Abstract
In endothelial cells, NF-κB is an important intracellular signaling molecule by which changes in wall shear stress are transduced into the nucleus to initiate downstream endothelial nitric oxide synthase (NOS3) gene expression. We investigated whether NF-κ light-chain gene enhancer in B cells 1 (NFKB1) promoter polymorphism (−94NFKB1 I/D, where I is the insertion allele and D is the deletion allele) was associated with 1) NOS3 gene expression in endothelial cells under physiological levels of unidirectional laminar shear stress (LSS) and 2) endothelial function in prehypertensive and stage I hypertensive individuals before and after a 6-mo supervised endurance exercise intervention. Competitive EMSAs revealed that proteins present in the nuclei of endothelial cells preferentially bound to the I allele NFKB1 promoter compared with the D allele. Reporter gene assays showed that the I allele promoter had significantly higher activity than the D allele. In agreement with these observations, homozygous II genotype cells had higher p50 expression levels than homozygous DD genotype cells. Cells with the homozygous II genotype showed a greater increase in NOS3 protein expression than did homozygous DD genotype cells under LSS. Functional experiments on volunteers confirmed higher baseline reactive hyperemic forearm blood flow, and, furthermore, the subgroup analysis revealed that DD homozygotes were significantly less prevalent in the exercise responder group compared with II and ID genotypes. We conclude that the −94NFKB1 I/D promoter variation contributes to the modulation of vascular function and adaptability to exercise-induced flow shear stress, most likely due to differences in NFKB1 gene transactivity.
Keywords: nuclear factor-κ light-chain gene enhancer in B cells 1, nitric oxide synthase 3, shear stress
Vascular endothelial cells are constantly exposed to hemodynamic shear stress, although the magnitude and pattern of the shear stress varies depending on the level of local blood flow and vascular geometry (6, 22). Endothelial cells sense the hemodynamic forces and respond by initiating the expression of downstream genes, in particular, the endothelial nitric oxide (NO) synthase gene (NOS3) (26).
Studies have shown that the p50/p65 NF-κB complex is an essential transcription factor in NOS3 gene expression in response to fluid shear stress in endothelial cells (7). In cultured endothelial cells, laminar shear stress (LSS) activates IκB kinase-α via c-Src and a MEK1/2-dependent signaling pathway. This increases proteosome-dependent degradation of IκB-α and induces nuclear translocation of the p50/p65 complex. Subsequently, the NF-κB complex binds to a shear stress response element (SSRE) in the human NOS3 gene promoter, resulting in increased gene expression (7, 12). Inhibition of the NF-κB-mediated signaling completely blocks the increase in NOS3 gene expression in endothelial cells exposed to shear stress (9).
The human NF-κ light-chain gene enhancer in B cells 1 gene (NFKB1) at 4q24 encodes a 105-kDa non-DNA-binding cytoplasmic precursor molecule (p105), which is cleaved to the 50-kDa DNA-binding protein (p50) (13). Recently, a 4-bp DNA sequence (ATTG) insertion (I)/deletion (D) variant (−94NFKB1 I/D) was described in the promoter of the NFKB1 gene. The allele frequencies are ∼60% and 40% for the I and D allele, respectively. −94NFKB1 I/D polymorphism has been shown to transcriptionally regulate NFKB1 gene expression (16).
Acute exercise acts as a physiological stressor that disturbs resting homeostasis, whereas chronic exercise elicits salutary effects through cardiovascular (CV) adaptations (11, 31). In the vasculature, exercise stimulus can be defined as endothelial wall shear stress (25). During endurance exercise, flow velocities are approximately double those observed during rest, and regions of the low-velocity/oscillatory shear stress that existed under resting condition are eliminated (4, 24). Animal studies have shown that exercise-induced flow-mediated shear stress increased NOS3 protein content in both coronary and small resistance arteries (19).
Therefore, we reasoned that the relevant functional polymorphism could modulate endothelial function with respect to NOS3 gene expression under LSS in human endothelial cells and that it may explain, in part, the interindividual variation in the direction and magnitude of the beneficial CV adaptations that occur with an exercise training intervention in individuals with CV risk factors. In the present study, we investigated the function of the −94NFKB1 I/D polymorphism in human endothelial cells under physiological levels of LSS. In addition, we assessed whether the polymorphism was associated with endothelial function and, furthermore, whether it was associated with adaptability in endothelial function after a 6-mo supervised exercise intervention in prehypertensive and stage I hypertensive individuals.
MATERIALS AND METHODS
Human subject recruitment and intervention
Prehypertensive and stage I hypertensive subjects responding to media advertisements and fliers were screened to assess their eligibility and provided written informed consents. The study protocol involving human subjects was approved by the Institutional Review Board of the University of Maryland (College Park, MD). Subjects were sedentary (aerobic exercise <2 times/wk, <20 min/session, sedentary job), 50–75 yr of age, nondiabetic, not on lipid-lowering medications, had prehypertension or stage I hypertension (systolic blood pressure: 120–159 mmHg, diastolic blood pressure: 80–99 mmHg), had no CV disease other than hypertension, and had a body mass index of <37 kg/m2. Qualified subjects could not have any medical conditions precluding their ability to perform vigorous exercise. Subjects using antihypertensive medications began a tapering schedule recommended by their physician, and a research study physician also supervised the medication tapering process. If a subject's systolic or diastolic blood pressure was >159 or >99 mmHg, respectively, for 3 consecutive weeks at any time during the study, they were excluded from further participation in the study and referred back to their personal physician. Qualified subjects underwent 6 wk of instruction in the principles of an American Heart Association low-fat (<30% total calorie intake) and <3 g/day sodium diet that they maintained for the entire study period. Subjects underwent three supervised endurance exercise sessions per week for 6 mo. The exercise session included warm-up and stretching periods and dynamic aerobic exercise. Heart rate was used to monitor each subject's training intensity. The initial exercise session consisted of 20 min of 50% maximal oxygen consumption (V̇o2max), and the training duration and intensity were gradually increased until 40 min of exercise at 70% V̇o2max was completed. After 10 wk, 1 day of walking exercise for 40 min was added to the exercise program.
Cell culture and shear stress experiments
Human umbilical vein endothelial cells (HUVECs) were grown in medium 199 (Mediatech) supplemented with 20% FBS, 16 U/ml heparin, 50 μg/ml endothelial growth supplement, 2 mmol/l glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin and cultured with 5% CO2 at 37°C in dishes coated with 0.1% gelatin. The unidirectional LSS experiments were performed as previously described (33). Briefly, confluent HUVECs grown in 100-mm tissue culture dishes were exposed to unidirectional LSS at 15 dyn/cm2 in shear medium (the growth medium without serum) by rotating a Teflon cone (0.5° cone angle) in a humidified environment with 5% CO2 at 37°C. All HUVEC experiments were conducted between the fourth and seventh passage.
Promoter reactivity analysis and EMSA
For I- and D-type promoter activity analyses, pGL3 (Promega) luciferase reporter plasmids containing 981 bp of the human NFKB1 gene promoter having either the I or D allele of the DNA sequence variation were transiently transfected into I/DHUVECs (16). Equal numbers of HUVECs (1 × 106 cells/dish) were plated in 100-mm culture dishes 18–24 h before transfection. These subconfluent (∼70–80%) HUVECs were transiently cotransfected with 1 μg of reporter vector and 250 ng of phRL-TK, as a cotransfection control, using 3 μl of FuGENE6 (Roche Molecular Biochemicals) according to the manufacturer's instructions. After exposure to 6 or 24 h of LPS (500 ng/ml), transfected cell lysates were harvested using passive lysis buffer (Promega) and stored at −80ÆC until used. A dual-luciferase system (Promega) was used for firefly and Renilla luciferase assays. A luminometer was programmed to perform a 2-s premeasurement delay, followed by a 20-s measurement period for each promoter assay, and data were presented as relative luciferase activity, which normalized to the Renilla luciferase activity. Results from three independent experiments were used for each analysis. Nuclear protein extracts were obtained from HUVECs using NE-PER (Pierce) as per the manufacturer's instructions. Oligonucleotide probes were synthesized based on the NFKB1 promoter sequence, and EMSAs were performed as previously described (16). Briefly, EMSA reactions contained 5 μg of protein, 1 ng of 32P-labeled probe, and 1 μg of poly(dI-dC). After 1 min, protein-DNA complexes were resolved by PAGE and detected by an autoradiography of the dried gel.
Analysis of NFKB1 and NOS3 gene expression
Western blot analysis was carried out according to standard procedures using monoclonal antibodies against NOS3 (BD Bioscience), p50/p105 (Cell Signaling Technology), p65 (BD Bioscience), a secondary antibody conjugated with alkaline phosphotase or horseradish peroxidase (for p50/p105 and p65), and the chemiluminescent reagent (Pierce). Loading and transfer of equal amounts of protein were normalized by reprobing the blots with a monoclonal anti-β-actin antibody (Santa Cruz Biotechnology). Scanning densitometry (Scion Image software) was used to quantify the signal density from the luminograms.
Genotyping
Genomic DNA was isolated from blood from the human subjects or HUVECs using the Genomic DNA Purification Kit (Gentra Systems). Genotyping for the −94NFKB1 (I/D) polymorphism was performed as described previously using a combined PCR-restriction digestion method (16). Genotyping accuracy was confirmed with direct sequencing on random samples. Based on the previous finding of a significant functional effect of the C allele in the T → C single-nucleotide substitution at −786 of the NOS3 gene on shear stress insensibility (3), this NOS3 gene polymorphism was also screened to exclude CC homozygote HUVECs in the present study. Briefly, the flanking region of the NOS3 gene was amplified using a pair of primers (forward: 5′-CACCCAGGCCCACCCCAACCT-3′ and reverse: 5′-GCCGCAGGTCGACAGAGAGACT-3′) through PCR. The PCR amplicon was digested overnight at 37°C using MspI (New England BioLabs) followed by electrophoresis for 4 h in a gel composed of 2% agarose + 1% Nusieve (FMC). The T allele (absence of the MspI restriction site) yields a fragment of 415 bp, and the C allele (presence of the MspI restriction site) yields fragments of 370 and 45 bp.
Measurement of forearm blood flow during reactive hyperemia
Forearm blood flow (FBF) was measured in the morning (7:00 ∼ 9:00 AM) after an overnight fast in the nondominant arm using venous occlusion plethysmography as previously described (28). For each measurement, a blood pressure cuff placed around the upper arm was inflated to 55 mmHg and connected to a rapid cuff inflator (Hokanson). A wrist cuff was inflated to suprasystolic pressure before each measurement. Baseline FBF was measured three times. Reactive hyperemia was then induced by inflating the upper arm cuff to 50 mmHg above the systolic blood pressure for 5 min to occlude the FBF. Immediately after the cuff was released, FBF was measured for 3 min at 15-s intervals (7-s measure + 8-s recovery).
Statistical analysis
Each variable was plotted and analyzed for variances to identify missing values, outliers, normality, and patterns of skewness in the distributions. Analysis of covariance (ANCOVA) with repeated measures was used to test for differences in reactive hyperemic FBF responses among genotype groups. Based on the results of previous studies, demographic (age, gender, and ethnicity) and biological (body weight, mean blood pressure, and body mass index) confounding factors were used as covariates in all FBF analyses. As no ethnicity by genotype or gender by genotype interactions were observed, these groups were combined for all the analyses. Student's t-tests were used to test if there were significant changes in subject characteristics between before and after exercise training. In the reporter gene expression analyses, Student's t-tests were used to test for differences in luciferase activities between the I- and D-type promoter constructs and for differences in NOS3 protein expression levels among genotyped HUVECs. In a subanalysis, subjects were grouped into responder and nonresponder groups based on the change in their reactive hyperemic response following exercise training. Fisher's exact test and odds ratio calculation were used to analyze differences in genotype frequencies between the responder and non-responder groups. The α-level was set at 0.05 for all hypothesis tests.
RESULTS
The I-type NFKB1 promoter has higher transcriptional activity
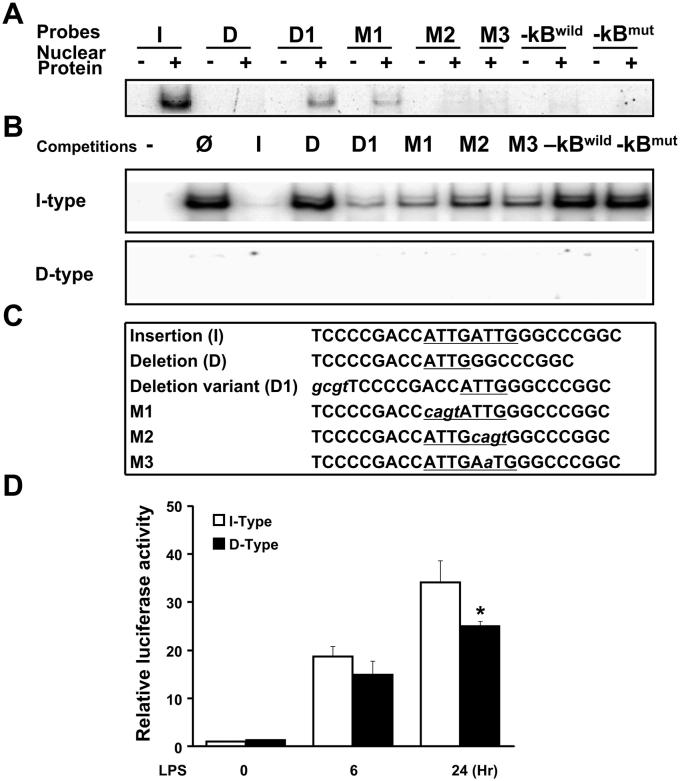
To determine whether −94NFKB1 I/D polymorphism affected DNA-protein binding activity, we conducted EMSAs with nuclear extracts from unstimulated HUVECs. Using wild-type (I), variant (D or D1), or mutant (M1, M2, or M3) 25-bp oligonucleotides representing the polymorphic region (Fig. 1C), we found that the I probe had higher binding activity compared with the D probe or size-matched D1 probe, and the mutant probes showed none or near-complete loss of binding to the nuclear protein (Fig. 1A). Competition assays, using a 50-fold excess of nonradioactive oligonucleotide probes, confirmed that the I allele formed a complex with higher affinity than the D allele (compare I with D and D1 competition; Fig. 1B). Oligonucleotides with the 5′-ATTG abolished (M1) and a single-point mutation in 3′-ATTG (M3) slightly competed for binding, whereas an oligonucleotide with the 3′-ATTG abolished (M2) did not compete. Overall, these results showed that proteins present in the nuclei of HUVECs bind to the I/D region of the NFKB1 promoter and preferentially bind to the I allele sequence, likely to 3′-ATTG.
Fig. 1.
The insertion (I)-type NF-κ light-chain gene enhancer in B cells 1 (NFKB1) gene promoter has higher transcriptional activity. Nuclear protein extract was prepared from basal human umbilical vein endothelial cells (HUVECs). A: protein binding activities of the 6 NFKB1 promoter mimic oligonucleaotide probes. B: protein binding activity of the I-type or deletion (D)-type NFKB1 gene promoter with competitors using excess nonradioactive (Ø), I, D, D1, M1, M2, and M3 probes (50 times higher concentration). A wild consensus or mutant (mut) -κB binding sequence was used as a positive control. C: sequences for the oligonucleotide probes. The underlined sequence represents the polymorphic region. The italicized lowercase letters are arbitrary sequences. D: the I-type NFKB1 promoter has significantly higher activity than the D-type promoter after LPS treatment. NFKB1 I- or D-type promoters with luciferase reporter gene constructs were transiently transfected into HUVECs, and the luciferase activity was measured following LPS stimulation. Data represent fold increases in adjusted luciferase activity over the control (0 h) observed at 6 or 24 h of LPS stimulation. Results from 3 independent experiments were used for each analysis. Data are presented as means ± SE. −, Free nuclear protein; +, nuclear protein added. *P < 0.05 vs. D-type.
To determine whether −94NFKB1 I/D polymorphism regulates the transcriptional activity of the NFKB1 promoter, we conducted reporter gene assays under LPS stimulation in HUVECs. Based on a previous study (16), transiently transfected HUVECs were subjected to 6 or 24 h of LPS (500 ng/μl) treatment. Both promoters were activated with LPS treatment by 25- to 33-fold compared with the control. The I-type promoter showed significantly higher activity than the D-type promoter after the 24-h LPS treatment (Fig. 1D).
p50/p105 NFKB1 I/D promoter polymorphism regulates NFKB1 gene expression
Since the above results suggested that −94NFKB1 I/D polymorphism affected NFKB1 promoter activity, we investigated whether NFKB1 polymorphism also affected protein expression levels in HUVECs. To test this, we obtained 17 primary-cultured HUVECs from different donors and identified II and DD homozygote cells. We tested the level of basal cytoplasmic p50/p105 proteins in different passages of these cells (passages 4–7). Higher p50-p105 protein levels were observed in II homozygote cells compared with DD homozygote cells (Fig. 2). These results showed that HUVECs with the I-type promoter had higher protein expression levels of NFKB1 gene products and, ultimately, a greater number of copies of the NF-κB complex in the cytoplasm, through which signaling transduction for downstream gene expression is likely to be facilitated in these cells.
Fig. 2.
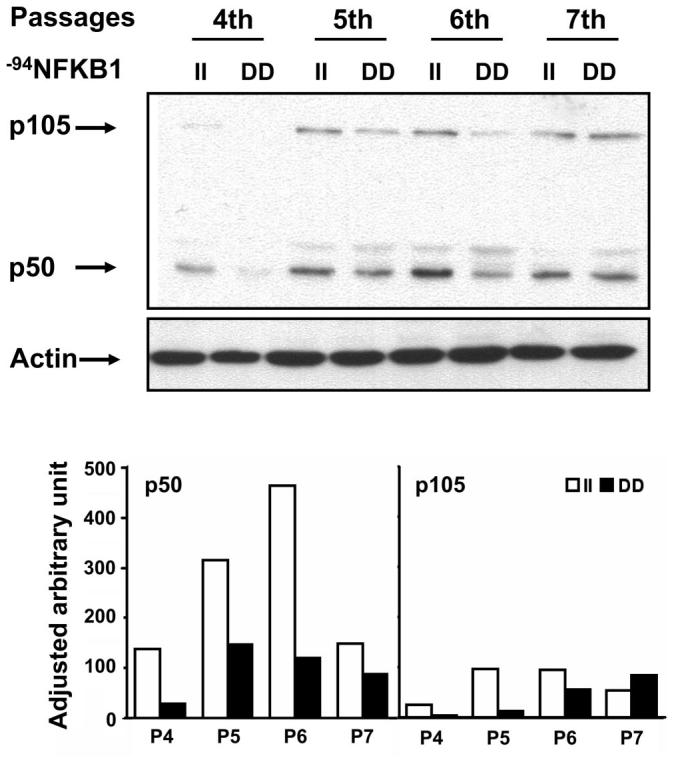
The p50/p105 NFKB1 I/D promoter regulates NFKB1 gene expression. Cytoplasmic proteins were extracted from genotyped homozygous II and DD HUVECs, and immunoblots were performed with monoclonal antibody against p50/p105 proteins. Higher basal p50/p105 protein levels were observed in II homozygote cells compared with DD homozygote cells. Actin was used as an internal control for equal sample loadings. P, passage.
II homozygote HUVECs are more sensitive to unidirectional LSS with respect to NOS3 protein expression
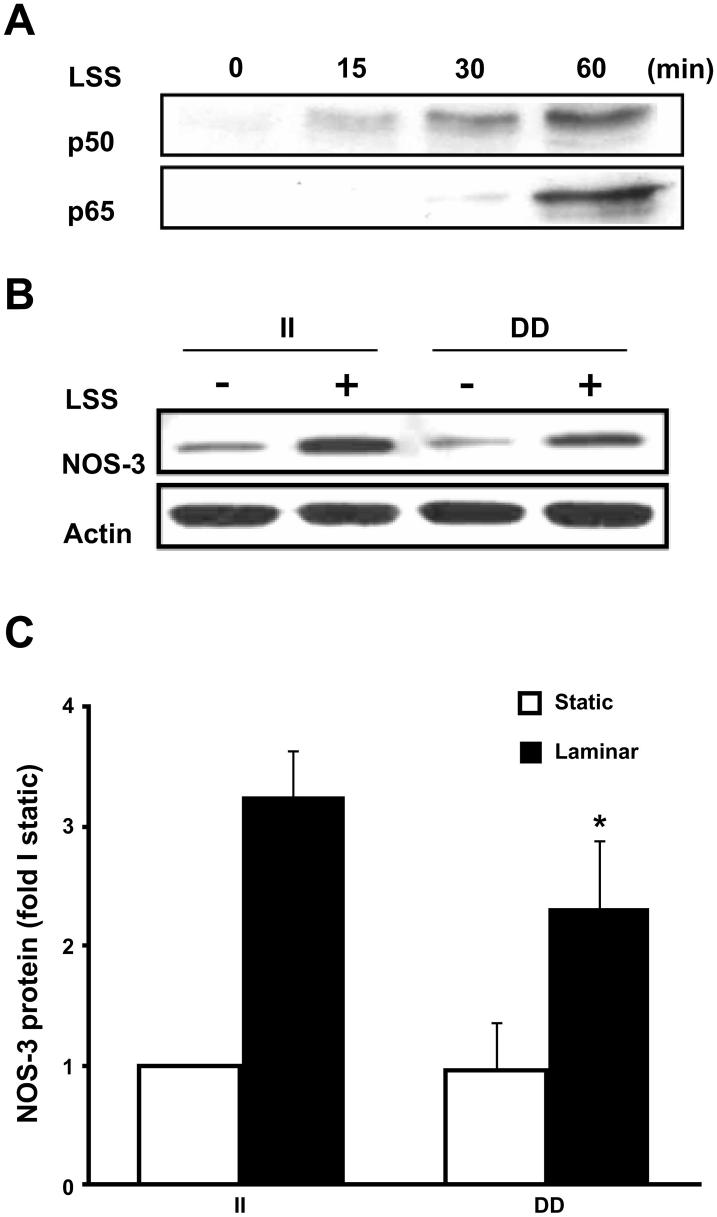
To clarify the involvement of unidirectional LSS in the activation of the NF-κB complex, we monitored the translocation of the NF-κB subunits, p50/p65, under unidirectional LSS by subsequent immunoblot assays. HUVECs were subjected to unidirectional LSS for up to 60 min, and nuclear extracts were prepared for p50/p65 protein immunoblot assays at the 0-, 15-, 30-, and 60-min time periods. In this experiment, gradual translocation of both p50 and p65 subunits was observed under unidirectional LSS (Fig. 3A). Figure 3A shows that unidirectional LSS activates the translocation of NF-κB p50/p65 in HUVECs. This observation was consistent with what we observed using LPS (100 ng/μl) stimulation (data not shown).
Fig. 3.
II homozygous cells are more responsive to unidirectional laminar shear stress (LSS) in the induction of endothelial nitric oxide synthase (NOS3) gene expression. A: both p50 and p65 NF-κB subunits are gradually translocated under unidirectional LSS in HUVECs. Primary cultured HUVECs were subjected to unidirectional LSS, and levels of p50 and p65 proteins were measured in nuclear extracts by Western blot analysis. B and C: genotyped HUVECs for NFKB1 I/D polymorphism were used for the experiments. HUVECs were subjected to unidirectinal LSS (15 dyn/cm2) for 24 h using a cone-plate apparatus, and NOS3 protein levels were measured in total extracts. B: example of the immunoblot assay performed for NOS3 protein levels in static or 24-h LSS-stimulated II or DD genotyped HUVECs. C: summary of the statistical analysis (n = 6) for NOS3 protein levels expressed as fold increases over II homozygote cells under static conditions. All protein levels were adjusted to actin protein levels. Values are means ± SE. *P < 0.05 vs. the DD change value.
Previous studies (7, 9) have demonstrated that the NF-κB signaling pathway is responsible for the upregulation of NOS3 in response to LSS. Because we found higher levels of NFKB1 gene products in NFKB1 II cells than in NFK1 DD cells, we tested whether −94NFKB1 I/D polymorphism affected the level of NOS3 gene expression in HUVECs in response to unidirectional LSS. II or DD homozygote HUVECs were subjected to 24 h of unidirectional LSS or static conditions, and total cell extracts were prepared. There were no differences in basal NOS3 levels between II or DD homozygote cells. However, there was a greater increase in NOS3 protein levels in II homozygote cells compared with DD homozygote cells (P < 0.05) after exposure to 24 h of LSS (Fig. 3, B and C). These results suggest that NFKB1 II homozygote cells are more responsive to unidirectional LSS in terms of the induction of NOS3 gene expression, likely due to the increased amounts of p50 and p105 in the cells.
The I allele is associated with higher reactive hyperemic FBF
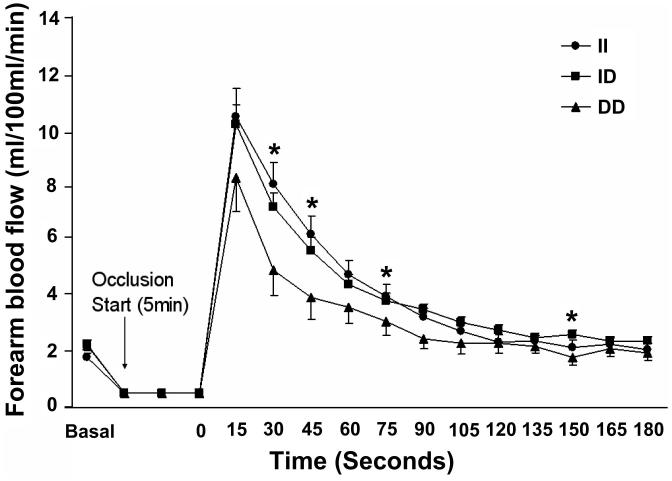
We examined arterial reactivity in subjects with each genotype at baseline and after 6 mo of exercise training. General characteristics of the subjects involved in this study are shown in Table 1. These genotype frequencies were similar to those observed in previous studies (2, 16). There was a significant increase in V̇O2max after exercise training (P < 0.05). There were no gender main effects observed on reactive hyperemic FBF responses. At baseline, there was a tendency for a similar reactive hyperemic FBF response between II and ID genotype groups. There was a significantly higher reactive hyperemic FBF response in the I allele carrier group than in the DD homozygote group (genotype main effect, P = 0.039; Fig. 4). In addition, there was a tendency for greater improvements in reactive hyperemic FBF in II and ID genotype groups compared with the DD homozygote group after 6 mo of exercise training (Table 2). To test the adaptability in reactive hyperemic FBF with exercise training, each subject was categorized as a responder or nonresponder based on the change in their reactive hyperemic FBF response following exercise training. Subjects who showed positive changes were categorized as responders, whereas subjects who did not change or exhibited a decreased response with exercise training were categorized as nonresponders. This subgroup analysis revealed that II homozygotes were more prevalent in the responder group (30.6%, odds ratio = 2.45, P = 0.047), and DD homozygotes were significantly less prevalent in the responder group (4.1%, odds ratio = 0.15, P = 0.006; Table 3).
Table 1.
Characteristics of patients in the human exercise training intervention experiments
| Baseline | Final | ||
|---|---|---|---|
| n | 47 | 36 | |
| Sex, women/men | 24/23 | 16/20 | |
| Ethnicity, Caucasian/non-Caucasian | 24/23 | 18/18 | |
| Age, yr | 58.5±0.8 | 58.7±0.7 | |
| Height, cm | 171.0±1.4 | 170.5±1.3 | |
| Weight, kg | 84.4+2.1 | 84.1±1.9 | |
| SBP, mmHg | 132.8±1.6 | 132.6±1.9 | |
| DBP, mmHg | 87.9±0.9 | 87.1±1.0 | |
| MBP, mmHg | 102.7±1.1 | 102.1±1.2 | |
| BMI, kg/m2 | 29.0±0.6 | 28.6±0.7 | |
| V̇o2 max, ml·kg−1·min−1 | 26.5±0.6 | 29.8±0.9 | |
| NFKB1 I/D, n | |||
| II | 12 (26%) | 8 (22%) | |
| ID | 26 (55%) | 23 (64%) | |
| DD | 9 (19%) | 5 (14%) | |
Values are mean ± SE except for frequency data; n, no. of subjects. Baseline, before exercise training; final, after exercise training; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; BMI, body mass index; V̇o2 max, maximal oxygen consumption; NFKB1, NF-κ light-chain gene enhancer in B cells 1; I, insertion allele; D, deletion allele.
Fig. 4.
The I allele carrier group had a higher reactive hyperemic forearm blood flow response than the DD homozygote group at baseline. *Significant difference between II/ID vs. DD. Data are adjusted least-significant means with error bars representing SEs. Age, gender, ethnicity, weight, mean blood pressure, and body mass index were used as covariates.
Table 2.
Percent changes in reactive hyperemic FBF responses after exercise training
|
NFKB1 I/D |
||||
|---|---|---|---|---|
| II | ID | DD | ||
| Percent change in basal FBF |
12.8±16.0 | 19.0±10.8 | −6.0±16.7 | |
| Percent change in reactive hyperemic FBF |
||||
| 1 min | 12.1±12.4 | 15.0±12.3 | −1.4±38.5 | |
| 2 min | 9.2±18.5 | 7.0±12.3 | −24.1±17.1 | |
| 3 min | 38.6±43.2 | 6.2±9.0 | −13.3±16.7 | |
Values are means ± SE. FBF, forearm blood flow.
Table 3.
NFKB1 genotype frequencies of responder and nonresponder groups
|
NFKB1 I/D |
||||
|---|---|---|---|---|
| II | ID | DD | Total Frequencies Among Genotype Groups | |
| Respondera | 15 (62.5%)* | 32 (46.4%)* | 2 (13.3%)* | 49 |
| Nonrespondera | 9 (37.5%) | 37 (53.6%) | 13 (86.7%) | 59 |
| All counts | 24 | 69 | 15 | 108 |
Net allele frequencies of responder or nonresponder groups at the 1st, 2nd, and 3rd minutes of reactive hyperemia.
Odds ratios (ORs) of responding versus nonresponding patients: II, OR = 2.45 (P < 0.05); ID, OR = 1.12 (not significant); and DD, OR = 0.15 (P < 0.05).
DISCUSSION
The major findings of the present study were that 1) proteins present in the nuclei of HUVECs preferentially bound to the I allele sequence compared with the D allele; 2) the I allele had significantly higher promoter activity than the D allele; 3) II homozygote cells had higher p50/p105 NFKB1 protein levels than DD homozygote cells; 4) II homozygote cells showed a significantly greater increase in NOS3 protein levels than DD homozygote cells under unidirectional LSS; and 5) the I-allele carrier group had a higher basal reactive hyperemic FBF response. Furthermore, the −94NFKB1 I allele was significantly associated with greater adaptability in endothelial function with long-term exercise training.
A healthy endothelium serves to 1) inhibit monocyte and platelet adhesion, 2) maintain profibrinolytic and antithrombotic activity, 3) prevent vascular smooth muscle cell proliferation, and 4) modulate the vasodilatory response. All of these protective functions of the endothelium are achieved through NOS3 and. ultimately, the release of NO. Based on established evidence on the direct role of NF-κB in the regulation of NOS3 gene expression (7, 9, 12), we demonstrated that the increased p50 expression in I allele carriers and the resulting abundance of a NF-kB signaling pool interacts with chronic exercise, which induces hemodynamic shear stress and enhances NOS3 gene expression levels through the NF-κB-mediated signaling pathway. This is translated into improved vascular function.
It is universally agreed upon that an increase in NOS3 content is a beneficial CV adaptation, as are many adaptations to aerobic exercise training. To our knowledge, there are only a few studies that have measured the NOS3 level directly in vessels obtained from human patients. Hambrecht et al. (10) showed a twofold increase in NOS3 protein levels in arteries obtained from coronary artery disease patients with aerobic exercise training. Accordingly, these patients also showed a significant increase in vasodilatory function by twofold. In the present study, we found a 30% difference in NOS3 protein levels in response to shear stress between genotyped cells. In the human data, the II homozygote group showed an ∼30% higher peak FBF compared with DD homozygotes at baseline. Based on the Hambrecht et al. study (10), our findings on DD homozygotes of NFKB1 promoter polymorphism, which nearly 20% of the population possesses, a decrease in arterial reactivity by 30% would appear to be functionally and physiologically significant.
In addition, endothelial dysfunction is a hallmark feature of most chronic diseases and is present before the onset of overt essential hypertension. A healthy endothelium is associated with good CV health and increased NOS3 content is synonymous with a healthy endothelium (1, 8, 15, 34). If a particular allele leads to enhanced NOS3 expression with exercise training, as was the case in the present study, then that allele would be considered to have a positive influence on CV health.
In the present study, we confirmed that this function of polymorphism is also present in human endothelial cells. The EMSA indicated that the I allele showed strong binding to nuclear protein extracts. In contrast, D and D1 alleles allowed no binding or minimal binding of proteins of similar mobility, respectively. Since the D1 variant contains the D allele with an additional 4 bp of genomic sequence added to the 5′ end, which creates a “size-matched” D allele, D1 is considered to be more pertinent to the endogenous function of the mutant promoter. Therefore, the D allele promoter binds to the unknown nuclear protein with noticeably less binding affinity, which results in reduced transcriptional activity rather than generating a completely nonfunctional promoter. This results were consistent with a previous studies (2, 16) showing that the I allele of the NFKB1 promoter previously showed higher protein binding activity than the D allele in immortalized cell lines. The specific polymorphic region may contain a core binding sequence of an unidentified nuclear protein. Identification of the specific binding protein remains to be elucidated.
In the promoter assays, we observed that NFKB1 promoter-reporter gene constructs containing the ATTG I-type allele showed significantly increased promoter activity in primary cultured HUVECs following 24 h of LPS treatment. A previous study (16) showed that the polymorphic promoter binds to normal human colonic cell lines, but there was no evidence of terminal ileal mucosa nuclear protein bound to the region. These results suggest that the polymorphism appears to have a tissue-specific effect. This led us to test the transactivational function of the I/D polymorphism in human endothelial cells. Our finding suggest that −94NFKB1 I/D polymorphism affects promoter activity of the NFKB1 gene in human endothelial cells.
Shear stress increases the nuclear translocation of NF-κB and elevates the transcriptional activity of promoters containing the -κB binding element in bovine aortic endothelial cells (17, 18, 32). In the present study, we showed that in HUVECs, the p50/p65 NF-κB complex translocated into the nucleus under a physiological level of unidirectional LSS (15 dyn/cm2) generated by a cone and plate apparatus. This result agrees with previous findings showing that p50/p65 NF-κB complex translocation occurred in response to 15 dyn/cm2 of LSS generated by a parallel plate flow chamber system incorporated into a closed-loop perfusion device (12).
In the present study, we demonstrated that II homozygote cells had higher cytoplasmic p50/p105 protein levels than DD homozygote cells and, accordingly, that II homozygote cells demonstrated a greater increase in NOS3 protein than DD homozygote cells after 24 h of unidirectional LSS. Several lines of evidence suggest that NF-κB is predominantly responsible for increasing the transcription of the NOS3 gene in response to LSS. Resnick et al. (29) identified a cis-acting SSRE (5′-GAGACC-3′) in promoter regions of shear-responsive genes. Supershift assays using p50 and p65 antibodies demonstrated that translocated NF-κB under flow shear stress binds to the SSRE in the promoter of target genes (12). Davis et al. (7) identified the SSRE (5′-GAGACC-3′) at position −990 to −984 bp upstream of the transcription start site of the NOS3 gene and demonstrated that shear activated p50/p65 NF-κB bound to the identified binding sequence. Moreover, Grumbach et al. (9) showed that a NF-κB inhibitor, panepoxy-done, completely prevented the increase in NOS3 mRNA levels in endothelial cells exposed to shear stress.
There were no significant differences in basal promoter activities between I- and D-type promoters. This result does not agree with our findings showing that II homozygote cells had higher basal p50/p105 cytoplasmic protein levels compared with DD homozygote cells. A definitive explanation of this discrepancy could not be made since it is beyond of the scope of this study. However, one possible explanation is that the gene expression level was regulated by an interaction between a protein binding to the polymorphic site and other transcription factors that bind to a cis-element distant from the transcription initiation site and, therefore, was not included in the reporter vectors we used for the promoter assays.
We found that the p105 protein level was affected by NFKB1 promoter polymorphism. Whether the upregulation of p105 protein affected NF-κB signaling and downstream NOS3 gene expression is controversial. p105 protein is considered a member of the IκB family because of the presence of ankyrin repeats in its COOH-terminus region (30); on the other hand, p105 protein is considered as a precursor protein for p50 protein. It has been demonstrated that p105 protein is constitutively processed via proteasomes, as opposed to degradation, through a cotranslational mechanism (20). However, the proteolysis of the precursor protein that generates p50 is also limited by the presence of a glycine-rich region between amino acids 376 and 404, which serves as a stop signal for proteolysis (21, 27). Therefore, the role of increased p105 in NOS3 gene expression is inconclusive.
In the vasculature, regions of low flow velocity and high oscillatory shear stress observed during resting conditions are abolished during a session of aerobic exercise. These regions experience higher flow, and the hemodynamic profile is converted from one that is oscillatory to one that is laminar. In vivo levels of vascular shear stress during exercise are difficult to measure. However, during cycling exercise in humans, shear stress levels between 7.3 and 16.5 dyn/cm2 have been shown (4). We appropriately used a shear stress level of 15 dyn/cm2 in the present study to simulate the level of shear stress experienced in humans during aerobic exercise.
Given the rationale that 1) flow shear stress on the endothelial cell is increased during aerobic exercise, 2) NF-κB plays a crucial role in intracellular signaling transduction for NOS3 gene expression in response to flow shear stress, and 3) −94NFKB1 I/D polymorphism transcriptionally regulates p50 NFKB1 gene expression, we hypothesized that the polymorphism may affect the endothelial function response to aerobic exercise training. Although overall the human results are consistent with our in vitro results, the weaker than expected relationship is not surprising. When assessing systemic functional phenotypes (end-point phenotypes) such as FBF or blood pressure, there are multiple factors that contribute to these biological responses. This means that there are other factors that contribute to the adaptation to exercise training, and it is improbable that a single-gene polymorphism will be the sole determinant of the adaptive response. However, our data demonstrated the importance of the gene polymorphism in vascular function, and this will help to better understand the molecular mechanisms involved in the adaptations to exercise training. In addition, there could be clinical relevance for preventive and interventional medicine as this polymorphism could be a good marker for predicting how an individual adapts to chronic exercise.
Reactive hyperemia refers to the phenomenon of increased blood flow that follows relief of ischemia and is a result of extended dilation of conduit arteries and resistance arterioles. Several factors have been implicated in the genesis of reactive hyperemia, including mechanical and neurogenic mechanisms, endothelium-derived NO, adenosine, and membrane-bound ion channels. Therefore, the measurement of reactive hyperemia is not solely endothelium dependent. However, several infusion studies using an NOS3 inhibitor (i.e., N-monomethyl-l-argi-nine) or a pharmacological agonist for NOS3 activator (i.e., acetylcholine) have revealed that 1) endothelium-derived NO accounts predominantly for the reactive hyperemic vasodilatory response (5, 23) and 2) there is a strong correlation (r = 0.89) observed between peak reactive hyperemic FBF and acetylcholine-induced peak vasodilation (14).
In conclusion, the present study identified a significant association between the −94NFKB1 5′-ATTG-3′ D allele and decreased reactive hyperemic FBF response, which was consistent with the effect of NFKB1 on downstream NOS3 gene expression found in HUVECs. These results have potential clinical implications for endothelial dysfunction that are related to the development and progression of atherosclerosis and CV diseases. Since >20% of the population is homozygous for the D allele, screening this polymorphism may provide an option for increasing the diagnostic capacity and improving the CV risk assessment in susceptible individuals. Additionally, our findings provide insight into the molecular mechanisms involved in the intracellular signaling transduction process of NOS3 gene expression and function of the NFKB1 gene promoter region.
ACKNOWLEDGMENTS
We thank Nancy Petro (University of Pittsburgh), Dr. SangJin Cao (University of Maryland), and Vascular Mechanics Lab staff members (Emory University) for the technical expertise and support.
GRANTS
This work was supported by an American Heart Association Predoctoral Fellowship Grant AHA-0415444U (to J. Y. Park) and by National Institutes of Health Grants AG-019640 (to M. D. Brown), AG-17474 (to J. M. Hagberg), AG-022791 (to S. M. Roth), DK-58189 (to S. R. Brant), and HL-071894 (to I. K. G. Farrance).
REFERENCES
- 1.Agnoletti L, Curello S, Bachetti T, Malacarne F, Gaia G, Comini L, Volterrani M, Bonetti P, Parrinello G, Cadei M, Grigolato PG, Ferrari R. Serum from patients with severe heart failure downregulates eNOS and is proapoptotic: role of tumor necrosis factor-alpha. Circulation. 1999;100:1983–1991. doi: 10.1161/01.cir.100.19.1983. [DOI] [PubMed] [Google Scholar]
- 2.Borm ME, van Bodegraven AA, Mulder CJ, Kraal G, Bouma G. A NFKB1 promoter polymorphism is involved in susceptibility to ulcerative colitis. Int J Immunogenet. 2005;32:401–405. doi: 10.1111/j.1744-313X.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 3.Cattaruzza M, Guzik TJ, Slodowski W, Pelvan A, Becker J, Halle M, Buchwald AB, Channon KM, Hecker M. Shear stress insensitivity of endothelial nitric oxide synthase expression as a genetic risk factor for coronary heart disease. Circ Res. 2004;95:841–847. doi: 10.1161/01.RES.0000145359.47708.2f. [DOI] [PubMed] [Google Scholar]
- 4.Cheng CP, Herfkens RJ, Taylor CA. Inferior vena caval hemodynamics quantified in vivo at rest and during cycling exercise using magnetic resonance imaging. Am J Physiol Heart Circ Physiol. 2003;284:H1161–H1167. doi: 10.1152/ajpheart.00641.2002. [DOI] [PubMed] [Google Scholar]
- 5.Dakak N, Husain S, Mulcahy D, Andrews NP, Panza JA, Waclawiw M, Schenke W, Quyyumi AA. Contribution of nitric oxide to reactive hyperemia: impact of endothelial dysfunction. Hypertension. 1998;32:9–15. doi: 10.1161/01.hyp.32.1.9. [DOI] [PubMed] [Google Scholar]
- 6.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis ME, Grumbach IM, Fukai T, Cutchins A, Harrison DG. Shear stress regulates endothelial nitric-oxide synthase promoter activity through nuclear factor kappaB binding. J Biol Chem. 2004;279:163–168. doi: 10.1074/jbc.M307528200. [DOI] [PubMed] [Google Scholar]
- 8.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 9.Grumbach IM, Chen W, Mertens SA, Harrison DG. A negative feedback mechanism involving nitric oxide and nuclear factor kappa-B modulates endothelial nitric oxide synthase transcription. J Mol Cell Cardiol. 2005;39:595–603. doi: 10.1016/j.yjmcc.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 11.Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342:454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 12.Hay DC, Beers C, Cameron V, Thomson L, Flitney FW, Hay RT. Activation of NF-kappaB nuclear transcription factor by flow in human endothelial cells. Biochim Biophys Acta. 2003;1642:33–44. doi: 10.1016/s0167-4889(03)00084-3. [DOI] [PubMed] [Google Scholar]
- 13.Heron E, Deloukas P, van Loon AP. The complete exon-intron structure of the 156-kb human gene NFKB1, which encodes the p105 and p50 proteins of transcription factors NF-kappa B and I kappa B-gamma: implications for NF-kappa B-mediated signal transduction. Genomics. 1995;30:493–505. doi: 10.1006/geno.1995.1270. [DOI] [PubMed] [Google Scholar]
- 14.Heylen E, Guerrero F, Berbari H, Gilard M, Saiag B, Mansourati J. Correlation between reactive hyperaemia and acetylcholine induced vasodilation in rat cutaneous microcirculation. Atherosclerosis. 2005;180:419–421. doi: 10.1016/j.atherosclerosis.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 16.Karban AS, Okazaki T, Panhuysen CI, Gallegos T, Potter JJ, Bailey-Wilson JE, Silverberg MS, Duerr RH, Cho JH, Gregersen PK, Wu Y, Achkar JP, Dassopoulos T, Mezey E, Bayless TM, Nouvet FJ, Brant SR. Functional annotation of a novel NFKB1 promoter polymorphism that increases risk for ulcerative colitis. Hum Mol Genet. 2004;13:35–45. doi: 10.1093/hmg/ddh008. [DOI] [PubMed] [Google Scholar]
- 17.Khachigian LM, Resnick N, Gimbrone MA, Jr, Collins T. Nuclear factor-kappa B interacts functionally with the platelet-derived growth factor B-chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J Clin Invest. 1995;96:1169–1175. doi: 10.1172/JCI118106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lan Q, Mercurius KO, Davies PF. Stimulation of transcription factors NF kappa B and AP1 in endothelial cells subjected to shear stress. Biochem Biophys Res Commun. 1994;201:950–956. doi: 10.1006/bbrc.1994.1794. [DOI] [PubMed] [Google Scholar]
- 19.Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol. 2001;90:501–510. doi: 10.1152/jappl.2001.90.2.501. [DOI] [PubMed] [Google Scholar]
- 20.Lin L, DeMartino GN, Greene WC. Cotranslational biogenesis of NF-kappaB p50 by the 26S proteasome. Cell. 1998;92:819–828. doi: 10.1016/s0092-8674(00)81409-9. [DOI] [PubMed] [Google Scholar]
- 21.Lin L, Ghosh S. A glycine-rich region in NF-kappaB p105 functions as a processing signal for the generation of the p50 subunit. Mol Cell Biol. 1996;16:2248–2254. doi: 10.1128/mcb.16.5.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 23.Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. Am J Physiol Heart Circ Physiol. 1996;270:H1435–H1440. doi: 10.1152/ajpheart.1996.270.4.H1435. [DOI] [PubMed] [Google Scholar]
- 24.Moore JE, Jr, Xu C, Glagov S, Zarins CK, Ku DN. Fluid wall shear stress measurements in a model of the human abdominal aorta: oscillatory behavior and relationship to atherosclerosis. Atherosclerosis. 1994;110:225–240. doi: 10.1016/0021-9150(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 25.Niebauer J, Cooke JP. Cardiovascular effects of exercise: role of endothelial shear stress. J Am Coll Cardiol. 1996;28:1652–1660. doi: 10.1016/S0735-1097(96)00393-2. [DOI] [PubMed] [Google Scholar]
- 26.Nishida K, Harrison DG, Navas JP, Fisher AA, Dockery SP, Uematsu M, Nerem RM, Alexander RW, Murphy TJ. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase. J Clin Invest. 1992;90:2092–2096. doi: 10.1172/JCI116092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orian A, Schwartz A, Israel A, Whiteside S, Kahana C, Ciechanover A. Structural motifs involved in ubiquitin-mediated processing of the NF-kappaB precursor p105: roles of the glycine-rich region and a downstream ubiquitination domain. Mol Cell Biol. 1999;19:3664–3673. doi: 10.1128/mcb.19.5.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 29.Resnick N, Collins T, Atkinson W, Bonthron DT, Dewey CF, Jr, Gimbrone MA., Jr. Platelet-derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element. Proc Natl Acad Sci USA. 1993;90:4591–4595. doi: 10.1073/pnas.90.10.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rice NR, MacKichan ML, Israel A. The precursor of NF-kappa B p50 has I kappa B-like functions. Cell. 1992;71:243–253. doi: 10.1016/0092-8674(92)90353-e. [DOI] [PubMed] [Google Scholar]
- 31.Shephard RJ, Balady GJ. Exercise as cardiovascular therapy. Circulation. 1999;99:963–972. doi: 10.1161/01.cir.99.7.963. [DOI] [PubMed] [Google Scholar]
- 32.Shyy JY, Li YS, Lin MC, Chen W, Yuan S, Usami S, Chien S. Multiple cis-elements mediate shear stress-induced gene expression. J Biomech. 1995;28:1451–1457. doi: 10.1016/0021-9290(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 33.Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem. 2003;278:31128–31135. doi: 10.1074/jbc.M300703200. [DOI] [PubMed] [Google Scholar]
- 34.Stein B, Eschenhagen T, Rudiger J, Scholz H, Forstermann U, Gath I. Increased expression of constitutive nitric oxide synthase III, but not inducible nitric oxide synthase II, in human heart failure. J Am Coll Cardiol. 1998;32:1179–1186. doi: 10.1016/s0735-1097(98)00399-4. [DOI] [PubMed] [Google Scholar]