/s0014-5793(00)02162-1. [DOI] [PubMed] [Google Scholar]
- Liu C, Chen S, Kamme F, Hu B R. Ischemic preconditioning prevents protein aggregation after transient cerebral ischemia. Neuroscience. 2005;134:69–80. doi: 10.1016/j.neuroscience.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradillo J M, Romera C, Hurtado O, Cárdenas A, Moro M A, Leza J C, Dávalos A, Castillo J, Lorenzo P, Lizasoain I. TNFR1 upregulation mediates tolerance after brain ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:193–203. doi: 10.1038/sj.jcbfm.9600019. [DOI] [PubMed] [Google Scholar]
- Meller R, Cameron J A, Torrey D J, Clayton C E, Ordonez A N, Henshall D C, Minami M, Schindler C K, Saugstad J A, Simon R P. Rapid degradation of Bim by the ubiquitin-proteasome pathway mediates short-term ischemic tolerance in cultured neurons. J Biol Chem. 2006;281:7429–7436. doi: 10.1074/jbc.M512138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A L. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- Powell S R. The ubiquitin-proteasome system in cardiac physiology and pathology. Am J Physiol Heart Circ Physiol. 2006;291:H1–H19. doi: 10.1152/ajpheart.00062.2006. [DOI] [PubMed] [Google Scholar]
- Gomes A V, Zong C, Ping P. Protein degradation by the 26S proteasome system in the normal and stressed myocardium. Antioxid Redox Signal. 2006;8:1677–1691. doi: 10.1089/ars.2006.8.1677. [DOI] [PubMed] [Google Scholar]
- Willis M S, Patterson C. Into the heart: the emerging role of the ubiquitin-proteasome system. J Mol Cell Cardiol. 2006;41:567–579. doi: 10.1016/j.yjmcc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Glickman M H, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- Baumeister W, Walz J, Zühl F, Seemüller E. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998;92:367–380. doi: 10.1016/s0092-8674(00)80929-0. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Zantopf D, Kraft R, Kostka S, Preissner R, Kloetzel P M. Sequence information within proteasomal prosequences mediates efficient integration of β-subunits into the 20 S proteasome complex. J Mol Biol. 1999;288:117–128. doi: 10.1006/jmbi.1999.2660. [DOI] [PubMed] [Google Scholar]
- Driscoll J, Brown M G, Finley D, Monaco J J. MHC-linked LMP gene products specifically alter peptidase activities of the proteasome. Nature. 1993;365:262–264. doi: 10.1038/365262a0. [DOI] [PubMed] [Google Scholar]
- Groettrup M, Ruppert T, Kuehn L, Seeger M, Standera S, Koszinowski U, Kloetzel P M. The interferon-γ-inducible 11 S regulator (PA28) and the LMP2/LMP7 subunits govern the peptide production by the 20 S proteasome in vitro. J Biol Chem. 1995;270:23808–23815. doi: 10.1074/jbc.270.40.23808. [DOI] [PubMed] [Google Scholar]
- Gaczynska M, Rock K L, Goldberg A L. γ-Interferon and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature. 1993;365:264–267. doi: 10.1038/365264a0. [DOI] [PubMed] [Google Scholar]
- Frentzel S, Kuhn-Hartmann I, Gernold M, Gött P, Seelig A, Kloetzel P M. Major-histocompatibility-complex-encoded β-type proteasome subunits LMP2 and LMP7: evidence that LMP2 and LMP7 are synthesized as proproteins and that cellular levels of both mRNA and LMP-containing 20S proteasomes are differentially regulated. Eur J Biochem. 1993;216:119–226. doi: 10.1111/j.1432-1033.1993.tb18123.x. [DOI] [PubMed] [Google Scholar]
- Barton L F, Cruz M, Rangwala R, Deepe G S, Jr, Monaco J J. Regulation of immunoproteasome subunit expression in vivo following pathogenic fungal infection. J Immunol. 2002;169:3046–3052. doi: 10.4049/jimmunol.169.6.3046. [DOI] [PubMed] [Google Scholar]
- Van Kaer L, Ashton-Rickardt P G, Eichelberger M, Gaczynska M, Nagashima K, Rock K L, Goldberg A L, Doherty P C, Tonegawa S. Altered peptidase and viral-specific T cell response in LMP2 mutant mice. Immunity. 1994;1:533–541. doi: 10.1016/1074-7613(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Ding Q, Martin S, Dimayuga E, Bruce-Keller A J, Keller J N. LMP2 knock-out mice have reduced proteasome activities and increased levels of oxidatively damaged proteins. Antioxid Redox Signal. 2006;8:130–135. doi: 10.1089/ars.2006.8.130. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sun L, Liang J, Yu W, Zhang Y, Wang Y, Chen Y, Li R, Sun X, Shang Y. The catalytic subunit of the proteasome is engaged in the entire process of estrogen receptor-regulated transcription. EMBO J. 2006;25:4223–4233. doi: 10.1038/sj.emboj.7601306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotamraju S, Matalon S, Matsunaga T, Shang T, Hickman-Davis J M, Kalyanaraman B. Upregulation of immunoproteasomes by nitric oxide: potential antioxidative mechanism in endothelial cells. Free Radic Biol Med. 2006;40:1034–1044. doi: 10.1016/j.freeradbiomed.2005.10.052. [DOI] [PubMed] [Google Scholar]
- Callahan M K, Wohlfert E A, Ménoret A, Srivastava P K. Heat shock up-regulates lmp2 and lmp7 and enhances presentation of immunoproteasome-dependent epitopes. J Immunol. 2006;177:8393–8399. doi: 10.4049/jimmunol.177.12.8393. [DOI] [PubMed] [Google Scholar]
- Michael L H, Entman M L, Hartley C J, Youker K A, Zhu J, Hall S R, Hawkins H K, Berens K, Ballantyne C M. Myocardial ischemia and reperfusion: a murine model. Am J Physiol Heart Circ Physiol. 1995;269:H2147–H2154. doi: 10.1152/ajpheart.1995.269.6.H2147. [DOI] [PubMed] [Google Scholar]
- Zong C, Gomes A V, Drews O, Li X, Young G W, Berhane B, Qiao X, French S W, Bardag-Gorce F, Ping P. Regulation of murine cardiac 20S proteasomes: role of associating partners. Circ Res. 2006;99:372–380. doi: 10.1161/01.RES.0000237389.40000.02. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Schmidtke G, Kloetzel P M. Structure and structure formation of the 20S proteasome. Mol Biol Rep. 1997;24:103–112. doi: 10.1023/a:1006826725056. [DOI] [PubMed] [Google Scholar]
- Griffin T A, Nandi D, Cruz M, Fehling H J, Kaer L V, Monaco J J, Colbert R A. Immunoproteasome assembly: cooperative incorporation of interferon γ (IFN-γ)-inducible subunits. J Exp Med. 1998;187:97–104. doi: 10.1084/jem.187.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H X, Wang H M, Lin H Y, Yang Q, Zhang H, Tsang B K, Zhu C. Proteasome subunit LMP2 is required for matrix metalloproteinase-2 and -9 expression and activities in human invasive extravillous trophoblast cell line. J Cell Physiol. 2006;206:616–623. doi: 10.1002/jcp.20508. [DOI] [PubMed] [Google Scholar]
- Reinheckel T, Sitte N, Ullrich O, Kuckelkorn U, Davies K J, Grune T. Comparative resistance of the 20S and 26S proteasome to oxidative stress. Biochem J. 1998;335:637–642. doi: 10.1042/bj3350637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfield W E, Tang R, Moss N C, Baldwin A S, Willis M S, Selzman C H. Proteasome inhibition promotes regression of left ventricular hypertrophy. Am J Physiol Heart Circ Physiol. 2008;294:H645–H650. doi: 10.1152/ajpheart.00196.2007. [DOI] [PubMed] [Google Scholar]
- Depre C, Wang Q, Yan L, Hedhli N, Peter P, Chen L, Hong C, Hittinger L, Ghaleh B, Sadoshima J, Vatner D E, Vatner S F, Madura K. Activation of the cardiac proteasome during pressure overload promotes ventricular hypertrophy. Circulation. 2006;114:1821–1828. doi: 10.1161/CIRCULATIONAHA.106.637827. [DOI] [PubMed] [Google Scholar]
- Schwartzbauer G, Robbins J. The tumor suppressor gene PTEN can regulate cardiac hypertrophy and survival. J Biol Chem. 2001;276:35786–35793. doi: 10.1074/jbc.M102479200. [DOI] [PubMed] [Google Scholar]
- Martin S, Gee J R, Bruce-Keller A J, Keller J N. Loss of an individual proteasome subunit alters motor function but not cognitive function or ambulation in mice. Neurosci Lett. 2004;357:76–78. doi: 10.1016/j.neulet.2003.10.085. [DOI] [PubMed] [Google Scholar]
- Huang S, Patterson E, Yu X, Garrett M W, De Aos I, Kem D C. Proteasome inhibition one hour following ischemia protects grk2 and prevents malignant ventricular tachyarrhythmias and SCD in a model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2008;294:H1298–H1303. doi: 10.1152/ajpheart.00765.2007. [DOI] [PubMed] [Google Scholar]
- Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus: implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- Powell S R, Wang P, Katzeff H, Shringarpure R, Teoh C, Khaliulin I, Das D K, Davies K J, Schwalb H. Oxidized and ubiquitinated proteins may predict recovery of postischemic cardiac function: essential role of the proteasome. Antioxid Redox Signal. 2005;7:538–546. doi: 10.1089/ars.2005.7.538. [DOI] [PubMed] [Google Scholar]