Abstract
The molecular and structural basis of anesthetic interactions with conformations and functionalities of cell surface receptors remains to be elucidated. We have demonstrated that the widely used volatile anesthetic isoflurane blocks the activation-dependent conformational conversion of integrin lymphocyte function associated antigen-1 (LFA-1), the major leukocyte cell adhesion molecule, to a high-affinity configuration. Perturbation of LFA-1 activation by isoflurane at clinically relevant concentrations leads to the inhibition of T-cell interactions with target cells as well as ligand-triggered intracellular signaling. Nuclear magnetic resonance spectroscopy reveals that isoflurane binds within a cavity in the LFA-1 ligand-binding domain, which is a previously identified drug-binding site for allosteric small-molecule antagonists that stabilize LFA-1 in a low-affinity conformation. These results provide a potential mechanism for the immunomodulatory properties of isoflurane.—Yuki, K., Astrof, N. S., Bracken, C., Yoo, R., Silkworth, W., Soriano, S. G., Shimaoka, M. The volatile anesthetic isoflurane perturbs conformational activation of integrin LFA-1 by binding to the allosteric regulatory cavity.
Keywords: NMR, structure, small-molecule antagonist, leukocyte, cell adhesion
Volatile anesthetics (VAs), which primarily target ligand-gated (i.e., GABA) channels in the central nervous system (CNS), have also displayed pleiotropic effects outside the CNS, such as immune modulation (1). For example, isoflurane, a widely used and clinically applied VA, has been shown to perturb leukocyte-endothelial cell interactions (2,3,4) as well as suppress leukocyte accumulation at sites of inflammation (5, 6). Notably, in the study using murine septic peritonitis, isoflurane treatment inhibits the tissue infiltration of leukocytes, subsequently improving survival (7). Despite potential clinical relevance, however, the mechanisms by which isoflurane, a small hydrophobic halogenated ether, exerts such exquisite control over the biological activities of key molecules regulating immune cells remain to be elucidated. One potential mechanism is the inhibition of functionally obligate conformational dynamics via the binding of anesthetic to protein cavities. This phenomenon has been observed in model systems involving either apomyoglobin (8) or a specially constructed 4-helical-bundle protein (9). In this study, we investigate how isoflurane affects the conformational dynamics of integrin lymphocyte function-associated antigen-1 (LFA-1), the major leukocyte adhesion molecule that mediates cell-cell interactions critical to immune responses and inflammation (10).
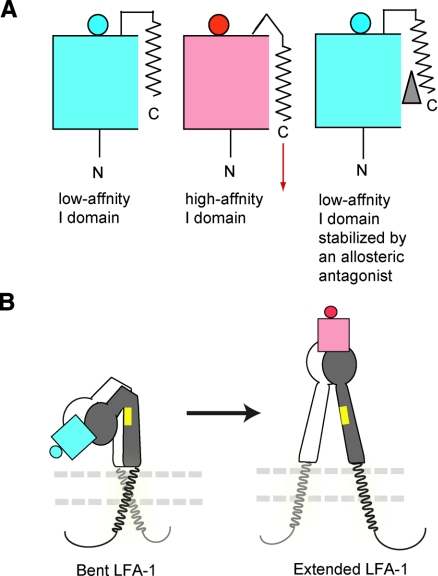
LFA-1 activity is dynamically up-regulated via activation-dependent global conformational changes linked to the conversion of the ligand-binding inserted (I) domain to the high-affinity form (10). On activation, the C-terminal helix of the I domain moves down, triggering conversion to a high-affinity configuration of the ligand-binding site at the top of the domain (Fig. 1A). Several small-molecule antagonists to LFA-1, known as α I allosteric antagonists, have been developed (10) such as the hydrophobic small-molecule derivatives of lovastatin (e.g., LFA703) (10,11,12). These can bind to the cavity beneath the C-terminal helix (the cavity termed “lovastatin site,” ref. 11) and allosterically stabilize the inactive conformation of the I domain (10) (Fig. 1A). Isoflurane’s propensity to bind to protein cavities leads us to hypothesize that, like α I allosteric antagonists, isoflurane would bind to the I domain cavity, specifically the lovastatin site, and perturb the functional dynamics needed for conversion to the active conformation. Using the engineered LFA-1 mutant that distinguishes the modes of inhibition (i.e., allosteric vs. competitive) as well as nuclear magnetic resonance (NMR) spectroscopy that maps the drug binding site, we have demonstrated that isoflurane directly binds to the allosteric pocket in LFA-1 and interferes with conformational conversion to the active form.
Figure 1.
A model for LFA-1 activation. A) Graphic drawings showing the conversion of the LFA-1 I domain from the low-affinity (left) to the high-affinity (middle) conformation, which is triggered by a downward shift of the C-terminal helix (shown as an arrow). A small-molecule allosteric LFA-1 antagonist such as lovastatin (gray triangle) binds underneath the C-terminal helix and perturbs the conversion to the high-affinity conformation (right). The body of the I domain is shown as a box, the ligand binding site at the top of the domain as a circle, and the C-terminal helix as a zigzag line. Inactive and active states are shown in blue and red, respectively. B) Global conformational changes of LFA-1 from the bent form (left, in the absence of activation) to the extended form (right, in the presence of activation). The extracellular domains are shown as graphic drawings: low-affinity I domain (blue), high-affinity I domain (red), α-subunit not containing the I domain (white), and β-subunit (gray). The plasma membrane is shown by two parallel dashed lines. Transmembrane and cytoplasmic domains for α and β subunits are shown in gray and back, respectively. The position of the KIM127 activation-dependent epitope is shown in yellow. Note that the KIM127 epitope was accessible only in the extended LFA-1.
MATERIALS AND METHODS
Cells
K562 transfectants expressing wild-type (WT) LFA-1 or mutant LFA-1 (K287C/K294C) and Jurkat cells were used as described previously (13). Peripheral blood mononuclear cells (PBMCs) were obtained from healthy volunteers using Ficoll-gradient sedimentation.
Isoflurane solution
Isoflurane was obtained from Baxter (Deerfield, IL, USA). Saturated isoflurane solution was prepared by adding excess drug to a sealed bottle containing buffer solution and then stirring overnight. The concentration of this saturated solution (15.3 mM) had been previously determined (14). This saturated solution was diluted using a gas-tight tube to yield the desired anesthetic concentration as described below.
Binding of soluble intercellular adhesion molecule 1 (ICAM-1) to LFA-1 on the cell surface
Flow cytometry to detect the binding of soluble ICAM-1-fragment crystallizable α (Fcα)/anti-immunoglobulin A-fluorescein isothiocyanate (IgA-FITC) to LFA-1 on K562 transfectants or PBMCs was performed as described previously (15) with minor modifications. Cells were harvested and washed once with HEPES-buffered saline (HBS) containing 10 mM EDTA and 3 times with HBS, and then resuspended in HBS. Cells (5×105) in 300 μl HBS were aliquoted to polymerase chain reaction (PCR) tubes (Axygen, Union City, CA, USA) and then centrifuged. Cell pellets were given a 150 μl aliquot of HBS, 2 mM MnCl2 containing isoflurane at 2× final concentration, and another 150 μl aliquot of HBS containing 10 μg/ml ICAM-1-Fcα fusion protein or control human IgA, 25 μg/ml FITC conjugated goat anti-human IgA antibody (Pierce, Rockford, IL, USA).
Tubes were immediately capped, incubated for 30 min at room temperature, and unbound ICAM-1-Fcα/anti-IgA-FITC was washed off with HBS. Bound ICAM-1 was detected by flow cytometry using a FACScan instrument (BD Bioscience, San Jose, CA USA). In some experiments, K562 cells expressing mutant LFA-1 (K287C/K294C) were pretreated with 20 mM dithiothreitol (DTT) for 10 min and then underwent continued treatment with 6.67 mM DTT during a 30-min incubation with soluble ICAM-1 (15). Immunofluorescent cytometry using TS1/22 or isotype control IgG1 X63 was performed as described previously (13).
Cell-cell conjugate assay
A cell-cell conjugate assay was performed as described previously (16) with modifications. Briefly, Jurkat and U937 cells were washed once with HBS containing 10 mM EDTA and 3 times with HBS. Jurkat and U937 cells were fluorescently labeled for 30 min at room temperature with 100 nM Mitotracker and 15 nM DiOC6(3) (Invitrogen, Carlsbad, CA, USA), respectively. After washing with HBS, 75 μl aliquots of each cell line containing 5 × 105 cells were mixed in PCR tubes (Axygen). Immediately after adding 150 μl HBS with 2 mM MnCl2 containing isoflurane (final concentration 1.5 mM), tubes were tightly capped, incubated for 30 min at room temperature, and subjected to flow cytometry analysis. Double-positive cells for FL1 [DiOC6(3)] and FL3 (Mitotracker) were regarded as conjugated cells. The conjugation ratio was calculated as the portion of DiOC6(3)/Mitotracker double-positive events within Mitotracker positive events.
KIM127 epitope exposure
Jurkat cells were harvested and washed once with HBS containing 10 mM EDTA and 3 times with HBS. Then 5 × 105 cells in 300 μl HBS were transferred to PCR tubes (Axygen) and centrifuged. Cell pellets were resuspended in 150 μl HBS, 2 mM MgCl2, 2 mM CaCl2, or 2 mM MnCl2 containing 3 mM isoflurane (final concentration 1.5 mM), and 150 μl HBS containing 10 μg/ml KIM127 or TS1/18. Tubes were immediately capped and incubated for 30 min at room temperature. Cells were washed and counterstained with anti-mouse Ig AlexaFluor488 (Invitrogen) for 30 min at room temperature. Flow cytometry analysis was performed immediately (15).
Western blot analysis
Jurkat cells that had been cultured in RPMI 1640 with 10% FBS were starved in RPMI 1640 with 0.1% BSA for 12 h. Cells were harvested and washed once with HBS containing 10 mM EDTA and 3 times with HBS. Cells (1×106) in 300 μl HBS were transferred to PCR tubes (Axygen) and centrifuged. Cell pellets were resuspended with 150 μl HBS, 2 mM MnCl2 containing isoflurane at 2× concentration, and 150 μl HBS containing 10 μg/ml ICAM-1 or TS1/22. Tubes were immediately capped and incubated for 30 min at room temperature. Cells were then washed and resuspended in ice-cold buffer containing 20 mM Tris-HCl (pH 7.4), 50 mM β-glycerophosphate, 1 mM sodium orthovanadate, 5 mM 2-ME, and the proteinase inhibitor cocktail Complete (Roche Applied Science, Indianapolis, IN, USA) and were sonicated 3 times on ice for 30 s. Cell lysates were centrifuged at 9000 g for 15 min, and supernatants were collected for analyses. Protein concentration was measured by the Bradford method, using a commercially prepared protein assay solution (Bio-Rad, Hercules, CA, USA) and BSA (fraction V, fatty acid-free; Sigma, St. Louis, MO, USA) as a standard. Protein (12 μg) was resolved by SDS-PAGE with 10% polyacrylamide separating gels and transferred to a Hybond enhanced chemiluminescence (ECL) nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ, USA). The membrane was blocked with 5% skim milk, probed with anti-p44/42 and anti-phospho p44/42 rabbit polyclonal antibodies (Cell Signaling, Danvers, MA, USA), and detected with horseradish peroxidase (HRP) -linked anti-rabbit IgG (Amersham Biosciences). Immunoreactive proteins were detected using ECL reagents (Amersham Biosciences) and X-ray film (Amersham Biosciences).
Soluble recombinant LFA-1
Soluble recombinant WT LFA-1 was used as described previously (15). A soluble form of recombinant disulfide-locked mutant high-affinity αL (K287C/K294C) was constructed, expressed, and purified as described previously for soluble recombinant WT LFA-1 (15). A soluble form of recombinant WT Mac-1 was kindly provided from Drs. Gang Song and Timothy A. Springer (Harvard Medical School, Boston, MA, USA).
Binding of soluble ICAM-1 to the immobilized extracellular part of the integrin
Soluble integrins (LFA-1 or Mac-1) (5 μg/ml) were immobilized indirectly on plastic ELISA plates with rabbit anti-Velcro C (antibody to the ACID BASE α-helical coiled coil) as described previously (15). Human ICAM-1-Fcα fusion protein (5 μg/ml) was added to immobilized integrin in HBS containing 1 mM MnCl2 and incubated for 1 h at room temperature in a closed chamber equilibrated with isoflurane at different concentrations using a vaporizer (Fluotec; Cyprane Ltd., Keighley, UK). The concentration of isoflurane was measured using infrared spectroscopy (Ultima; Datex Instrument Corp., Helsinki, Finland). The plates were then removed from the chamber, and unbound ICAM-1 was washed off with Tris-buffered saline. Bound ICAM-1 was detected by peroxidase-labeled goat anti-human IgA and substrate (Zymed, South San Francisco, CA, USA).
NMR
15N-labeled LFA-1 I domain (G128-Y307) protein was expressed as inclusion bodies in Escherichia coli BL21(DE3) on M9 medium containing 15NH4Cl (Cambridge Isotope Labs, Andover, MA, USA). The I domain was purified to homogeneity as described previously (17). Recombinant I domain was exchanged into a buffer containing 10 mM sodium phosphate (pH 7.2), 10 mM MgSO4, 150 mM NaCl, 0.05% NaN3, and 10% v/v 2H20. The NMR sample contained 600 μl of protein solution at 150 μM concentration. All NMR experiments were acquired at 22°C using a Varian Inova spectrometer operating at an 1H frequency of 599.763 MHz and equipped with a triple-resonance, single-axis gradient probe (Varian Systems, Palo Alto, CA, USA). 15N-heteronuclear single quantum coherence (HSQC) experiments (18) were acquired with 200 × 1024 points to obtain a spectral width of 2.02 × 10 kHz. The chemical shifts were indirectly referenced to a sample of DSS in sample buffer (19). Previously published chemical shift resonance assignments (20, 21) were used to identify which amino acid residues of the I domain were affected by the binding to isoflurane. A subset of resonances (N163, K188, R221, I258, K268, F299) was excluded from consideration due to overlap and/or uncertainty in the chemical shift assignment during titration. An additional set of resonances (I259, T300, I306) that underwent changes in exchange broadening during titration was explicitly not included in the analysis. An isoflurane [2-chloro-2-(difluoromethoxy)-1,1,1-trifluoroethane] titration was performed by adding aliquots of neat anesthetic (Halocarbon, River Edge, NJ, USA) through a rubber septum into a screw-cap NMR tube (Wilmad-Labglass, Buena, NJ, USA) via a gas-tight syringe (SGE Analytical, Ringwood, VIC, Australia). The sample was gently mixed by inverting it and allowed to equilibrate for 1 h prior to the acquisition of each NMR spectrum. A supraclinical concentration of isoflurane was used in the NMR studies to obtain complete occupancy of the binding pocket and to exclude the presence of alternative binding sites. The concentration of added isoflurane was determined by dividing the requisite quantity of added anesthetic by the protein solution volume (fixed at 600 μl). This method assumes 100% transfer of volatile anesthetic and is likely to overestimate the final concentration of isoflurane in the NMR tube. Data were processed using NMRPipe (22) and analyzed in Sparky.
Statistical analysis
Data were analyzed using Student’s t test or ANOVA with Tukey post hoc pairwise comparisons. Statistical significance was defined as P < 0.05.
RESULTS
Isoflurane at a clinically relevant concentration suppresses LFA-1-mediated adhesive interactions
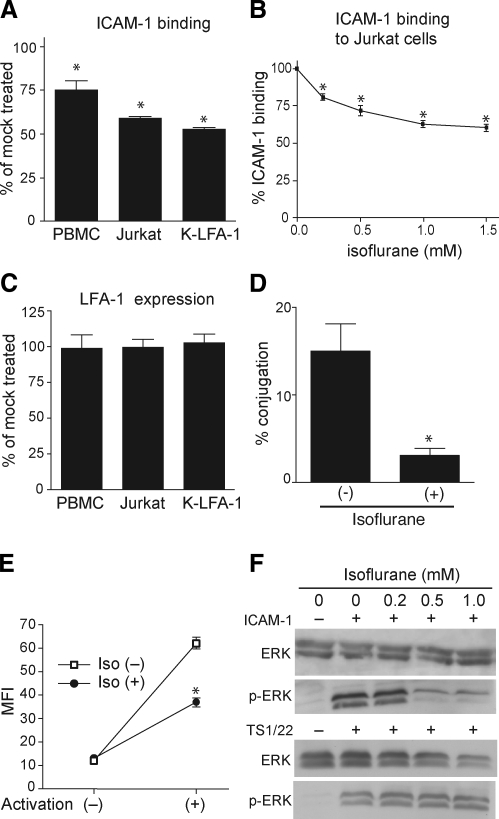
We studied the effects of isoflurane on the ligand binding of LFA-1 stimulated with the agonist Mn2+. Isoflurane at clinically relevant concentrations (up to 1.5 mM) blocked any binding to the LFA-1 ligand ICAM-1 of primary leukocytes, T-lymphocyte cell-line Jurkat cells, and K562 transfectants expressing LFA-1 in a dose-dependent manner (Fig. 2A, B and data not shown). Isoflurane did not affect the cell-surface expression of LFA-1 (Fig. 2C).
Figure 2.
Isoflurane at clinically relevant concentrations inhibits LFA-1 function. A–C) Isoflurane blocked ICAM-1 binding to LFA-1 (A, B) without altering its cell surface expression probed by mAb TS1/22 (C) and TS1/18 (data not shown), as determined by flow cytometry on PBMCs, Jurkat T-cell line, and K562 transfectant expressing WT LFA-1 (K-LFA-1). D) Isoflurane (1.5 mM) suppressed LFA-1-ICAM-1-dependent cell-cell conjugate formation of Jurkat and U937 cells. E) Isoflurane (1.5 mM) suppressed Mn2+-induced transition of LFA-1 from the bent to the extended conformation, as shown by the inhibition of KIM127 epitope exposure. MFI, mean fluorescence intensity. F) Isoflurane blocked phosphorylation of ERK in Mn2+-stumulated Jurkat cells induced by ICAM-1 but not by mAb TS1/22. Cells were treated for 30 min with ICAM-1 (5 μg/ml) or TS 1/22 (5 μg/ml) in the absence or presence of isoflurane. Cell lysate was subjected to Western blot analysis probed with anti-p44/42 (ERK) or anti-phospho p44/42 (p-ERK) antibodies. A representative result is shown. Data are expressed as means ± se of 3 independent experiments. *P < 0.05 vs. mock-treated samples.
LFA-1 on T lymphocytes plays a pivotal role in forming a stable cell-cell contact (i.e., immunological synapse) with antigen-presenting cells (APCs). In this cell-cell contact, LFA-1 transmits costimulatory signals that modulate T-cell functions (23). To study the biological relevance of the LFA-1 inhibition exerted by isoflurane, we investigated its impact on the stable cell-cell contact formation of Jurkat cells with a model APC, U937 cells. Consistent with isoflurane’s capacity to interfere with ICAM-1 binding to LFA-1, it potently blocked the Jurkat-U937 conjugate formation (Fig. 2D).
Isoflurane at a clinically relevant concentration suppresses activation-dependent global conformational changes of, and signal transmission though, LFA-1
The agonist-induced conformational changes of LFA-1 involve the transition from the bent to the extended form, thus triggering the conversion of the ligand-binding I domain to the high-affinity state (Fig. 1B) (10). The transition to the extended conformation exposes a mAb KIM127 epitope that is buried in the bent form and accessible only in the extended LFA-1 (15) (Fig. 1B). We found that isoflurane suppressed Mn2+-induced exposure of the KIM127 epitope in Jurkat cells, implying that the transition to the extended LFA-1 is perturbed (Fig. 2E). In contrast, isoflurane did not affect expression of TS1/22 and TS1/18 epitopes, which remain accessible independent of LFA-1 activation, thereby excluding the possibility that it merely weakens antibody binding in a nonspecific manner (Fig. 2C and data not shown).
The extension of LFA-1 is coupled with the dissociation of cytoplasmic domains (Fig. 1B), triggering intracellular signaling cascades such as protein phosphorylation (10, 23). We therefore reasoned that suppression of the LFA-1 extension by isoflurane would lead to inhibition of its downstream signaling. We studied ICAM-1-induced ERK phosphorylation in Mn2+-stimulated Jurkat cells. Isoflurane suppressed this ERK phosphorylation (Fig. 2F). The suppression was most likely not due to a nonspecific effect of isoflurane on protein phosphorylation, since it did not affect mAb TS1/22-induced ERK phosphorylation (Fig. 2F). These data suggest that isoflurane blocks the ligand binding of LFA-1 and subsequent signaling to the cytoplasm by suppressing the transition to the extended conformation.
Isoflurane allosterically inhibits LFA-1 function by perturbing the conformational conversion to the active state
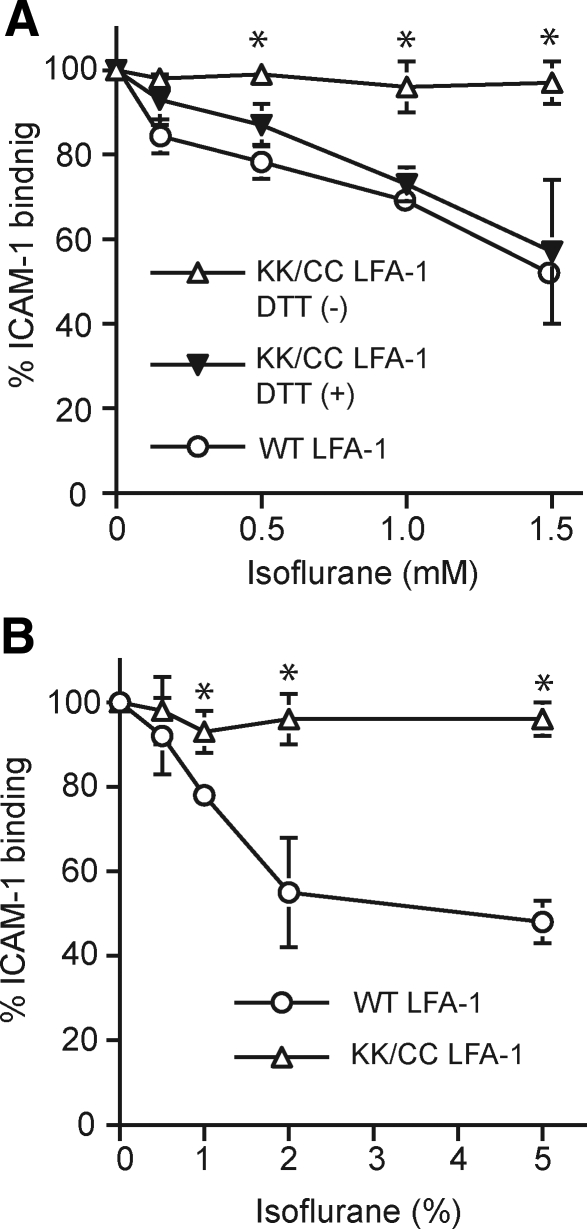
Two potential modes by which isoflurane inhibits LFA-1 function are 1) direct competition with ligand binding and 2) inhibition of the allosteric transition from the inactive to the active conformation. To distinguish between allosteric and competitive inhibition of LFA-1, we utilized K562 transfectants expressing the engineered disulfide bond-locked high-affinity LFA-1 (K287C/K294C). This mutant LFA-1 is blocked by direct competitors but not by allosteric inhibitors (13, 15). The engineered disulfide bond can be reduced by DTT, converting the LFA-1 to the WT conformation. Like the true WT LFA-1, the DTT-treated mutant can bind ICAM-1 on the addition of Mn2+ and is blocked by both direct and allosteric inhibition (13, 15). We have shown that isoflurane up to 1.5 mM failed to inhibit locked high-affinity LFA-1 (Fig. 3A). In contrast, after DTT treatment, isoflurane could block LFA-1 (K287C/K294C) as well as WT LFA-1 (Fig. 3A). These results demonstrate that isoflurane inhibits LFA-1 function allosterically by perturbing the conformational conversion to the active state.
Figure 3.
Isoflurane inhibits LFA-1 allosterically. A) Binding of ICAM-1 to WT and locked high-affinity LFA-1 K287C/K294C (KK/CC LFA-1) on K562 cells was examined using flow cytometry in the presence or absence of different concentrations of isoflurane. Isoflurane failed to block locked KK/CC LFA-1; however, on DTT reduction, isoflurane blocked Mn2+-stimulated KK/CC LFA-1 as well as Mn2+-stimulated WT LFA-1. B) Isoflurane inhibited ICAM-1 binding to LFA-1 in a cell-free system. Binding of ICAM-1 to the extracellular part of WT LFA-1 or KK/CC LFA-1 immobilized on plates was studied using a colorimetric ELISA-type assay while exposed to different concentrations of isoflurane in a vaporous closed chamber. Bound ICAM-1 was detected colorimetrically at an optical density (OD) of 405 nm using peroxidase-labeled goat anti-human Fc and substrate. Data represent means ± se of 3 independent experiments and are expressed as percentage of Mock-treated samples. *P < 0.05 vs. other treatments at the same isoflurane concentration.
Isoflurane inhibits affinity up-regulation of LFA-1 by acting on the extracellular domains
Direct interaction of isoflurane with the plasma membrane and intracellular proteins could inhibit LFA-1 clustering. This would consequently result in a lower ligand-binding strength of LFA-1 for ICAM-1 without affecting the conformational up-regulation of affinity. To distinguish between these two alternatives, we used a cell-free system with the extracellular part of LFA-1 attached to the artificial coiled-coil transmembrane domains immobilized on plates (15). We examined the binding of ICAM-1, both to WT and to the locked high-affinity LFA-1 stimulated with Mn2+, while exposed to various concentrations of isoflurane in a closed chamber. Isoflurane dose-dependently inhibited ICAM-1 binding to WT LFA-1 (Fig. 3B). In contrast, isoflurane failed to block ICAM-1 binding to the locked high-affinity mutant LFA-1 (Fig. 3B). These results confirmed that isoflurane perturbs the conformational activation of LFA-1 by binding to its extracellular domains, possibly including the I domain. However, this does not necessarily exclude the possibility that isoflurane exerts other effects in vivo due to its binding to the plasma membrane, to the transmembrane domains, or to intracellular signaling molecules.
NMR spectroscopy identifies isoflurane binding to the LFA-1 I domain
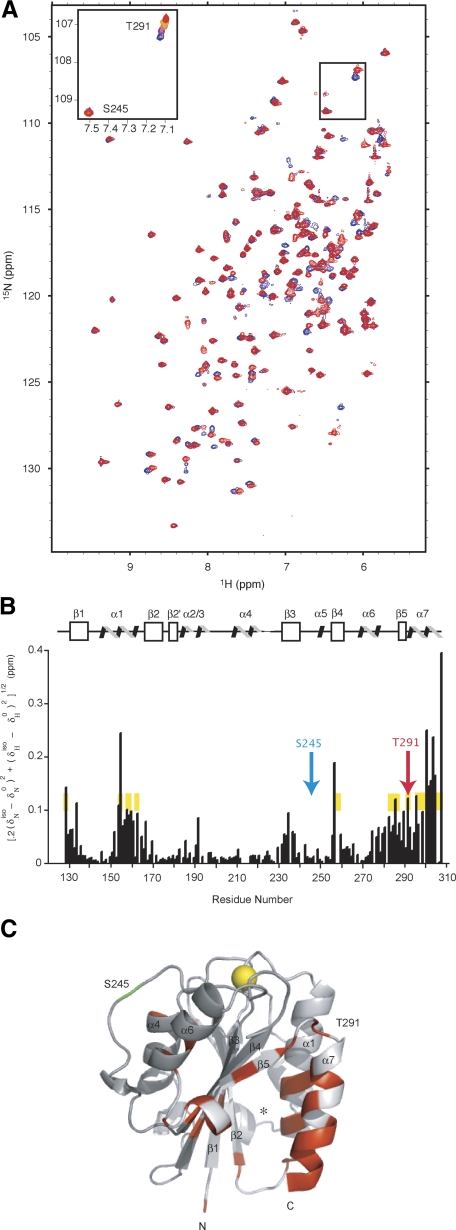
To map isoflurane binding sites in the LFA-1 I domain, we utilized heteronuclear 1H,15N-HSQC-NMR spectroscopy, an established technique for the identification of small-molecule interaction sites on isotopically labeled biomolecules, including anesthetic interactions with soluble protein domains (24,25,26). An overlay of two HSQCs taken at the endpoints of an isoflurane titration (0 and 12 mM isoflurane, Fig. 4A) not only revealed that a number of resonances undergo shifts in resonance frequency but also unambiguously identified the LFA-1 I domain as a binding site for isoflurane. The chemical shift of most residues was unchanged (Δδ≤0.01 ppm), whether at the lowest (0 mM) or the highest (ca. 12 mM) concentration of isoflurane (e.g., S245, Fig. 4A, inset). However, several resonances, such as T291 (Fig. 4A, inset), underwent a dose-dependent shift in resonance frequency (≥0.07 ppm over the full range of added isoflurane), indicating an interaction and/or change in the electronic environment or local structure of the protein.
Figure 4.
NMR spectroscopy to study isoflurane-binding sites in the LFA-1 I domain. A) Overlay experiments of 1H,15N-HSQC acquired without (blue) and with (red) 12 mM isoflurane. Inset: isoflurane titration series of T291 (top right) and S245 (bottom left). Colors correspond to: 0 mM (blue), 3 mM (purple), 6 mM (orange), 9 mM (yellow), and 12 mM (red) isoflurane. B) Scaled chemical-shift perturbation of 12 mM isoflurane mapped onto the LFA-1 I domain protein sequence and secondary structure. Chemical-shift perturbation calculated as [0.2(δNiso − δN0)2+(δHiso − δH0)2]1/2. Inset residues from A are shown in blue (S245) and red (T291), respectively. Secondary structure assignments (36): β1 (130–137), α1 (144–160), β2 (166–173), β2′ (177–181), α2 (183–188), α3 (192–196), α4 (208–218), β3 (231–238), α5 (249–251), β4 (255–261), α6 (268–277), β5 (286–289), α7 (293–305). For comparison, residues that shifted on addition of the LFA-1 allosteric inhibitor lovastatin are highlighted in yellow (27). C) Structure of the LFA-1 I domain (residues 127–307, PDB file 1ZOP; ref. 36) showing amide nitrogen residues affected (δppm≥0.05 ppm) by the addition of 12 mM isoflurane. Gray represents residues unperturbed by isoflurane; red represents residues that met or exceeded the threshold for perturbation. Helices and strands are labeled. Residues T291 (red) and S245 (green) are labeled. Yellow spheres represent the Mg2+ ion at the ICAM-1 binding site, termed the metal ion-dependent adhesion site (MIDAS). Note that the residues near the MIADS were not affected and that the affected residues clustered near the cavity formed between the α1 and α7 helices and the central β strands. This figure was created using PYMOL.
The magnitude of the isoflurane-induced shift was mapped onto the sequence and secondary structure (Fig. 4B). Significant chemical-shift perturbation occurred in 6 regions of the protein sequence: 1) the N-terminal segment, 2) the C-terminal portion of α1, 3) the loop between α2 and α3, 4) β3, 5) β5, and 6) the C-terminal segment incorporating β5-α7. Residues perturbed by the addition of the α I allosteric antagonist lovastatin (27) (visible as a uniform yellow background, Fig. 4B) showed a good correlation with those by isoflurane. The largest deviation in chemical shifts occurred with the aromatic resonances F153 and Y307, which could indicate a direct interaction between the halogenated hydrocarbons and the aromatic moieties, as previously suggested (24).
Residues with chemical shifts that were most perturbed by the binding of isoflurane were examined within the three-dimensional structure (Fig. 4C). Significantly, the distribution of residues was exceptionally homogenous and distant from the ICAM-1 ligand-binding site that coordinates the Mg2+ ion (shown by a yellow sphere in Fig. 4C). The most significantly perturbed residues formed a “crevice” (shown by an asterisk in Fig. 4C) between α1 and α7 on one side and between β1 and β4 on the other, which enclosed a partially buried hydrophobic cavity with polar side chains at the periphery. An excellent overlap was found between the two spatially proximate residue clusters with chemical shifts made by the addition of isoflurane and lovastatin, which suggests that these two dramatically different chemical moieties interact with a common site on the I domain (Fig. 4B). From the NMR titration, we have estimated the KD of isoflurane binding to the I domain as ∼800 μM. However, the actual binding to the LFA-1 holoreceptor may be tighter due to a more structured C-terminal helix, which forms part of the isoflurane binding site, in the intact integrin as opposed to the isolated I domain (28).
Isoflurane inhibits the related leukocyte integrin Mac-1 (αMβ2)
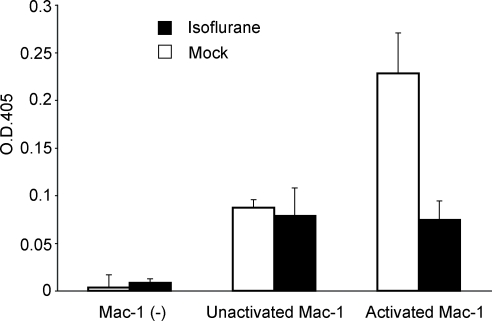
The structure, as well as the mode of conformational regulation, for the αL I domain are well conserved in other I domains such as the one in a related leukocyte integrin Mac-1 (29,30,31). To study the ability of isoflurane to suppress other I domain-containing integrins, we examined the effects of isoflurane on Mac-1 in the aforementioned ELISA-type cell-free assay system. We found that isoflurane at 5% potently blocked ICAM-1 binding to Mn2+-activated Mac-1 (Fig. 5), supporting the potential general property of isoflurane to perturb the conformational activation of integrin I domains.
Figure 5.
Isoflurane inhibits ICAM-1 binding to Mac-1. Binding of ICAM-1-Fc to immobilized extracellular part of WT Mac-1 in the presence or absence of 5% isoflurane was studied by an ELISA-type assay as in Fig. 3B. Bound ICAM-1 was detected colorimetrically at an OD of 405 nm using peroxidase-labeled goat anti-human Fc and substrate. Isoflurane suppressed ICAM-1 binding to Mn2+-activated Mac-1 to the basal level (i.e., binding to unactivated Mac-1). ICAM-1 binding was specific to Mac-1, as a background binding of ICAM-1 [i.e., Mac-1 (–)] was negligible. Data are mean and difference from the mean of triplicate samples in two independent experiments.
DISCUSSION
In this study, we have demonstrated that isoflurane at anesthetic concentrations blocks activation-dependent conformational conversion of integrin LFA-1. The NMR analysis identified the I domain as an isoflurane binding site. Consistent with the results of the functional experiments using the locked high-affinity LFA-1, residues at the ICAM-1 binding face (top) of the I domain are unaffected by the addition of isoflurane, demonstrating that the anesthetic does not act as a competitive antagonist by directly blocking the LFA-1:ICAM-1 binding interface. Important to note, spatial clustering of affected residues indicates that isoflurane does not inhibit LFA-1 function via a global destabilization of the I domain, for which a more homogeneous distribution of affected residues would be affected. The residues line a cavity obligate for protein function, previously identified by the binding of small molecule LFA-1 inhibitors such as lovastatin and its derivatives, suggesting a similar mode of allosteric inhibition; i.e., perturbation of the conformational conversion to the active form.
Our results provide important insight into how the relatively featureless halocarbon ether isoflurane can mimic the behavior of structurally complex LFA-1 allosteric inhibitors. Using the model protein system apomyoglobin, Eckenhoff and co-workers demonstrated that the binding of halothane, a similar volatile anesthetic, to apomyoglobin could destabilize the native state of the protein by stabilizing less-populated interconverting conformations (such as an enlarged hydrophobic cavity) (8). A similar mechanism may account for the I domain inhibition exerted by isoflurane; i.e., the binding of isoflurane to the I domain may favor alternate conformations of secondary and/or tertiary structures that disfavor any transition from the closed (inactive) to the open (active) I domain conformation. This is achieved by strengthening contacts that favor the closed state and/or attenuating contacts that facilitate the open conformation. This issue should be addressed by future investigations using NMR analyses of protein side chains.
Plasma isoflurane is weakly bound to transport proteins such as albumin (KD∼1 mM) (32). However, the concentration distribution of exceptionally heterogeneous volatile anesthetics is predominately located in the membrane interfacial region, as has been demonstrated by NMR and molecular dynamics studies (33, 34). The anisotropic distribution of isoflurane is significant in terms of the mechanism underlying the anesthetic-mediated inhibition of LFA-1 function. The integrin LFA-1 holo-receptor is thought to undergo global conformational changes from the inactive (bent) to the active (extended) form (35). Whereas the I domain in the extended form is located distally to the plasma membrane, the I domain in the bent form is located close to the membrane and is likely immersed in a dense layer of isoflurane (Fig. 1B). Because the conformation of the I domain is interconnected to the global LFA-1 conformation, binding of the α I allosteric antagonists favors the bent conformation (10). Thus, isoflurane binding to LFA-1 is likely to favor the bent conformation. This property might act as a positive feedback loop to enhance the inhibitory properties of isoflurane, thereby raising the threshold for integrin activation.
The allosteric inhibition by isoflurane of the transition to the active integrin conformer shown in this study might help explain the mechanism of immune suppression associated with general anesthesia. Conversely, we anticipate that our findings will contribute to a growing awareness of the potential therapeutic applications of VA and their derivatives outside of the CNS. Given the potential to suppress multiple integrin I domains (e.g., LFA-1 and Mac-1), isoflurane might be used as the lead compound for the development of a novel class of allosteric antagonists for leukocyte integrins.
Acknowledgments
We thank G. Song and T. A. Springer (Harvard Medical School, Boston, MA, USA) for providing reagents; T. Shimizu (University of Tokyo, Tokyo, Japan) for providing reagents and insightful comments; E. J. Park, D. Peer, and Y. Imai for technical support; S. Piva for insightful comments and stimulating our interest in integrin-isoflurane interactions; and T. Zimmerman and F. J. Blanco for sending the unpublished NMR assignments. This work was supported by grants from the National Institutes of Health, AI063421 and HL048675 (M.S.). K.Y. designed research, performed research, analyzed data, and wrote the paper; N.S.A. designed research, performed research, analyzed data, and wrote the paper; C.B. performed research and analyzed data; R.Y. performed research; W.S. performed research; S.G.S. designed research, analyzed data, and wrote the paper; M. S. designed research, performed research, analyzed data, and wrote the paper. The authors declare no conflicts of interest.
References
- McBride W T, Armstrong M A, McBride S J. Immunomodulation: an important concept in modern anaesthesia. Anaesthesia. 1996;51:465–473. doi: 10.1111/j.1365-2044.1996.tb07793.x. [DOI] [PubMed] [Google Scholar]
- Heindl B, Reichle F M, Zahler S, Conzen P F, Becker B F. Sevoflurane and isoflurane protect the reperfused guinea pig heart by reducing postischemic adhesion of polymorphonuclear neutrophils. Anesthesiology. 1999;91:521–530. doi: 10.1097/00000542-199908000-00027. [DOI] [PubMed] [Google Scholar]
- Mobert J, Zahler S, Becker B F, Conzen P F. Inhibition of neutrophil activation by volatile anesthetics decreases adhesion to cultured human endothelial cells. Anesthesiology. 1999;90:1372–1381. doi: 10.1097/00000542-199905000-00022. [DOI] [PubMed] [Google Scholar]
- Hayes J K, Havaleshko D M, Plachinta R V, Rich G F. Isoflurane pretreatment supports hemodynamics and leukocyte rolling velocities in rat mesentery during lipopolysaccharide-induced inflammation. Anesth Analg. 2004;98:999–1006. doi: 10.1213/01.ANE.0000104584.91385.1D. [DOI] [PubMed] [Google Scholar]
- Chiang N, Schwab J M, Fredman G, Kasuga K, Gelman S, Serhan C N. Anesthetics impact the resolution of inflammation. PLoS ONE. 2008;3:e1879. doi: 10.1371/journal.pone.0001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H T, Kim M, Kim N, Billings F T, IV, D'Agati V D, Emala C W., Sr Isoflurane protects against renal ischemia and reperfusion injury and modulates leukocyte infiltration in mice. Am J Physiol Renal Physiol. 2007;293:F713–F722. doi: 10.1152/ajprenal.00161.2007. [DOI] [PubMed] [Google Scholar]
- Lee H T, Emala C W, Joo J D, Kim M. Isoflurane improves survival and protects against renal and hepatic injury in murine septic peritonitis. Shock. 2007;27:373–379. doi: 10.1097/01.shk.0000248595.17130.24. [DOI] [PubMed] [Google Scholar]
- Eckenhoff R G, Pidikiti R, Reddy K S. Anesthetic stabilization of protein intermediates: myoglobin and halothane. Biochemistry. 2001;40:10819–10824. doi: 10.1021/bi010691r. [DOI] [PubMed] [Google Scholar]
- Liu R, Loll P J, Eckenhoff R G. Structural basis for high-affinity volatile anesthetic binding in a natural 4-helix bundle protein. FASEB J. 2005;19:567–576. doi: 10.1096/fj.04-3171com. [DOI] [PubMed] [Google Scholar]
- Shimaoka M, Springer T A. Therapeutic antagonists and the conformational regulation of integrin structure and function. Nat Rev Drug Disc. 2003;2:703–716. doi: 10.1038/nrd1174. [DOI] [PubMed] [Google Scholar]
- Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, Cottens S, Takada Y, Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- Frenette P S. Locking a leukocyte integrin with statins. N Engl J Med. 2001;345:1419–1421. doi: 10.1056/NEJM200111083451911. [DOI] [PubMed] [Google Scholar]
- Lu C, Shimaoka M, Ferzly M, Oxvig C, Takagi J, Springer T A. An isolated, surface-expressed I domain of the integrin αLβ2 is sufficient for strong adhesive function when locked in the open conformation with a disulfide. Proc Natl Acad Sci U S A. 2001;98:2387–2392. doi: 10.1073/pnas.041606398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N P, Lieb W R. Stereospecific effects of inhalational general anesthetic optical isomers on nerve ion channels. Science. 1991;254:427–430. doi: 10.1126/science.1925602. [DOI] [PubMed] [Google Scholar]
- Shimaoka M, Salas A, Yang W, Weitz-Schmidt G, Springer T A. Small molecule integrin antagonists that bind to the β2 subunit I-like domain and activate signals in one direction and block them in another. Immunity. 2003;19:391–402. doi: 10.1016/s1074-7613(03)00238-3. [DOI] [PubMed] [Google Scholar]
- Chen X, Trivedi P P, Ge B, Krzewski K, Strominger J L. Many NK cell receptors activate ERK2 and JNK1 to trigger microtubule organizing center and granule polarization and cytotoxicity. Proc Natl Acad Sci U S A. 2007;104:6329–6334. doi: 10.1073/pnas.0611655104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimaoka M, Lu C, Palframan R, von Andrian U H, Takagi J, Springer T A. Reversibly locking a protein fold in an active conformation with a disulfide bond: integrin αL I domains with high affinity and antagonist activity in vivo. Proc Natl Acad Sci U S A. 2001;98:6009–6014. doi: 10.1073/pnas.101130498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleucher J, Schwendinger M, Sattler M, Schmidt P, Schedletzky O, Glaser S J, Sorensen O W, Griesinger C. A general enhancement scheme in heteronuclear multidimensional NMR employing pulsed field gradients. J Biomol NMR. 1994;4:301–306. doi: 10.1007/BF00175254. [DOI] [PubMed] [Google Scholar]
- Wishart D S, Bigam C G, Yao J, Abildgaard F, Dyson H J, Oldfield E, Markley J L, Sykes B D. 1H, 13C and 15N chemical shift referencing in biomolecular NMR. J Biomol NMR. 1995;6:135–140. doi: 10.1007/BF00211777. [DOI] [PubMed] [Google Scholar]
- Kriwacki R W, Legge G B, Hommel U, Ramage P, Chung J, Tennant L L, Wright P E, Dyson H J. Assignment of 1H, 13C and 15N resonances of the I-domain of human leukocyte function associated antigen-1. J Biomol NMR. 2000;16:271–272. doi: 10.1023/a:1008358912334. [DOI] [PubMed] [Google Scholar]
- Zimmerman T, Oyarzabal J, Sebastian E S, Majumdar S, Tejo B A, Siahaan T J, Blanco F J. ICAM-1 peptide inhibitors of T-cell adhesion bind to the allosteric site of LFA-1. An NMR characterization. Chem Biol Drug Des. 2007;70:347–353. doi: 10.1111/j.1747-0285.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister G W, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Perez O D, Mitchell D, Jager G C, South S, Murriel C, McBride J, Herzenberg L A, Kinoshita S, Nolan G P. Leukocyte functional antigen 1 lowers T cell activation thresholds and signaling through cytohesin-1 and Jun-activating binding protein 1. Nat Immunol. 2003;4:1083–1092. doi: 10.1038/ni984. [DOI] [PubMed] [Google Scholar]
- Manderson G A, Johansson J S. Role of aromatic side chains in the binding of volatile general anesthetics to a four-alpha-helix bundle. Biochemistry. 2002;41:4080–4087. doi: 10.1021/bi0160718. [DOI] [PubMed] [Google Scholar]
- Yonkunas M J, Xu Y, Tang P. Anesthetic interaction with ketosteroid isomerase: insights from molecular dynamics simulations. Biophys J. 2005;89:2350–2356. doi: 10.1529/biophysj.105.063396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Tao Y X, He F, Zhang M, Levine C F, Mao P, Tao F, Chou C L, Sadegh-Nasseri S, Johns R A. Synaptic PDZ domain-mediated protein interactions are disrupted by inhalational anesthetics. J Biol Chem. 2003;278:36669–36675. doi: 10.1074/jbc.M303520200. [DOI] [PubMed] [Google Scholar]
- Kallen J, Welzenbach K, Ramage P, Geyl D, Kriwacki R, Legge G, Cottens S, Weitz-Schmidt G, Hommel U. Structural basis for LFA-1 inhibition upon lovastatin binding to the CD11a I-domain. J Mol Biol. 1999;292:1–9. doi: 10.1006/jmbi.1999.3047. [DOI] [PubMed] [Google Scholar]
- Salas A, Shimaoka M, Chen S, Carman C V, Springer T A. Transition from rolling to firm adhesion is regulated by the conformation of the I domain of the integrin LFA-1. J Biol Chem. 2002;277:50255–50262. doi: 10.1074/jbc.M209822200. [DOI] [PubMed] [Google Scholar]
- Lee J-O, Bankston L A, Arnaout M A, Liddington R C. Two conformations of the integrin A-domain (I-domain): a pathway for activation? Structure. 1995;3:1333–1340. doi: 10.1016/s0969-2126(01)00271-4. [DOI] [PubMed] [Google Scholar]
- Xiong J-P, Li R, Essafi M, Stehle T, Arnaout M A. An isoleucine-based allosteric switch controls affinity and shape shifting in integrin CD11b A-domain. J Biol Chem. 2000;275:38762–38767. doi: 10.1074/jbc.C000563200. [DOI] [PubMed] [Google Scholar]
- Shimaoka M, Shifman J M, Jing H, Takagi J, Mayo S L, Springer T A. Computational design of an integrin I domain stabilized in the open, high affinity conformation. Nat Struct Biol. 2000;7:674–678. doi: 10.1038/77978. [DOI] [PubMed] [Google Scholar]
- Dubois B W, Evers A S. 19F-NMR spin-spin relaxation (T2) method for characterizing volatile anesthetic binding to proteins. Analysis of isoflurane binding to serum albumin. Biochemistry. 1992;31:7069–7076. doi: 10.1021/bi00146a007. [DOI] [PubMed] [Google Scholar]
- Tang P, Yan B, Xu Y. Different distribution of fluorinated anesthetics and nonanesthetics in model membrane: a 19F NMR study. Biophys J. 1997;72:1676–1682. doi: 10.1016/S0006-3495(97)78813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemparala S, Saiz L, Eckenhoff R G, Klein M L. Partitioning of anesthetics into a lipid bilayer and their interaction with membrane-bound peptide bundles. Biophys J. 2006;91:2815–2825. doi: 10.1529/biophysj.106.085324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida N, Xie C, Shimaoka M, Cheng Y, Walz T, Springer T A. Activation of leukocyte beta2 integrins by conversion from bent to extended conformations. Immunity. 2006;25:583–594. doi: 10.1016/j.immuni.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Qu A, Leahy D J. Crystal structure of the I-domain from the CD11a/CD18 (LFA-1, αLβ2) integrin. Proc Natl Acad Sci U S A. 1995;92:10277–10281. doi: 10.1073/pnas.92.22.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]