Summary
Background
Prader-Willi syndrome (PWS) is associated with failure to thrive in infancy and progressive hyperphagia and obesity in childhood. This progressive weight gain is associated with hyperghrelinaemia and increased insulin sensitivity. The role of ghrelin excess in the pathogenesis of obesity is unclear.
Objective
To determine if high ghrelin levels precede the onset of obesity in young PWS children.
Design and methods
A cross-sectional study of 33 infants with PWS and 28 healthy control subjects (C). Fasting ghrelin and other satiety hormones were measured.
Results
Median total serum ghrelin in young children with PWS trended higher, but did not differ significantly from those in C of similar age, weight-for-age z-score and sex. However, there was more variability in ghrelin concentrations of young PWS. Eleven of 33 PWS subjects had ghrelin levels greater than the 95th percentile for ghrelin values in the C subjects (> 2871 pg/ml). Six of the PWS subjects with high ghrelin levels had weight-for-age z-scores < 0. Ghrelin concentrations in PWS and C infants exceeded those in older children. In youngsters with PWS, leptin was higher, suggesting a relative excess of fat to lean body mass and plasma adiponectin was increased.
Conclusions
Young infants with PWS who have not yet developed hyperphagia or obesity have median fasting ghrelin levels similar to controls. However, a subset (33%) of young PWS is hyperghrelinaemic; approximately one-half of those with hyperghrelinaemia have BMI z-score < 0. The age-related decline in ghrelin is blunted in PWS.
Introduction
Prader-Willi syndrome (PWS) is a genetic syndrome that results from lack of expression of paternal (imprinted) genes located on chromosome 15q11-q13. In the newborn period, children with PWS have hypotonia, poor suck, decreased arousal, and failure-to-thrive and often require tube feedings for several weeks to months. By 1-6 years of age, such patients develop insatiable appetite, progressive weight gain, short stature, hypogonadism, cognitive and motor delayed development, behavioural difficulties and sleep disturbances.1
It is not known what triggers the switch from a state of failure-to-thrive during infancy to that of insatiable appetite and morbid obesity in later childhood. Previous studies from our group and other centres have shown that adolescents and adults with PWS have high circulating concentrations of ghrelin, an orexigenic hormone produced by the stomach. In contrast, ghrelin is low in children and adults with nonsyndromic obesity.2-5 Ghrelin administration increases body weight in both rodents and humans,6-10 while ghrelin knockout mice are hypophagic and resistant to dietinduced obesity.11,12 These findings suggest that hyperghrelinaemia might contribute to the hyperphagia and excessive weight gain in PWS.
If ghrelin does mediate, in part, the hyperphagia and obesity seen in PWS, we would predict that elevations in serum ghrelin concentrations would precede or coincide with the development of hyperphagia and food-seeking in PWS children. The objectives of the current study were: (i) to determine if fasting serum ghrelin is higher in infants and young children with PWS than in control (C) subjects of equivalent age, weight-for-age z-score and sex; (ii) to examine the relationship between serum ghrelin and weight-for-age z-score, age and measures of insulin sensitivity including fasting insulin, glucose, HOMA-IR and adiponectin; (iii) to follow a subset of children with PWS longitudinally to assess changes in ghrelin during early childhood development; and (iv) to compare fasting serum ghrelin concentrations of control and PWS infants and young children to that of older control and PWS subjects.
Subjects and methods
Subjects
This study was approved by the Institutional Review Board of Duke University Medical Center. For studies of both young and older children, we mailed a recruitment letter to all paediatricians in North Carolina, Maryland, Washington, DC and Northern Virginia, and posted an advertisement on the Prader-Willi Syndrome Association (USA) and the Foundation for Prader-Willi Research (FPWR) websites. A parent or legal guardian of each child gave consent and children ≥ 12 years gave assent before entry into the study.
Infants and young children study
Thirty-three children with PWS (median age and weight-for-age z-score: 22·4 months and -0·12) and 28 normal controls (C) (median age and weight-for-age z-score: 34·0 months and 0·31) of comparable weight-for-age z-score, age and sex were studied (Table 1). Subjects with chronic secondary illnesses such as diabetes mellitus, liver or kidney disease or active malignancy or those taking investigational drugs were excluded. Some of the normal C subjects were siblings or friends of the young PWS subjects. A minority of subjects were healthy controls who were undergoing unrelated medical procedures (such as placement of myringotomy tubes); blood samples were obtained at the time of insertion of the intravenous line. The diagnosis of PWS was confirmed by history and chart review. Nineteen subjects had a deletion in chromosome 15q11-q13, 12 subjects had uniparental disomy of chromosome 15, and two had PWS confirmed by methylation testing only (undetermined form). The majority of study subjects were non-Hispanic Caucasians (one Hispanic PWS; five African American (AA) C; three Asian C; one Asian/AA C). Twenty-nine of 33 PWS subjects were taking a stable dose of GH at the time of study. Referring physicians had made the initial decisions whether or not to treat with GH. Age, sex, weight, height (three measurements) and time of last meal were determined. Anthropometric measurements were obtained by standard methods. Weight-for-age and weight-for-length z-scores and percentiles were calculated using EpiInfo Version 3·3·2 (Centers for Disease Control and Prevention).
Table 1.
Study subject characteristics
| Young |
Older |
|||||
|---|---|---|---|---|---|---|
| PWS (n = 33) | C (n = 28) | P1 value | PWS (n = 14) | C (n = 14) | p2 value | |
| Age (months) | 22·40 (13·30, 34·00) | 34·00 (18·05, 44·60) | 0·12 | - | - | - |
| Age (years) | - | - | - | 11·39 (7·13, 14·70) | 12·01 (10·58, 14·22) | 0·45 |
| Sex (M/F) | 16/17 | 13/15 | 1·0 | 5/9 | 6/8 | 1·0 |
| Weight-for-age percentile | 45·07 (12·02, 82·12) | 62·03 (26·63, 85·65) | 0·20 | - | - | - |
| Weight-for-age z-score | -0·12 (-1·17, 0·92) | 0·31 (-0·63, 1·07) | 0·20 | - | - | - |
| Weight-for-length percentile | 39·45 (24·54, 95·25) | 72·10 (40·50, 85·79) | 0·54 | - | - | - |
| Weight-for-length z-score | -0·27 (-0·69, 1·67) | 0·59 (-0·24, 1·04) | 0·55 | - | - | - |
| BMI z-score | - | - | - | 2·17 (1·53, 2·52) | 2·33 (1·82, 2·65) | 0·84 |
| Chromosome (Deletion/UPD/Undetermined) | 19/12/2 | - | - | 9/4/1 | - | - |
| GH treatment (Yes/No/Undetermined) | 29/3/1 | - | - | 9/5/0 | - | - |
All data are expressed as medians (1st quartile, 3rd quartile).
p1 = P-value for comparison of young PWS vs. young C.
p2 = P-value for comparison of old PWS vs. old C.
Older children study
Fourteen children with PWS (median age and body mass index (BMI)-z-score: 11·35 years and 2·15, respectively) and 14 BMI-matched control children without PWS (median age and BMI z-score 11·97 years and 2·35, respectively) were studied. Some initial baseline results from this study of older children were previously published and details of methods are available.13 Briefly, BMI z-scores were calculated using EpiInfo Version 3·3·2 (Centers for Disease Control and Prevention). Subjects with chronic secondary illnesses such as diabetes mellitus, liver or kidney disease or active malignancy or those taking investigational drugs were excluded. Nine of 14 PWS subjects were taking a stable dose of GH at the time of study. Referring physicians made the initial decisions whether or not to treat with GH.
One PWS subject was on thyroid replacement to treat central hypothyroidism; all PWS subjects had free T4 and TSH levels in the normal range. Four subjects with PWS had uniparental disomy; all others had a deletion in chromosome 15. C subjects were recruited from the insulin resistance and obesity clinics at Duke University Medical Center. None of the C subjects were receiving medication for treatment of insulin resistance at the time of study.
Blood sample collection
Blood samples were collected by venipuncture (between 08·00 and 10·00 h) following an overnight fast (10-12 h for subjects > 2 years; 6-8 h for subjects < 2 years). Both serum and plasma were obtained. Aprotinin, a protease inhibitor (500 KIU per ml of blood, Roche, Indianapolis, IN) was added to each vacutainer. Serum samples were allowed to clot on ice for 30 min and each sample was centrifuged and the serum or plasma was removed and stored at -70 °C until time of the assay. All analytes were measured in duplicate.
Blood measurements
Ghrelin analysis
Serum samples were assayed for immunoreactive total ghrelin concentration using a commercial RIA (Linco, St Charles, MO). The intra-assay and interassay coefficients of variation (CV) were < 10% and < 18%, respectively. The lower and upper limits of detection for this assay were 100 and 6000 pg/ml, respectively. The assay measures both active (octanoylated) and inactive ghrelin. Although only acylated ghrelin is bioactive,14 total ghrelin is a reasonable surrogate for the acylated form because the ratio of the two levels is constant under a wide variety of conditions.15,16
Leptin analysis
Plasma leptin concentrations were determined using a commercially available RIA (Linco, St Charles, MO) with a detection limit of 0·5 ng/ml (upper limit 100 ng/ml), and intra-assay and interassay CVs of each < 10%.
Insulin analysis
Serum insulin concentrations were measured by RIA (Linco, St Charles, MO). The intra-assay and interassay CV were each < 10%. The normal range of fasting plasma insulin is 2-100 μU/ml. HOMA-IR was calculated using the formula: Fasting glucose (mg/dL) × Fasting insulin (μIU/ml)/405.17
Adiponectin analysis
Fasting plasma total adiponectin concentrations and the high molecular weight (HMW) isoform for adiponectin were measured by EIA (Multimeric adiponectin EIA, Alpco Diagnostics, Salem, NH).18 The intra-assay CV was 5·4% for total adiponectin, and 5·0% for HMW adiponectin while the interassay CV was 5·0% for total adiponectin and 5·7% for HMW adiponectin. The sensitivity was 0·075 ng/ml for both total and HMW adiponectin.
Statistical analysis
Because of deviations (i.e. non-normality and/or outliers) from standard parametric distributional assumptions, all values are summarized using the median and 1st and 3rd quartiles. Between group comparisons were performed using the nonparametric Wilcoxon Rank-Sum test. Spearman rank correlation coefficients were used to measure monotonic associations between variables. Robust MM regression19 was used to determine the association between ghrelin and weight-for-age z-score, age and HOMA-IR. Separate regression models were run for each of the studies (infants and young children and older children) that incorporated both PWS and C subjects to allow for formal comparisons of slope coefficients. Robust regression techniques (e.g. MM regression) provide estimates with the same interpretation as standard ordinary least squares (OLS) regression, but are more reliable in the presence of deviations to standard regression assumptions. Statistical analyses were performed using sas 9·1 for Windows (SAS Institute, Cary, NC) and Sigmastat (SPSS, Inc., Chicago, IL). Two-sided P-values at the standard 0·05 level were used to determine statistical significance.
Results
Infants and young children study
We studied 33 PWS and 28 healthy C subjects who were of comparable age, weight-for-age percentile and z-score, and weight-for-length percentile and z-score and sex (P = 0·12, 0·20 and 0·20, 0·54 and 0·55 and 1·0, respectively) (Table 1).
Fasting ghrelin, leptin, adiponectin, insulin and HOMA-IR in young children
Median fasting total serum ghrelin levels in young children with PWS trended higher, but did not differ significantly from ghrelin levels in controls [2217·0 (1646·0, 3274·0) vs. 1964·0 (1647·7, 2251·5) pg/ml, respectively; P = 0·11]. However, there was much greater variability in serum ghrelin levels in young PWS subjects than in C, and 11 of 33 (33%) PWS subjects had ghrelin levels higher than the 95th percentile of ghrelin values of C (> 2871 pg/ml). (see Fig. 1a). Interestingly, 6 of the 11 PWS subjects with ghrelin levels exceeding 2871 pg/ml had weight for age z-score below median for age (z score < 0); only one young PWS subject with high ghrelin levels was overweight (z score > 1·64). Consequently, there was no significant correlation between serum ghrelin and weight for age z-score in either PWS or C subjects during infancy and early childhood. Fasting ghrelin levels in young PWS and C children exceeded those in older children (Fig. 2 and Table 2).
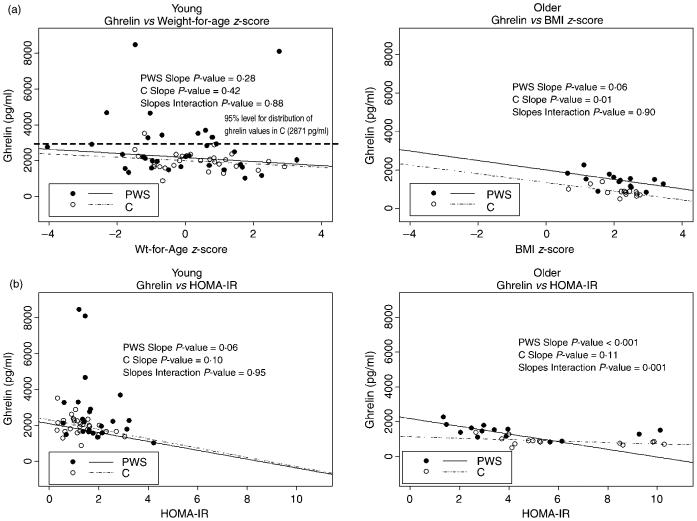
Fig. 1.
Robust regression models between ghrelin and weight-for-age z-score (young) or BMI-z-score (older) and HOMA-IR in young and older children. Individual P-values are shown for relationship between ghrelin and parameters in either PWS or C. The slopes interaction P × value represents differences between PWS and C.
Fig. 2.

Boxplots of ghrelin values in the four groups (PWS young, C young, PWS older and C older). The square symbol represents the mean and the horizontal line represents the median. *P < 0·05.
Table 2.
Hormonal profile of study subjects
| Young |
Older |
Young PWS vs. older PWS | Young C vs. older C | |||||
|---|---|---|---|---|---|---|---|---|
| PWS (n = 33) | C (n = 28) | P1 value | PWS (n = 14) | C (n = 14) | P2 value | P3 value | P4 value | |
| Total ghrelin (pg/ml) | 2217·0 (1646·00, 3274·00) | 1964·00 (1647·70, 2251·50) | 0·11 | 1468·45 (1145·71, 1619·32) | 839·7 (719·8, 889·7) | < 0·001 | < 0·001 | < 0·001 |
| Leptin (ng/ml) | 6·25 (2·83, 7·06) | 3·93 (2·65, 5·13) | 0·04 | 31·45 (12·92, 42·67) | 32·2 (15·6, 54·5) | 0·80 | < 0·001 | < 0·001 |
| Glucose (mg/dl) | 85·10 (76·60, 88·60) | 78·80 (74·80, 84·80) | 0·05 | 87·75 (81·75, 101·50) | 94·9 (89·0, 99·5) | 0·45 | 0·08 | < 0·001 |
| Insulin (μIU/ml) | 8·42 (6·30, 10·64) | 5·96 (4·88, 7·60) | 0·01 | 13·49 (10·17, 29·38) | 23·2 (17·2, 34·7) | 0·03 | 0·002 | < 0·001 |
| HOMA-IR | 1·62 (1·35, 2·16) | 1·19 (0·89, 1·59) | 0·01 | 3·20 (2·49, 5·68) | 5·1 (4·1, 8·6) | 0·03 | 0·001 | < 0·001 |
| Total adiponectin (ng/ml) | 16330·00 (12636·00, 21223·00) | 11105·00 (9379·00, 17824·50) | 0·03 | 8769·00 (6363·50, 15045·50) | 5798·0 (3216·5, 7352·5) | 0·01 | 0·002 | < 0·001 |
| HMW adiponectin (ng/ml) | 11342·00 (9208·00, 12725·00) | 6826·0 (5610·00, 11610·50) | 0·03 | 5370·50 (2763·00, 11867·50) | 2523·3 (819·0, 3147·0) | 0·01 | 0·02 | < 0·001 |
| HMW : total adiponectin ratio | 0·66 (0·59, 0·78) | 0·61 (0·55, 0·69) | 0·12 | 0·71 (0·48, 0·84) | 0·40 (0·27, 0·51) | 0·03 | 0·92 | 0·005 |
| Total adiponectin : leptin ratio | 2812·71 (1676·73, 6264·45) | 3467·68 (1977·38, 4974·79) | 0·61 | 257·05 (126·49, 1105·55) | 132·31 (62·13, 366·18) | 0·15 | < 0·001 | < 0·001 |
| HMW adiponectin : leptin ratio | 2812·71 (1676·73, 6264·45) | 2150·21 (1195·74, 3536·42) | 0·19 | 172·056 (66·54, 846·29) | 59·47 (21·65, 176·12) | 0·05 | < 0·001 | < 0·001 |
All data are expressed as medians (1st quartile, 3rd quartile); P-values calculated using Wilcoxon rank-sum test.
P1 = P-value for comparison of young PWS vs. young NC.
P2 = P-value for comparison of old PWS vs. old NC.
P3 = P-value for comparison of young PWS vs. older PWS.
P4 = P-value for comparison of young C vs. older C.
Plasma leptin was higher in young PWS subjects than in controls, suggesting a relative excess of fat to lean body mass. Plasma total and HMW adiponectin levels were higher in young PWS children than in young controls [Total adiponectin: 16330·0 (12636·0, 21223·0) vs. 11105·0 (9379·0, 17824·5) ng/ml, P = 0·03 and HMW adiponectin: 11342·0 (9208·0, 12725·0) vs. 6826·0 (5610·0, 11610·5), P = 0·03], despite mild increases in glucose, insulin and HOMA-IR (P = 0·05, 0·01, 0·01, respectively). However, the HMW: total adiponectin ratio, total adiponectin: leptin ratio, HMW adiponectin: leptin ratio in young PWS subjects were comparable to those in young controls (P = 0·12, 0·61, and 0·19, respectively) (Table 2).
Correlation of ghrelin with other metabolic parameters in young children
We analysed the Spearman correlations between fasting serum ghrelin and age, weight-for-age z-score, leptin, glucose, insulin, HOMA-IR, total adiponectin, HMW adiponectin and HMW: total adiponectin ratio. In young PWS children, total ghrelin correlated negatively with insulin (r = -0·36; P < 0·05). In young control (C) children, total ghrelin correlated negatively with leptin (r = -0·42; P < 0·05) (Table 3). Robust regression results are also presented between fasting serum ghrelin and weight-for-age z-score and HOMA-IR (Fig. 1a,b). The results show that serum ghrelin and the above parameters correlated very weakly overall (overall R2 is very low) in the young children. There were no differences in these relationships in young PWS compared to young C children.
Table 3.
Correlations between total ghrelin and other variables in young and older PWS children and weight for age or BMI z-score equivalent controls (C)
| For PWS children: | ||
|---|---|---|
| Total ghrelin |
||
| Young | Older | |
| Age | 0·02 | -0·73** |
| Weight-for-age percentage | -0·20 | - |
| Weight-for-age z-score | -0·20 | - |
| Weight-for-length percentage | 0·021 | - |
| Weight-for-length z-score | 0·020 | - |
| BMI z-score | - | -0·60* |
| Leptin | 0·35 | -0·51 |
| Glucose | -0·16 | -0·17 |
| Insulin | -0·36* | -0·48 |
| HOMA-IR | -0·29 | -0·55* |
| Total adiponectin | -0·02 | 0·49 |
| HMW adiponectin | -0·01 | 0·49 |
| HMW : total adiponectin ratio | -0·19 | 0·48 |
| For C children: | ||
|---|---|---|
| Total ghrelin |
||
| Young | Older | |
| Age | 0·14 | -0·42 |
| Weight-for-age percentage | -0·17 | - |
| Weight-for-age z-score | -0·17 | - |
| Weight-for-length percentage | -0·26 | - |
| Weight-for-length z-score | -0·26 | - |
| BMI z-score | - | -0·58* |
| Leptin | -0·42* | -0·49 |
| Glucose | -0·25 | -0·51 |
| Insulin | -0·25 | -0·37 |
| HOMA-IR | -0·36 | -0·53* |
| Total adiponectin | 0·07 | 0·49 |
| HMW adiponectin | 0·03 | 0·58* |
| HMW : total adiponectin ratio | -0·10 | 0·41 |
Spearman rank correlations reported.
P < 0·05.
P < 0·01.
Spearman rank correlations reported.
P < 0·05.
Longitudinal analysis of ghrelin in infants and young children
We studied a subset of young PWS infants and children (n = 9) [median age: 11·30 months (9·58, 15·70); median weight for age z-score: -1·09 (-1·78, - 0·65); 4 females/5 males; all Caucasians] every 3 months for a total of 18 months in order to assess changes in serum ghrelin concentrations during early development. Boxplots for ghrelin values in subjects at each time-point (0-18 months) are shown graphically in Fig. 3(a). Ghrelin levels did not appear to change significantly over the course of the 18 months of follow-up in this age group. Again, we observed considerable variability in ghrelin in the young PWS children at each of these time-points; this variability increased over time. Boxplots of weight-for-age z-score are also shown graphically in Fig. 3(b). The median weight-for-age z-score also did not change significantly over the course of 18 months.
Fig. 3.

(a) Boxplots for ghrelin values in nine PWS young children at each time-point (0-18 months). The upper and lower whiskers in the boxplots represent the maximum and minimum values at that visit. The connecting line is drawn between the median point of each boxplot. (b) Boxplots for weight-for-age z-score values in nine PWS young children at each time-point (0-18 months). The upper and lower whiskers in the boxplots represent the maximum and minimum values at that visit. The connecting line is drawn between the median point of each boxplot.
Older children study
We studied 14 PWS and 14 healthy C subjects of similar age, BMI z-score and sex (P = 0·51, 0·70 and 1·0, respectively) (Table 1).
Fasting ghrelin, leptin, adiponectin, insulin and HOMA-IR in older children
Fasting total serum ghrelin levels were higher in the older PWS subjects than in older C subjects [1468·5 (1145·7, 1619·3) vs. 839·7 (719·8, 889·7) pg/ml; P < 0·001]. Ghrelin levels in both the PWS and control older subjects were lower than those seen in the younger children (P < 0·001) (Fig. 2).
Plasma leptin was similar in the older PWS and older C (P = 0·8) and was significantly higher (P < 0·001) than plasma leptin in the young children. Despite comparable BMI z-score and leptin levels, the fasting insulin levels and HOMA-IR index of PWS were significantly lower (P = 0·03) than in BMI-matched controls. Total plasma adiponectin, HMW adiponectin, HMW: total adiponectin ratio and HMW adiponectin: leptin ratio were all significantly higher in older PWS than in C subjects (P = 0·01, 0·01, 0·03 and 0·05, respectively), consistent with higher insulin sensitivity in the older PWS subjects. In general, plasma total adiponectin and the isoforms and ratios were lower in the older PWS and C subjects than in their younger counterparts.
Correlation of ghrelin with other metabolic parameters in older children
In older PWS children, Spearman correlations were examined between total ghrelin and other parameters. In older PWS children, total ghrelin correlated negatively with age (r = -0·73; P < 0·01), BMI z-score (r = -0·60; P < 0·05) and HOMA-IR (r = -0·55; P < 0·05). In older C children, total ghrelin correlated negatively with BMI z-score (r = -0·58; P < 0·05) and HOMA-IR (r = -0·53; P < 0·05) and positively with HMW-adiponectin (r = 0·58; P < 0·05). Robust regression models were also examined in the older children; these models showed much stronger relationships in general between ghrelin and all parameters examined (higher overall R2 values) (Fig. 1a,b). In older children, there were negative relationships demonstrated between ghrelin and BMI z-score, age and HOMA-IR in both groups; these were significant for ghrelin and BMI z-score only in C (P = 0·01), and significant for ghrelin and age (P < 0·001) and HOMA-IR (P < 0·001) only in PWS.
Ghrelin and other metabolic parameters in young vs. older children
Total ghrelin in the young children was significantly higher than in their older counterparts (Fig. 2 and Table 2). In older C, ghrelin was 57% lower than in young C (P < 0·001). In older PWS, ghrelin was 34% lower than in young PWS (P < 0·001). Thus, the decline in ghrelin with age was more striking in C subjects than in PWS subjects. There was a negative relationship between total ghrelin and age in older PWS children (r = -0·7; P < 0·01), but not younger PWS subjects or controls. It is possible that the relationship between ghrelin and age might not be detected in younger children because of the narrow time frame assessed (Table 3).
Insulin and HOMA-IR were higher in the older PWS and C than in their younger counterparts (Table 2). However, at young ages, C subjects were more insulin sensitive (26% as estimated by HOMA-IR) than PWS subjects; at older ages, the PWS subjects appeared more insulin sensitive than older C subjects. Total adiponectin, HMW adiponectin, total adiponectin: leptin ratio and HMW: leptin ratio were all lower in the older PWS and C than in younger counterparts (Table 2). Of interest, the HMW: total adiponectin ratio in the older PWS subjects did not differ from that in young PWS subjects. Plasma adiponectin levels correlated inversely with HOMA-IR in older PWS subjects but not in younger PWS subjects.
A negative relationship between total ghrelin vs. insulin was present in all groups, but was significant only in young PWS (r = -0·36, P < 0·05) and not in young C or older PWS or C (Table 3). No significant relationships between total ghrelin vs. glucose were identified in either PWS or C at any age. A negative relationship between total ghrelin and HOMA-IR was detected in all groups, but was significant only in older PWS and C (r = -0·55; P < 0·05 and r = -0·53; P < 0·05), suggesting that this relationship requires developmental maturation (Table 3).
There appears to be a significant negative relationship between ghrelin and BMI z-score only in the older children (both PWS and C) (Table 3 and Fig. 1a). This finding suggests that serum ghrelin in young children is regulated by factors other than body mass index.
Discussion
In our study, fasting total serum ghrelin in young children with PWS trended higher, but did not differ significantly from those in controls of comparable age, weight-for-age percentile and z-score, and weight-for-length percentile and z-score and sex. However, there was far more variability in ghrelin concentrations in the young children with PWS than in young controls, and 11 of 33 PWS subjects (but only 1 of 28 C subjects) had ghrelin levels higher than the 95th percentile of ghrelin values in the normal controls (> 2871 pg/ml). Thus, hyperghrelinaemia is detected in approximately one-third of PWS subjects at an early age.
Longitudinal assessment of ghrelin in a subset of nine young PWS children showed no significant changes over 18 months. Total ghrelin in young PWS and control children was significantly higher than in their older counterparts. Interestingly, the decline in ghrelin with age was more exaggerated in C subjects than in PWS subjects. A negative relationship between total ghrelin and age was present in the older PWS and controls, but not in the young age groups. A negative relationship between total ghrelin and insulin was present in all groups, but significant only in young PWS subjects. A negative relationship between ghrelin and BMI z-score was found in the older but not in the younger children (both PWS and C).
A previous study of plasma ghrelin in a small group (n = 9) of young children with PWS showed no significant elevation of total or acylated plasma ghrelin relative to controls of similar age, BMI and sex.20 A more recent investigation shows increased ghrelin levels in young PWS children. However, careful review of the data in the latter study indicates that increases in ghrelin are found only in a subset of subjects; these findings are therefore consistent with our own.21 We also provide additional longitudinal data of ghrelin levels in young children with PWS.
Interestingly, 6 of the 11 young PWS subjects in our study with high ghrelin levels had weight-for-age z-score below median for age (z score < 0). At the present time, we cannot explain the hyperghrelinaemia found in a subset of young PWS subjects. We found no significant difference in serum ghrelin between those with a deletion of chromosome 15 and those with uniparental disomy. Likewise, GH therapy does not appear to explain the divergence in ghrelin concentrations; the vast majority (29/33) of young PWS children were taking GH, yet only a subset (11/33) had hyperghrelinaemia. Moreover, neither GH deficiency nor GH replacement therapy alters ghrelin concentrations in either GH deficient or PWS subjects.22-26 Finally, hyperghrelinaemia was detected in the majority of older PWS children though only 9 of 14 were receiving GH therapy.
The relationship between ghrelin and the hyperphagia and obesity of PWS also remains unclear. On one hand, there was no relationship between ghrelin and weight-for-age z-score in young infants and none of the hyperghrelinaemic PWS subjects were obviously hyperphagicby parental report. Moreover, only 1 of 11 young hyperghrelinaemic PWS subjects was obese; indeed 6 of 11 had weight-for-age z-score < 0. These findings suggest that the relationship between ghrelin and appetite/weight gain might be established only sometime in late childhood or early adolescence. Alternatively, there may be no clear relationship between ghrelin and weight gain in the young PWS because parents control (and may even restrict) food intake in young children with PWS. This young group includes subjects at various ‘stages’ of development; some very young PWS children require gavage feeding while others are of low-normal or normal weight and eat freely on their own. In theory, hyperghrelinaemia might induce weight gain only when PWS children are capable of controlling their own food intake independently. Because ghrelin levels are increased in response to caloric deprivation, the restriction of food intake in early childhood could explain the increase in plasma ghrelin levels in a subset of the thinnest young PWS subjects.
Ghrelin concentrations sampled every 3 months for a total of 18 months in a subset (n = 9) of young PWS subjects did not change significantly over 18 months. However, a high degree of variability of ghrelin levels in the young PWS was observed at each time-point; this variability increased with age. Ghrelin levels in younger children with PWS were higher than those in the older PWS subjects. In both groups, ghrelin declined with age and weight gain. However, the suppression of ghrelin by weight gain was blunted in the PWS children.
In young PWS children, the correlations between serum ghrelin and other parameters evaluated were in general weak. The most striking finding was a negative correlation of total ghrelin with insulin (P < 0·05). Total ghrelin correlated negatively with BMI z-score and HOMA-IR in older, but not younger, C and PWS children. Thus, serum ghrelin may be regulated differentially in young and older children. Some previous studies showed a negative relationship between total ghrelin and BMI in older children and adults with PWS.3,27-29 In contrast, other investigations found that the negative relationship between total ghrelin and BMI or adiposity is absent in young PWS infants and in older PWS children and adults.2,4 These discrepant results might be related to differences in ages of the various samples, limited sample sizes and perhaps differences in genetic subtypes of PWS individuals in each cohort.
Our previously published study showed that for any given BMI z-score, older PWS children had higher concentrations of fasting total and HMW adiponectin and higher HMW: total adiponectin ratios than age and BMI-matched controls. The HMW: total adioponectin ratio remained relatively high in children with PWS at high degrees of obesity.13 In the current study, we found that total adiponectin, HMW adiponectin, total adiponectin: leptin ratio and HMW: leptin ratio were all lower in the older PWS and C than in their younger counterparts. Additionally, at young ages, C subjects were more insulin sensitive (26% as estimated by HOMA-IR) than PWS subjects, and plasma adiponectin levels in young PWS children, in contrast to older PWS children, did not correlate inversely with HOMA-IR. It should be noted, however, that insulin sensitivity in young PWS and young C subjects is considerably higher than that in older children, making the relationship between HOMA-IR and adiponectin more difficult to interpret in the younger group. The wide variability in BMI z-score and weight gain (i.e. some subjects are failing to thrive while others are gaining weight) in the young PWS compounds the difficulty with interpretation of this relationship. The high ratio of HMW: total adiponectin ratio was maintained with age in older PWS, but not in obese controls. This observation reflects the relative higher insulin sensitivity in older PWS compared to older C subjects. The adiponectin: leptin ratio is considered to be more closely associated with both the prevalence of metabolic syndrome and atherogenic tendency than either adiponectin or leptin alone.30-32 The trend of higher adiponectin: leptin ratio suggests that older PWS subjects might be relatively protected from the development of insulin resistance in comparison to equally obese control children.33-35
Several previous studies showed that insulin is able to suppress ghrelin levels in humans.36-38 We speculate that changes in insulin sensitivity with age in the C and PWS children play a role in the changes in serum ghrelin seen in our cohort. Ghrelin falls in both C and PWS children with age as insulin reciprocally increases (and insulin sensitivity declines). However, insulin does not rise as dramatically in PWS children as in controls. The relative hypoinsulinaemia, and/or increase in insulin sensitivity, may explain in part the blunted suppression of ghrelin with age in the older PWS compared to C subjects.
There are several limitations of this study. First, we were not able to strictly one-to-one match the young PWS subjects to the young controls in age, weight-for-age z-score and gender because of difficulty in recruitment of normal control infants and toddlers. However, there is striking overlap between the young PWS and control groups and no statistical difference in any of these parameters between the two groups. Thus, comparison of the two groups is valid. Second, the correlations between ghrelin and leptin and BMI in the PWS subjects might be confounded by other factors including the use of GH therapy. For reasons specified previously, the available evidence suggests that GH therapy had little or no effect on ghrelin concentrations in either young or older children.
In summary, total serum ghrelin in young children with PWS trended higher, but did not differ significantly from ghrelin in controls of similar age, weight-for-age z-score and sex. However, there was marked variability in the measurements of ghrelin concentrations in the young PWS children compared to controls, and one-third of young PWS subjects were hyperghrelinaemic. The hyperghrelinaemia in this subset was observed in the absence of reported hyperphagia. Six of the 11 hyperghrelinaemic young PWS subjects had BMI z-scores below the median for age. Moreover, there was no significant relationship between ghrelin and weight-for-age z-score during infancy and early childhood.
Hyperghrelinaemia in young PWS children might be a response to failure to thrive or food restriction in infancy. Chronic or persistent hyperghrelinaemia might eventually promote hyperphagia, leading to progressive weight gain in PWS. Further longitudinal analysis of ghrelin in PWS children might provide insight into why hyperghrelinaemia persists in older PWS children and adults.
Acknowledgements
The authors acknowledge Ms Juanita Cuffee and Ms Donnetta McCain (study coordinators) for help with recruitment of study subjects. We thank all the families and children who participated in these studies.
This study was conducted through the Duke University Medical Center, General Clinical Research Center (MO1-RR-30, National Center for Research Resources, Clinical Research Centers Program, NIH) and was supported by 1K23-RR-021979 (to A.M.H.) and NIDDK R01 DK071161 (to J.Q.P.), Z01-HD-00641 from the Intramural Research Program of the NICHD, NIH (to J.A.Y.) and additional funding from the Lawson Wilkins Pediatric Endocrine Society and the Sarah W. Stedman Nutrition and Metabolism Center.
References
- 1.Goldstone AP. Prader-Willi syndrome: advances in genetics, pathophysiology and treatment. Trends in Endocrinology and Metabolism. 2004;15:12–20. doi: 10.1016/j.tem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS, Schwartz MW, Basdevant A, Weigle DS. Elevated plasma ghrelin levels in Prader-Willi syndrome. Nature Medicine. 2002;8:643–644. doi: 10.1038/nm0702-643. [DOI] [PubMed] [Google Scholar]
- 3.DeIParigi A, Tschop M, Heiman ML, Salbe AD, Vozarova B, Sell SM, Bunt JC, Tataranni PA. High circulating ghrelin: a potential cause for hyperphagia and obesity in prader-Willi syndrome. Journal of Clinical Endocrinology and Metabolism. 2002;87:5461–5464. doi: 10.1210/jc.2002-020871. [DOI] [PubMed] [Google Scholar]
- 4.Haqq AM, Farooqi IS, O’Rahilly S, Stadler DD, Rosenfeld RG, Pratt KL, LaFranchi SH, Purnell JQ. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader-Willi syndrome. Journal of Clinical Endocrinology and Metabolism. 2003;88:174–178. doi: 10.1210/jc.2002-021052. [DOI] [PubMed] [Google Scholar]
- 5.Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 6.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 7.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 8.Dornonville de la Cour C, Lindqvist A, Egecioglu E, Tung YC, Surve V, Ohlsson C, Jansson JO, Erlanson-Albertsson C, Dickson SL, Hakanson R. Ghrelin treatment reverses the reduction in weight gain and body fat in gastrectomised mice. Gut. 2005;54:907–913. doi: 10.1136/gut.2004.058578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. Journal of Clinical Endocrinology and Metabolism. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 10.Druce MR, Wren AM, Park AJ, Milton JE, Patterson M, Frost G, Ghatei MA, Small C, Bloom SR. Ghrelin increases food intake in obese as well as lean subjects. International Journal of Obesity. 2005;29:1130–1136. doi: 10.1038/sj.ijo.0803001. [DOI] [PubMed] [Google Scholar]
- 11.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK. Mice lacking ghrelin receptors resist the development of diet-induced obesity. Journal of Clinical Investigation. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wortley KE, del Rincon JP, Murray JD, Garcia K, Iida K, Thorner MO, Sleeman MW. Absence of ghrelin protects against early-onset obesity. Journal of Clinical Investigation. 2005;115:3573–3578. doi: 10.1172/JCI26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haqq AM, Muehlbauer M, Svetkey LP, Newgard CB, Purnell JQ, Grambow SC, Freemark MS. Altered distribution of adiponectin isoforms in children with Prader-Willi syndrome (PWS): association with insulin sensitivity and circulating satiety peptide hormones. Clinical Endocrinology. 2007;67:944–951. doi: 10.1111/j.1365-2265.2007.02991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 15.Murakami N, Hayashida T, Kuroiwa T, Nakahara K, Ida T, Mondal MS, Nakazato M, Kojima M, Kangawa K. Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. Journal of Endocrinology. 2002;174:283–288. doi: 10.1677/joe.0.1740283. [DOI] [PubMed] [Google Scholar]
- 16.Ariyasu H, Takaya K, Hosoda H, Iwakura H, Ebihara K, Mori K, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K. Delayed short-term secretory regulation of ghrelin in obese animals: evidenced by a specific RIA for the active form of ghrelin. Endocrinology. 2002;143:3341–3350. doi: 10.1210/en.2002-220225. [DOI] [PubMed] [Google Scholar]
- 17.Conwell LS, Trost SG, Brown WJ, Batch JA. Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care. 2004;27:314–319. doi: 10.2337/diacare.27.2.314. [DOI] [PubMed] [Google Scholar]
- 18.Ebinuma H, Miyazaki O, Yago H, Hara K, Yamauchi T, Kadowaki T. A novel ELISA system for selective measurement of human adiponectin multimers by using proteases. Clinica Chimica Acta. 2006;372:47–53. doi: 10.1016/j.cca.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Yohai VJ. High breakdown point and high efficiency robust estimates for regression. Annals of Statistics. 1987;15:642–656. [Google Scholar]
- 20.Erdie-Lalena CR, Holm VA, Kelly PC, Frayo RS, Cummings DE. Ghrelin levels in young children with Prader-Willi syndrome. The Journal of Pediatrics. 2006;149:199–204. doi: 10.1016/j.jpeds.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Feigerlova E, Diene G, Conte-Auriol F, Molinas C, Gennero I, Salles JP, Arnaud C, Tauber M. Hyperghrelinemia precedes obesity in Prader-Willi syndrome. Journal of Clinical Endocrinology and Metabolism. 2008;93:2800–2805. doi: 10.1210/jc.2007-2138. [DOI] [PubMed] [Google Scholar]
- 22.Barkan AL, Dimaraki EV, Jessup SK, Symons KV, Ermolenko M, Jaffe CA. Ghrelin secretion in humans is sexually dimorphic, suppressed by somatostatin, and not affected by the ambient growth hormone levels. Journal of Clinical Endocrinology and Metabolism. 2003;88:2180–2184. doi: 10.1210/jc.2002-021169. [DOI] [PubMed] [Google Scholar]
- 23.Janssen JA, van der Toorn FM, Hofland LJ, van Koetsveld P, Broglio F, Ghigo E, Lamberts SW, Jan van der Lely A. Systemic ghrelin levels in subjects with growth hormone deficiency are not modified by one year of growth hormone replacement therapy. European Journal of Endocrinology. 2001;145:711–716. doi: 10.1530/eje.0.1450711. [DOI] [PubMed] [Google Scholar]
- 24.Haqq AM, Stadler DD, Jackson RH, Rosenfeld RG, Purnell JQ, LaFranchi SH. Effects of growth hormone on pulmonary function, sleep quality, behavior, cognition, growth velocity, body composition, and resting energy expenditure in Prader-Willi syndrome. Journal of Clinical Endocrinology and Metabolism. 2003;88:2206–2212. doi: 10.1210/jc.2002-021536. [DOI] [PubMed] [Google Scholar]
- 25.Hoybye C, Barkeling B, Espelund U, Petersson M, Thoren M. Peptides associated with hyperphagia in adults with Prader-Willi syndrome before and during GH treatment. Growth Hormone and IGF Research. 2003;13:322–327. doi: 10.1016/s1096-6374(03)00077-7. [DOI] [PubMed] [Google Scholar]
- 26.Malik IA, English PJ, Ghatei MA, Bloom SR, MacFarlane IA, Wilding JP. The relationship of ghrelin to biochemical and anthropometric markers of adult growth hormone deficiency. Clinical Endocrinology. 2004;60:137–141. doi: 10.1111/j.1365-2265.2004.01929.x. [DOI] [PubMed] [Google Scholar]
- 27.Goldstone AP, Thomas EL, Brynes AE, Castroman G, Edwards R, Ghatei MA, Frost G, Holland AJ, Grossman AB, Korbonits M, Bloom SR, Bell JD. Elevated fasting plasma ghrelin in Prader-Willi syndrome adults is not solely explained by their reduced visceral adiposity and insulin resistance. Journal of Clinical Endocrinology and Metabolism. 2004;89:1718–1726. doi: 10.1210/jc.2003-031118. [DOI] [PubMed] [Google Scholar]
- 28.Paik KH, Jin DK, Song SY, Lee JE, Ko SH, Song SM, Kim JS, Oh YJ, Kim SW, Lee SH, Kim SH, Kwon EK, Choe YH. Correlation between fasting plasma ghrelin levels and age, body mass index (BMI), BMI percentiles, and 24-hour plasma ghrelin profiles in Prader-Willi syndrome. Journal of Clinical Endocrinology and Metabolism. 2004;89:3885–3889. doi: 10.1210/jc.2003-032137. [DOI] [PubMed] [Google Scholar]
- 29.Butler MG, Bittel DC. Plasma obestatin and ghrelin levels in subjects with Prader-Willi syndrome. American Journal of Medical Genetics. Part A. 2007;143:415–421. doi: 10.1002/ajmg.a.31687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue M, Maehata E, Yano M, Taniyama M, Suzuki S. Correlation between the adiponectin-leptin ratio and parameters of insulin resistance in patients with type 2 diabetes. Metabolism. 2005;54:281–286. doi: 10.1016/j.metabol.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Satoh N, Naruse M, Usui T, Tagami T, Suganami T, Yamada K, Kuzuya H, Shimatsu A, Ogawa Y. Leptin-to-adiponectin ratio as a potential atherogenic index in obese type 2 diabetic patients. Diabetes Care. 2004;27:2488–2490. doi: 10.2337/diacare.27.10.2488. [DOI] [PubMed] [Google Scholar]
- 32.Norata GD, Raselli S, Grigore L, Garlaschelli K, Dozio E, Magni P, Catapano AL. Leptin: adiponectin ratio is an independent predictor of intima media thickness of the common carotid artery. Stroke. 2007;38:2844–2846. doi: 10.1161/STROKEAHA.107.485540. [DOI] [PubMed] [Google Scholar]
- 33.Krochik AG, Ozuna B, Torrado M, Chertkoff L, Mazza C. Characterization of alterations in carbohydrate metabolism in children with Prader-Willi syndrome. Journal of Pediatric Endocrinology and Metabolism. 2006;19:911–918. doi: 10.1515/jpem.2006.19.7.911. [DOI] [PubMed] [Google Scholar]
- 34.Goldstone AP, Thomas EL, Brynes AE, Bell JD, Frost G, Saeed N, Hajnal JV, Howard JK, Holland A, Bloom SR. Visceral adipose tissue and metabolic complications of obesity are reduced in Prader-Willi syndrome female adults: evidence for novel influences on body fat distribution. Journal of Clinical Endocrinology and Metabolism. 2001;86:4330–4338. doi: 10.1210/jcem.86.9.7814. [DOI] [PubMed] [Google Scholar]
- 35.Mogul HR, Lee PD, Whitman B, Zipf WB, Frey M, Myers SE, Cahan M, Pinyerd B. Preservation of insulin sensitivity and paucity of metabolic syndrome symptoms in Prader-Willi Syndrome adults: preliminary data from the US multicenter study. Obesity Research. 2004;12:171. [Google Scholar]
- 36.Flanagan DE, Evans ML, Monsod TP, Rife F, Heptulla RA, Tamborlane WV, Sherwin RS. The influence of insulin on circulating ghrelin. American Journal of Physiology, Endocrinology and Metabolism. 2003;284:E313–E316. doi: 10.1152/ajpendo.00569.2001. [DOI] [PubMed] [Google Scholar]
- 37.Mohlig M, Spranger J, Otto B, Ristow M, Tschop M, Pfeiffer AF. Euglycemic hyperinsulinemia, but not lipid infusion, decreases circulating ghrelin levels in humans. Journal of Endocrinological Investigation. 2002;25:RC36–RC38. doi: 10.1007/BF03344062. [DOI] [PubMed] [Google Scholar]
- 38.Zou CC, Liang L, Zhao ZY. Factors associated with fasting plasma ghrelin levels in children and adolescents. World Journal of Gastroenterology. 2008;14:790–794. doi: 10.3748/wjg.14.790. [DOI] [PMC free article] [PubMed] [Google Scholar]