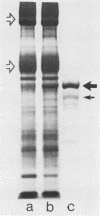
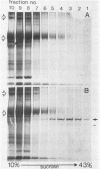
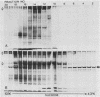
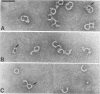
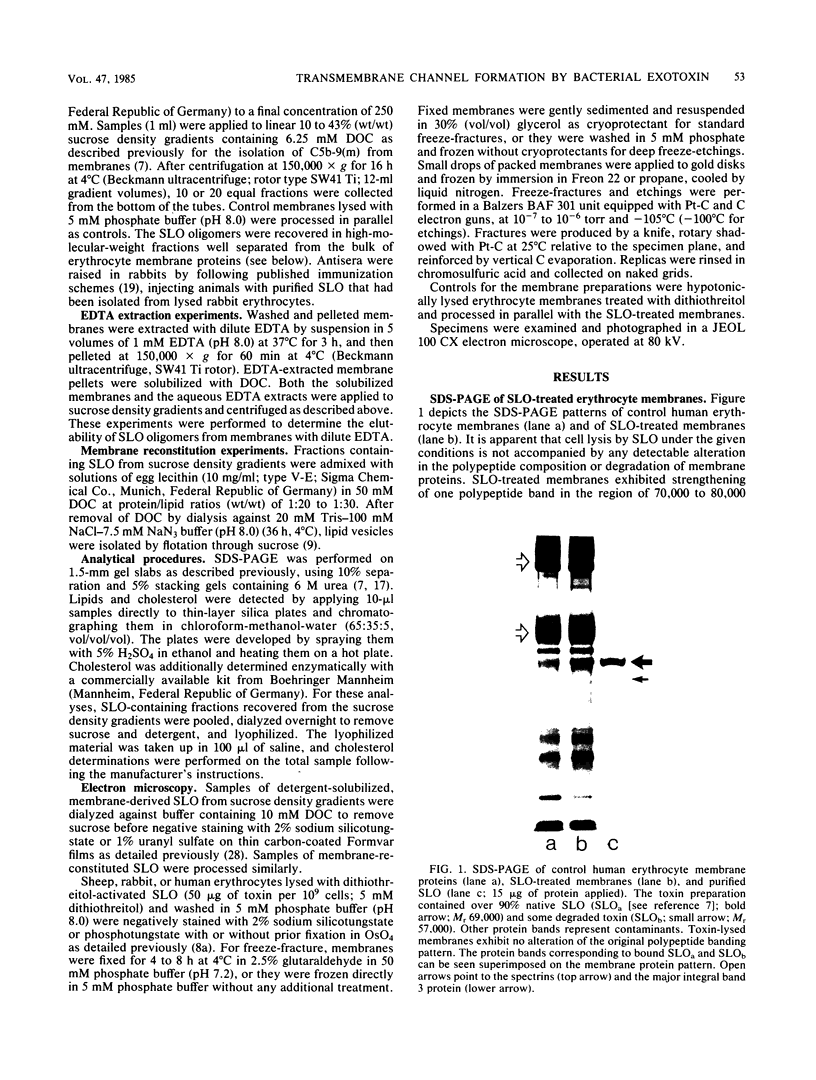
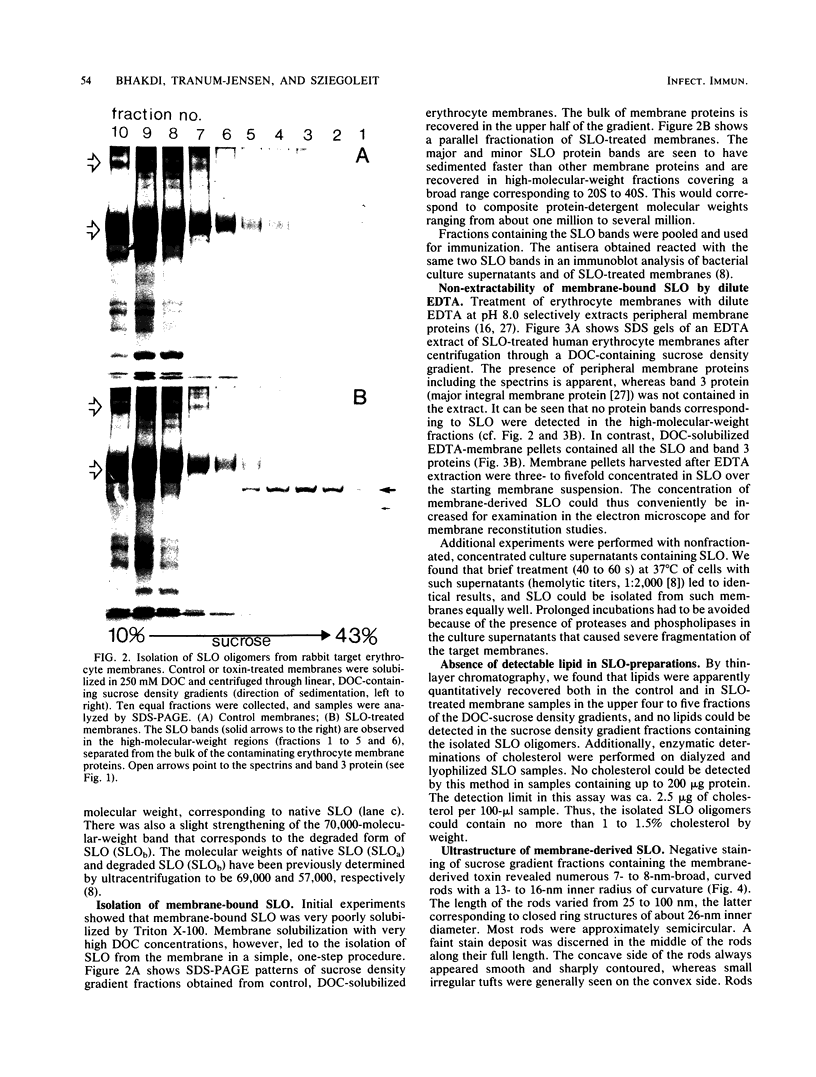
Abstract
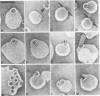
Streptolysin-O (SLO) is a thiol-activated, membrane-damaging protein toxin of Mr 69,000 that is produced by most strains of beta-hemolytic group A streptococci. Native, primarily water-soluble toxin molecules bind to cholesterol-containing target membranes to assemble into supramolecular curved rod structures (25 to 100 nm long by ca. 7.5 nm wide), forming rings and arcs that penetrate into the apolar domain of the bilayer. Electron microscopic analyses of toxin polymers in their native and reconstituted membrane-bound form indicate that the convex surface of the rod structures is a hydrophobic, lipid-binding domain, whereas the concave surfaces appear to be hydrophilic. The embedment of the rings and arcs generates large transmembrane slits or pores of up to 30-nm diameter that can be directly visualized by negative staining and freeze-fracture electron microscopy. SLO oligomers were isolated in extensively delipidated form in detergent solution, and cholesterol was found not to detectably contribute to the observed rod structures. The rods are stable structures that resist prolonged exposure to trypsin and chymotrypsin. They can be reincorporated into cholesterol-free phosphatidylcholine liposomes to generate lesions identical to those observed on erythrocytes lysed by native SLO. Thus, although cholesterol plays a key role in the initial binding of SLO to the membrane, it does not directly participate in the formation of the membrane-penetrating toxin channels. Membrane damage by SLO is basically analogous to that mediated by previously studied channel formers, namely, the C5b-9 complement complex and staphylococcal alpha-toxin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alouf J. E. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxin). Pharmacol Ther. 1980;11(3):661–717. doi: 10.1016/0163-7258(80)90045-5. [DOI] [PubMed] [Google Scholar]
- Arbuthnott J. P., Freer J. H., Bernheimer A. W. Physical states of staphylococcal alpha-toxin. J Bacteriol. 1967 Oct;94(4):1170–1177. doi: 10.1128/jb.94.4.1170-1177.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Füssle R. Correlation between toxin binding and hemolytic activity in membrane damage by staphylococcal alpha-toxin. Infect Immun. 1984 Nov;46(2):318–323. doi: 10.1128/iai.46.2.318-323.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Roth M. Preparation and isolation of specific antibodies to complement components. Methods Enzymol. 1983;93:409–420. doi: 10.1016/s0076-6879(83)93054-9. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Roth M., Sziegoleit A., Tranum-Jensen J. Isolation and identification of two hemolytic forms of streptolysin-O. Infect Immun. 1984 Nov;46(2):394–400. doi: 10.1128/iai.46.2.394-400.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Mechanism of complement cytolysis and the concept of channel-forming proteins. Philos Trans R Soc Lond B Biol Sci. 1984 Sep 6;306(1129):311–324. doi: 10.1098/rstb.1984.0092. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Molecular nature of the complement lesion. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5655–5659. doi: 10.1073/pnas.75.11.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. On the cause and nature of C9-related heterogeneity of terminal complement complexes generated on target erythrocytes through the action of whole serum. J Immunol. 1984 Sep;133(3):1453–1463. [PubMed] [Google Scholar]
- Buckingham L., Duncan J. L. Approximate dimensions of membrane lesions produced by streptolysin S and streptolysin O. Biochim Biophys Acta. 1983 Mar 23;729(1):115–122. doi: 10.1016/0005-2736(83)90462-5. [DOI] [PubMed] [Google Scholar]
- Cowell J. L., Kim K. S., Bernheimer A. W. Alteration by cereolysin of the structure of cholesterol-containing membranes. Biochim Biophys Acta. 1978 Feb 21;507(2):230–241. doi: 10.1016/0005-2736(78)90419-4. [DOI] [PubMed] [Google Scholar]
- Dourmashkin R. R., Rosse W. F. Morphologic changes in the membranes of red blood cells undergoing hemolysis. Am J Med. 1966 Nov;41(5):699–710. doi: 10.1016/0002-9343(66)90031-3. [DOI] [PubMed] [Google Scholar]
- Duncan J. L. Characteristics of streptolysin O hemolysis: kinetics of hemoglobin and 86rubidium release. Infect Immun. 1974 Jun;9(6):1022–1027. doi: 10.1128/iai.9.6.1022-1027.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. L., Schlegel R. Effect of streptolysin O on erythrocyte membranes, liposomes, and lipid dispersions. A protein-cholesterol interaction. J Cell Biol. 1975 Oct;67(1):160–174. doi: 10.1083/jcb.67.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN H., BARROW P., GOLDBERG B. Effect of antibody and complement on permeability control in ascites tumor cells and erythrocytes. J Exp Med. 1959 Nov 1;110:699–713. doi: 10.1084/jem.110.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Rottem S., Cole R. M., Habig W. H., Barile M. F., Hardegree M. C. Structural characteristics of tetanolysin and its binding to lipid vesicles. J Bacteriol. 1982 Nov;152(2):888–892. doi: 10.1128/jb.152.2.888-892.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARS D. A., WEED R. I., SWISHER S. N. DIFFERENCES IN THE MECHANISM OF IN VITRO IMMUNE HEMOLYSIS RELATED TO ANTIBODY SPECIFICITY. J Clin Invest. 1964 May;43:975–985. doi: 10.1172/JCI104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J Cell Biol. 1980 Dec;87(3 Pt 1):828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Freer J. H., Arbuthnott J. P. Interaction of Clostridium perfringens theta-haemolysin, a contaminant of commercial phospholipase C, with erythrocyte ghost membranes and lipid dispersions. A morphological study. Biochim Biophys Acta. 1975 Apr 8;382(4):479–493. doi: 10.1016/0005-2736(75)90216-3. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranum-Jensen J., Bhakdi S., Bhakdi-Lehnen B., Bjerrum O. J., Speth V. Complement lysis: the ultrastructure and orientation of the C5b-9 complex on target sheep erythrocyte membranes. Scand J Immunol. 1978;7(1):45–46. doi: 10.1111/j.1365-3083.1978.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Tschopp J., Müller-Eberhard H. J., Podack E. R. Formation of transmembrane tubules by spontaneous polymerization of the hydrophilic complement protein C9. Nature. 1982 Aug 5;298(5874):534–538. doi: 10.1038/298534a0. [DOI] [PubMed] [Google Scholar]