Abstract
The cytochrome P450 (CYP) enzymes participate in a wide range of biochemical functions, including metabolism of arachidonic acid and steroid hormones. Mouse CYP2J5 is abundant in the kidney where its products, the cis-epoxyeicosatrienoic acids (EETs), modulate sodium transport and vascular tone. To define the physiological role of CYP2J5 in the kidney, knockout mice were generated using a conventional gene targeting approach. Cyp2j5 (−/−) mice develop normally and exhibit no overt renal pathology. While renal EET biosynthesis was apparently unaffected by the absence of CYP2J5, deficiency of this CYP in female mice was associated with increased blood pressure, enhanced proximal tubular transport rates, and exaggerated afferent arteriolar responses to angiotensin II and endothelin I. Interestingly, plasma 17β-estradiol levels were reduced in female Cyp2j5 (−/−) mice and estrogen replacement restored blood pressure and vascular responsiveness to normal levels. There was no evidence of enhanced estrogen metabolism, or altered expression or activities of steroidogenic enzymes in female Cyp2j5 (−/−) mice, but their plasma levels of luteinizing hormone and follicle stimulating hormone were inappropriately low. Together, our findings illustrate a sex-specific role for CYP2J5 in regulation of blood pressure, proximal tubular transport, and afferent arteriolar responsiveness via an estrogen-dependent mechanism.—Athirakul, K., Bradbury, J. A., Graves, J. P., DeGraff, L. M., Ma, J., Zhao, Y., Couse, J. F., Quigley, R., Harder, D. R., Zhao, X., Imig, J. D., Pedersen, T. L., Newman, J. W., Hammock, B. D., Conley, A. J., Korach, K. S., Coffman, T. M., Zeldin, D. C. Increased blood pressure in mice lacking cytochrome P450 2J5.
Keywords: hypertension, estrogen, proximal tubule, vascular responsiveness, eicosanoid
The cytochrome P450 (CYP) enzymes have diverse physiological functions that contribute to sodium homeostasis, control of vasomotor tone, and hormone metabolism (1,2,3). A role for CYPs in blood pressure regulation was initially suggested based on their ability to generate biologically active metabolites of arachidonic acid (AA) with the capacity to modulate renal tubular function and vascular tone (1, 2, 4). For example, 20-hydroxyeicosatetranoic acid (HETE), which is produced by CYP4A subfamily enzymes, is a potent vasoconstrictor and inhibits sodium retention in the kidney (4,5,6). In contrast, cis-epoxyeicosatrienoic acids (EETs), which are produced by members of the CYP2C and CYP2J subfamilies, dilate blood vessels and have potent natriuretic effects (4, 7, 8). While the biological actions of EETs and HETEs suggest a capacity for these eicosanoids to participate in blood pressure homeostasis, direct evidence to support the involvement of CYP epoxygenases and CYP ω-hydroxylases in the pathogenesis of hypertension is somewhat limited. Renal CYP epoxygenases are under regulatory control by dietary salt (9), and spontaneously hypertensive rats (SHR) have altered renal CYP epoxygenase and ω-hydroxylase expression and activity (10, 11). Moreover, urinary excretion of EET metabolites is increased during pregnancy-induced hypertension in humans (12). Additionally, studies in mice demonstrate that disruption of the Cyp4a14 gene causes spontaneous hypertension associated with increased renal 20-HETE formation (13), and disruption of the Cyp4a10 gene causes salt-sensitive hypertension associated with reduced urinary EET excretion (14). Further, recent genetic studies reveal that single nucleotide polymorphisms in the CYP4A11 and CYP2J2 genes are associated with hypertension in humans (15, 16). Nevertheless, the role of CYP enzymes in blood pressure homeostasis and control of renal function in vivo remains controversial and in need of further investigation.
We previously identified mouse CYP2J5 and showed that it is expressed primarily in the kidney and is particularly abundant in renal proximal tubules (17). Moreover, the recombinant CYP2J5 protein is active in the metabolism of AA to EETs (17). The distinctive tissue distribution and enzymatic properties of CYP2J5 suggest that it may have a role in regulating renal function and blood pressure. Hence, the present study was designed to investigate the physiological functions of this enzyme using mice with targeted disruption of the Cyp2j5 gene.
MATERIALS AND METHODS
Generation of Cyp2j5 deficient mice
The Cyp2j5 gene was cloned from a strain 129/SvEv mouse genomic library and sequenced (GenBank AF218853, AF218854). The gene is 27 kb in length, contains 9 exons and 8 introns, and is located on chromosome 4. The gene targeting strategy (Fig. 1A) involved replacement of exon 9, including the splice signal, with a neomycin resistance cassette such that if a protein were made from the resulting disrupted gene, it would not be functional because it lacks the critical heme binding peptide. Embryonic stem (ES) cells (129/SvEv) were grown, electroporated, and screened using standard methods (18). Targeted ES cell clones were identified by Southern blotting and confirmed by polymerase chain reaction (PCR) analysis of ES cell genomic DNA using specific probes and primers (see below). Targeted ES cells were microinjected into C57BL/6 blastocysts using standard techniques and implanted into pseudopregnant female mice. Male chimeras were mated with 129/SvEv females to identify germline-competent chimeras that were capable of transferring the genetically modified ES cell genome to their offspring. The targeted Cyp2j5 allele was detected by Southern blotting of tail genomic DNAs and confirmed by PCR analysis. Progeny that were heterozygous for the null mutation [Cyp2j5 (+/−)] were intercrossed to obtain mice that were homozygous null [Cyp2j5 (−/−)] and wild type [Cyp2j5 (+/+)] for the targeted allele. All studies used F3–F10 generation Cyp2j5 (−/−) mice and isogenic Cyp2j5 (+/+) littermates as controls. All studies were in accordance with principles outlined in the NIH Guide for the Care and Use of Laboratory Animals and were approved by the animal care and use committees at the respective institutions.
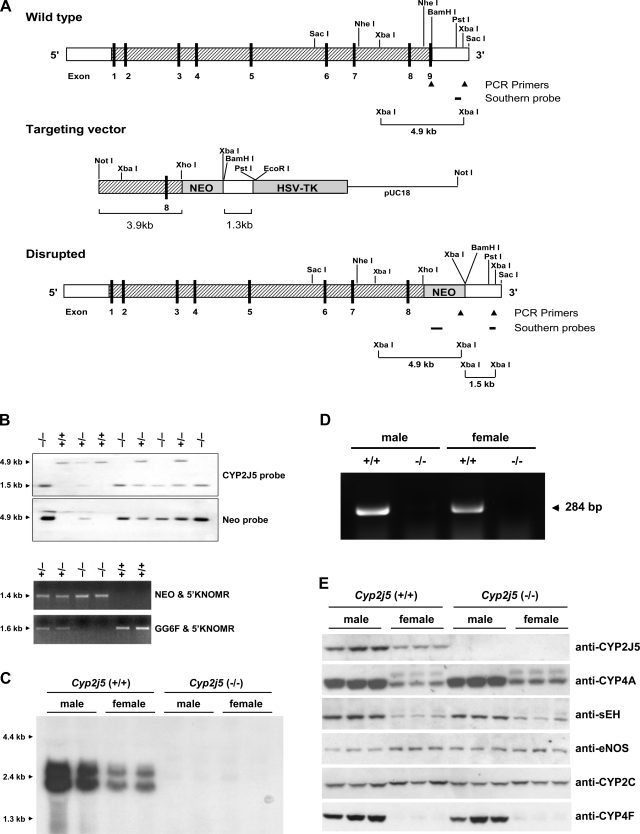
Figure 1.
Development and initial characterization of Cyp2j5 (−/−) mice. A) Gene targeting strategy. The intron/exon organization of the Cyp2j5 gene and the locations of selected restriction sites, PCR primers and Southern probes are shown. In the targeting vector, exon 9 is replaced by the neomycin resistance gene. B) Genotyping by Southern blotting and PCR analysis of tail genomic DNAs. Wild-type (+/+), heterozygous (+/−), and homozygous null (−/−) genotypes are shown. C) Northern blot analysis showing absence of CYP2J5 transcripts in male and female Cyp2j5 (−/−) kidneys. D) RT-PCR analysis confirming absence of CYP2J5 mRNA in male and female Cyp2j5 (−/−) kidneys. E) Immunoblotting showing absence of CYP2J5 protein in male and female Cyp2j5 (−/−) kidneys. There were no differences in the expression of CYP4A, CYP2C, or CYP2F subfamily P450s, or in the expression of eNOS and sEH between the genotypes in either males or females.
Genotyping Cyp2j5 deficient mice
Southern blotting of mouse genomic DNA was performed using a modification of the protocol described by Sambrook (19). Briefly, genomic DNAs were digested with XbaI, electrophoresed on 0.8% agarose gels, and transferred by capillary pressure to HybondN nylon membranes (Amersham Biosciences, Inc., Piscataway, NJ, USA). Blots were hybridized with either a 0.24 kb PstI-XbaI Cyp2j5 probe, which corresponds to genomic sequence 3′ of the short arm of the targeting construct (Fig. 1A), or a 0.63 kb neomycin probe in QuikHyb Solution (Stratagene, La Jolla, CA, USA). Probes were purified using the QIAquick Gel Extraction Kit (Qiagen, Valencia, CA, USA), labeled with [α-32P]ATP using the Random Primed DNA Labeling Kit (Roche Applied Science, Indianapolis, IN, USA) and purified through NICK Columns (Amersham). The Cyp2j5 probe yielded a single 4.9 kb band in Cyp2j5 (+/+) mice, a single 1.5 kb band in Cyp2j5 (−/−) mice, and both 4.9 kb and 1.5 kb bands in Cyp2j5 (+/−) mice (Fig. 1B). A single 4.9 kb band was present with the neomycin probe in both Cyp2j5 (−/−) and Cyp2j5 (+/−) mice (Fig. 1B).
Three primers were developed for PCR-based genotyping (Fig. 1A). The forward primer GG6F (5′-AAGAGCTTGTCTTGGAGAACAGT-3′) for identification of the wild-type allele is located in exon 9 of the Cyp2j5 gene. The forward primer NEO (5′-GCAGCCTCTGTTCCACATACAC-3′) for identification of the disrupted Cyp2j5 allele is located within the neomycin cassette. The reverse primer 5′KNOMR (5′-GCATTCATATGTAAGAACTGGCAG-3′) is located in the 3′ untranslated region of the Cyp2j5 gene distal to the XbaI site. Mouse genomic DNA (200 ng) was used for each 50 μl PCR reaction with final concentrations as follows: 0.16 mM dNTPs, 1X reaction buffer (Applied Biosystems, Foster City, CA, USA), 1.92 mM MgCl2, 0.001 mM of each primer, and 2.5 U of AmpliTaq® Gold DNA polymerase (Applied Biosystems). The cycling protocol was as follows: 95°C, 15 min (1 cycle); 95°C, 25 s; 64°C, 30 s; 72°C, 2 min (35 cycles); and 72°C, 7 min (1 cycle). PCR products were separated on 1.2% agarose gels. The NEO & 5′KNOMR primers yielded a single 1.4 kb band in Cyp2j5 (+/−) and Cyp2j5 (−/−) mice, whereas the GG6F & 5′KNOMR primers yielded a single 1.6 kb band in Cyp2j5 (+/−) and Cyp2j5 (+/+) mice (Fig. 1B).
Blood pressure measurements and transthoracic echocardiography
Mice were maintained in the animal care facility at the National Institute of Environmental Health Sciences (NIEHS) and Duke University Medical Center according to U.S. National Institutes of Health (NIH) guidelines. Mice were fed a normal-salt diet (0.4% NaCl, Harlan-Teklad, Madison, WI, USA) and had free access to water. For some studies, mice were fed either a low-salt (<0.04% NaCl) or a high-salt (6% NaCl) diet. All studies were done with adult mice (12–18 wk).
Systolic blood pressure (SBP) was measured noninvasively in conscious Cyp2j5 (+/+) and Cyp2j5 (−/−) mice using a computerized tail cuff system equipped with a photoelectric sensor (Visitech Systems, Cary, NC, USA) (20). This system allows pressure to be measured in 4 mice simultaneously and minimizes the potential for observer bias (20). Before the study was initiated, mice were trained for adaptation to the apparatus for at least 5 days. Blood pressure measurements were recorded daily and averaged over 21 days for each mouse. The validity of this system has been established previously, and we have demonstrated its correlation with intra-arterial blood pressure measurements in several experimental systems (20). For some studies, blood pressure was also measured invasively via an indwelling right carotid artery catheter connected to a pressure transducer. Left ventricular mass was measured by 2-dimensional M-mode echocardiography in conscious mice using an HDI 5000 echocardiograph (ATL, Bothell, WA, USA), as described elsewhere (21). Investigators who performed blood pressure and echocardiographic analyses were masked to mouse genotype.
Sex hormones assays and hormonal manipulations
Blood samples were drawn from retro-orbital veins during the morning hours. Plasma concentrations of 17β-estradiol, testosterone, and luteinizing hormone (LH) were determined by ultrasensitive radioimmunoassays (Diagnostic Systems Laboratories, Inc., Webster, TX, USA) using the manufacturer’s protocols. Plasma concentrations of follicle stimulating hormone (FSH) were determined using an immunoradiometric assay from Alpco Diagnostics (Salem, NH, USA). For some studies, 17β-estradiol was administered by subcutaneous placement of 60-day timed release pellets (0.18 mg/pellet, Innovative Research, Sarasota, FL, USA). This dose has been previously shown to provide physiological levels of estrogen in mice (22). Some mice also underwent bilateral ovariectomy. Control mice received placebo pellets and underwent sham ovariectomy. Blood pressure measurements were repeated 3 wk following surgery and pellet implantation.
In vitro microperfusion flux studies
To assess the physiological effects of Cyp2j5 disruption on renal tubular function, we measured proximal tubular transport rates in mice. Juxtamedullary and midcortical proximal convoluted tubules from Cyp2j5 (+/+) and Cyp2j5 (−/−) mice were perfused in vitro as described elsewhere (23). Briefly, tubules were dissected in ice-cold modified Hank’s solution containing 137 mM NaCl, 5 mM KCl, 0.8 mM MgSO4, 0.33 mM Na2HPO4, 0.44 mM KH2PO4, 1 mM MgCl2, 10 mM Tris-HCl (pH 7.4), 0.25 mM CaCl2, 2 mM glutamine, and 2 mM l-lactate bubbled with 100% O2. Tubules were then transferred to a 1.2 ml temperature-controlled bathing chamber and perfused via concentric glass pipettes at 37°C. The perfusion solution contained 110 mM NaCl, 25 mM NaHCO3, 2.3 mM Na2HPO4, 10 mM NaOAc, 1.8 mM CaCl2, 1 mM MgSO4, 5 mM KCl, 8.3 mM glucose, and 5 mM alanine. The bathing solution was identical to the perfusion solution except that it also contained 6 g/dl albumin. Solutions were bubbled with 95% O2:5% CO2 and had pH 7.4. Osmolalities of the perfusion and bathing solutions were adjusted to 295 mosmol/kg H2O by the addition of water or NaCl. The osmolalities of the perfusion and bathing solutions were measured with a Wide Range Osmometer (Advanced Instruments, Norwood, MA, USA). The bathing solution was exchanged at a rate of 0.5 ml/min to keep the osmolality and pH constant. Volume absorption (Jv, in nl/min/mm) was measured as the difference between the perfusion and collection rates, and normalized per millimeter of tubule length. The collection rate was determined by timed collections using a constant volume pipette. Exhaustively dialyzed [methoxy-3H]inulin (New England Nuclear, Wellesley, MA, USA) was added to the perfusate at a concentration of 50 μCi/ml so that the perfusion rate could be calculated. The tubule length was measured using an eyepiece micrometer.
Afferent arteriolar responses to vasoactive compounds
The in vitro perfused juxtamedullary nephron preparation was used to assess afferent arteriolar responses to angiotensin II and endothelin I, as described elsewhere (7). Briefly, after pentobarbital anesthesia (50 mg/kg i.p.) and midline laparotomy, the right renal artery was cannulated through the superior mesenteric artery, and the kidney was immediately perfused with a Tyrode’s solution containing 6% albumin and a mixture of l-amino acids. The tissue surface was continuously superfused with a Tyrode’s solution containing 1% albumin. Following a 20-min equilibration period, an afferent arteriole was chosen for study and baseline diameter was measured. The control vascular diameter at a renal perfusion pressure of 100 mmHg over a 5-min period was determined. Angiotensin II (0.01–10 nM) was subsequently delivered by superfusion for 5 min and a cumulative concentration curve was obtained. The vessel was allowed to recover and the response to endothelin I (1–10 nM) was determined. Steady-state diameters attained within 2 min were utilized for statistical analysis.
AA metabolism assays
Microsomal fractions were prepared from freshly isolated male and female mouse kidneys, as described elsewhere (17). Reaction mixtures containing 50 mM Tris-Cl buffer (pH 7.5), 150 mM KCl, 10 mM MgCl2, 8 mM sodium isocitrate, 0.5 IU/ml isocitrate dehydrogenase, 1 mM NADPH, 100 μM [1-14C]AA (25 μCi/μmol), and 1–2 mg microsomal protein/ml were incubated at 37°C with constant mixing. At different time points, aliquots were withdrawn, and the reaction products were extracted into diethyl ether and analyzed by reverse-phase HPLC, as described elsewhere (17). All products were identified by comparing their HPLC properties with those of authentic EET and HETE standards. We also measured levels of endogenous EETs and HETEs in mouse urine. Mice were individually housed in metabolic cages (Hatteras Instruments, Cary, NC, USA) and 24-h urine specimens were collected. Urine was analyzed for levels of epoxy, monohydroxy, and dihydroxy fatty acid derivatives of AA and linoleic acid using established HPLC/MS/MS methods (24).
Estradiol metabolism assays
Aromatase activity was measured from the release of tritiated water following the metabolism of [1β-3H]androstenedione to estrone as previously described and validated (25). Assays used 100 μg of crude tissue lysate with 300 nM substrate incubated for 6 h at 37°C. The metabolism of estradiol (10 μM final concentration of authentic standard with [6,7-3H]17β-estradiol, 50 Ci/mmol, American Radiochemicals, St. Louis, MO, USA) was examined in tissue lysates (100 μg) incubated at 37°C for 6 h with a generating system as described above. Ascorbic acid (10 mM final concentration) was added at the end of the incubation and the reaction was extracted with 5 ml methylene chloride. Extracts were dried, reconstituted together with authentic standards (2-OH, 4-OH, and 6-OH estradiol) in ethyl acetate, and spotted onto silica gel plates (Whatman, Inc., Clifton, NJ, USA) for thin-layer chromatography. Plates were developed once with a mobile phase of chloroform, cyclohexane, and acetic acid (2:2:1), dried, placed overnight on tritium detection screens, and scanned on a phosphorimager.
Northern blotting, reverse transcriptase-PCR (RT-PCR), ribonuclease protection assays, and Western blotting
Tissues were perfused in situ with ice-cold phosphate-buffered saline, dissected free of surrounding tissue, frozen in liquid nitrogen, and stored at −80°C until use. RNA was prepared from frozen tissues using TRIreagent (Molecular Research Center, Cincinnati, OH, USA) according to the manufacturer’s instructions. Northern blot analysis was performed using the full-length CYP2J5 cDNA probe or specific 3′-untranslated end cDNA probes to CYP4A10, CYP4A12, and CYP4A14, as described elsewhere (13, 17). Northern blot analysis results were independently confirmed by RT-PCR using CYP2J5 sequence-specific oligonucleotide primers and the GeneAmp RNA PCR Kit (PerkinElmer), as described elsewhere (17). For real-time RT-PCR, first-strand cDNA was synthesized from DNaseI treated total RNA using the High Capacity cDNA Archive Kit (Applied Biosystems) according to the manufacturer’s instructions. The cDNA levels were detected using the TaqMan Universal PCR Master Mix (Applied Biosystems) on an ABI Prism 7700 HT Sequence Detection System (Applied Biosystems). The following specific probes and primers were designed and synthesized by Applied Biosystems: CYP2J5 (Mm00487292_m1), CYP2J9 (Mm00466426_m1), kidney androgen regulated protein (Mm00495104_m1), and insulin-like growth factor 2 (Mm00439563_m1). For cDNA amplification, 50 ng of cDNA was combined with 25 μl of TaqMan Universal Master Mix, 2.5 μl of the primer/probe mix, and RNase-free water up to a total volume of 50 μl. Samples were analyzed in quadruplicate with no template controls and serially diluted concentrations of cDNA sample, from which a standard curve was generated. Amplifications were as follows: 50°C, 2 min; 95°C, 10 min; 40 cycles of 95°C, 15 s; and 60°C, 1 min. Data were analyzed using the 2–ΔΔCt method (26). Results were normalized to an internal control transcript that encodes glyceraldehyde-3-phosphate dehydrogenase using TaqMan Rodent GAPDH control reagents. Ovarian expression of steroidogenic acute regulatory protein (StAR), cholesterol side-chain cleavage enzyme (CYP11A1), 17α-hydroxylase (CYP17), 3β-hydroxysteroid dehydrogenase/isomerase type 1 (3β-HSD1), 17β-hydroxysteroid dehydrogenase type 1 (17β-HSD1), and aromatase (CYP19A1) were evaluated by RNase protection assay (RPA), as described elsewhere (27). RPA gels were exposed to a phosphorimager screen and data analyzed using a Storm 860 and accompanying ImageQuant software (GE Healthcare, Milwaukee, WI, USA). Because CYP19A1 levels were at or below the level of detection for the RPA, the same samples were also assayed by semiquantitative RT-PCR, as described previously (28).
Polyclonal rabbit anti-mouse CYP2J5 were prepared as described elsewhere (17). This antibody has been shown to strongly react with recombinant mouse CYP2J5 but does not cross-react with other mouse P450s (17). Polyclonal goat anti-rat CYP4A1, which cross-reacts with mouse CYP4A isoforms, was purchased from BD Gentest (San Jose, CA, USA). Polyclonal rabbit anti-human soluble epoxide hydrolase (sEH), which cross-reacts with mouse sEH, was from Dr. Bruce Hammock (University of California, Davis, CA, USA). Polyclonal rabbit anti-endothelial nitric oxide synthase (anti-eNOS) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Polyclonal antibodies against the CYP2C-specific peptide RGKLPPGPTPLPII (anti-CYP2C 1548) were prepared as described elsewhere (17). This antibody reacts primarily with mouse CYP2C29, CYP2C37, CYP2C40, CYP2C44 and, to a lesser extent, with other mouse CYP2C isoforms, but not with non-CYP2C isoforms. Polyclonal rabbit anti-CYP4F was a gift from Dr. Jerome Lasker (Institute for Biomedical Research, Hackensack, NJ, USA). For immunoblotting, microsomal fractions were electrophoresed in 8–16% Tris glycine gels (80×80×1 mm) purchased from Invitrogen (Carlsbad, CA, USA) and the resolved proteins transferred electrophoretically onto nitrocellulose membranes. Membranes were immunoblotted using primary antibodies (1:1000 or 1:2000 dilution), goat anti-rabbit immunoglobulin G (IgG) or rabbit anti-goat IgG conjugated to horseradish peroxidase (1:2000) (Santa Cruz Biotechnology), and the enhanced chemiluminescence (ECL) Western blotting detection system (Amersham Biosciences), as described elsewhere (17).
Statistical analysis
Data were analyzed by investigators who were masked to genotype and treatment group assignment. Values are expressed as means ± se. Data were analyzed by ANOVA or Student’s t test using Systat software (Systat Inc., San Jose, CA, USA). Values of P < 0.05 were considered significant.
RESULTS
Generation and initial characterization of Cyp2j5 (−/−) Mice
Germline chimeras carrying the Cyp2j5 mutant allele were generated as shown in Fig. 1A and mated to 129/SvEv mice to generate isogenic progeny that were heterozygous for the null mutation. These mice were intercrossed to obtain additional Cyp2j5 (+/−) breeders and to generate Cyp2j5 (−/−) and Cyp2j5 (+/+) mice for study. Cyp2j5 (−/−) mice developed normally and lacked overt symptoms of disease or organ malformation. Their body weight, serum creatinine, urine output, and urine osmolality were not significantly different from Cyp2j5 (+/+) littermate controls (Table 1). Microscopic examination of all tissues including liver, kidney, adrenal, and reproductive organs revealed no significant pathology. Northern blotting with the CYP2J5 cDNA probe (Fig. 1C) and RT-PCR using sequence-specific oligonucleotide primers (Fig. 1D) showed complete absence of CYP2J5 transcripts in Cyp2j5 (−/−) kidney RNA. Protein immunoblotting with a specific CYP2J5 antibody (Fig. 1E) showed complete absence of CYP2J5 protein in Cyp2j5 (−/−) kidney microsomes, thus confirming functional disruption of the Cyp2j5 gene.
TABLE 1.
Phenotypic characterization of Cyp2j5 (−/−) mice
| Characteristic | Cyp2j5 (+/+) | Cyp2j5 (−/−) |
|---|---|---|
| Body weight (g) | 26.8 ± 0.6 | 27.2 ± 0.6 |
| Urine osmolarity, basal (mosmol/L) | 2083 ± 123 | 1934 ± 145 |
| Urine osmolarity, 24 h water deprivation (moosmol/L) | 3343 ± 579 | 2937 ± 186 |
| Urine output, normal salt diet (ml) | 1.3 ± 0.1 | 1.3 ± 0.1 |
| Urine output, high salt diet (ml) | 3.8 ± 0.6 | 3.6 ± 0.3 |
| Urine output, low salt diet (ml) | 0.9 ± 0.1 | 0.8 ± 0.2 |
| Serum creatinine (mg/dl) | 0.19 ± 0.04 | 0.18 ± 0.04 |
Values are means + se; n = 14–17 mice/group. Urine output was measured in a metabolic cage. Urine osmolality was measured with an osmometer. Serum creatinine was measured using a kit from Sigma-Aldrich.
The proportions of surviving Cyp2j5 (+/+), Cyp2j5 (+/−), and Cyp2j5 (−/−) mice from heterozygous matings were similar to expected Mendelian proportions. Moreover, Cyp2j5 (−/−) mice had a normal life span, suggesting that disruption of Cyp2j5 did not significantly affect survival. However, when Cyp2j5 (−/−) females were continuously mated with Cyp2j5 (+/+) males for 5 months, litter sizes were significantly smaller than when Cyp2j5 (+/+) females were continuously mated with Cyp2j5 (+/+) males (4.4±0.4 vs. 6.4±0.4 pups/litter; n=8 mice/group; P=0.0003) suggesting reduced female fertility. There were no significant differences in the number of litters per mouse (4.6±0.5 vs. 4.4±0.7; P=0.77) or in the period between consecutive litters (28.5±1.7 vs. 25.9±0.8 days; P=0.16).
Female Cyp2j5 (−/−) mice have increased blood pressure
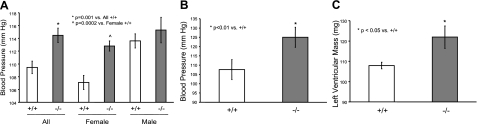
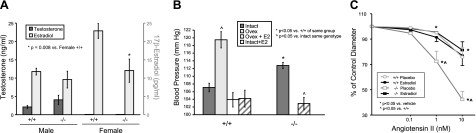
To determine the contribution of CYP2J5 to blood pressure homeostasis, we compared blood pressure values measured noninvasively by tail cuff in Cyp2j5 (−/−) and Cyp2j5 (+/+) mice. Interestingly, Cyp2j5 (−/−) mice had significantly higher SBP than their corresponding littermate control Cyp2j5 (+/+) mice (114.5±1.1 vs. 109.5±1.0 mmHg; n=26–28 mice/group; P=0.001) (Fig. 2A). Because of known sex differences in the prevalence of hypertension in rodents and in humans (29,30,31), we also analyzed blood pressure data separately for male and female mice. The differences in blood pressure in the entire cohort were driven largely by differences in female mice. Indeed, female Cyp2j5 (−/−) mice had significantly higher SBP than female Cyp2j5 (+/+) mice (112.8±0.8 vs. 107.1±1.1 mmHg; n=12–18 mice/group; P=0.0002) (Fig. 2A). In contrast, there were no significant differences in SBP between male Cyp2j5 (−/−) and male Cyp2j5 (+/+) mice (Fig. 2A). The hypertensive phenotype of Cyp2j5 (−/−) mice was confirmed by invasive blood pressure measurements. Thus, female Cyp2j5 (−/−) mice had significantly higher SBP than female Cyp2j5 (+/+) mice (125.0±5.4 vs. 107.6±5.4 mmHg; n=7–8 mice/group; P<0.01) (Fig. 2B). Moreover, the increased SBP in female Cyp2j5 (−/−) mice was accompanied by increased left ventricular mass (122±6 vs. 108±2 mg in Cyp2j5 (−/−) and Cyp2j5 (+/+) mice, respectively; n=6–7 mice/group; P<0.05) (Fig. 2C), consistent with the presence of cardiac hypertrophy. Differences in blood pressure between female Cyp2j5 (−/−) and female Cyp2j5 (+/+) mice persisted when animals were maintained on low-salt (114.2±2.2 vs. 105.9±2.3 mmHg; n=6–10 mice/group; P=0.01) and high-salt diets (113.2±3.7 vs. 101.8±2.3 mmHg; n=6–10 mice/group; P=0.01). Together, these data suggest a role for CYP2J5 in blood pressure regulation in females.
Figure 2.
Cyp2j5 (−/−) female mice have increased blood pressure and left ventricular mass. A) Systolic blood pressure measured in conscious mice via tail cuff is significantly higher in Cyp2j5 (−/−) mice than in Cyp2j5 (+/+) littermate controls. When analyzed separately by sex, only female Cyp2j5 (−/−) mice have elevated blood pressure relative to their female Cyp2j5 (+/+) counterparts; blood pressure is similar in male Cyp2j5 (−/−) and male Cyp2j5 (+/+) mice. Values are means ± se; n = 26 Cyp2j5 (−/−) mice (12 females, 14 males); n = 28 Cyp2j5 (+/+) mice (18 females, 10 males). *P = 0.001 vs. all Cyp2j5 (+/+) mice; ^P = 0.0002 vs. female Cyp2j5 (+/+) mice. B) Systolic blood pressure measured by invasive methods is significantly higher in female Cyp2j5 (−/−) mice than in female Cyp2j5 (+/+) littermate controls. Values are means ± se; n = 7–8 mice/group. *P < 0.01 vs. Cyp2j5 (+/+) mice. C) Left ventricular mass measured by two-dimensional M-mode echocardiography is significantly higher in Cyp2j5 (−/−) female mice than in Cyp2j5 (+/+) female littermate controls. Values are means ± se; n = 6–7 mice/group. *P < 0.05 vs. female Cyp2j5 (+/+) mice.
Female Cyp2j5 (−/−) mice have increased proximal tubular transport rates
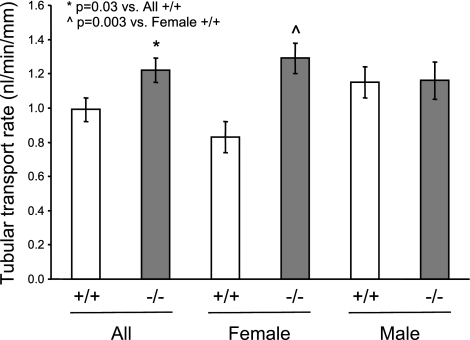
To determine if disruption of the Cyp2j5 gene caused alterations in renal tubular function, we compared proximal tubular transport rates in Cyp2j5 (−/−) and Cyp2j5 (+/+) animals. Transport rates from lumen to bath were significantly higher in Cyp2j5 (−/−) mice compared to Cyp2j5 (+/+) littermates (1.22±0.07 vs. 0.99±0.07 nl/min/mm; n=14 mice/group; P=0.03) (Fig. 3A). However, as in the case of blood pressure, differences between the groups were driven largely by differences in female mice. Thus, female Cyp2j5 (−/−) mice had significantly higher tubular transport rates than female Cyp2j5 (+/+) mice (1.29±0.09 vs. 0.83±0.09 nl/min/mm; n=7 mice/group; P=0.003) (Fig. 3A). In contrast, there were no significant differences in proximal tubular transport rates between male Cyp2j5 (−/−) and male Cyp2j5 (+/+) mice (Fig. 3A). Together, these data suggest a role for CYP2J5 in regulation of proximal tubular transport in females.
Figure 3.
Cyp2j5 (−/−) female mice have increased proximal tubular transport rates. Proximal tubular transport rates from lumen to bath are significantly higher in Cyp2j5 (−/−) mice than in Cyp2j5 (+/+) littermate controls. When analyzed separately by sex, only female Cyp2j5 (−/−) mice have elevated tubular transport rates relative to their female Cyp2j5 (+/+) counterparts; transport rates are comparable in male Cyp2j5 (−/−) and male Cyp2j5 (+/+) mice. Values are means ± se; n = 14 Cyp2j5 (−/−) mice (7 females, 7 males); n = 14 Cyp2j5 (+/+) mice (7 females, 7 males). *P = 0.03 vs. all Cyp2j5 (+/+) mice; P̂ = 0.003 vs. female Cyp2j5 (+/+) mice.
Female Cyp2j5 (−/−) mice have increased afferent arteriolar responses to endogenous vasoconstrictors
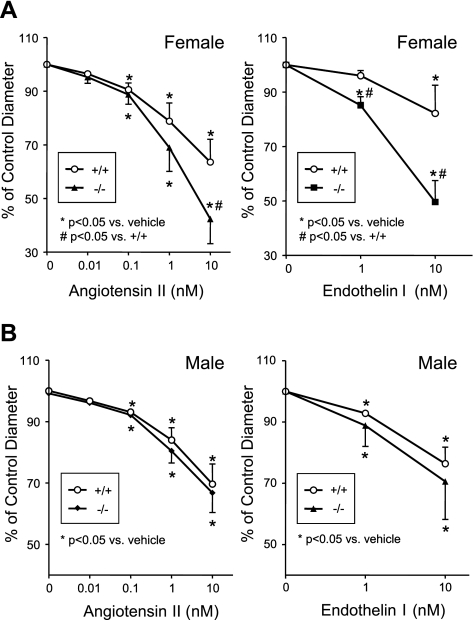
To study the role of CYP2J5 in modulation of vascular tone and responsiveness, we examined afferent arteriolar responses to 2 endogenous vasoconstrictors, angiotensin II and endothelin I. At baseline, afferent arteriolar diameters were similar in female Cyp2j5 (−/−) and female Cyp2j5 (+/+) mice (17.8±0.6 vs. 18.4±0.7 μm; n=8–9; P=NS, nonsignificant). Both angiotensin II and endothelin I caused significant, dose-dependent constriction of female afferent arterioles; however, responses to both agents were significantly enhanced in arterioles from female Cyp2j5 (−/−) mice as compared to arterioles from female Cyp2j5 (+/+) mice (Fig. 4A). Baseline afferent arteriolar diameters were also similar in male Cyp2j5 (−/−) and male Cyp2j5 (+/+) mice (17.3±0.5 vs. 16.7±0.5 μm; n=9–11; P=NS), and both angiotensin II and endothelin I caused significant, dose-dependent vasoconstriction of male afferent arterioles. However, in contrast to female responses, responses to both agents were similar in arterioles from male Cyp2j5 (−/−) mice and male Cyp2j5 (+/+) mice (Fig. 4B). Together, these data suggest a role for CYP2J5 in regulation of vascular responsiveness to endogenous vasoconstrictors in females.
Figure 4.
Cyp2j5 (−/−) female mice have increased afferent arteriolar responses to angiotensin II and endothelin I. Afferent arteriolar responses to increasing doses of angiotensin II (0.01–10 nM) and endothelin I (1–10 nM) in Cyp2j5 (−/−) mice (solid symbols) and Cyp2j5 (+/+) littermate controls (open symbols) are shown for females (A) and males (B). Results are reported as percentage of control diameter; values are means ± se, n = 8–11 mice/group. *P < 0.05 vs. vehicle, #P < 0.05 vs. Cyp2j5 (+/+) mice.
Female Cyp2j5 (−/−) mice have reduced circulating estrogen levels
Given the sexual dimorphism in blood pressure, proximal tubular transport, and afferent arteriolar response phenotypes of Cyp2j5 (−/−) mice, we postulated that disruption of the Cyp2j5 gene might alter circulating sex-hormone levels. Interestingly, plasma 17β-estradiol levels were significantly lower in female Cyp2j5 (−/−) mice than in female Cyp2j5 (+/+) littermate controls (12.0±3.2 vs. 22.9±2.0 pg/ml; n=15–17 mice/group; P=0.008) (Fig. 5A). In contrast, there were no significant differences in circulating 17β-estradiol levels between male Cyp2j5 (−/−) and male Cyp2j5 (+/+) mice (Fig. 5A). Plasma testosterone levels were similar in female Cyp2j5 (−/−) and female Cyp2j5 (+/+) mice (0.078±0.005 vs. 0.081±0.003 ng/ml, respectively; n=8–12; P=NS). Plasma testosterone levels were significantly higher in males than in females, but not significantly different between the 2 genotypes (Fig. 5A). Consistent with the reduced circulating estrogen levels, renal expression of estrogen-inducible genes such as insulin-like growth factor 2 and kidney androgen-regulated protein were down-regulated 5-fold (P<0.01) and 2-fold (P<0.05), respectively, in Cyp2j5 (−/−) females (data not shown).
Figure 5.
Cyp2j5 (−/−) female mice have reduced circulating estrogen levels, and estrogen replacement normalizes their blood pressure and afferent arteriolar response. A) Plasma 17β-estradiol levels are significantly lower in female Cyp2j5 (−/−) mice than in female Cyp2j5 (+/+) littermates. There are no significant differences in 17β-estradiol levels in males and no significant differences in circulating testosterone levels in males or females of either genotype. Values are means ± se; n = 15–17 mice/group for 17β-estradiol; 8–12 mice/group for testosterone; *P = 0.008 vs. female Cyp2j5 (+/+) mice. B) Blood pressure was measured by tail cuff in intact female mice with (hatched bars) or without (dark gray bars) estrogen supplementation, and in ovariectomized mice with (open bar) or without (light gray bar) estrogen supplementation. Value are means ± se; n = 7–12 mice/group. *P < 0.05 vs. Cyp2j5 (+/+) mice of same treatment group; ^P < 0.05 vs. intact mice of same genotype. C) Effect of estrogen replacement on afferent arteriolar responsiveness to angiotensin II in female mice. Female Cyp2j5 (−/−) mice (squares) and Cyp2j5 (+/+) littermate controls (circles) were treated with placebo (open symbols) or 17β-estradiol containing pellets (solid symbols) and afferent arteriolar responsiveness to angiotensin II was determined. Values are means ± se; n = 7 mice/group; *P < 0.05 vs. vehicle; ^P < 0.05 vs. Cyp2j5 (+/+).
To determine whether the reduced plasma 17β-estradiol levels in Cyp2j5 (−/−) females were accompanied by changes in pituitary hormone secretion, we measured circulating levels of LH and FSH. Plasma LH and FSH levels were similar in Cyp2j5 (−/−) and Cyp2j5 (+/+) females (LH: 1.44±0.06 vs. 1.44±0.05 ng/ml, respectively, n=15–16, P=NS; FSH: 3.58±0.53 vs. 3.81±0.52 ng/ml, respectively, n=14–15, P=NS), suggesting an inappropriate pituitary response to the reduced circulating estrogen (32, 33).
Effects of estrogen replacement on blood pressure and afferent arteriolar responses
To begin to elucidate the relationship between CYP2J5, estrogen, and blood pressure regulation, we initially examined female Cyp2j5 (+/+) mice that had undergone ovariectomy or sham surgery, with or without estrogen supplementation. As shown in Fig. 5B, systolic blood pressure was significantly higher in ovariectomized Cyp2j5 (+/+) female mice relative to Cyp2j5 (+/+) female mice with intact ovaries (119.5±2.1 vs. 107.1±1.1; n=10–25; P<0.001). In contrast, systolic blood pressures remained essentially normal in ovariectomized Cyp2j5 (+/+) female mice that received estrogen replacement therapy and in intact Cyp2j5 (+/+) mice that received estrogen supplementation (103.9±1.9 and 104.2±2.3 mmHg; n=7–10; P=NS vs. intact Cyp2j5 (+/+) mice that did not receive estrogen supplementation) (Fig. 5B). Importantly, whereas intact Cyp2j5 (−/−) mice had elevated systolic blood pressures (112.8±0.8 mmHg; n=24; P<0.001 vs. intact Cyp2j5 (+/+) mice), estrogen supplementation significantly reduced their systolic blood pressure to normal levels (102.9±1.6 mmHg; n=12; P<0.0001 vs. intact Cyp2j5 (−/−) mice; P=NS vs. intact Cyp2j5 (+/+) mice) (Fig. 5B). Together, these data indicate that 1) wild-type female mice exhibit increased blood pressure following ovariectomy only in the absence of estrogen replacement therapy; 2) estrogen supplementation alone has minimal effects on blood pressure in intact animals; and 3) the increased blood pressure in female Cyp2j5 (−/−) mice is estrogen-responsive.
To determine whether the increased vascular responsiveness in female Cyp2j5 (−/−) mice is also estrogen-responsive, we examined afferent arteriolar responses to angiotensin II. As shown in Fig. 5C, whereas estrogen supplementation had little effect on afferent arteriolar responses in Cyp2j5 (+/+) females, afferent arteriolar responses were markedly attenuated following treatment of female Cyp2j5 (−/−) mice with estrogen. Thus, the vascular responsiveness phenotype of female Cyp2j5 (−/−) mice is also estrogen responsive.
Estrogen biosynthesis and metabolism in female Cyp2j5 (−/−) mice
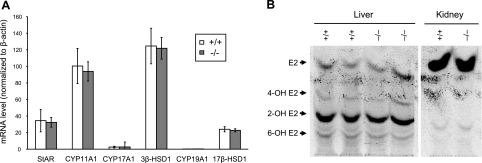
Since circulating estrogen levels were reduced in female Cyp2j5 (−/−) mice, we examined the expression of a variety of enzymes involved in sex-hormone biosynthesis at the mRNA level. Ovarian expression of StAR, CYP11A1, CYP17A1, 3β-HSD1, 17β-HSD1, and CYP19 were not significantly different between female Cyp2j5 (−/−) and female Cyp2j5 (+/+) mice (Fig. 6A). Likewise, aromatase activities were not significantly different in ovaries of Cyp2j5 (−/−) and Cyp2j5 (+/+) mice (1037±154 vs. 851±174 fmol/mg/h, respectively; P =NS). Based on this data, we conclude that estrogen biosynthesis is not likely altered in female Cyp2j5 (−/−) mice.
Figure 6.
Estrogen biosynthesis and metabolism is unchanged in female Cyp2j5 (−/−) mice. A) Ovarian expression of StAR, CYP11A1, CYP17A1, 3β-HSD1, CYP19A1, and 17β-HSD1 in Cyp2j5 (−/−) and Cyp2j5 (+/+) female mice. Values are means ± se; n = 8 mice/group. B) Metabolism of 17β-estradiol by liver and kidney microsomes form Cyp2j5 (−/−) and Cyp2j5 (+/+) female mice. Retention of authentic standards for 17β-estradiol and its hydroxylated derivatives is marked by arrows. Results are representative of 3 independent experiments with identical results.
Estradiol can be further metabolized via oxidation by a number of P450 enzymes, including members of the CYP1A, CYP1B, CYP2C, CYP2C, CYP2D, and CYP3A subfamilies (34). We did not detect significant changes in expression of any of these CYP enzymes in ovaries, kidneys, or livers of Cyp2j5 (−/−) mice relative to Cyp2j5 (+/+) controls (data not shown). Moreover, renal and hepatic microsomal metabolism of estradiol was similar in Cyp2j5 (−/−) and Cyp2j5 (+/+) mice (Fig. 6B). Notably, neither recombinant CYP2J5 nor recombinant CYP2J9 (see below) catalyzed estrogen oxidation (data not shown). Based on this data, we conclude that estrogen metabolism is also not likely altered in female Cyp2j5 (−/−) mice.
AA metabolism in Cyp2j5 (−/−) mice
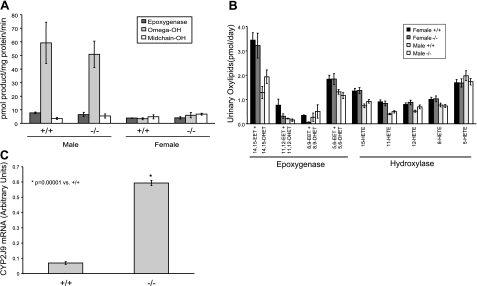
Since CYP2J5 is active in the metabolism of AA to eicosanoids that can influence renal vascular tone and tubular transport processes (17), we examined AA metabolism in Cyp2j5 (−/−) mice and Cyp2j5 (+/+) littermate controls. Surprisingly, as shown in Fig. 7A, while there were some differences in renal microsomal AA metabolism between male and female mice, there were no significant differences between Cyp2j5 (−/−) and Cyp2j5 (+/+) mice of either sex. Moreover, urinary excretion of AA epoxygenase (EET) and hydroxylase (HETE) products was also similar between the 2 genotypes in both males and females (Fig. 7B). Based on this data, we conclude that renal arachidonic metabolism is not significantly altered in Cyp2j5 (−/−) mice.
Figure 7.
Arachidonic acid metabolism is unchanged in Cyp2j5 (−/−) mice. A) Renal microsomal AA epoxygenase, midchain hydroxylase and ω-hydroxylase activity is similar in Cyp2j5 (−/−) and Cyp2j5 (+/+) mice of either sex. Results are means ± se; n = 3–4 mice/group. B) Urinary levels of AA epoxygenase and hydroxylase metabolites are similar in Cyp2j5 (−/−) and Cyp2j5 (+/+) mice of either sex. Values are means ± se; n = 12 mice/group. C) Cyp2j5 (−/−) female mice have increased CYP2J9 mRNA levels compared to Cyp2j5 (+/+) littermate controls. Values are means ± se; n = 3 mice/group.
Disruption of a single gene can lead to compensatory changes in expression of other genes, resulting in altered fatty-acid metabolism. For example, recent studies have demonstrated that up-regulation of Cyp4a12 in Cyp4a14 (−/−) mice is associated with increased renal AA ω-hydroxylase activity, and down-regulation of Cyp2c44 in Cyp4a10 (−/−) mice is associated with reduced renal AA epoxygenase activity (13, 14). To examine whether absence of CYP2J5 alters expression of other renal enzymes involved in AA metabolism, we performed immunoblotting of kidney microsomes prepared from Cyp2j5 (−/−) and Cyp2j5 (+/+) mice. As shown in Fig. 1D, while there were sex differences in the expression of CYP2C, CYP4A and CYP4F subfamily enzymes, and in the expression of sEH, renal expression of these proteins was similar in Cyp2j5 (−/−) and Cyp2j5 (+/+) mice of either sex. Similarly, CYP4A10, CYP4A12, and CYP4A14 transcript levels were not significantly different between the genotypes (data not shown). Moreover, expression of eNOS, which modifies renal P450 expression and activity (35), was comparable in Cyp2j5 (−/−) and Cyp2j5 (+/+) mice. Mouse CYP2J9 is expressed in kidney and is active in the metabolism of AA to EETs (36). Interestingly, CYP2J9 expression was significantly higher in kidneys from Cyp2j5 (−/−) mice than in kidneys from Cyp2j5 (+/+) littermate controls (Fig. 7C). Based on these data, we conclude that there is a compensatory up-regulation of Cyp2j9 in Cyp2j5 (−/−) mice and that this may account for the absence of observed changes in EET biosynthesis in Cyp2j5 (−/−) kidneys.
DISCUSSION
Control of blood pressure requires the complex integration of multiple regulatory systems. In addition to the renin-angiotensin-aldosterone system and endothelial-derived relaxing factors (e.g., eNOS, prostacyclin), there is growing support for the involvement of CYP enzymes and their eicosanoid metabolites in blood pressure regulation (1,2,3, 37). Indeed, disruption of the Cyp4a10 and Cyp4a14 genes in mice causes increased blood pressure (13, 14) and a polymorphism in the CYP4A11 gene in humans is associated with essential hypertension (15). In the current study, we disrupted the Cyp2j5 gene in mice to determine its physiological function. Our main finding is that female Cyp2j5 (−/−) mice have increased blood pressure compared with their female wild-type counterparts. The magnitude of the increased blood pressure is comparable to that observed in angiotensinogen gene duplication transgenic mice (38). Furthermore, the increased blood pressure phenotype in Cyp2j5 (−/−) females is associated with increased left ventricular mass, increased proximal tubular transport rates, and enhanced renal vascular responsiveness to endogenous vasoconstrictors including angiotensin II and endothelin I. Importantly, the increased blood pressure in Cyp2j5 (−/−) females is associated with reduced circulating estrogen and estrogen replacement restores blood pressure and vascular responsiveness to normal levels. Together, our data provides direct evidence of a role for Cyp2j5 in blood pressure regulation in females and indicates that the vascular phenotypes in Cyp2j5 (−/−) mice are estrogen responsive.
Sex differences in blood pressure have been observed in humans and other species. In humans, blood pressure is lower in premenopausal females than in age-matched males; however, blood pressure rises with age in females such that the prevalence of hypertension is higher in older, postmenopausal females than in older males (39, 40). In the SHR, higher blood pressure has been noted in males compared to females (29, 30). Similarly, sex differences in salt-induced hypertension have been reported in Dahl salt-sensitive rats (41). We and others have reported sex differences in blood pressure in multiple mouse strains, including Balb/c, DBA/2, 129/SvEv, and 129/SvJ (13, 42). Sex hormones have been implicated in the pathogenesis of hypertension in both rodents and humans. For example, castration of male SHR reduces blood pressure to female levels and treatment of female SHR with androgen increases blood pressure to male levels (30). Moreover, treatment of male SHR with an androgen receptor antagonist lowers blood pressure (43). Likewise, hypertension in Cyp4a14 null male mice is associated with increased circulating androgen levels and castration normalizes blood pressure (13). In contrast, estrogen has been proposed to control blood flow and peripheral vascular resistance via alterations in the release of endothelial mediators, such as nitric oxide, prostaglandins, and endothelium-derived hyperpolarizing factor (44). Estrogen treatment attenuates the development of hypertension and ovariectomy increases blood pressure in SHR and Dahl salt-sensitive rats (45,46,47). Furthermore, estrogen treatment of postmenopausal women leads to a reduction in blood pressure (48,49,50,51). Together, these studies suggest that while androgens are generally prohypertensive, estrogens tend to have antihypertensive effects.
Plasma 17β-estradiol levels of female Cyp2j5 (−/−) mice are reduced to ∼50% of the levels seen in female Cyp2j5 (+/+) mice of similar age. Indeed, the estrogen levels observed in young adult Cyp2j5 (−/−) mice are similar to those normally observed in older, postmenopausal, wild-type mice (52). In general, estrogen levels do not differ significantly between mouse strains. For example, estrogen levels in wild-type female mice on a C57BL/6 background are comparable to those observed in wild-type mice on a 129/SvEv background in our study (27). To determine whether the blood pressure and vascular responsiveness phenotypes in the Cyp2j5 (−/−) mice were related to their reduced circulating estrogen levels, we implanted sustained-release pellets containing 17β-estradiol at a dose that has previously been shown to provide physiological levels of estrogen (22). Importantly, estrogen replacement reduced blood pressure and vascular responsiveness in female Cyp2j5 (−/−) mice to levels that were comparable to those observed in female Cyp2j5 (+/+) controls. Moreover, ovariectomy to reduce circulating estrogen caused an increase in blood pressure in Cyp2j5 (+/+) mice, an effect that was abrogated by estrogen replacement. Interestingly, estrogen supplementation did not significantly alter blood pressures in intact female Cyp2j5 (+/+), mice suggesting that the antihypertensive properties of estrogen only occur when estrogen levels are reduced, as in the case of Cyp2j5 (−/−) mice. Enhanced renal vascular sensitivity to angiotensin II has been demonstrated in the early phase of hypertension in rodents and humans (53, 54). It has also been previously shown that vasoconstrictive effects of angiotensin II, endothelin I, and phenylephrine can be opposed by estrogen (55, 56). Estrogen mediates its physiological actions via 2 estrogen receptors (ERα and ERβ). Both receptors are present in vascular endothelial and smooth muscle cells, where they exert vasodilatory effects through eNOS-dependent mechanisms (57,58,59). Disruption of ERβ in mice has been shown to enhance vasoconstrictive responses to phenylephrine and cause hypertension (60). Whether the estrogen responsiveness of the blood pressure and vascular phenotypes in Cyp2j5 (−/−) mice is mediated by ERα or ERβ or through nonreceptor mechanisms remains unknown.
CYP enzymes have been shown to be involved in estrogen biosynthesis and metabolism (34, 61, 62). Indeed, many of the enzymes involved in steroidogenesis are CYPs including members of the CYP11, CYP17, and CYP19 subfamilies (61). Furthermore, estrogens are eliminated from the body by conversion to inactive metabolites that are excreted in the urine and/or feces. The first step in the metabolism of estrogens is hydroxylation catalyzed by members of the CYP1A, CYP1B, CYP2C, CYP2C, CYP2D, and CYP3A subfamilies (34, 62). CYP2J5 is not expressed in reproductive tissues (17) (unpublished results) and we did not observe any changes in the expression and/or activity of known steroidogenic pathway enzymes in Cyp2j5 (−/−) ovaries, suggesting that estrogen biosynthesis is not likely altered in these mice. Moreover, we did not detect significant changes in the expression of CYP1A, CYP1B, CYP2C, CYP2D, or CYP3A subfamily enzymes in ovaries, kidneys, or livers of Cyp2j5 (−/−) mice, we did not detect any alterations in renal or hepatic microsomal metabolism of 17β-estradiol in Cyp2j5 (−/−) mice, and neither CYP2J5 nor CYP2J9 catalyzed the hydroxylation of 17β-estradiol, suggesting that estrogen metabolism is also unlikely to be altered in female Cyp2j5 (−/−) mice. Therefore, the precise molecular mechanisms responsible for reduced circulating estrogen in Cyp2j5 (−/−) mice remain unknown. We postulate that catalytic turnover by CYP2J5 generates a yet-to-be-characterized mediator that modulates the levels of circulating estrogens. Our findings are reminiscent of the sexually dimorphic hypertension in Cyp4a14 (−/−) mice that is associated with increased androgen levels via unknown mechanisms (13).
Estrogen biosynthesis in the ovary is tightly regulated by pituitary gonadotropins. Indeed, the reduced estrogen state that characterizes menopause is accompanied by increased pituitary gonadotropin secretion, and primary pituitary abnormalities can lead to reduced circulating estrogen levels and infertility (32, 33, 63). In this regard, we found that LH and FSH levels were similar in female Cyp2j5 (−/−) and Cyp2j5 (+/+) mice, despite the reduced circulating estrogen in the Cyp2j5 (−/−) females. Thus, female Cyp2j5 (−/−) mice had inappropriately low gonadotropin levels for their level of estrogen, suggesting the presence of an altered pituitary-ovarian axis. Capdevila and co-workers (64, 65) have previously shown that EETs can act as secretagogues in the pituitary. Indeed, it remains possible that the impact of CYP2J5 deficiency is limited to the pituitary. Interestingly, the inappropriately low gonadotropin levels and reduced circulating estrogen levels in Cyp2j5 (−/−) mice were accompanied by reduced fertility.
EETs are potent vasodilators in a variety of vascular beds (2, 4, 7). EETs have also been shown to modulate sodium and water transport in different portions of the nephron (2, 4, 66, 67). For example, EETs inhibit sodium transport in the proximal tubule and inhibit sodium reabsorption and vasopressin-stimulated water reabsorption in the cortical collecting duct (68, 69). We observed that female Cyp2j5 (−/−) mice have increased proximal tubular transport rates, which could result in increased intravascular volume and elevated blood pressure. However, salt loading had no significant effect on blood pressure in Cyp2j5 (−/−) females. Moreover, serum and urinary sodium (data not shown) and water conservation (measured by the ability to increase urine osmolarity following a period of water deprivation) were normal in female Cyp2j5 (−/−) mice. These findings suggest that the altered tubular transport rates likely contributed minimally to the elevated blood pressure in this model.
Although CYP2J5 is abundant in both kidney and liver and active in the metabolism of AA to EETs (17), we did not observe significant differences in renal or hepatic microsomal metabolism of AA in Cyp2j5 (−/−) mice, nor did their urinary excretion of EETs or HETEs differ significantly. CYP-derived eicosanoids have a relatively short half-life and their biological effects are often limited to cells in close proximity to their site of biosynthesis. Current methods to quantify eicosanoid levels at the whole body or tissue level are not sufficiently sensitive to detect changes in local concentrations of these metabolites. Thus, measuring EET production in whole kidney microsomes and endogenous EET levels in urine may have masked local changes that could potentially explain the observed phenotypic outcomes. In addition, several different CYPs may contribute to EET and HETE biosynthesis in kidney and liver (2). Interestingly, we found that expression of CYP2J9, which is an active AA epoxygenase (36), is increased more than 8-fold in female Cyp2j5 (−/−) kidneys. We postulate that this compensatory increase in CYP2J9 expression explains, at least in part, our inability to detect changes in renal EET biosynthesis and urinary EET excretion in Cyp2j5 (−/−) mice.
We previously reported sex differences in renal CYP2J5 expression in adult mice such that males had more CYP2J5 than females (70). Castration of males resulted in decreased renal CYP2J5 expression, and treatment of castrated males or females with testosterone increased CYP2J5 expression (70). In contrast, treatment of ovariectomized females or castrated males with estradiol caused a further reduction in CYP2J5 expression (70). Thus, renal CYP2J5 expression is sex hormone-regulated. These findings are particularly interesting given the current findings of a sex-specific role for CYP2J5 in regulation of blood pressure via an estrogen-dependent mechanism.
In conclusion, our studies provide direct evidence for a role of Cyp2j5 in control of blood pressure, proximal tubular transport and afferent arteriolar responsiveness in female mice. Our data are also consistent with a role of Cyp2j5 in regulating circulating estrogen levels and implicate estrogen in the pathogenesis of the blood pressure and vascular responsiveness phenotypes of these mice. Further studies with the Cyp2j5 (−/−) mice may lead to a better understanding of the complex interrelationships between the blood pressure, sex hormones, and renal eicosanoids.
Acknowledgments
We thank Drs. Michael Fessler and Robert Langenbach for helpful comments during the preparation of this manuscript, Dr. Howard Rockman for assistance with echocardiography, and Dr. Beverly Koller for assistance with generation of the Cyp2j5 (−/−) mice. This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences Z01 ES025034 (K.S.K., D.C.Z.), and NIH grants HL6876 (D.R.H.), DK38226 (J.D.I.), ES004699 (B.D.H.), ES02710 (B.D.H.), MH39917 (A.J.C.) and DK069896 (T.M.C.).
References
- Capdevila J H, Falck J R, Harris R C. Cytochrome P450 and arachidonic acid bioactivation: molecular and functional properties of the arachidonate monooxygenase. J Lipid Res. 2000;41:163–181. [PubMed] [Google Scholar]
- Roman R J. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Zeldin D C. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- McGiff J C, Quilley J. 20-hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids and blood pressure. Curr Opin Nephrol Hypertens. 2001;10:231–237. doi: 10.1097/00041552-200103000-00012. [DOI] [PubMed] [Google Scholar]
- Imig J D, Zou A P, Stec D E, Harder D R, Falck J R, Roman R J. Formation and actions of 20-hydroxyeicosatetraenoic acid in rat renal arterioles. Am J Physiol. 1996;270:R217–227. doi: 10.1152/ajpregu.1996.270.1.R217. [DOI] [PubMed] [Google Scholar]
- Escalante B, Omata K, Sessa W, Lee S G, Falck J R, Schwartzman M L. 20-hydroxyeicosatetraenoic acid is an endothelium-dependent vasoconstrictor in rabbit arteries. Eur J Pharmacol. 1993;235:1–7. doi: 10.1016/0014-2999(93)90812-v. [DOI] [PubMed] [Google Scholar]
- Imig J D, Falck J R, Wei S, Capdevila J H. Epoxygenase metabolites contribute to nitric oxide-independent afferent arteriolar vasodilation in response to bradykinin. J Vasc Res. 2001;38:247–255. doi: 10.1159/000051053. [DOI] [PubMed] [Google Scholar]
- Oltman C L, Weintraub N L, VanRollins M, Dellsperger K C. Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ Res. 1998;83:932–939. doi: 10.1161/01.res.83.9.932. [DOI] [PubMed] [Google Scholar]
- Makita K, Takahashi K, Karara A, Jacobson H R, Falck J R, Capdevila J H. Experimental and/or genetically controlled alterations of the renal microsomal cytochrome P450 epoxygenase induce hypertension in rats fed a high salt diet. J Clin Invest. 1994;94:2414–2420. doi: 10.1172/JCI117608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman M L, da Silva J L, Lin F, Nishimura M, Abraham N G. Cytochrome P450 4A expression and arachidonic acid omega-hydroxylation in the kidney of the spontaneously hypertensive rat. Nephron. 1996;73:652–663. doi: 10.1159/000189154. [DOI] [PubMed] [Google Scholar]
- Yu Z, Huse L M, Adler P, Graham L, Ma J, Zeldin D C, Kroetz D L. Increased CYP2J expression and epoxyeicosatrienoic acid formation in spontaneously hypertensive rat kidney. Mol Pharmacol. 2000;57:1011–1020. [PubMed] [Google Scholar]
- Catella F, Lawson J A, Fitzgerald D J, FitzGerald G A. Endogenous biosynthesis of arachidonic acid epoxides in humans: increased formation in pregnancy-induced hypertension. Proc Natl Acad Sci U S A. 1990;87:5893–5897. doi: 10.1073/pnas.87.15.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holla V R, Adas F, Imig J D, Zhao X, Price E, Jr, Olsen N, Kovacs W J, Magnuson M A, Keeney D S, Breyer M D, Falck J R, Waterman M R, Capdevila J H. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci U S A. 2001;98:5211–5216. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa K, Holla V R, Wei Y, Wang W H, Gatica A, Wei S, Mei S, Miller C M, Cha D R, Price E, Jr, Zent R, Pozzi A, Breyer M D, Guan Y, Falck J R, Waterman M R, Capdevila J H. Salt-sensitive hypertension is associated with dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J Clin Invest. 2006;116:1696–1702. doi: 10.1172/JCI27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainer J V, Bellamine A, Dawson E P, Womble K E, Grant S W, Wang Y, Cupples L A, Guo C Y, Demissie S, O'Donnell C J, Brown N J, Waterman M R, Capdevila J H. Functional variant of CYP4A11 20-hydroxyeicosatetraenoic acid synthase is associated with essential hypertension. Circulation. 2005;111:63–69. doi: 10.1161/01.CIR.0000151309.82473.59. [DOI] [PubMed] [Google Scholar]
- King L M, Gainer J V, David G L, Dai D, Goldstein J A, Brown N J, Zeldin D C. Single nucleotide polymorphisms in the CYP2J2 and CYP2C8 genes and the risk of hypertension. Pharmacogenet Genomics. 2005;15:7–13. doi: 10.1097/01213011-200501000-00002. [DOI] [PubMed] [Google Scholar]
- Ma J, Qu W, Scarborough P E, Tomer K B, Moomaw C R, Maronpot R, Davis L S, Breyer M D, Zeldin D C. Molecular cloning, enzymatic characterization, developmental expression, and cellular localization of a mouse cytochrome P450 highly expressed in kidney. J Biol Chem. 1999;274:17777–17788. doi: 10.1074/jbc.274.25.17777. [DOI] [PubMed] [Google Scholar]
- Koller B H, Smithies O. Altering genes in animals by gene targeting. Annu Rev Immunol. 1992;10:705–730. doi: 10.1146/annurev.iy.10.040192.003421. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch E F, Maniatis T. Cold Spring Harbor, NY, USA: Cold Spring Harbor Press; 1989 [Google Scholar]
- Krege J H, Hodgin J B, Hagaman J R, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- Esposito G, Rapacciuolo A, Naga Prasad S V, Takaoka H, Thomas S A, Koch W J, Rockman H A. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002;105:85–92. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- Tang A C, Nakazawa M, Romeo R D, Reeb B C, Sisti H, McEwen B S. Effects of long-term estrogen replacement on social investigation and social memory in ovariectomized C57BL/6 mice. Horm Behav. 2005;47:350–357. doi: 10.1016/j.yhbeh.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Quigley R, Baum M, Reddy K M, Griener J C, Falck J R. Effects of 20-HETE and 19(S)-HETE on rabbit proximal straight tubule volume transport. Am J Physiol. 2000;278:F949–953. doi: 10.1152/ajprenal.2000.278.6.F949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J W, Watanabe T, Hammock B D. The simultaneous quantification of cytochrome P450 dependent linoleate and arachidonate metabolites in urine by HPLC-MS/MS. J Lipid Res. 2002;43:1563–1578. doi: 10.1194/jlr.d200018-jlr200. [DOI] [PubMed] [Google Scholar]
- Corbin C J, Trant J M, Conley A J. Porcine gonadal and placental isozymes of aromatase cytochrome P450: sub-cellular distribution and support by NADPH-cytochrome P450 reductase. Mol Cell Endocrinol. 2001;172:115–124. doi: 10.1016/s0303-7207(00)00373-7. [DOI] [PubMed] [Google Scholar]
- Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Couse J F, Yates M M, Walker V R, Korach K S. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- Couse J F, Yates M M, Sanford R, Nyska A, Nilson J H, Korach K S. Formation of cystic ovarian follicles associated with elevated luteinizing hormone requires estrogen receptor-beta. Endocrinology. 2004;145:4693–4702. doi: 10.1210/en.2004-0548. [DOI] [PubMed] [Google Scholar]
- Reckelhoff J F, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension. 2000;35:480–483. doi: 10.1161/01.hyp.35.1.480. [DOI] [PubMed] [Google Scholar]
- Reckelhoff J F, Zhang H, Srivastava K, Granger J P. Gender differences in hypertension in spontaneously hypertensive rats: role of androgens and androgen receptor. Hypertension. 1999;34:920–923. doi: 10.1161/01.hyp.34.4.920. [DOI] [PubMed] [Google Scholar]
- Wiinberg N, Hoegholm A, Christensen H R, Bang L E, Mikkelsen K L, Nielsen P E, Svendsen T L, Kampmann J P, Madsen N H, Bentzon M W. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens. 1995;8:978–986. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Chakravarti S, Collins W P, Forecast J D, Newton J R, Oram D H, Studd J W. Hormonal profiles after the menopause. Br Med J. 1976;2:784–787. doi: 10.1136/bmj.2.6039.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalch D S, Parlow A F, Boon R C, Reichlin S. Measurement of human luteinizing hormone in plasma by radioimmunoassay. J Clin Invest. 1968;47:665–678. doi: 10.1172/JCI105762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A J, Cai M X, Thomas P E, Conney A H, Zhu B T. Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology. 2003;144:3382–3398. doi: 10.1210/en.2003-0192. [DOI] [PubMed] [Google Scholar]
- Oyekan A O, Youseff T, Fulton D, Quilley J, McGiff J C. Renal cytochrome P450 omega-hydroxylase and epoxygenase activity are differentially modified by nitric oxide and sodium chloride. J Clin Invest. 1999;104:1131–1137. doi: 10.1172/JCI6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu W, Bradbury J A, Tsao C C, Maronpot R, Harry G J, Parker C E, Davis L S, Breyer M D, Waalkes M P, Falck J R, Chen J, Rosenberg R L, Zeldin D C. Cytochrome P450 CYP2J9, a new mouse arachidonic acid omega-1 hydroxylase predominantly expressed in brain. J Biol Chem. 2001;276:25467–25479. doi: 10.1074/jbc.M100545200. [DOI] [PubMed] [Google Scholar]
- McGiff J C. Cytochrome P-450 metabolism of arachidonic acid. Annu Rev Pharmacol Toxicol. 1991;31:339–369. doi: 10.1146/annurev.pa.31.040191.002011. [DOI] [PubMed] [Google Scholar]
- Kim H S, Krege J H, Kluckman K D, Hagaman J R, Hodgin J B, Best C F, Jennette J C, Coffman T M, Maeda N, Smithies O. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci U S A. 1995;92:2735–2739. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins D, Nelson K, Pan D, Tareen N, Norris K. The effect of gender on age-related blood pressure changes and the prevalence of isolated systolic hypertension among older adults: data from NHANES III. J Gend Specif Med. 2001;4:10–13, 20. [PubMed] [Google Scholar]
- August P, Oparil S. Hypertension in women. J Clin Endocrinol Metab. 1999;84:1862–1866. doi: 10.1210/jcem.84.6.5724. [DOI] [PubMed] [Google Scholar]
- Bayorh M A, Socci R R, Eatman D, Wang M, Thierry-Palmer M. The role of gender in salt-induced hypertension. Clin Exp Hypertens. 2001;23:241–255. doi: 10.1081/ceh-100102663. [DOI] [PubMed] [Google Scholar]
- Gurley S B, Clare S E, Snow K P, Hu A, Meyer T W, Coffman T M. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol. 2006;290:F214–222. doi: 10.1152/ajprenal.00204.2005. [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Ito O, Omata K, Ito S. Role of androgens in the renal production of 20-hydroxyeicosatetraenoic acid in spontaneously hypertensive rats. Nippon Jinzo Gakkai Shi. 2004;46:685–692. [PubMed] [Google Scholar]
- Huang A, Kaley G. Gender-specific regulation of cardiovascular function: estrogen as key player. Microcirculation. 2004;11:9–38. doi: 10.1080/10739680490266162. [DOI] [PubMed] [Google Scholar]
- Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood J R, Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension. 2004;44:405–409. doi: 10.1161/01.HYP.0000142893.08655.96. [DOI] [PubMed] [Google Scholar]
- Hoeg J M, Willis L R, Weinberger M H. Estrogen attenuation of the development of hypertension in spontaneously hypertensive rats. Am J Physiol. 1977;233:H369–373. doi: 10.1152/ajpheart.1977.233.3.H369. [DOI] [PubMed] [Google Scholar]
- Peng N, Clark J T, Wei C C, Wyss J M. Estrogen depletion increases blood pressure and hypothalamic norepinephrine in middle-aged spontaneously hypertensive rats. Hypertension. 2003;41:1164–1167. doi: 10.1161/01.HYP.0000065387.09043.2E. [DOI] [PubMed] [Google Scholar]
- Seely E W, Walsh B W, Gerhard M D, Williams G H. Estradiol with or without progesterone and ambulatory blood pressure in postmenopausal women. Hypertension. 1999;33:1190–1194. doi: 10.1161/01.hyp.33.5.1190. [DOI] [PubMed] [Google Scholar]
- Jespersen C M, Arnung K, Hagen C, Hilden T, Nielsen F, Nielsen M D, Giese J. Effects of natural oestrogen therapy on blood pressure and renin-angiotensin system in normotensive and hypertensive menopausal women. J Hypertens. 1983;1:361–364. doi: 10.1097/00004872-198312000-00007. [DOI] [PubMed] [Google Scholar]
- Mercuro G, Zoncu S, Pilia I, Lao A, Melis G B, Cherchi A. Effects of acute administration of transdermal estrogen on postmenopausal women with systemic hypertension. Am J Cardiol. 1997;80:652–655. doi: 10.1016/s0002-9149(97)00444-x. [DOI] [PubMed] [Google Scholar]
- Manhem K, Ahlm H, Milsom I, Svensson A. Transdermal oestrogen reduces daytime blood pressure in hypertensive women. J Hum Hypertens. 1998;12:323–327. doi: 10.1038/sj.jhh.1000563. [DOI] [PubMed] [Google Scholar]
- Gee D M, Flurkey K, Finch C E. Aging and the regulation of luteinizing hormone in C57BL/6J mice: impaired elevations after ovariectomy and spontaneous elevations at advanced ages. Biol Reprod. 1983;28:598–607. doi: 10.1095/biolreprod28.3.598. [DOI] [PubMed] [Google Scholar]
- Imig J D. Afferent arteriolar reactivity to angiotensin II is enhanced during the early phase of angiotensin II hypertension. Am J Hypertens. 2000;13:810–818. doi: 10.1016/s0895-7061(00)00264-8. [DOI] [PubMed] [Google Scholar]
- Simon G, Abraham G, Cserep G. Pressor and subpressor angiotensin II administration. Two experimental models of hypertension. Am J Hypertens. 1995;8:645–650. doi: 10.1016/0895-7061(95)00047-S. [DOI] [PubMed] [Google Scholar]
- Nickenig G, Baumer A T, Grohe C, Kahlert S, Strehlow K, Rosenkranz S, Stablein A, Beckers F, Smits J F, Daemen M J, Vetter H, Bohm M. Estrogen modulates AT1 receptor gene expression in vitro and in vivo. Circulation. 1998;97:2197–2201. doi: 10.1161/01.cir.97.22.2197. [DOI] [PubMed] [Google Scholar]
- Dubey R K, Jackson E K, Keller P J, Imthurn B, Rosselli M. Estradiol metabolites inhibit endothelin synthesis by an estrogen receptor-independent mechanism. Hypertension. 2001;37:640–644. doi: 10.1161/01.hyp.37.2.640. [DOI] [PubMed] [Google Scholar]
- Widder J, Pelzer T, von Poser-Klein C, Hu K, Jazbutyte V, Fritzemeier K H, Hegele-Hartung C, Neyses L, Bauersachs J. Improvement of endothelial dysfunction by selective estrogen receptor-alpha stimulation in ovariectomized SHR. Hypertension. 2003;42:991–996. doi: 10.1161/01.HYP.0000098661.37637.89. [DOI] [PubMed] [Google Scholar]
- Darblade B, Pendaries C, Krust A, Dupont S, Fouque M J, Rami J, Chambon P, Bayard F, Arnal J F. Estradiol alters nitric oxide production in the mouse aorta through the alpha-, but not beta-, estrogen receptor. Circ Res. 2002;90:413–419. doi: 10.1161/hh0402.105096. [DOI] [PubMed] [Google Scholar]
- Muller-Delp J M, Lubahn D B, Nichol K E, Philips B J, Price E M, Curran E M, Laughlin M H. Regulation of nitric oxide-dependent vasodilation in coronary arteries of estrogen receptor-alpha-deficient mice. Am J Physiol Heart Circ Physiol. 2003;285:H2150–2157. doi: 10.1152/ajpheart.00966.2002. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bian Z, Lu P, Karas R H, Bao L, Cox D, Hodgin J, Shaul P W, Thoren P, Smithies O, Gustafsson J A, Mendelsohn M E. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science. 2002;295:505–508. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]
- Conley A J, Bird I M. The role of cytochrome P450 17 alpha-hydroxylase and 3 beta-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the delta 5 and delta 4 pathways of steroidogenesis in mammals. Biol Reprod. 1997;56:789–799. doi: 10.1095/biolreprod56.4.789. [DOI] [PubMed] [Google Scholar]
- Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005;227:115–124. doi: 10.1016/j.canlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Christin-Maitre S, Laveille C, Collette J, Brion N, Reginster J Y. Pharmacodynamics of follicle stimulating hormone (FSH) in postmenopausal women during pulsed estrogen therapy: Evidence that FSH release and synthesis are controlled by distinct pathways. J Clin Endocrinol Metab. 2003;88:5405–5413. doi: 10.1210/jc.2003-030094. [DOI] [PubMed] [Google Scholar]
- Negro-Vilar A, Snyder G D, Falck J R, Manna S, Chacos N, Capdevila J. Involvement of eicosanoids in release of oxytocin and vasopressin from the neural lobe of the rat pituitary. Endocrinology. 1985;116:2663–2668. doi: 10.1210/endo-116-6-2663. [DOI] [PubMed] [Google Scholar]
- Snyder G, Lattanzio F, Yadagiri P, Falck J R, Capdevila J. 5,6-Epoxyeicosatrienoic acid mobilizes Ca2+ in anterior pituitary cells. Biochem Biophys Res Commun. 1986;139:1188–1194. doi: 10.1016/s0006-291x(86)80303-5. [DOI] [PubMed] [Google Scholar]
- Maier K G, Roman R J. Cytochrome P450 metabolites of arachidonic acid in the control of renal function. Curr Opin Nephrol Hypertens. 2001;10:81–87. doi: 10.1097/00041552-200101000-00013. [DOI] [PubMed] [Google Scholar]
- Wei Y, Lin D H, Kemp R, Yaddanapudi G S, Nasjletti A, Falck J R, Wang W H. Arachidonic acid inhibits epithelial Na channel via cytochrome P450 (CYP) epoxygenase-dependent metabolic pathways. J Gen Physiol. 2004;124:719–727. doi: 10.1085/jgp.200409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhun Z T, Goldthwait D A, McKay D, Hopfer U, Douglas J G. An epoxygenase metabolite of arachidonic acid mediates angiotensin II-induced rises in cytosolic calcium in rabbit proximal tubule epithelial cells. J Clin Invest. 1991;88:456–461. doi: 10.1172/JCI115325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt D L, Capdevila J, Falck J R, Breyer M D, Jacobson H R. Cytochrome P450 metabolites of arachidonic acid are potent inhibitors of vasopressin action on rabbit cortical collecting duct. J Clin Invest. 1989;84:1805–1812. doi: 10.1172/JCI114365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Graves J, Bradbury J A, Zhao Y, Swope D L, King L, Qu W, Clark J, Myers P, Walker V, Lindzey J, Korach K S, Zeldin D C. Regulation of mouse renal CYP2J5 expression by sex hormones. Mol Pharmacol. 2004;65:730–743. doi: 10.1124/mol.65.3.730. [DOI] [PubMed] [Google Scholar]