Abstract
Our objectives were 1) to characterize angiogenesis in the MDA-MB-435 xenograft mouse model with three-dimensional (3D) MR molecular imaging using α5β1(RGD)- or irrelevant RGS-targeted paramagnetic nanoparticles and 2) to use MR molecular imaging to assess the antiangiogenic effectiveness of α5β1(ανβ3)- vs. ανβ3-targeted fumagillin (50 μg/kg) nanoparticles. Tumor-bearing mice were imaged with MR before and after administration of either α5β1(RGD) or irrelevant RGS-paramagnetic nanoparticles. In experiment 2, mice received saline or α5β1(ανβ3)- or ανβ3-targeted fumagillin nanoparticles on days 7, 11, 15, and 19 posttumor implant. On day 22, MRI was performed using α5β1(ανβ3)-targeted paramagnetic nanoparticles to monitor the antiangiogenic response. 3D reconstructions of α5β1(RGD)-signal enhancement revealed a sparse, asymmetrical pattern of angiogenesis along the tumor periphery, which occupied <2.0% tumor surface area. α5β1-targeted rhodamine nanoparticles colocalized with FITC-lectin corroborated the peripheral neovascular signal. α5β1(ανβ3)-fumagillin nanoparticles decreased neovasculature to negligible levels relative to control; ανβ3-targeted fumagillin nanoparticles were less effective (P>0.05). Reduction of angiogenesis in MDA-MB-435 tumors from low to negligible levels did not decrease tumor volume. MR molecular imaging may be useful for characterizing tumors with sparse neovasculature that are unlikely to have a reduced growth response to targeted antiangiogenic therapy.—Schmieder, A. H., Caruthers, S. D., Zhang, H., Williams, T. A., Robertson, J. D., Wickline, S. A., Lanza, G. M. Three-dimensional MR mapping of angiogenesis with α5β1(ανβ3)-targeted theranostic nanoparticles in the MDA-MB-435 xenograft mouse model.
Keywords: magnetic resonance imaging, fumagillin, cancer, molecular imaging, antiangiogenic
The benefit of antiangiogenesis therapy in conjunction with established chemotherapy or radiation therapy has become well established (1) for lung, colon, and breast cancer (2,3,4). However, the effectiveness of antiangiogenic pretreatment is not universal among patients, and the clinical timing of the treatment is not always optimal. Given the projected annual expense (>$100,000) of these therapies and the potential adverse effects (i.e., hypertension, proteinuria, hemorrhage, thrombosis, wound healing complications, and gastrointestinal perforation) (5,6,7,8), a compelling need exists to better risk stratify candidate patients for treatment.
Clinical techniques are available to help stage and monitor response to treatment, and include dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and 18fluorodeoxyglucose (18FDG) positron emission tomography (PET). However, these techniques have not proved effective in substratifying patients into treatment regimens based on tumor neovascularity. Radiolabeled arginine-glycine-aspartic acid (RGD) peptides and antibodies, particularly directed to the ανβ3-integrin, have been used to target and characterize tumor angiogenesis by microPET (9) and single photon emission computed tomography (SPECT) (10). However, these small molecules readily permeate beyond the tumor and, like 18FDG, bind many cell types, including macrophages and tumor cells, which diminishes the signal specificity for angiogenesis per se (10, 11). DCE-MRI can detect changes in tumor microvasculature permeability to MR blood pool contrast agents (12,13,14), and some studies have correlated these kinetic estimates with traditional measures such as microvessel density (MVD), but initial clinical trials have yielded inconsistent results either due to insufficient standardization of the endpoints or technique issues (15, 16).
Among a cadre of MR molecular imaging agents that have been invented and studied over the past several years, lipid-based paramagnetic particles, such as perfluorocarbon (PFC) emulsions (17,18,19), liposomes (20, 21), or micelles (22) targeted to biomarkers by antibody, small peptides, or peptidomimetics have shown particular effectiveness in monitoring ανβ3-integrin endothelial expression before and after treatment. Of these, PFC nanoparticles have proven to be a robust theranostic technology for quantitative MR monitoring and antiangiogenic treatment in rabbit models of early atherosclerosis (23) and in Vx2 adenocarcinoma (24).
The present research explores the utility of α5β1-integrin as a biomarker for angiogenesis in cancer in the MDA-MB-435 xenograft mouse model. α5β1-integrin, like ανβ3-integrin, is an important adhesion molecule, which regulates endothelial cell migration and survival during neovascularization (25). α5β1-integrin is poorly expressed on normal quiescent blood vessels, but its expression is induced on tumor blood vessels and in response to angiogenic factors (26), including basic fibroblast growth factor, interleukin-8, tumor necrosis factor-alpha, and the angiomatrix protein Del-1. Integrin α5β1 and its ligand fibronectin are coordinately up-regulated on human tumor blood vessels. Similar to ανβ3-integrin, α5β1-integrin regulates human endothelial cell vacuolation and lumen formation in three-dimensional (3D) fibrin matrices, and these morphogenic events are completely inhibited by the simultaneous addition of anti-ανβ3-integrin and anti-α5-integrin antibodies (27). The relevance of α5β1-fibronectin interactions is further exemplified by the enhancement of angiogenesis induced by the addition of fibronectin during the chick chorioallantoic membrane (CAM) assay, as well as the converse suppression of neovascularity by antibody, peptides, and nonpeptide antagonists of α5β1-integrin in CAM and human xenograft tumor models (25). However, while the role of ανβ3-integrin is well documented in aggressive melanoma and breast cancer metastasis, α5β1-integrin is frequently expressed by low malignant potential tumors, in addition to aggressive carcinomas, e.g., in ovarian cancers. In glioblastomas, endothelial proliferations have been phenotypically characterized into three subtypes based on variable integrin expression at different steps in the endothelial maturation progression. Although ανβ3-integrin is an important biomarker of interest, MR theranostic agents directed against a spectrum of biomarkers will likely be required to properly characterize, segment, and treat the widely heterogeneous population of tumors and associated neovasculature.
The overarching hypothesis of this research was to determine potential utility of α5β1-PFC nanoparticles as MR theranostics to characterize tumor angiogenesis and response to antiangiogenic therapy. The objective of the first study was to quantify and visually characterize the 3D spatial distribution angiogenesis in the MDA-MB-435 xenograft model using MR molecular imaging of α5β1-peptide-targeted nanoparticles. In a second study, the antiangiogenic effects of fumagillin nanoparticles (50 μg/kg) targeted by a dual α5β1(ανβ3)-peptidomimetic vs. the ανβ3-peptidomimetic alone were investigated using 3D MR neovascular mapping of α5β1(ανβ3)-targeted paramagnetic nanoparticles to assess tumor response.
MATERIALS AND METHODS
Nanoparticle formulation
Paramagnetic PFC nanoparticles were prepared similar to previously described methods (19, 24). Briefly, the emulsions comprised 20% (v/v) perfluorooctylbromide (PFOB), 2.0% (w/v) of a surfactant comixture, and 1.7% (w/v) glycerin in distilled, deionized water. Targeted nanoparticles were prepared by incorporating either a peptide or a peptidomimetic targeting ligand. The surfactant comixture of peptidomimetic nanoparticles included 68 mol% lecithin (Avanti Polar Lipids, Alabaster, AL, USA), 0.1 mol% of either peptidomimetic ανβ3- or α5β1-integrin antagonist, conjugated to PEG2000-phosphatidylethanolamine (Kereos, St. Louis, MO, USA), 1.9 mol% phosphatidylethanolamine (Avanti Polar Lipids) and 30% (w/v) gadolinium-tetraazacyclododecanetetraacetic acid–phosphatidylethanolamine (Gd-DOTA-PE; Kereos). Nontargeted nanoparticles excluded the integrin homing ligand from the surfactant, which was replaced with equimolar phosphatidylethanolamine, a neutral phospholipid. The surfactant components were combined with the PFOB, water, and glycerin; the pH was adjusted to 7.5; and the mixture was emulsified (Microfluidics, Newton, MA, USA) at 20,000 psi for 4 min. Peptide-targeted nanoparticles were functionalized by inclusion of 0.1 mol% carboxy-PEG2000-phosphatidylethanolamine in the surfactant commixture in lieu of the peptidomimetic-lipid conjugate for coupling of the ligand after emulsification. For nonparamagnetic nanoparticles, the gadolinium phospholipid chelate was substituted with lecithin on an equimolar basis. Rhodamine-labeled nanoparticles included 0.1 mol% rhodamine phosphatidylethanolamine in the surfactant mix at the expense of lecithin. Fumagillin nanoparticles included 0.2 mol% fumagillin (30 μg/ml of emulsion; National Cancer Institute, Bethesda, MD, USA), which was added to the surfactant mixture at the proportionate expense of lecithin.
Nominal particle sizes of the targeted and nontargeted nanoparticles were 282 ± 14 nm with a polydispersity of 0.18 ± 0.1 (Brookhaven Instrument Corp., Holtsville, NY, USA). Gd concentrations were determined by neutron activation analysis (University of Missouri Research Reactor Center, Columbia, MO, USA), and the number of Gd3+/nanoparticles was ∼130,000. T1 ionic relaxivities assessed at 0.47 T and 40°C with a Minispec Analyzer (Bruker, Milton, ON, Canada) were similar: r1 = 12.5 (s·mM Gd3+)−1 for targeted particles and 13.1 (s·mM Gd3+)−1 for control; particulate relaxivities were greater than 1.6 × 106 (s·mM nanoparticle)−1.
Homing ligands
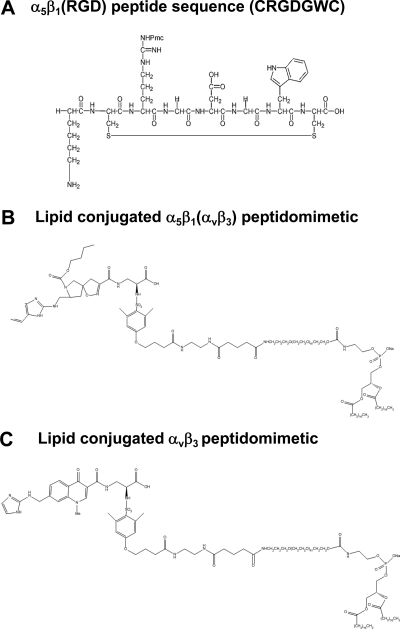
In study 1, a cyclic RGD peptide ligand previously reported and characterized (IC50<10μM in the binding of α5-expressing cells to fibronectin) (28, 29) was synthesized (JPT Peptide Technologies, Springfield, VA, USA) and covalently coupled to carboxy-PEG(2000)-phosphotidylethanolamine (Avanti Polar Lipids) through a short spacer (Fig. 1A) to test the potential utility of homing to the α5β1-integrin. An irrelevant RGS peptide was used for the nontargeted, control paramagnetic emulsion and coupled to the nanoparticle with identical chemistry. In study 2, a small molecule peptidomimetic α5β1(ανβ3)-antagonist (30) was used and compared to a similar peptidomimetic ανβ3-antagonist (31) (Fig. 1B, C). The α5β1(ανβ3)-integrin antagonist is a quinalone nonpeptide developed by Bristol-Myers Squibb Medical Imaging (PCT/US1998/012010) with an IC50 of 390 pM for α5β1 and 87 nM for ανβ3. The mimetic has no effective cross-reactivity with ανβ5 or GPIIb/IIIa (Kereos, Inc., unpublished data). The ανβ3-integrin antagonist is also a quinalone nonpeptide developed by Bristol-Myers Squibb Medical Imaging (U.S. patent 6,511,648 and related patents), which was initially reported and characterized as the 111In-DOTA conjugate RP478 and cyan 5.5 homolog TA145 (32). The specificity of the ανβ3-ligand mirrors that of LM609, as assessed by staining and flow cytometry, and it has a 15-fold preference for the Mn2+ activated receptor (21 nM) (33). The IC50 for ανβ5, α5β1, and GP IIbIIIa was determined to be >10 μM (BMSMI; Billerica, MA, USA, unpublished data).
Figure 1.
Chemical structures of integrin homing ligands. A) α5β1(RGD) peptide used in study 1. B) α5β1(ανβ3) peptidomimetic coupled to PEG(2000)-phosphatidylethanolamine used in study 2. C) ανβ3-targeted peptidomimetic coupled to PEG(2000)-phosphatidylethanolamine used in study 2.
In vitro competitive cell adhesion assay
The binding of the α5β1-targeted PFC nanoparticles was confirmed in vitro using an inhibitory cell adhesion assay, as described previously (19). Suspensions of HT1080 human fibrosarcoma cells (WUMS Tissue Culture Center, St. Louis, MO, USA), which express the α5β1-integrin, were incubated with serial dilutions of the nanoparticle emulsions and aliquoted (100 μl/well) onto a 96-well plate coated with human fibronectin (1 μg/well; Chemicon, Temecula, CA, USA). HT1080 cells were allowed to adhere for 1 h, unbound cells were removed by washing with PBS, and adherent cells were stained with crystal violet. The absorbance (570 nm) of crystal violet retained by cells adhered to the fibronectin-coated wells was adjusted for background, and correlated to a titrated curve of dye retained by bound HT1080 cells with no inhibitor. ανβ3-targeting was tested in an analogous manner using C32 human melanoma cell (American Type Culture Collection, Manassas, VA, USA) adhesion to vitronectin (50 ng/well; Chemicon). Data analysis was performed by curve-fitting the cell binding data with a dose-response logistic fit in Kaleidagraph (Synergy Software, Reading, PA, USA).
Cell binding to targeted contrast agent
The specific binding of nanoparticles was further evaluated by flow cytometry and fluorescence microscopy. 2F2B mouse endothelial cells (American Type Culture Collection) expressing the α5β1-integrin were plated onto glass coverslips and incubated with rhodamine labeled α5β1(ανβ3)-targeted or nontargeted particles for 2 h at 37°C. The cells were then washed with PBS and fixed with 4% paraformaldehyde for observation by fluorescence microscopy. For flow cytometry, cells were grown in tissue culture flasks and incubated with nanoparticles for 30 min at 4°C. Cells were washed with TBS + 1% BSA, resuspended by removal with nonenzymatic Cell Dissociation Solution (Sigma, St. Louis, MO, USA), and analyzed on a CyAn ADP flow cytometer (Dako, Carpinteria, CA, USA).
MDA-MB-435 xenograft model studies
All animal studies were conducted in accordance with a protocol approved by the Washington University Animal Studies Committee.
Experiment 1: MRI of tumor neovasculature with α5β1-peptide-targeted nanoparticles
Athymic nude mice were implanted with 1 × 106 MDA-MB-435 human breast carcinoma cells in the caudal mammary fat-pad. On day 14, mice were sedated with ketamine/xylazine, and jugular vein access was established for injection of contrast agent. Following baseline acquisition of MR T1-weighted tumor images, mice received 1 ml/kg (0.03 nmol particles/kg) of either α5β1(RGD)-targeted paramagnetic nanoparticles (n=7) or irrelevant RGS-targeted paramagnetic control (n=6). MR images were acquired dynamically at 0, 30, 60, 90, and 120 min postinjection. Anesthesia was maintained with 1.0% isoflurane in oxygen.
Experiment 2: monitoring response to fumagillin therapy by α5β1-targeted imaging
Athymic mice were implanted with 2 × 106 MDA-MB-435 cells as described previously. On day 6 postimplantation, tumor volumes were estimated by T2-weighted MRI, and animals with tumor volumes within 2 sd of the mean were randomized to 3 treatment groups. On days 7, 11, 15, and 19 postimplantation, animals were administered either ανβ3-targeted fumagillin nanoparticles (1 ml/kg, equivalent to 50 μg/kg fumagillin) (n=5), α5β1(ανβ3)-targeted fumagillin nanoparticles (n=5), or saline control (n=4) via tail-vein injection. On day 22, MR images were obtained at baseline and 120 min following injection of α5β1(ανβ3)-targeted paramagnetic nanoparticles (see Table 1).
TABLE 1.
Experimental design for animal studies
| Experiment
|
Day
|
Treatment
|
MR imaging
|
|
|---|---|---|---|---|
| Time point | Contrast agent | |||
| 1 | None | Day 14 | α5β1(RGD)-targeted paramagnetic nanoparticles (n= 7) | |
| Control irrelevant RGS-targeted paramagnetic nanoparticles (n=6) | ||||
| 2 | 7, 11, 15, 19 | ανβ3-targeted fumagillin nanoparticles (n=5) | Day 22 | α5β1(ανβ3)-targeted paramagnetic nanoparticles |
| α5β1(ανβ3)-targeted fumagillin nanoparticles (n=5) | ||||
| Saline control (n=4) | ||||
MR imaging
Image acquisition
In experiment 1, animals were imaged in a clinical 1.5 T MR scanner (Philips Gyroscan NT Intera, Philips MedicalSystems, Best, Netherlands) using a high-resolution, T1-weighted, fat-suppressed, 3D gradient echo sequence and a 47-mm coil. The imaging parameters were 55 mm field of view (FOV), 0.5-mm slice thickness, 256 × 256 matrix, TR/TE = 46/5.8 ms, 65° flip angle, and 4 signal averages. For tumor volume measurements, T2-weighted TSE scans were used with the following imaging parameters: 2.0-mm slice thickness, TR/TE = 2000/60 ms, 90° flip angle, and 2 signal averages. In experiment 2, mice were imaged in a clinical 3T MR scanner (Philips Achieva) with a SENSE-Flex-M coil again using a similar high-resolution, T1-weighted, fat-suppressed, 3D gradient echo sequence. The imaging parameters were: 100 mm FOV, 0.5-mm slice thickness, 256 × 256 matrix, TR/TE = 46/3.9 ms, 40° flip angle. A Gd-DTPA doped water standard was placed within the field of view of each image and used as a signal reference standard. T2-weighted images were obtained as above.
Image analysis
The pixel intensities from the dynamic, T1-weighted images were analyzed with MATLAB software (The MathWorks, Natick, MA, USA). The image signal intensity at each time point was normalized to the baseline image via the reference gadolinium standard. For each animal, a region-of-interest (ROI) was manually placed along the tumor edge on each baseline slice, and the sd of the average tumor signal was calculated. Subsequent, serial images were spatially coregistered using a cross-correlation routine, and the tumor ROI mask was copied to each time point. Pixels at 120 min with MR signal enhancement at least 3 sd above the baseline tumor signal were considered significant. The tumor ROI was automatically partitioned into a rim and core region using an automated method developed in MATLAB. The core region was approximately half of the tumor volume. The average signal increase, the percentage area of enhancement, and the contiguity of enhancing voxels were determined in each region.
High-resolution 3D maps were reconstructed from the 3D image data set to present the spatial distribution of neovasculature using MATLAB. The overall 3D structure of the tumor was displayed as a mesh surface plot using isosurface rendering and a smoothing filter. A surface plot of the enhancing voxels was reconstructed similarly, and overlaid in blue onto the tumor volume. These renderings were rotated in space to better appreciate the 3D distribution of neovascular enhancement.
Optical microscopy
Immunohistochemistry
After MR imaging, animals were euthanized, and tumors were resected, weighed, and quickly frozen in optimal cutting temperature (OCT) compound or fixed in formalin for immunohistochemistry. Frozen 14-day tumors from experiment 1 were sectioned (8 μm), fixed in acetone, and costained for α5β1-integrin and endothelium. Nonspecific binding of antibodies was blocked by incubation with donkey or rabbit serum. The sections were incubated overnight with rat anti-mouse CD31 monoclonal antibody (BD Pharmingen, San Jose, CA, USA), then washed and incubated with Cy3 donkey anti-rat immunoglobulin G (IgG) (Jackson ImmunoResearch, West Grove, PA, USA). Sections were then costained by incubating with monoclonal fluorescein isothiocyanate (FITC) rat anti-mouse integrin α5 subunit (anti-CD49e, clone 5H10–27; Southern Biotech, Birmingham, AL, USA), for 2 h. Microscopic images were obtained with a Nikon E800 microscope using a Nikon DXM 1200 digital camera (Nikon, Tokyo, Japan) and analyzed with Olympus MicroSuite software (Olympus America, Center Valley, PA, USA).
Frozen tumor sections from animals treated with either saline, ανβ3-targeted fumagillin, or α5β1(ανβ3)-targeted fumagillin in experiment 2 were immunostained with CD31 monoclonal antibody using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA). Five high-power fields were identified on each section, with 3 sections per tumor and 2 tumors per treatment group. Areas of necrosis were excluded. On each tissue section, the percentage area occupied by CD31-positive vessels was quantified using Image J software (National Institutes of Health, Bethesda, MD, USA).
Ex vivo microscopy of fluorescent nanoparticles and lectins
Ex vivo fluorescence microscopy was used to assess the physiological distribution of the integrin-targeted nanoparticles with respect to the vasculature. Tumor-bearing mice were anesthetized, as described above, and administered the α5β1-targeted, rhodamine-labeled nanoparticles (1 ml/kg) by tail-vein injection. After 2 h, 100 μl FITC-lycopersicon esculentum lectin (Vector Laboratories), a general stain for vascular endothelium, was slowly injected through the tail vein and allowed to circulate for 10 min. The mouse vasculature was then extensively perfused with saline to remove unbound labels. The tumor was excised, frozen in OCT, sectioned in 10- and 30-μm slices, and imaged by fluorescence microscopy.
In vivo 5-bromo-2′-deoxyuracil (BrdU) labeling of cells
In experiment 2, the effect of integrin-targeted antiangiogenic therapy on proliferation was assessed by the in vivo incorporation of the pyrimidine analog BrdU into DNA. Several hours before imaging, mice (n=2/treatment) from each group were administered 50 mg/kg BrdU. Formalin-fixed tissue slices from three different regions of each tumor were stained for BrdU using BD Pharmingen In-Situ Detection Kit. Microscopic images were manually analyzed for percentage of tumor area occupied by BrdU-positive cells.
Statistical analyses
In experiment 1, data were analyzed by ANOVA using the general linear model (GLM) procedure in SAS (SAS Institute, Cary, NC, USA). The least squares difference (LSD) method was applied for multiple comparisons where appropriate. In experiment 2, data were analyzed by the NPAR1WAY nonparametric SAS procedure that calculates simple linear rank statistics on the basis of Van der Waerden scores. Significant differences were declared at P ≤ 0.05. Values are means ± se.
RESULTS
In vitro targeting
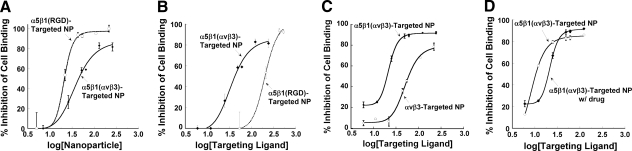
Binding of the α5β1-targeted paramagnetic nanoparticles was confirmed in vitro using competitive inhibition of HT1080 cell adhesion to fibronectin. Both α5β1-targeted nanoparticles (i.e., RGD peptide or mimetic) increasingly inhibited HT1080 cell adhesion to fibronectin-coated plates as nanoparticle concentration, and the equivalent ligand concentration, increased (Fig. 2A, B). ανβ3 -targeted nanoparticles inhibited HT1080 cell adhesion to fibronectin less than the cross-reactive α5β1(ανβ3)-targeted nanoparticles (Fig. 2C). The ανβ3-targeted nanoparticles inhibited binding of C32 cells to vitronectin, demonstrating affinity for their target integrin, as previously reported (19). The addition of fumagillin to the nanoparticle formulation did not interfere with binding activity (Fig. 2D).
Figure 2.
Bioactivity of integrin-targeted contrast agents. The binding of α5β1(RGD)-targeted nanoparticles (study 1) or peptidomimetic α5β1(ανβ3)- or (ανβ3)-targeted nanoparticles (study 2) was confirmed by a competitive cell binding assay. Preincubation of HT1080 cells, which express the α5β1-integrin, with targeted nanoparticles inhibited cell adhesion to fibronectin-coated wells, reflecting the competitive blockage of the fibronectin receptor (i.e., the α5β1-integrin) by the particles. Cell binding in the absence of nanoparticles was used to determine 100% binding. Results were analyzed by curve-fitting the dose-response data to a logistic curve fit. A) On a per-nanoparticle basis, nanoparticles prepared with the RGD peptide bound cells more effectively than those with α5β1 peptidomimetic ligand, due to the high copy number of peptide on the particle. B) As a function of ligand concentration, α5β1(ανβ3) peptidomimetic nanoparticles were more effective at inhibiting cell adhesion, which reflects the higher affinity of the mimetic. C) HT1080 cells also express the ανβ3-integrin, which is capable of binding to fibronectin. α5β1(ανβ3)-targeted nanoparticles resulted in inhibition over and above that of the ανβ3-targeted nanoparticles, which reflects their cross-reactivity with the ανβ3 integrin. D) The addition of fumagillin to the nanoparticle formulation did not interfere with binding ability. NP, nanoparticle. Error bars indicate se; n = 2–4.
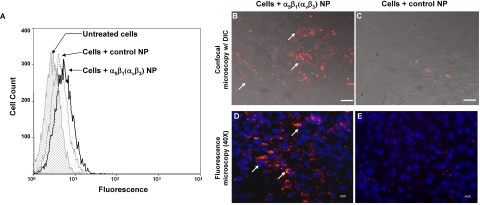
Specificity of targeted nanoparticles was further confirmed by direct visualization of fluorescent nanoparticle binding to cells by flow cytometry and with fluorescence microscopy imaging. Rhodamine-labeled α5β1(ανβ3)-targeted nanoparticles positively stained a greater percentage of 2F2B cells compared to nontargeted nanoparticles, demonstrating the specific binding of the targeted nanoparticles (Fig. 3A). Confocal imaging further confirmed strong, specific binding. There was substantial labeling of the cells with targeted nanoparticles, but negligible fluorescence was detected from the nontargeted nanoparticles (Fig. 3B, C).
Figure 3.
Flow cytometry and confocal imaging were used to assess the nanoparticle-cell binding. A) Flow cytometry analysis revealed increased binding of α5β1(ανβ3)-targeted nanoparticles (solid black line) to mouse endothelial cells expressing the α5β1-integrin compared to nontargeted nanoparticles (dotted line). B–E) Confocal micrographs (B, C) with simultaneous differential interference contrast imaging and routine fluorescence microscopy (D, E) with cell nuclei counterstained by DAPI show substantially greater binding of rhodamine-labeled α5β1(ανβ3)-targeted nanoparticles to α5β1-integrin-expressing cells (B, D) compared to control nontargeted nanoparticles (C, E). Scale bars = 5 μm.
MRI characterization of tumor angiogenesis with α5β1(RGD) nanoparticles
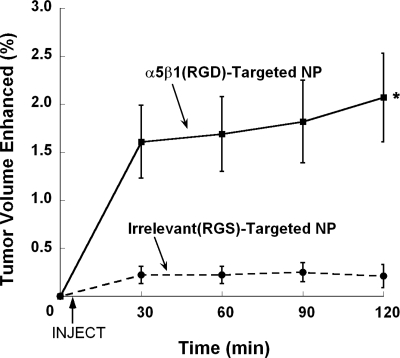
In experiment 1, athymic mice bearing MDA-MB-435 tumors were administered α5β1(RGD)-targeted or control (i.e., irrelevant RGS peptide-targeted) paramagnetic nanoparticles and imaged dynamically by MR over a 2 h period. At 2 h postinjection, α5β1-targeted paramagnetic nanoparticle contrast increased in 1.8 ± 0.4% (P<0.05) of the tumor volume by 117 ± 10% (P<0.05) in comparison with the irrelevant control, which enhanced 0.2 ± 0.1% of the tumor volume by 51 ± 11%. Area of contrast enhancement from the α5β1-targeted nanoparticle was increased (P<0.05) relative to control after 30 min and continued to rise over 2 h (Fig. 4); contrast enhancement in the control group was low and almost unchanged over the study window. In the α5β1-targeted nanoparticle group, ∼90% of the enhancing voxels were located in the rim area of the tumor, which comprised 2.0 ± 0.5% of the periphery. The remainder was scattered within the core. In the control group, less (P<0.05) rim area (0.2±0.5%) was enhanced, and no voxels exceeded the signal threshold in the core.
Figure 4.
Percentage of peripheral (rim) tumor volume with significant contrast enhancement. The number of enhanced voxels in the tumor rim, comprising the area in and around the tumor border, was calculated for targeted and nontargeted groups. The α5β1(RGD)-targeted contrast particles resulted in a significantly larger volume enhanced at each time point vs. the irrelevant RGS-targeted nanoparticles. *P < 0.05; n = 5.
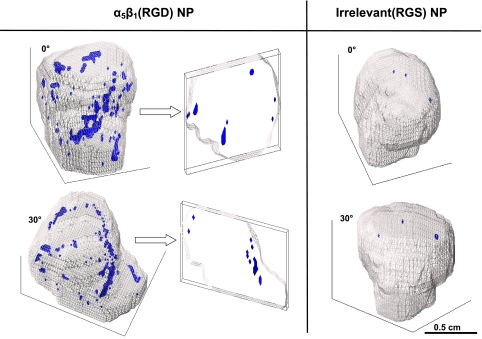
High-resolution 3D neovascular maps were created, which depicted the asymmetric distribution of α5β1-integrin neovascular expression (Fig. 5). These maps showed the sparse, predominantly peripheral distribution of angiogenesis, which comprised a few high-density voxel regions with a surrounding speckled, reticular network of enhanced pixels. Negligible coherent contrast enhancement was detected in the tumor interior, and large regions of the tumor surface were devoid of signal enhancement. Clusters of up to 100 adjacent, enhanced voxels (1.39 mm3) were observed in the α5β1-targeted tumors, whereas enhanced voxels in control tumors were generally noncontiguous or in small clusters, of which the largest grouping comprised 5 voxels (0.13 mm3).
Figure 5.
3D reconstructions of MR signal enhancement reveal tumor neovascular morphology. The tumor volume is outlined in gray, and voxels meeting the enhancement threshold at 2 h postinjection of contrast agent are shown in blue. Left panel: two rotated views of an α5β1(RGD)-targeted tumor. The cross sections at right demonstrate the paucity of angiogenesis in the core. Right panel: minimal enhancement associated with irrelevant RGS-targeted contrast agent.
Microscopic examination of α5β1-integrin expression
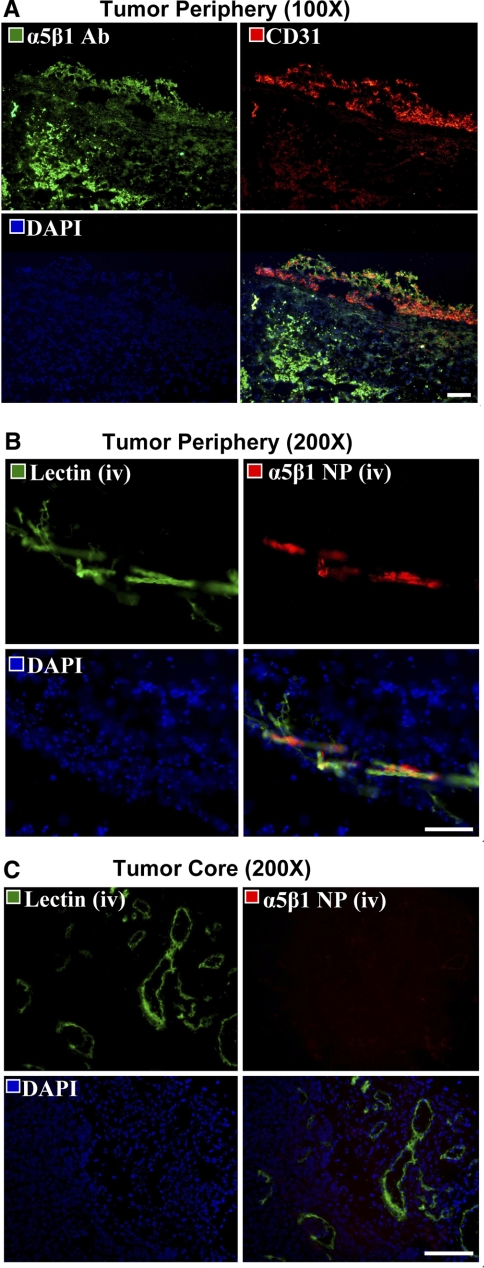
Routine immunohistological staining of tumors revealed the prevalence of α5β1-integrin expression within both vascular and extravascular tissues (Fig. 6A). In contrast, α5β1-integrin-targeted rhodamine nanoparticles were spatially distributed in the tumor periphery, constrained to the vasculature, and colocalized with endothelial FITC-lectin contrast. Within the tumor core, prominent fluorescent signal from microvessel bound lectin but not the α5β1-integrin-targeted rhodamine nanoparticles was observed (Fig. 6B, C). The pattern of α5β1-targeted paramagnetic nanoparticle enhancement observed in the reconstructed images paralleled these microscopic observations. Although the α5β1-integrin is expressed by extravascular cells within the tumor (34), the distribution of α5β1-integrin-targeted nanoparticles was conspicuously constrained within the peripheral microvasculature and notably absent from larger, more established vessels within the rim and core of the tumors.
Figure 6.
Histological examination of α5β1-integrin expression and nanoparticle localization. A) Fluorescent microscopy images (×200) of conventional immunohistochemistry of 8-μm sections of tumor border stained for α5β1-integrin (green) and endothelium (red), with nuclei counterstained blue. There is prevalent expression of α5β1-integrin throughout the tissue section, both within and outside of the vasculature. B, C) Images of 30-μm tumor sections from mice intravenously injected with nanoparticles (NP, red) and lectin (green). B) The merged images confirm that the nanoparticles colocalized with the angiogenic vasculature, and did not reach the α5β1-integrin expressed by tumor and other cells in the extravascular matrix. C) Nanoparticles are absent from the mature vasculature in the tumor core, consistent with MR findings. Scale bars = 200 μm.
Assessment of antiangiogenic response to integrin-targeted fumagillin
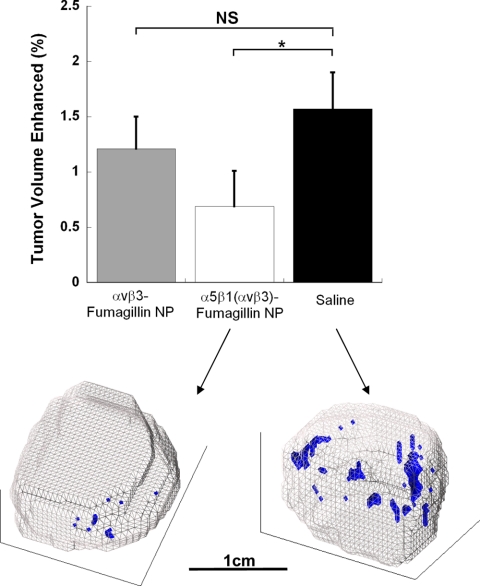
MR molecular imaging with α5β1(ανβ3)-targeted paramagnetic nanoparticles was used to assess the response to fumagillin nanoparticles homed with either the dual α5β1(ανβ3)-peptidomimetic or ανβ3-peptidomimetic alone. α5β1(ανβ3)-targeted fumagillin nanoparticles elicited a greater decrease (P<0.05) in tumor neovasculature relative to the saline control than the ανβ3-targeted fumagillin nanoparticles (Fig. 7A). High-resolution 3D neovascular mapping illustrated that the decrease in angiogenesis by treatment with α5β1(ανβ3)-targeted fumagillin nanoparticles manifested morphologically as smaller, contracted islands of neovasculature and diminished appearance of speckled angiogenic signal (Fig. 7B). This effect was validated by histological analysis of the decrease in angiogenesis. The percentage area of CD31-positive vessels in tumor sections from the α5β1(ανβ3)-targeted fumagillin group was less than half that of the saline group (1.8 vs. 4.1% of tissue area). Although α5β1(ανβ3)-targeted fumagillin nanoparticles decreased the already sparse neovasculature of the MDA-MB-435 xenograft to negligible levels, no differences in tumor volume were appreciated between the control (202±55 mm3) and fumagillin-treated mice (199±71 mm3). Similarly, no difference in tumor proliferation, as assessed microscopically by BrdU staining, was noted between the saline (40.1±8.5%) and α5β1(ανβ3)-targeted fumagillin treatment (44.6±5.9%) group.
Figure 7.
Assessment of the antiangiogenic response to integrin-targeted fumagillin nanoparticles with α5β1(ανβ3)-targeted MR contrast agent. Top: the extent of neovascularity was quantified by calculating the amount of signal enhancement in the tumor periphery. α5β1(ανβ3)-targeted fumagillin nanoparticles reduced peripheral tumor neovascularity relative to control (P<0.05, n=5). ανβ3-targeted fumagillin nanoparticles had no significant effect on angiogenesis, compared to control. Bottom: the effect of α5β1(ανβ3)-targeted fumagillin nanoparticles on tumor neovascular morphology is clearly apparent on 3D reconstructions of MR signal enhancement. Tumor volume is outlined in gray; contrast-enhanced pixels are blue.
DISCUSSION
In this study, we have demonstrated the use of a new paramagnetic nanoparticle formulation targeted to α5β1-integrin positive neovasculature for both delivering antiangiogenic therapy and detecting the tumor response. In addition, unique 3D reconstructions of the MR signal enhancement revealed the spatial organization of neovessels, which predominantly were confined to the tumor periphery, and consisted of merged regions of neovasculature interspersed with a sparser, reticular pattern of angiogenic positive voxels. This technique allowed for real-time, comprehensive analysis of the state of the tumor neovasculature following antiangiogenic therapy.
α5β1(ανβ3)-targeted nanoparticles containing fumagillin decreased the amount of enhancement in the peripheral tumor volume to an almost negligible level when characterized at 22 days with α5β1(ανβ3)-targeted paramagnetic nanoparticles. ανβ3-fumagillin-nanoparticles followed this trend but did not significantly decrease angiogenesis relative to control. The differential angiogenic response suggests that the cross-reactive α5β1(ανβ3)-ligand reaches a subset of neovessels that are not targeted with ανβ3 alone and that the dual targeting of both integrins increased delivery of fumagillin to cells, which improved antiangiogenic efficacy.
Neovascularity in this study associated with α5β1(ανβ3)-expression is markedly different from results observed in a previous rabbit Vx2 tumor model study (24). In that experiment, Vx2 tumor-bearing rabbits were treated on days 6, 9, and 12 postimplantation with ανβ3-targeted fumagillin nanoparticles (30 μg/kg), ανβ3-targeted nanoparticles without drug, nontargeted fumagillin nanoparticles (30 μg/kg), or saline. On day 16, MR molecular imaging with ανβ3-targeted paramagnetic nanoparticles revealed a predominant peripheral distribution of neovascularity representing 7.2% of the tumor rim volume of control rabbits (no fumagillin), which decreased to 2.8% (P<0.05) with ανβ3-targeted fumagillin nanoparticle treatment. Tumor volume was reduced among rabbits receiving ανβ3-targeted fumagillin nanoparticles (470 ±120 mm3) compared with the three control groups (ave. 1143±166 mm3).
The effectiveness of targeted antiangiogenic therapies logically requires adequate expression of the neovascular biomarker and a dependence of the tumor on the neovasculature for rapid development. In contradistinction to Vx2 tumor model, the MDA-MB-435 tumor model had a very sparse neovasculature, and reduction of angiogenesis to negligible levels elicited no change in tumor volume. As opposed to the syngeneic Vx2 tumor, the MDA-MB-435 xenograft tumor was relatively slow growing and did not display an “angiogenic switch” phenotype associated with accelerated growth.
From the clinical perspective, angiogenic characterization of small tumors, particularly breast, lung, or colon, may better stratify patients into medical strategies involving anti-VEGF treatment in combination with chemo or radiation therapy. The presence of neovasculature at some threshold, could justify the risk and cost of these treatments for a likely benefit; whereas, in patients without the neovascular target a different course of treatment would potentially have greater benefit. Although both current PET/CT and DCE-MR imaging techniques may help follow anticancer treatment response, neither approach has been shown to segment patient populations based on angiogenesis.
Histological examination of the distribution of nanoparticles and lectin-stained vasculature confirmed that the α5β1(ανβ3)-PFC nanoparticles (∼250 nm) were constrained by size to endothelial epitopes accessible from the blood pool (23, 24, 35). This is consistent with previous studies using ανβ3 -targeted particles, and with observations of other similar-sized nanoparticles (36, 37). Numerous radiolabeled integrin antagonists peptides and peptidomimetics have been explored as tumor vasculature targeting imaging agents, but these freely penetrate into the tumor, where binding to macrophages, platelets, lymphocytes, smooth muscle cells, and tumor cells confounds the source of signal enhancement.
Fumagillin, a mycotoxin produced by Aspergillus fumigatus, is an angiogenesis inhibitor that exerts its effect by binding to methionine aminopeptidase 2 (MetAP2), an intracellular enzyme necessary for protein myristolation, thus preventing translocation of membrane proteins to the cell surface. The antitumor effectiveness of TNP-470, a water-soluble analog of fumagillin, has been well demonstrated in animal models at serial dosages of 30 mg/kg (38,39,40,41), and clinical anecdotal data suggest potential efficacy with serial doses of 100 mg/m2 in the treatment of cancer but with significant, transient neurocognitive side effects (42,43,44,45). So a reduced dosage that still retains efficacy is required for clinical translation. In experiment 2, integrin-targeted nanoparticles were used to deliver fumagillin at a dosage 1000-fold less than that used for systemic TNP-470 treatment clinically, which still maintains antiangiogenic efficacy (24). This nanomedicine approach should have fewer side effects than previous oral delivery methods.
The biodistribution and pharmacokinetics of integrin-targeted nanoparticles have been previously studied and reported, which show that the majority of the nanoparticle dose is cleared by the reticuloendothelial system, particularly, the liver and spleen (35). Before the last imaging session (day 22) in experiment 2, blood was drawn to assess treatment effects on electrolytes, liver function, and hematology, and all measures were within normal range regardless of study group. Alternative approaches to antineovascular therapy have been considered by others and include Abegrin, a humanized anti-ανβ3-integrin monoclonal antibody conjugated 64Cu-DOTA (46), and beta-emitting nuclides conjugated to peptides against ovarian cancer or incorporated into integrin-targeted liposomes (47, 48). These have found limited clinical success due to minimal efficacy and radioactive dose-limiting toxicity. RGD-targeted liposomal doxorubicin has been shown to be somewhat more effective than the nontargeted agent (49), but the improved potency is not strictly antiangiogenic, and the overall toxicity profile is unchanged.
Whether the neovascular response observed in the MDA-MB-435 adenocarcinoma model is unique or general for xenograft models should be considered further. The magnitude of angiogenesis and peripheral distribution of neovasculature presented in the 3D neovascular maps in this study are markedly different with the estimates based on summed 2D slices by other investigators using RGD-targeted paramagnetic liposomes (21). Whether these differences relate to the animal model variability or the pharmacokinetics/pharmacodynamics of the liposome contrast effects vs. the paramagnetic PFC nanoparticles is unclear.
In the present study, we used the α5β1(ανβ3)-peptidomimetic paramagnetic nanoparticles to assess neovascular response to α5β1(ανβ3)-targeted or ανβ3-targeted fumagillin treatment to reduce animal number. However, we could not resolve whether the improved response observed in the α5β1(ανβ3)-targeted treatment group resulted from targeting a broader distribution of endothelial cells or from increased drug dosage to cells afforded by the dual integrin targeting. Because only 4 or 5 animals per treatment group were used, it is likely that the study may not be powered enough to detect the antiangiogenesis effectiveness of the ανβ3-targeted therapy.
The RGD peptide was used in experiment 1 due to the fact that the peptidomimetic was not yet available. Even with this lower affinity peptide, by incorporating a high copy number onto the nanoparticles, we were able to demonstrate α5β1-targeted imaging of tumor angiogenesis. In experiment 2, peptidomimetic targeting ligands became available and were utilized to characterize tumor angiogenesis and its response to targeted fumagillin therapy. Because of the markedly higher affinity for the α5β1-integrin than the RGD peptide of experiment 1, the proof of concept study of experiment 1 was not repeated. The patterns and density of peripheral angiogenesis assessed as with α5β1(ανβ3)-targeted nanoparticles in the saline control group did not differ appreciably from the earlier results obtained with the α5β1(RGD) nanoparticles, which reflects the compensatory benefit of increased particle avidity achieved by the incorporation of thousands of ligands per nanoparticle (Fig. 2A). However, a major advantage of the peptidomimetic was its resilience to chemical processing and heat sterilization, which would afford the requisite chemical synthesis control for GMP process scale-up and clinical translation.
CONCLUSIONS
Angiogenesis associated with the expression of α5β1-integrin or ανβ3-integrin in MDA-MB-435 tumors was sparse whether MR molecular imaging was guided with RGD -peptide or peptidomimetic ligands. 3D neovascular mapping of tumor neovascularity demonstrated that 90% of the new vessel signal was peripherally distributed, asymmetric, and generally sparse, with no neovessel signal appreciated over large portions of the tumor surface. Immunohistochemistry revealed that α5β1-integrin expression was expressed abundantly by cells in the extravascular tumor matrix, but targeted rhodamine-labeled nanoparticles were bound and colocalized with FITC-lectin in the peripheral microvasculature only and not in the tumor core. α5β1(ανβ3)-targeted fumagillin nanoparticles decreased the sparse neovascularity more than ανβ3-targeted nanoparticle counterpart relative to control. In contradistinction to previous reports in the Vx2 tumor model, the MDA-MB-435 tumor model had a sparse neovasculature and no growth response to antiangiogenic therapy despite a further reduction in peripheral neovessels. The xenograft tumor was relatively slow growing and did not display an aggressive angiogenic switch phenotype associated with accelerated growth. These results suggest that characterization of angiogenesis with MR molecular imaging may identify tumors with low levels of neovasculature that may respond poorly to antiangiogenic therapy.
Acknowledgments
We thank Kereos, Inc. for the gift of the peptidomimetic integrin antagonists, Grace Hu for assistance with formulation chemistry, and John S. Allen and Cordelia Caradine for support of the animal studies. A.H.S., T.W., H.Z., S.A.W., and G.L. are supported by the National Cancer Institute; National Heart, Lung, and Blood Institute; National Institute of Biomedical Imaging and BioEngineering (HL-78631, HL-73646, and CA-119342); and Philips Healthcare. S.A.W. and G.M.L. are scientific cofounders of Kereos, Inc. S.D.C. is employed by Philips Healthcare.
References
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- Zetter B R. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407–424. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Izzedine H, Rixe O, Billemont B, Baumelou A, Deray G. Angiogenesis inhibitor therapies: focus on kidney toxicity and hypertension. Am J Kidney Dis. 2007;50:203–218. doi: 10.1053/j.ajkd.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Giles F J, Bellamy W T, Estrov Z, O'Brien S M, Verstovsek S, Ravandi F, Beran M, Bycott P, Pithavala Y, Steinfeldt H, Reich S D, List A F, Yee K W. The anti-angiogenesis agent, AG-013736, has minimal activity in elderly patients with poor prognosis acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) Leuk Res. 2006;30:801–811. doi: 10.1016/j.leukres.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Zakarija A, Soff G. Update on angiogenesis inhibitors. Curr Opin Oncol. 2005;17:578–583. doi: 10.1097/01.cco.0000183672.15133.ab. [DOI] [PubMed] [Google Scholar]
- Eremina V, Jefferson J A, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp J B, Kabir M G, Backx P H, Gerber H P, Ferrara N, Barisoni L, Alpers C E, Quaggin S E. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Park R, Tohme M, Shahinian A H, Bading J R, Conti P S. MicroPET and autoradiographic imaging of breast cancer alpha v-integrin expression using 18F- and 64Cu-labeled RGD peptide. Bioconjug Chem. 2004;15:41–49. doi: 10.1021/bc0300403. [DOI] [PubMed] [Google Scholar]
- Liu S, Hsieh W Y, Jiang Y, Kim Y S, Sreerama S G, Chen X, Jia B, Wang F. Evaluation of a (99m)Tc-labeled cyclic RGD tetramer for noninvasive imaging integrin alpha (v)beta3-positive breast cancer. Bioconjug Chem. 2007;18:438–446. doi: 10.1021/bc0603081. [DOI] [PubMed] [Google Scholar]
- Zitzmann S, Ehemann V, Schwab M. Arginine-glycine-aspartic acid (RGD)-peptide binds to both tumor and tumor-endothelial cells in vivo. Cancer Res. 2002;62:5139–5143. [PubMed] [Google Scholar]
- Wilmes L J, Pallavicini M G, Fleming L M, Gibbs J, Wang D, Li K L, Partridge S C, Henry R G, Shalinsky D R, Hu-Lowe D, Park J W, McShane T M, Lu Y, Brasch R C, Hylton N M. AG-013736, a novel inhibitor of VEGF receptor tyrosine kinases, inhibits breast cancer growth and decreases vascular permeability as detected by dynamic contrast-enhanced magnetic resonance imaging. Magn Reson Imaging. 2007;25:319–327. doi: 10.1016/j.mri.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Liu G, Rugo H S, Wilding G, McShane T M, Evelhoch J L, Ng C, Jackson E, Kelcz F, Yeh B M, Lee F T, Jr, Charnsangavej C, Park J W, Ashton E A, Steinfeldt H M, Pithavala Y K, Reich S D, Herbst R S. Dynamic contrast-enhanced magnetic resonance imaging as a pharmacodynamic measure of response after acute dosing of AG-013736, an oral angiogenesis inhibitor, in patients with advanced solid tumors: results from a phase I study. J Clin Oncol. 2005;23:5464–5473. doi: 10.1200/JCO.2005.04.143. [DOI] [PubMed] [Google Scholar]
- Jayson G C, Zweit J, Jackson A, Mulatero C, Julyan P, Ranson M, Broughton L, Wagstaff J, Hakannson L, Groenewegen G, Bailey J, Smith N, Hastings D, Lawrance J, Haroon H, Ward T, McGown A T, Tang M, Levitt D, Marreaud S, Lehmann F F, Herold M, Zwierzina H. Molecular imaging and biological evaluation of HuMV833 anti-VEGF antibody: implications for trial design of antiangiogenic antibodies. J Natl Cancer Inst. 2002;94:1484–1493. doi: 10.1093/jnci/94.19.1484. [DOI] [PubMed] [Google Scholar]
- Leach M O, Brindle K M, Evelhoch J L, Griffiths J R, Horsman M R, Jackson A, Jayson G C, Judson I R, Knopp M V, Maxwell R J, McIntyre D, Padhani A R, Price P, Rathbone R, Rustin G J, Tofts P S, Tozer G M, Vennart W, Waterton J C, Williams S R, Workman P. The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: issues and recommendations. Br J Cancer. 2005;92:1599–1610. doi: 10.1038/sj.bjc.6602550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylton N. Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J Clin Oncol. 2006;24:3293–3298. doi: 10.1200/JCO.2006.06.8080. [DOI] [PubMed] [Google Scholar]
- Flacke S, Fischer S, Scott M J, Fuhrhop R J, Allen J S, McLean M, Winter P, Sicard G A, Gaffney P J, Wickline S A, Lanza G M. Novel MRI contrast agent for molecular imaging of fibrin: implications for detecting vulnerable plaques. Circulation. 2001;104:1280–1285. doi: 10.1161/hc3601.094303. [DOI] [PubMed] [Google Scholar]
- Winter P M, Caruthers S D, Kassner A, Harris T D, Chinen L K, Allen J S, Lacy E K, Zhang H, Robertson J D, Wickline S A, Lanza G M. Molecular imaging of angiogenesis in nascent Vx-2 rabbit tumors using a novel alphavbeta3-targeted nanoparticle and 1.5 tesla magnetic resonance imaging. Cancer Res. 2003;63:5838–5843. [PubMed] [Google Scholar]
- Schmieder A H, Winter P M, Caruthers S D, Harris T D, Williams T A, Allen J S, Lacy E K, Zhang H, Scott M J, Hu G, Robertson J D, Wickline S A, Lanza G M. Molecular MR imaging of melanoma angiogenesis with alphavbeta3-targeted paramagnetic nanoparticles. Magn Reson Med. 2005;53:621–627. doi: 10.1002/mrm.20391. [DOI] [PubMed] [Google Scholar]
- Sipkins D A, Cheresh D A, Kazemi M R, Nevin L M, Bednarski M D, Li K C. Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat Med. 1998;4:623–626. doi: 10.1038/nm0598-623. [DOI] [PubMed] [Google Scholar]
- Mulder W J, Strijkers G J, Habets J W, Bleeker E J, van der Schaft D W, Storm G, Koning G A, Griffioen A W, Nicolay K. MR molecular imaging and fluorescence microscopy for identification of activated tumor endothelium using a bimodal lipidic nanoparticle. FASEB J. 2005;19:2008–2010. doi: 10.1096/fj.05-4145fje. [DOI] [PubMed] [Google Scholar]
- Frias J C, Williams K J, Fisher E A, Fayad Z A. Recombinant HDL-like nanoparticles: a specific contrast agent for MRI of atherosclerotic plaques. J Am Chem Soc. 2004;126:16316–16317. doi: 10.1021/ja044911a. [DOI] [PubMed] [Google Scholar]
- Winter P M, Neubauer A M, Caruthers S D, Harris T D, Robertson J D, Williams T A, Schmieder A H, Hu G, Allen J S, Lacy E K, Zhang H, Wickline S A, Lanza G M. Endothelial alpha (v) beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2103–2109. doi: 10.1161/01.ATV.0000235724.11299.76. [DOI] [PubMed] [Google Scholar]
- 24.Winter, P. M., Schmieder, A. H., Caruthers, S. D., Keene, J. L., Zhang, H., Wickline, S. A., and Lanza, G. M. (2008) Minute dosages of alphavbeta3-targeted fumagillin nanoparticles impair Vx-2 tumor angiogenesis and development in rabbits. [E-pub ahead of print] FASEB J. 10.1096/fj.07-103929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Bell K, Mousa S A, Varner J A. Regulation of angiogenesis in vivo by ligation of integrin alpha5beta1 with the central cell-binding domain of fibronectin. Am J Pathol. 2000;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau N J, Varner J A. The homeobox transcription factor Hox D3 promotes integrin α5β1 expression and function during angiogenesis. J Biol Chem. 2004;279:4862–4868. doi: 10.1074/jbc.M305190200. [DOI] [PubMed] [Google Scholar]
- Bayless K J, Salazar R, Davis G E. RGD-dependent vacuolation and lumen formation observed during endothelial cell morphogenesis in three-dimensional fibrin matrices involves the alpha (v) beta(3) and alpha(5)beta(1) integrins. Am J Pathol. 2000;156:1673–1683. doi: 10.1016/s0002-9440(10)65038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivunen E, Wang B, Ruoslahti E. Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD-directed integrins. Bio/Technology (N Y) 1995;13:265–270. doi: 10.1038/nbt0395-265. [DOI] [PubMed] [Google Scholar]
- Humphries J D, Askari J A, Zhang X P, Takada Y, Humphries M J, Mould A P. Molecular basis of ligand recognition by integrin α5β 1. II. Specificity of arg-gly-Asp binding is determined by Trp157 of the α subunit. J Biol Chem. 2000;275:20337–20345. doi: 10.1074/jbc.M000568200. [DOI] [PubMed] [Google Scholar]
- Cue D, Southern S O, Southern P J, Prabhakar J, Lorelli W, Smallheer J M, Mousa S A, Cleary P P. A nonpeptide integrin antagonist can inhibit epithelial cell ingestion of Streptococcus pyogenes by blocking formation of integrin α 5β 1-fibronectin-M1 protein complexes. Proc Natl Acad Sci U S A. 2000;97:2858–2863. doi: 10.1073/pnas.050587897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T D, Kalogeropoulos S, Nguyen T, Liu S, Bartis J, Ellars C, Edwards S, Onthank D, Silva P, Yalamanchili P, Robinson S, Lazewatsky J, Barrett J, Bozarth J. Design, synthesis, and evaluation of radiolabeled integrin α v β 3 receptor antagonists for tumor imaging and radiotherapy. Cancer Biother Radiopharm. 2003;18:627–641. doi: 10.1089/108497803322287727. [DOI] [PubMed] [Google Scholar]
- Meoli D F, Sadeghi M M, Krassilnikova S, Bourke B N, Giordano F J, Dione D P, Su H, Edwards D S, Liu S, Harris T D, Madri J A, Zaret B L, Sinusas A J. Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J Clin Invest. 2004;113:1684–1691. doi: 10.1172/JCI20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi M M, Krassilnikova S, Zhang J, Gharaei A A, Fassaei H R, Esmailzadeh L, Kooshkabadi A, Edwards S, Yalamanchili P, Harris T D, Sinusas A J, Zaret B L, Bender J R. Detection of injury-induced vascular remodeling by targeting activated αvβ3 integrin in vivo. Circulation. 2004;110:84–90. doi: 10.1161/01.CIR.0000133319.84326.70. [DOI] [PubMed] [Google Scholar]
- Parsons-Wingerter P, Kasman I M, Norberg S, Magnussen A, Zanivan S, Rissone A, Baluk P, Favre C J, Jeffry U, Murray R, McDonald D M. Uniform overexpression and rapid accessibility of α5β1 integrin on blood vessels in tumors. Am J Pathol. 2005;167:193–211. doi: 10.1016/s0002-9440(10)62965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Lijowski M, Zhang H, Partlow K C, Caruthers S D, Kiefer G, Gulyas G, Athey P, Scott M J, Wickline S A, Lanza G M. Imaging of Vx-2 rabbit tumors with α(nu)β3-integrin-targeted 111In nanoparticles. Int J Cancer. 2007;120:1951–1957. doi: 10.1002/ijc.22581. [DOI] [PubMed] [Google Scholar]
- Kong G, Braun R D, Dewhirst M W. Hyperthermia enables tumor-specific nanoparticle delivery: effect of particle size. Cancer Res. 2000;60:4440–4445. [PubMed] [Google Scholar]
- Weissleder R, Bogdanov A, Jr, Tung C H, Weinmann H J. Size optimization of synthetic graft copolymers for in vivo angiogenesis imaging. Bioconjug Chem. 2001;12:213–219. doi: 10.1021/bc000091p. [DOI] [PubMed] [Google Scholar]
- Bergers G, Javaherian K, Lo K M, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;284:808–812. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- Castronovo V, Belotti D. TNP-470 (AGM-1470): mechanisms of action and early clinical development. Eur J Cancer. 1996;32A:2520–2527. doi: 10.1016/s0959-8049(96)00388-7. [DOI] [PubMed] [Google Scholar]
- Konno H, Tanaka T, Kanai T, Maruyama K, Nakamura S, Baba S. Efficacy of an angiogenesis inhibitor, TNP-470, in xenotransplanted human colorectal cancer with high metastatic potential. Cancer. 1996;77:1736–1740. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1736::AID-CNCR48>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Shusterman S, Grupp S A, Barr R, Carpentieri D, Zhao H, Maris J M. The angiogenesis inhibitor tnp-470 effectively inhibits human neuroblastoma xenograft growth, especially in the setting of subclinical disease. Clin Cancer Res. 2001;7:977–984. [PubMed] [Google Scholar]
- Bhargava P, Marshall J L, Rizvi N, Dahut W, Yoe J, Figuera M, Phipps K, Ong V S, Kato A, Hawkins M J. A Phase I and pharmacokinetic study of TNP-470 administered weekly to patients with advanced cancer. Clin Cancer Res. 1999;5:1989–1995. [PubMed] [Google Scholar]
- Kudelka A P, Verschraegen C F, Loyer E. Complete remission of metastatic cervical cancer with the angiogenesis inhibitor TNP-470. N Engl J Med. 1998;338:991–992. doi: 10.1056/NEJM199804023381412. [DOI] [PubMed] [Google Scholar]
- Logothetis C J, Wu K K, Finn L D, Daliani D, Figg W, Ghaddar H, Gutterman J U. Phase I trial of the angiogenesis inhibitor TNP-470 for progressive androgen-independent prostate cancer. Clin Cancer Res. 2001;7:1198–1203. [PubMed] [Google Scholar]
- Offodile R, Walton T, Lee M, Stiles A, Nguyen M. Regression of metastatic breast cancer in a patient treated with the anti-angiogenic drug TNP-470. Tumori. 1999;85:51–53. doi: 10.1177/030089169908500111. [DOI] [PubMed] [Google Scholar]
- Cai W, Wu Y, Chen K, Cao Q, Tice D A, Chen X. In vitro and in vivo characterization of 64Cu-labeled Abegrin, a humanized monoclonal antibody against integrin alpha v beta 3. Cancer Res. 2006;66:9673–9681. doi: 10.1158/0008-5472.CAN-06-1480. [DOI] [PubMed] [Google Scholar]
- Janssen M, Frielink C, Dijkgraaf I, Oyen W, Edwards D S, Liu S, Rajopadhye M, Massuger L, Corstens F, Boerman O. Improved tumor targeting of radiolabeled RGD peptides using rapid dose fractionation. Cancer Biother Radiopharm. 2004;19:399–404. doi: 10.1089/cbr.2004.19.399. [DOI] [PubMed] [Google Scholar]
- Li L, Wartchow C A, Danthi S N, Shen Z, Dechene N, Pease J, Choi H S, Doede T, Chu P, Ning S, Lee D Y, Bednarski M D, Knox S J. A novel antiangiogenesis therapy using an integrin antagonist or anti-Flk-1 antibody coated 90Y-labeled nanoparticles. Int J Radiat Oncol Biol Phys. 2004;58:1215–1227. doi: 10.1016/j.ijrobp.2003.10.057. [DOI] [PubMed] [Google Scholar]
- Holig P, Bach M, Volkel T, Nahde T, Hoffmann S, Muller R, Kontermann R E. Novel RGD lipopeptides for the targeting of liposomes to integrin-expressing endothelial and melanoma cells. Protein Eng Des Sel. 2004;17:433–441. doi: 10.1093/protein/gzh055. [DOI] [PubMed] [Google Scholar]