Abstract
Mice deficient in corticotropin releasing factor receptor 2 (CRF2) (C57BL/6J:129Sv background) exhibit impaired maternal defense (protection of offspring) and are more reactive to stressors than wild-type mice. To further understand CRF2’s role in maternal behavior, we crossed the knockout mice with a line bred for high maternal defense that also has elevated maternal care relative to inbred lines. Maternal care was normal in knockout mice (relative to wild-type). Maternal defense was impaired as previously observed. Exposure to a mild stressor (15 min restraint) did not trigger deficits in maternal defense in either genotype as determined by a two-way repeated measures ANOVA analysis. However, when examining difference scores between unrestrained and restrained conditions, knockout mice exhibited significant decreases in maternal defense with stress, suggesting knockouts are more susceptible to a mild stressor’s effects. To gain possible insights into brain activity differences between WT and KO mice, we examined c-Fos expression in association with stress. Unrestrained KO mice exhibited significantly lower c-Fos levels relative to unrestrained WT mice in 9 regions, including lateral septum and periaqueductal gray. For WT mice, restraint stress triggered c-Fos activity increases in 3 regions while for KO mice, restraint stress triggered c-Fos increases in 16 regions. Taken together, our results suggest both altered behavioral and c-Fos responses to stress in lactating CRF2 KO mice.
Keywords: stress, lactation, maternal aggression, maternal defense, corticotropin-releasing factor, corticotropin-releasing factor receptor
Introduction
Maternal defense is a highly aggressive behavior performed by lactating females in protection of their offspring. This behavior has been shown to be inhibited by central administration of the anxiogenic peptide corticotropin releasing factor (CRF) and its related family of peptides, urocortin (Ucn) 1 and Ucn 3 (Gammie, Negron et al. 2004; D’Anna, Stevenson et al. 2005), possibly suggesting decreased neurotransmission of these peptides during lactation to facilitate the normal display of maternal defense. Furthermore, during lactation, the CNS is less responsive to CRF and stress (da Costa, Wood et al. 1996; da Costa, Kampa et al. 1997), suggesting that the activation of this G-protein coupled receptor family, including both CRF receptor 1 (CRF1) and CRF receptor (CRF2), may be altered during lactation in order to support full maternal care. Interestingly, the CRF-related peptides have varying binding affinities for these receptors. CRF and Ucn 1 bind to both CRF1 and CRF2, whereas Ucn 3 specifically activates CRF2 (as well as Ucn 2) (Hauger, Grigoriadis et al. 2003). It has been demonstrated that CRF2 activation alone is sufficient to impair the behavior as evidenced by central injections of Ucn 3 alone diminishing maternal defense. Thus, the regulation of CRF2 activation during lactation likely influences the normal display of this behavior.
One way to assess the relative contribution of CRF2 to maternal defense behavior is to investigate genetically altered mice lacking CRF2. Mice deficient in CRF2 (C57BL/6J:129Sv background) exhibit deficits in maternal defense (Gammie, Hasen et al. 2005) and display a heightened stress reactivity in various experimental paradigms (Bale, Contarino et al. 2000). The basis of this effect is unknown. However, CRF overproduction in these mice has been documented (Bale, Contarino et al. 2000) and a CRF1 antagonist mitigates aspects of their high stress reactivity (Bale and Vale 2003). Thus, it has been hypothesized that overproduction of CRF acting on an intact CRF1 may account for the effects on heightened stress reactivity and may underlie the low maternal aggression in these CRF2 knockout (KO) mice. Thus, CRF2 KO mice with heightened CRF1 activation may have difficulty producing normal maternal behaviors, including protection of offspring.
Although our previous CRF2 KO study examined maternal defense regulation, an inbred background was used and effects on other maternal behavior were not determined. For this study, we crossed KO mice with mice selected for high maternal defense that show excellent maternal profiles (Gammie, Garland et al. 2006) and we directly looked at the loss of the CRF2 and its role in the stress-induced impairment of maternal aggression in these stress sensitive mice. Because central CRF can be released in association with stress (Givalois, Arancibia et al. 2000; Makino, Hashimoto et al. 2002) and 30 min of acute restraint applied postpartum impaired maternal aggression in outbred mice (Gammie and Stevenson 2006), one possibility is that central CRF release is a mechanism for the negative regulation of maternal defense by stress. Whether a shorter duration restraint stress (mild) could differentially alter maternal aggression in mice with different stress reactivities had not been tested. Thus, we hypothesized that increased stress sensitivity makes an aggressive lactating female more susceptible to the inhibitory effects of stressors and that increased stress reactivity may also alter other maternal behaviors. The goals of this study were to examine CRF2 loss on maternal defense and maternal care in a robust genetically diverse background, to test the behavioral effects of a mild stressor in wild-type versus KO dams, and to examine neuronal c-Fos activity with and without a stressor to understand how gene loss leads to an altered response to stress.
Results
Effect of genotype on maternal behavior on postpartum Days 2 and 3
On postpartum Days 2 and 3, mice were examined for maternal behaviors for 60 minutes. CRF2 mutant mice and their WT counterparts did not exhibit a difference in any measure maternal care (Table 1).
Table 1.
Maternal Behavior postpartum Days 2 and 3
| Maternal Behavior | Day 2 | Day 3 | ||||
|---|---|---|---|---|---|---|
| WT | CRF2 KO | p value | WT | CRF2 KO | p value | |
| On-nest | 5.8±1.4 | 4.3±0.7 | p = 0.509 | 6.3±2.0 | 3.8±0.6 # | p = 0.536 |
| Off-nest | 6.1±1.9 | 8.4±1.5 | p = 0.335 | 10.8±3.6 | 10.5±2.5 # | p = 0.782 |
| Nursing | 47.4±2.4 | 45.9±1.6 | p = 0.619 | 42.5±3.7 | 45.5±2.6 | p = 0.508 |
| Nest building | 1.6±0.6 | 2.9±0.7 # | p = 0.134 | 1.5±0.5 | 2.4±0.8 # | p = 0.510 |
| Pup licking | 4.9±0.7 | 6.1±0.7 | p = 0.272 | 4.1±0.6 | 4.6±0.7 | p = 0.604 |
| Self-grooming | 4.3±0.6 | 3.9±0.6 | p = 0.641 | 3.8±0.6 | 3.6±0.6 # | p = 0.807 |
| Eating/drinking | 3.0±0.7 | 4.1±1.1 # | p = 0.986 | 4.0±1.3 | 2.8±0.6 # | p = 0.944 |
Mean (±SEM) bouts of maternal behavior (recorded every 30 seconds) performed in lactating WT (n =18) or CRF2 KO (n =18) mice on postpartum Days 2 and 3. No treatments were performed. No values differed significantly between WT or CRF2 KO mice on either postpartum Day 2 or 3.
data non-normal, analyzed with the Kruskal-Wallis One Way Analysis of Variance on Ranks
There was no difference in mean pup weight (H (1) = 0.866, p = 0.352; ANOVA on Ranks) or mean dam weight (F (1,32) = 0.00156, p=0.969; ANOVA) on postpartum Day 1 between WT and KO dams nor did WT and KO dams differ in mean litter size (F (1,31) = 0.309, p=0.582; ANOVA).
Effect of genotype on maternal aggression and pup retrieval on postpartum Day 4
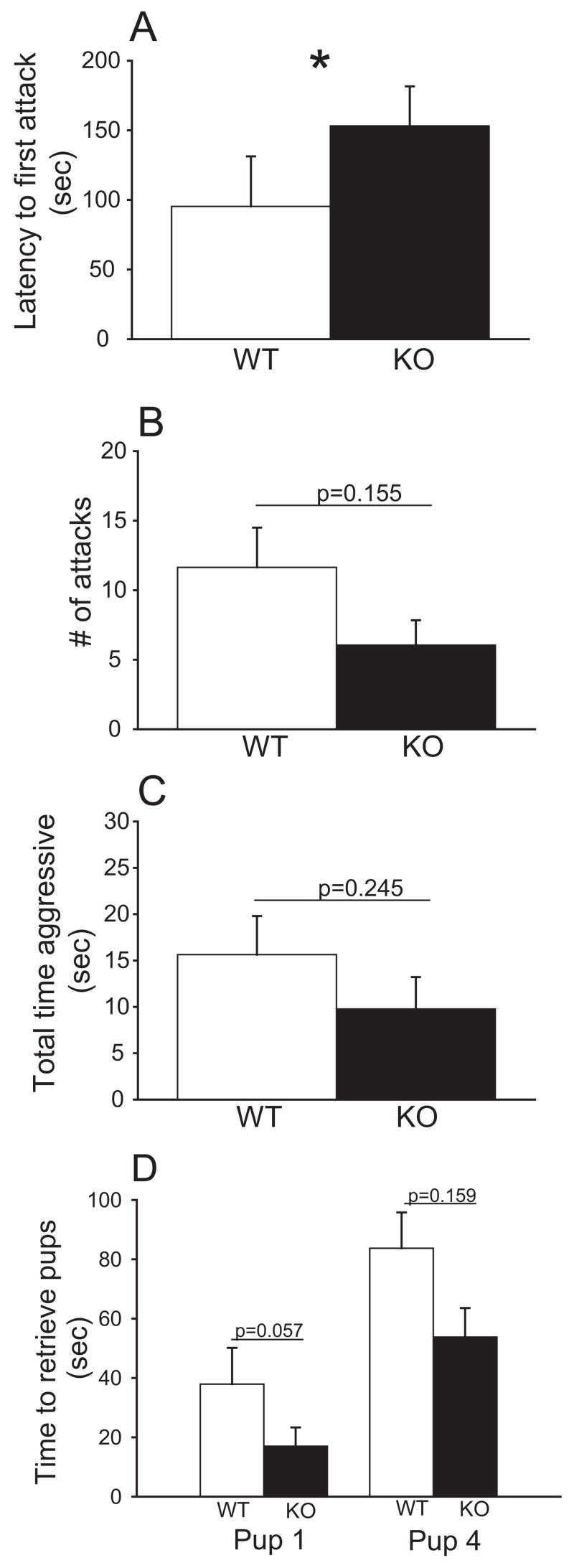
There was a significant effect of genotype in one of the maternal defense behavioral measures (Fig 1A–C). There was a significant effect of genotype on latency to first attack (F (1,30) = 5.433, p = 0.027; ANOVA) and post-hoc tests revealed an increased latency to first attack in KO dams relative to WT mice (t=2.331 , p=0.027; Holm-Sidak) (WT, mean±SEM: 3.4±0.4; KO, mean±SEM: 4.5±0.3). To allow for the performance of parametric tests, data were natural log (ln)-transformed. However, there was not an effect of genotype on number of attacks (F (1,30) = 2.125, p = 0.155; ANOVA) (WT, mean±SEM: 2.8±0.5; KO, mean±SEM: 1.8±0.4) or total time aggressive (F (1,30) = 1.409, p = 0.245; ANOVA) (WT, mean±SEM: 3.2±0.6; KO, mean±SEM: 2.2±0.5). Data were square root-transformed.
Fig. 1.
Effects of genotype on maternal aggression in CRF2 KO and WT mice. Bars represent non-transformed mean differences ± SEM within each genotype. Statistics represent transformed data (see Results). A) Mean latency to first attack was significantly longer in CRF2 KO dams relative to WT dams (ANOVA). B) Mean number of attacks was not significantly altered in CRF2 KO dams relative to WT dams (ANOVA). C) Mean total time aggressive was not significantly altered in CRF2 KO dams relative to WT dams (ANOVA). D) mean time to retrieve first and fourth pup was not significantly altered in CRF2 KO dams relative to WT (ANOVA). * p<0.05.
Additionally there was no significant effect of genotype (WT vs. KO dams) on pup retrieval behavior, including time to fourth pup (F (1,30) = 2.083, p = 0.159; ANOVA) (Fig 1D). However, while not significant, time to retrieve first pup exhibited a strong trend towards KO dams retrieving their pups faster than WT mice ( F (1,30)= 3.916, p=0.057; ANOVA). Data on time to first pup were ln-transformed.
Effects of mild stressor and genotype on maternal defense behavior on postpartum Days 5 and 6
A two way RM ANOVA revealed there was a significant effect of genotype on mean number of attacks, such that KO dams exhibited fewer attacks (F (1,30)=5.877, p=0.021). There was no significant genotype by restraint interaction on latency to first attack ( F (1,31)= 0.0930, p=0.911) or mean total time aggressive (F (1,31)= 0.956, p=0.390), but there was a strong trend in terms of number of attacks (F(1,31)= 3.071 , p=0.054) with restrained CRF2 KO dams displaying fewer attacks than WT mice. Table 2 shows statistics and means and standard errors for all maternal defense behaviors examined.
Table 2.
Maternal defense behavior following restraint
| Maternal defense | WT (n=10) | CRF2 KO (n=10) | Two Way | RM ANOVA | |||
|---|---|---|---|---|---|---|---|
| Unrestrained | Restrained | Unrestrained | Restrained | Genotype p value | Restraint p value | Interaction p value | |
| Latency to first attack | 68.8±16.4 | 91.4±16.4 | 119.7±15.0 | 128.9±15.0 | 0.292 | 0.066 | 0.911 |
| # attacks | 15.8±1.1 | 13.7±1.1 | 6.1±1.0 | 5.0±1.0 | 0.021 | 0.069 | 0.054 |
| Total time aggressive | 19.7±2.6 | 19.8±2.6 | 14.9±2.4 | 9.1±2.4 | 0.262 | 0.128 | 0.390 |
| Time to first pup | 31.7±6.4 | 37.3±6.3 | 16.1±5.8 | 11.4±5.8 | 0.050 | 0.944 | 0.408 |
| Time to fourth pup | 76.9±6.6 | 83.7±6.6 | 55.3±6.0 | 53.8±6.0 | 0.077 | 0.676 | 0.508 |
| Time to all pups | 842.5±132.4 | 1051.8±132.4 | 480.2±120.9 | 446.2±120.9 | 0.039 | 0.494 | 0.345 |
Mean (±SEM) time(s) of maternal defense behavior and pup retrieval performed by lactating CRF2 KO and WT dams exposed to mild restraint stress or no restraint. P-values less than 0.05 are shown in bold
When only animals with robust baselines of maternal aggression (>10 sec, as determined on Day 4) were analyzed as a change from non-restraint to restraint treatment, there was an overall significant effect of the mild stressor on CRF2 KO mice in 2 of the 3 maternal defense measures. The rationale for the 10 sec cutoff is that if one is testing the hypothesis that stress decreases aggression, then mice with low aggression cannot be properly be evaluated due to a floor effect. The mild stressor significantly affected latency to first attack (H (1)= 3.982, p=0.046; ANOVA on Ranks) and post-hoc tests revealed an increased change in latency to first attack from non-restraint to restraint treatment in KO dams relative to WT mice (Q = 1.960, p< 0.05; Dunn’s Method) (Fig. 2A). The mild stressor did not significantly affect number of attacks, however there was a trend towards decreased change in the number of attacks exhibited by KO vs. WT mice (F (1,14) = 3.611, p = 0.080; ANOVA) (Fig 2B). The mild stressor also significantly affected total time aggressive F (1,14) = 4.709, p = 0.049; ANOVA) and post-hoc tests revealed a decreased change in total time aggressive from non-restraint to restraint treatment in KO dams relative to WT (t = 2.17, p=0.049, Holm-Sidak) (Fig 2C).
Fig. 2.
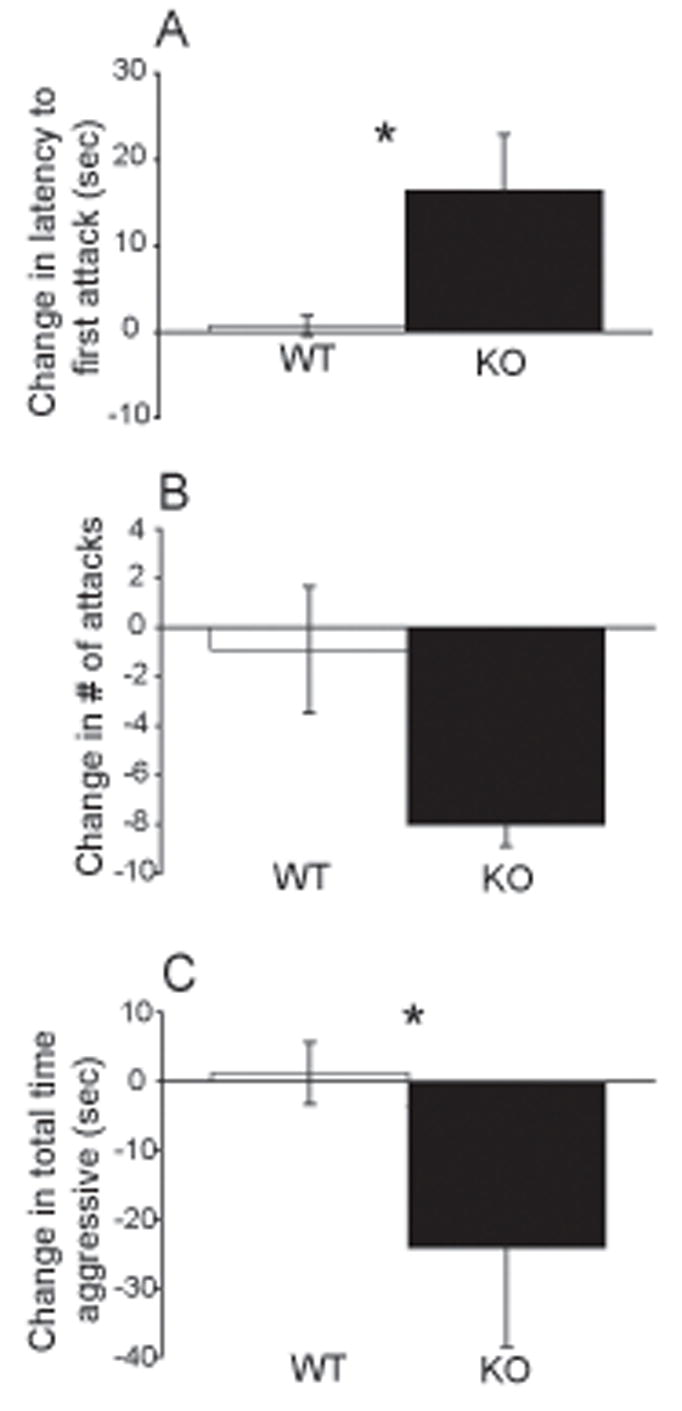
Effects of mild restraint stress on maternal aggression in CRF2 KO and WT mice with a baseline of aggression ≥ 10 seconds. Bars represent mean conditions (non-restraint) and stress conditions (15 min restraint) within each genotype. A) Mean latency to first attack was significantly delayed by restraint stress in CRF2 KO dams relative to WT dams (ANOVA on Ranks). B) Mean number of attacks was not significantly altered by restraint stress in either CRF2 KO dams relative to WT dams (ANOVA). C) Mean total time aggressive was significantly decreased by restraint stress in CRF2 KO dams relative to WT dams (ANOVA). * p<0.05
Effects of mild stressor and genotype on maternal behavior
Pup retrieval, when analyzed with a two way RM ANOVA, was not significantly affected by the mild stressor on WT or CRF2 mutant mice in any of the 3 measures of pup retrieval. There was not an effect of restraint on WT and KO mice based on the differences in time to retrieve first pup (F (1, 31) = 0.703, p = 0.408), fourth pup (F (1,31) = 0.448, p = 0.508) or all pups (F (1,31) = 0.921, p = 0.345) (Table 2). However, there was an effect of genotype on time to retrieve first pup (F (1, 31) = 4.164, p = 0.050; Two Way ANOVA) and post-hoc tests reveal that KO mice (regardless of restraint type) retrieve their first pup faster than WT dams (t=2.041, p=0.050; Holm-Sidak). In addition, there was a significant effect of genotype on time to retrieve all pups (F (1, 31) = 4.668, p = 0.039; Two Way ANOVA) and post-hoc tests reveal that KO mice (regardless of restraint type) retrieve all their pups faster than WT dams (t= 2.160, p=0.039; Holm-Sidak).
There was not a significant effect of the mild stressor on WT or CRF2 KO mice in any measure of maternal behavior (Table 3). While not significant, restrained KO dams did exhibit a strong trend towards decreased time spent arched-back nursing relative to WT dams( F (1,31) = 3.124, p = 0.053; Two Way RM ANOVA).
Table 3.
Maternal behavior following restraint
| Maternal Behavior | WT (n=15) | CRF2 KO (n= 18) | Two Way | RM ANOVA | |||
|---|---|---|---|---|---|---|---|
| Unrestrained | Restrained | Unrestrained | Restrained | Genotype p value | Restraint p value | Interaction p value | |
| On-nest | 0.1±0.0 | 0.1±0.0 | 0.1±0.0 | 0.1±0.0 | 0.759 | 0.786 | 0.233 |
| Off-nest | 0.4±0.1 | 0.4±0.1 | 0.4±0.0 | 0.5±0.0 | 0.619 | 0.157 | 0.334 |
| Nursing | 0.3±0.1 | 0.3±0.1 | 0.4±0.0 | 0.2±0.0 | 0.854 | 0.071 | 0.053 |
| Nest building | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.877 | 0.559 | 0.331 |
| Licking | 0.0±0.0 | 0.1±0.0 | 0.1±0.0 | 0.1±0.0 | 0.858 | 0.336 | 0.103 |
| Self-grooming | 0.0±0.0 | 0.1±0.0 | 0.1±0.0 | 0.1±0.0 | 0.317 | 0.063 | 0.576 |
Mean (±SEM) maternal behavior expression performed in lactating WT or CRF2 KO mice following restraint stress or no restraint. No values differed significantly between WT or CRF2 KO mice.
Effects of mild stressor and genotype on elevated plus maze performance
There was a limited effect of mild stress on WT and CRF2 KO mice on elevated plus maze performance. Two-way ANOVA analysis did not reveal a significant genotype by restraint interaction in any of the measures. However, the two way ANOVA revealed that restrained mice (regardless of genotype) entered the open arms more than the unrestrained mice ( F (1,26) = 5.702, p=0.024; Two Way ANOVA). Table 4 contains means and standard errors for all measures on elevated plus maze.
Table 4.
Elevated plus maze behavior
| EPM behavior | WT | CRF2 KO | Two Way | ANOVA | |||
|---|---|---|---|---|---|---|---|
| Unrestrained (n=9) | Restrained (n=6) | Unrestrained (n=7) | Restrained (n=8) | Genotype p value | Restraint p value | Interaction p value | |
| Total time open | 4.8±4.0 | 20.8±10.8 | 9.6±5.6 | 27.0±12.4 | 0.536 | 0.066 | 0.938 |
| #open | 0.3±0.2 | 1.5±0.8 | 0.7±0.4 | 2.4±0.8 | 0.299 | 0.024 | 0.680 |
| Total time closed | 179.3±16.5 | 158.2±39.7 | 179.9±29.8 | 165.5±20.4 | 0.881 | 0.499 | 0.896 |
| # closed | 9.1±1.4 | 11.3±1.6 | 11.1±2.1 | 12.0±0.7 | 0.376 | 0.313 | 0.652 |
| Total time middle | 106.3±19.5 | 74.2±14.2 | 110.0±28.4 | 101.8±22.8 | 0.493 | 0.377 | 0.600 |
| #middle | 11.8±1.8 | 13.3±2.1 | 11.7±1.8 | 13.4±0.8 | 0.995 | 0.345 | 0.975 |
Mean (±SEM) elevated plus maze (EPM) performed in lactating WT or CRF2 KO mice following restraint stress or not. Measures of EPM performance with p<0.05 that reach significance are shown in bold.
Effects of Fos immunoreactivity (Fos-IR) in association with a mild exposure and genotype
On postpartum Day 8 dams were either restrained or unrestrained for 15 min, returned to their home cage with their pups and brains collected 105 min later (±5 min). No behavioral tests were performed. To analyze how restraint affected c-Fos activity, 31 mice were randomly assigned to 2 groups: unrestrained (WT, n= 6; KO, n= 8) or restrained (WT, n= 8; KO, n= 9) to determine changes within the CNS using Fos-IR.
One brain region, caudal periaqueductal gray (cPAG), out of the 44 regions examined showed a significant increase in Fos-IR in the restrained CRF2 KO, but not WT mice, when evaluated with a two way ANOVA to assess the interaction between genotype and restraint type ( F(1,24) = 6.585, p = 0.017; Two Way ANOVA) (Table 5; Fig 4). However, 2 regions showed a significant effect of genotype, the bed nucleus of the stria terminalis dorsal (BNSTd) (F (1,26) = 7.693, p=0.010) and dentate gyrus (DG) (F (1,23) = 9.296; p = 0.006), while 10 regions showed a significant effect of restraint, infralimbic cortex (IL) (F (1,24) = 4.650; p = 0.041), nucleus accumbens core (NuAbC) (F (1,24) = 6.237, p = 0.020), lateral preoptic area (LPO) (F (1,24) = 5.872, p=0.023), BNSTd (F (1,26) = 4.620, p=0.041), paraventricular hypothalamic nucleus (PVN) (F (1,26) = 35.311, p<0.001), anterior hypothalamic area anterior (AHA) (F (1,23) = 12.820, p = 0.002), ventromedial hypothalamus (VMH) (F (1,21) = 6.391, p = 0.020), anterior hypothalamic area posterior (AHP) (F (1,23) = 6.358, p = 0.019), dorsomedial hypothalamus (DM) (F (1,27) = 15.608; p<0.001), and perifornical nucleus (PeF) ( F(1,26) = 24.443, p<0.001) (Table 5). Table 5 contains means and standard errors for all brain regions examined.
Table 5.
Changes in c-Fos activity in WT and KO lactating dams
| Region | WT | CRF2 KO | Two Way Genotype p-value | ANOVA Restraint p-value | ||
|---|---|---|---|---|---|---|
| Unrestrained (n) | Restrained (n) | Unrestrained (n) | Restrained (n) | |||
| AOM | 11.3±4.8 (3) a | 14.1±7.3 (7) | 0.8±0.4 (5) a,b | 11.0±4.8 (9) # b | 0.245 | 0.267 |
| AOP | 10.0±4.7 (3) a | 14.7±5.3 (7) | 1.20±0.4 (5) a,b | 9.3±3.4 (9) # b | 0.133 | 0.172 |
| VTT | 17.5±0.5 (2) a | 31.8±10.0 (5) | 3.8±0.6 (4) a,b | 21.9±5.6 (8) # b | 0.240 | 0.099 |
| F Cg Cx | 52.3±13.5 (4) | 91.1±28.1 (7) | 27.4±10.9 (7) | 59.9±13.6 (9) | 0.133 | 0.062 |
| IL | 80.3±25.8 (4) | 108.4±26.2 (7) | 41.7±12.8 (7) | 93.2±12.9 (9) | 0.152 | 0.041 |
| NuAcbC | 28.8±4.3 (4) | 65.9±22.8 (7) # | 17.1±4.6 (7) | 52.0±13.3 (9) # | 0.308 | 0.020 |
| NuASh | 26.8±15.9 (4) a | 33.3±15.7 (7) # | 4.9±1.6 (7) a | 19.6±6.2 (9) # | 0.068 | 0.212 |
| MFC | 56.8±21.4 (5) a | 102.3±35.1 (7) | 14.7±5.6 (7) a,b | 89.4±36.7 (9) # b | 0.480 | 0.089 |
| Pir Cx | 113.20±24.5 (5) | 136.7±20.5 (7) | 98.3±34.2 (8) | 117.6±19.9 (9) | 0.525 | 0.423 |
| LS1 | 43.8±14.2 (4) | 45.4±15.9 (7) # | 55.7±17.5 (7) | 55.4±19.7 (9) | 0.446 | 0.756 |
| MS/VDB | 15.6±9.1 (5) | 19.9±8.3 (8) | 19.2±6.1 (6) | 22.8±7.6 (9) # | 0.828 | 0.767 |
| LS2 | 77.6±17.5 (5) | 71.9±15.2 (7) | 52.8±11.2 (8) | 93.6±28.4 (9) # | 0.727 | 0.290 |
| LSV1 | 122.8±15.8 (5) a | 131.9±38.6 (7) | 63.0±12.1 (8) a,b | 136.8±22.0 (9) b | 0.215 | 0.078 |
| MPOM | 28.2±3.0 (5) | 79.9±21.0 (8) | 66.1±21.2 (7) | 62.3±13.3 (8) | 0.582 | 0.201 |
| MPA | 24.0±5.0 (5) | 51.3±10.3 (8) | 42.4±9.0 (7) | 40.5±10.2 (8) | 0.699 | 0.210 |
| LPO | 9.8±3.6 (6) | 36.8±9.9 (8) | 17.4±4.7 (7) | 26.0±6.0 (8) | 0.833 | 0.023 |
| BNSTd | 29.0±6.7(6) | 56.4±9.6 (7) | 21.4±7.0 (8) | 24.6±4.9 (9) | 0.010 | 0.041 |
| BNSTv | 27.5±8.5 (6) | 28.1±4.1 (7) | 13.5±3.6 (8) | 23.2±4.4 (9) | 0.076 | 0.321 |
| Cg Cx | 57.5±26.7 (6) | 127.8±36.8 (6) | 86.3±28.8 (8) | 115.7±35.5 (9) | 0.834 | 0.125 |
| LS3 | 52.3±15.4 (4) | 57.7±13.1 (7) | 28.8±5.5 (8) | 58.3±19.5 (9) # | 0.444 | 0.253 |
| LSV2 | 90.0±34.1 (4) | 118.3±17.4 (7) | 80.5±19.4 (8) | 93.9±15.6 (9) | 0.422 | 0.322 |
| SCN | 63.0±17.4 (6) c | 136.3±23.1 (7) c | 98.7±36.1 (7) | 132.9±15.5 (9) | 0.351 | 0.070 |
| PVN | 48.0±10.7 (5) c | 205.3±39.6 (7) c # | 43.4±9.4 (8) b | 204.4±28.2 (9) # b | 0.962 | <0.001 |
| AHA | 18.0±3.7 (5) | 31.1±5.2 (7) | 10.2±1.7 (6) b | 25.3±3.7 (8) b | 0.100 | 0.002 |
| PVA | 232.4±57.2 (5) | 262.1±51.2 (7) | 161.5±33.5 (8) b | 264.9±14.2 (9) b | 0.528 | 0.419 |
| SON | 16.3±4.9 (6) | 17.3±4.3 (6) | 27.1±14.6 (7) | 17.8±6.2 (9) # | 0.479 | 0.588 |
| CeAmy | 104.8±37.1 (4) | 152.3±32.5 (8) | 61.4±26.9 (8) | 124.7±19.8 (7) | 0.256 | 0.082 |
| MeAmy | 23.8±4.6 (4) | 26.0±6.6 (8) | 15.8±3.6 (8) | 22.8±3.7 (8) | 0.290 | 0.382 |
| LH | 56.3±12.8 (4) | 70.6±9.9 (8) | 46.9±11.1 (8) | 67.9±8.6 (7) | 0.587 | 0.122 |
| VMH | 13.0±6.0 (4) | 25.5±9.8 (6) | 5.8±1.5 (8) b | 30.1±8.6 (7) # b | 0.860 | 0.020 |
| AHP | 85.3±23.1 (4) | 106.6±11.7 (8) | 60.1±9.3 (8) b | 116.3±18.9 (7) b | 0.620 | 0.019 |
| CA1 | 5.3±2.6 (4) | 15.3±9.8 (7) # | 6.6±3.6 (7) | 4.8±1.6 (9) # | 0.439 | 0.487 |
| CA2 | 11.3±1.7 (4) | 16.9±6.3 (7) | 10.4±5.5 (7) | 13.0±3.4 (9) # | 0.657 | 0.440 |
| CA3 | 24.8±0.6 (4) | 31.9±8.7 (7) # | 18.7±4.1 (6) | 22.8±3.9 (9) | 0.222 | 0.363 |
| DG | 44.3±8.7 (4) a | 32.4±8.7 (7) | 18.4±3.8 (7) a | 22.0±2.2 (9) | 0.006 | 0.495 |
| DM | 47.3±19.6 (6) | 117.7±25.9 (7) # | 44.8±8.2 (8) b | 101.2±11.6 (9) b | 0.548 | <0.001 |
| PeF | 26.3±5.6 (6) c | 46.1±6.2 (7) c | 15.3±2.8 (7) b | 50.2±6.2 (9) b | 0.568 | <0.001 |
| PH | 150.0±37.0 (5) | 157.0±17.6 (6) | 115.6±32.6 (7) b | 193.6±19.0 (9) b | 0.886 | 0.139 |
| VTA | 41.4±9.6 (5) a | 39.0±9.5 (7) | 18.5±5.5 (8) a,b | 41.8±8.3 (9) b | 0.344 | 0.322 |
| cPAG | 109.0±5.6 (4) a | 86.7±10.9 (8) | 63.8±8.9 (8) a,b | 110.9±15.8 (9) # b | 0.444 | 0.367 |
| DRD | 89.0±9.9 (4) | 106.9±17.6 (7) | 60.5±11.7 (8) b | 118.7±20.4 (9) b | 0.654 | 0.050 |
| LDTg | 38.6±12.0 (5) | 31.3±7.5 (7) | 24.3±6.1 (7) | 17.1±5.3 (7) | 0.066 | 0.301 |
| PB | 26.0±11.3 (5) | 27.0±9.2 (7) | 19.9±8.5 (7) | 15.2±4.8 (6) | 0.644 | 0.694 |
| LC | 71.8±12.9 (5) | 35.1±14.1 (7) | 30.9±15.7 (7) | 44.1±15.5 (7) | 0.533 | 0.727 |
Mean (±SEM) number of c-Fos-positive cells in brain regions for WT and KO mice that were either unrestrained as a control or exposed to a mild stressor for 15 minute using a two-way ANOVA. Planned comparisons with a one-way ANOVA are also represented.
An effect of genotype (p<0.05) between unrestrained WT and KO mice.
An effect of restraint stress treatment within KO mice.
An effect of restraint stress treatment within WT mice.
Restraint did not alter c-Fos activity in WT mice. Regions with p<0.05 are shown in bold.
cPAG was the only brain region to show a significant effect of genotype x restraint (p=0.017, see Results).
data non-normal, analyzed with the Kruskal-Wallis One Way ANOVA on Ranks.
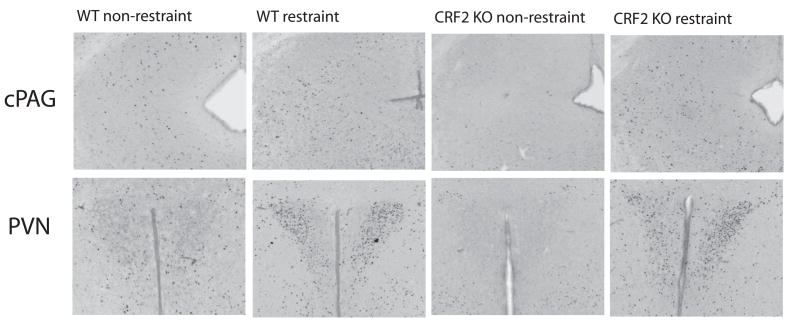
Fig. 4.
Examples of c-Fos immunoreactivity (Fos-IR) following either the restraint or non-restraint condition for WT and CRF2 KO mice. Restraint or non-restraint were performed 105 min (±5 min) before brain fixation. Unrestrained CRF2 KO mice exhibit significantly lower levels of Fos-IR relative to unrestrained WT mice in cPAG. In addition, with restraint, CRF2 KO mice show significant increases in Fos-IR relative to their unrestrained state in cPAG. These differences in cPAG were photographed on the right side of the brain. PVN shows significant increases in Fos-IR with restraint for both WT and CRF2 KO mice (see Results for more details).
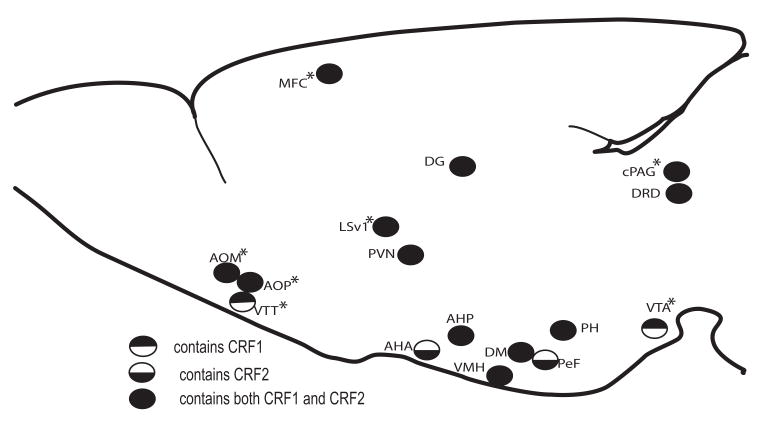
In addition, with planned comparisons 3 brain regions out of the 44 examined showed a significant increase in Fos-IR in unrestrained relative to restrained WT mice, including suprachiasmatic nucleus (SCN), PVN, and PeF (Table 5). However, following planned comparisons, sixteen brain regions out of the 44 regions examined showed a significant increase in Fos-IR in restrained relative to unrestrained KO mice including, anterior olfactory nucleus medial (AOM), anterior olfactory nucleus posterior (AOP), ventral tenia tecta (VTT), medial frontal cortex (MFC), lateral septum ventral (LSV)1, PVN, AHA, paraventricular thalamic nucleus anterior (PVA), VMH, AHP, DM, perifornical nucleus (PeF), posterior hypothalamic area (PH), ventral tegmental area (VTA), caudal periaqueductal gray (cPAG), dorsal raphe nucleus dorsal (DRD) (Table 5; Fig 3).
Fig. 3.
Schematic of brain regions exhibiting a significant increase in c-Fos activity in CRF2-deficient dams following exposure to a mild stressor (p<0.05) and their relative receptor contributions. Regions showing significantly lower c-Fos activity in KO animals in the unrestrained condition relative to WT are indicated with * (p<0.05). WT dams exhibited increased in c-Fos activity following exposure to a mild stressor in three regions; PVN, SCN and PeF (not highlighted on drawing).
In addition, 9 brain regions out of the 44 examined demonstrated a significant increase in baseline Fos-IR levels in WT mice relative to KO mice in the unrestrained condition (Table 5). These regions include AOM, AOP, VTT, nucleus accumbens shell (NuASh), MFC, LSV1, DG, VTA, cPAG. However, no brain regions out off the 44 examined demonstrated a significant increase in Fos-IR in WT relative to KO mice in the restrained condition.
As a way to examine whether previous experience altered final c-Fos expression, all significant brain regions were additionally analyzed with the restraint treatment the animal had received the previous day as a covariate. No effect of previous experience on c-Fos expression was found in any of the significant regions (data not shown).
Discussion
Mice deficient in CRF2 were used to evaluate the contribution of CRF2 to the regulation of maternal defense behavior. This study found that baseline levels of maternal aggression were lower in CRF2 KO lactating dams in a mixed background and exposure to a mild stressor significantly impaired maternal defense in KO dams relative to WT without altering other maternal behaviors. In the unrestrained condition, WT mice exhibited significantly higher levels of c-Fos relative to KO mice in 9 regions. In response to stress, WT dams exhibited c-Fos increases in three regions, while KO dams exhibited increases in c-Fos activity in 16 regions. An important caveat of the c-Fos results is that all mice had previously been examined for offspring protection and exposed to restraint, so it is not known whether or how this previous experience altered final c-Fos expression.
To ensure robust levels of maternal aggression and maternal care, inbred mice (C57BL/6J:129Sv) with the CRF2 deletion were bred into a strain selected for high maternal aggression within our laboratory (Gammie, Garland et al. 2006). Previously, our laboratory (Gammie and Stevenson 2006; Gammie, Bethea et al. 2007) along with others (Sibille, Pavlides et al. 2000; Olivier, Pattij et al. 2001; Toth 2003; Van Bogaert, Oosting et al. 2006), have successfully placed deletions into more varied backgrounds to measure behavior. By varying the background in which the deletion was bred, our measures could be more sensitive to changes in maternal aggression levels and better represent how a gene may work in humans where alleles have not been completely fixed (as with inbred mice) (Toth 2003). In addition, levels of maternal defense were weak in the inbred mouse as evidenced in the previous study evaluating maternal defense in CRF2 KOs (Gammie, Hasen et al. 2005). Inbred WT mice exhibited one-third the amount of maternal aggression (15 sec of maternal aggression in 10 min) (Gammie, Hasen et al. 2005) as outbred S females (45.6 sec of maternal aggression in 3 min) (S.A. Stevenson and S.C. Gammie, unpublished observations). Thus, by creating a new mixed background, maternal aggression levels of WT dams in this study were able to correspond to the robust levels in outbred S mice.
Baseline levels of maternal defense were lower in KO dams relative to WT in this study consistent with the previous study with inbred CRF2 KO mice (Gammie, Hasen et al. 2005). However, following a mild stressor (15 min restraint), there was not a restraint by genotype interaction on latency to first attack or total time aggressive, but there was a strong trend towards stressed CRF2 KO dams displaying less number of attacks relative to WT (p=0.054). In addition, when animals with robust levels of defense maternal defense (>10 sec) were analyzed as a difference between restraint treatment, mild restraint significantly reduced the maternal defense behavior of CRF2 KO dams. On average, CRF2 KO dams exposed to 15 min restraint exhibited a decrease in maternal defense of 20 sec, whereas WT dams showed almost no change in maternal aggression to this mild stressor. This finding suggests that heightened stress reactivity is detrimental to maternal care because even a mild stressor can negatively impact protective behavior. Previous work has suggested that an overproduction of the anxiogenic peptide, CRF, acting on CRF1 underlies the heightened stress sensitivity of CRF2 KO mice (Bale, Contarino et al. 2000) and a recent study suggested the importance of an intact CRF1 in modulating aggressive behavior in hamsters (Farrokhi, Blanchard et al. 2004). Thus, one possible explanation of our results may be that the behavioral deficits seen in our KO mice are due to altered activation of CRF1 and naturally released CRF in response to the stressor may be acting on this intact receptor. However, it is also possible that some basal activation of CRF2 is required for full maternal defense, even though at higher levels of activation this receptor inhibits the behavior (D’Anna, Stevenson et al. 2005).
In the present study, no differences were observed in any measure of maternal behavior in CRF2 KO dams relative to WT and pup weight was similar between genotypes. KO dams showed a trend toward faster retrieval of the first pup (p=0.057) under baseline conditions and when examined using a two way ANOVA (regardless of restraint treatment), KO mice were quicker to retrieve their first pup and all pups relative to WT dams. This finding is consistent with pup retrieval results obtained on Day 6 in the previous study where time to retrieve first pup almost reached significance for KO mice (Gammie, Hasen et al. 2005). Why a deletion of CRF2 would result in faster retrieval times is not known.
Analysis of CRF2 KO and WT lactating females in the elevated plus maze as an indirect measure of anxiety did not reveal an effect of genotype, consistent with the previous performance of inbred KO dams on the elevated plus maze (Gammie, Hasen et al. 2005), nor did it reveal an interaction between genotype and restraint (Table 4). There was significant effect of restraint increasing the number of entries to the open arms of the maze (regardless of genotype), suggesting restraint stress here promoted an increase in locomotion or exploration. However, it should be noted that baseline level of entries and time in open arms was extremely low in both unrestrained groups. Both WT and KO mice average less than 2 entries to the open arms and less than 10 sec on the open arms for a 3 min test. Also, plus maze results are not always consistent (Hogg 1996) and outcome can change depending on light levels (Handley, McBlane et al. 1993). Thus, the elevated plus maze results should be interpreted with caution. Future work examining short restraint and anxiety would need to involve a more reliable baseline measure of anxiety on an independent group of CRF2 KO and WT mice without previous behavioral experience.
In terms of changes in brain activity, a two way analysis found a significant genotype by restraint interaction in one brain region, cPAG, in which restrained CRF2 KO, but not WT mice, exhibited increases in Fos-IR (Table 5; Fig 4). Increases in Fos-IR have been shown in this region following various stressors both outside of the postpartum period (Cullinan, Herman et al. 1995; Gavrilov, Perekrest et al. 2008) and during lactation (Gammie and Stevenson 2006), suggesting its involvement in the general regulation of stress. cPAG is also implicated in the regulation of maternal aggression (Lonstein and Gammie 2002; Gammie 2005; Hasen and Gammie 2005; Hasen and Gammie 2006), suggesting it may be a critical node in the negative regulation of maternal defense behavior modulated by stressors.
Unrestrained Fos-IR levels were significantly higher in nine regions in WT relative to KO mice, including within AOM, AOP, VTT, NuASh, MFC, LSV1, DG, VTA, cPAG (ANOVA; Table 5). These unrestrained differences may be associated with the heightened stress sensitivity of CRF2 KO mice. However, c-Fos expression in WT lactating dams may already be induced in many regions, and therefore they are non-responsive to restraint during lactation, whereas in the stress sensitive CRF2 KO dams, c-Fos expression is already low, and therefore more responsive to stress. These differences in c-Fos activity in WT and KO mice may be related to their previous behavioral experience or may also reflect a differential response of these mice to pup separation since an additional group without pup separation was not included in this study. While these differences seen in c-Fos activity are interesting, this data should be considered preliminary and be used as a point of reference for future studies to determine which brain regions underlie the behavioral phenotype of CRF2 KO mice.
WT brain activity increased in response to stress in three brain regions including SCN, PVN and PeF (ANOVA; Table 5; Fig 4 for PVN results). All these brain regions show changes following stressful encounters. Increases in Fos-IR have been observed in PVN following both restraint stress (Gammie and Stevenson 2006) and immobilization stress in lactating females (da Costa, Wood et al. 1996) and male rats (Cullinan, Herman et al. 1995; Yokoyama and Sasaki 1999). In addition, both the PVN and PeF have been associated with cellular changes following stress-induced immunosuppression in rats (Gavrilov, Perekrest et al. 2008). Likewise, increases in the regulatory peptide vasopressin are seen in SCN following footshock (Handa, Zoeller et al. 2007). Thus, this study demonstrates distinct changes in the brain activity of WT mice following mild restraint stress in three stress-related brain regions. However, it is possible the previous behavioral manipulations of the WT mice in this experiment concealed other stress-induced effects of c-Fos expression observed in other studies (Schreiber, Tocco et al. 1991; Yokoyama and Sasaki 1999). It also is possible that the central nervous activity of a lactating WT dam is less responsive to stress in general. Previous studies have shown that the brain activity of intact lactating rats are less responsive (relative to virgins) to both stress (da Costa, Wood et al. 1996) and CRF (da Costa, Kampa et al. 1997), thus a lack of a robust c-Fos response to stress may be characteristic of WT lactating mice. It would be interesting to perform another experiment on mice without previous behavioral experience to confirm the low levels of c-Fos activity in WT mice observed following a mild stressor.
Bouts of restraint stress that impaired maternal defense, triggered increases in Fos-IR in 16 brain regions in KO dams, including: AOM, AOP, VTT, MFC, LSV1, PVN, AHA, PVA, VMH, AHP, DM, PeF, PH, VTA, cPAG, DRD (ANOVA; Table 5; Fig 3), six of which also exhibited increased c-Fos with restraint (regardless of genotype) in the two-way ANOVA (PVN, AHA, VMH, AHP, DM, PeF). Increases in Fos-IR have been observed in some of these regions following 30 min restraint in outbred lactating mice (LS, cPAG, DRD, and PVN) (Gammie and Stevenson 2006) and after 30 min immobilization stress in virgin, pregnant, and lactating female rats (LS, PVN, and VTA) (da Costa, Wood et al. 1996). Some regions, including DRD (Hammack, Richey et al. 2001; Hammack, Richey et al. 2003; Hammack, Schmid et al. 2003), LS (Henry, Vale et al. 2006) and PVN have been specifically linked to how an animal responds to a stressor. Many of these regions also exhibit cellular changes in response to stress, including PVN, VMH, DMH, PH and various anterior hypothalamic nuclei (Gavrilov, Perekrest et al. 2008). c-Fos changes occur following either restraint or swim stress in LS, PVN, AHA, DMH and cPAG (Cullinan, Herman et al. 1995). Any of these 16 regions could be a critical site for the modulation of maternal defense in CRF2 KO dams. Since CRF2KO mice overproduce CRF (Bale, Contarino et al. 2000), it is possible that CRF is released by mildly stressed CRF2 KO mice and the peptide alters maternal aggression by acting on CRF1 in a number of regions. All of the highlighted regions contain CRF1, including LSV, DM, VMH, PVN (Potter, Sutton et al. 1994; Chalmers, Lovenberg et al. 1995), DRD (Chalmers, Lovenberg et al. 1995), VTA, PH, and the olfactory pathway (AOM, AOP, VTT) (Potter, Sutton et al. 1994) (Fig 3) as based on previous studies in rats. While thought to be similar, it is not certain if the distribution of CRF1 receptors is absolutely identical in mice and rats. Many of these same regions contain CRF2, including AOM, AOP, LSV, DM, VMH, PVN, AHP, DRD, VTA, PH and cPAG (Potter, Sutton et al. 1994; Chalmers, Lovenberg et al. 1995; Van Pett, Viau et al. 2000) (Fig 3), however, CRF2 is not present in these mice. Thus, it is possible that in the present study that CRF, a potent inhibitor of maternal defense, is naturally released in response to the mild stressor and acts on an intact CRF1 at the regions highlighted by this analysis to decrease maternal defense. However, under normal conditions, CRF, and its related peptides Ucn 1 and 3, could be acting at either receptor in these regions to facilitate the stress-induced impairment of maternal defense.
A subset of the regions identified in this analysis have been implicated in the regulation of maternal aggression, including LS, PVN, VMH, AHP, DM, and cPAG (Lonstein and Gammie 2002; Gammie 2005; Hasen and Gammie 2005; Hasen and Gammie 2006). Thus, altered activity within these regions due to mild stress in KO mice may be directly affecting key components of the maternal defense circuitry. Increases in c-Fos with mild stress do not indicate whether a neuron received inhibitory or excitatory input, only that a change in second messenger activity occurred (Dragunow and Faull 1989). Thus, restraint stress may inhibit maternal defense by acting on or near maternal aggression circuitry neurons, but this is unknown.
In humans, CRF hyperactivity, similar to CRF2 KO mice, is linked to depression and stressful life events that often precede depressive episodes (Anisman and Zacharko 1982). There is also evidence supporting the role of CRF in the relationship between postpartum depression and stressful life events (O’Hara, Schlechte et al. 1991; Stevens-Simon, Kelly et al. 2000). Our findings support an important role for CRF-related peptides and their receptors in postpartum stress reactivity and demonstrate how that can modify protection of offspring in a mouse model.
Experimental Procedures
Mice
CRF2-deficient mice on a 50:50 mixed C57BL/6Jx129Sv inbred background strain were used (originally a kind gift of Dr. Wylie Vale, Salk Institute) (Bale, Contarino et al. 2000; Gammie, Hasen et al. 2005). Mutant males were crossed with females selected for high maternal aggression (outbred S) that were generated in our lab using a within-family selection approach (starting stock was outbred hsd:ICR (CD-1) mice) (Gammie, Garland et al. 2006). The mice used for this study were from generation 13 mice and averaged 45.6 s of maternal aggression in a 3 minute test (S.A. Stevenson and S.C. Gammie, unpublished observations). The heterozygote CRF2 (+/−) mice (with a 50:50 mix of these two backgrounds) produced from this cross were then bred to produce the wild-type (WT) and KO female mice used in this study. Offspring were weaned at 21 days and housed until pairing as adults. All genotyping occurred after 21 days. Thus, WT and KO female siblings were exposed to the same maternal environment. All females (~45 days old) regardless of genotype were housed with a single outbred breeder male (Mus musculus:~ 42–56 days postpartum) (Harlan, Madison, WI). Following impregnation (~2 weeks), each female was housed individually for the remainder of the study. Polypropylene cages were cleaned weekly prior to parturition, but afterwards cages were unchanged until the end of the experiment. Mice were given ad lib access to Breeder Chow (Harlan) and tap water. Sexually naïve male mice of the same age and strain (hsd:ICR) were used as intruders during maternal aggression tests. Intruder males were group housed (4 animals/cage) and never used more than once daily and for ~3 total tests each. Over the course of testing, each female was exposed to three different intruder males. Due to the counterbalanced nature of the study (see below), males with similar testing experience were used equally for all groups. All animals were housed on a 14:10 light/dark cycle with lights on at 0600 CST. All testing was performed between 0930 and 1500 h. All procedures followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Animal Care and Use Committee of the University of Wisconsin.
Genotyping
Mice were genotyped by PCR using sense WT (5′-TGTGG-AAGCTGATCCTGGTG-3′), sense KO (5′-GGGAACTTCCTG-ACTAGGGG-3′) and a common antisense primer (5′-CCT-GATGCCTGCTGAGTTGA-3′). Reactions were run with purified DNA and analyzed as previously described (Bale, Contarino et al. 2000; Gammie, Hasen et al. 2005).
Maternal behavior examination
All animals in this study underwent the series of behavioral tests described below. On postpartum Days 2 and 3 (parturition is postpartum Day 0) each dam was observed for an hour between 1000 and 1200 h. Dams were observed within their home cages in the homeroom (WT, n= 16; KO, n=18). Every minute for one hour, observers blind to treatment noted the maternal behavior of the dam. The behaviors examined included: arched-back nursing (females adopting a crouched position over pups), supine nursing (the dam on her side next to pups), nest building, pup-directed licking, on-nest, off-nest, self-grooming, and eating/drinking. Off-nest, on-nest and nursing are all mutually exclusive behavioral categories. Therefore, we also analyzed whether the dam participated in other maternal behavior while off-nest, on-nest or nursing (i.e. pup licking, nest building while nursing). Latency to nurse, on-nest behavior, and pup licking were quantified by measuring the time elapsed to the onset of the mentioned behaviors.
Maternal aggression and behavior testing
On postpartum Days 4, 5 and 6 each female was exposed to an intruder male for 5 min in her home cage between 900 and 1300 h. Females were moved into the testing room and tested in their home cages, an environment kept constant during the experiment. The maternal aggression test began by introducing a male intruder for 5 min. After the intruder male was removed, the pups were scattered evenly away from the nest allowing the female to retrieve and interact with her pups for 30 minutes. The total testing process lasted for 35 minutes. Each test session was recorded on videotape and subsequently analyzed off-line by individuals blind to testing conditions. For quantification of maternal aggression the following features were measured: latency to first attack, number of attacks, and total duration of attacks (total time aggressive) (Gammie and Nelson 1999; Gammie, Huang et al. 2000). Pup retrieval was quantified by measuring the time elapsed to retrieval of first and fourth (Gammie, Negron et al. 2004; D’Anna, Stevenson et al. 2005) and time to retrieve all pups. Other maternal behavior measures (see above) were surveyed every 30 seconds.
Restraint stress
Restraint stress occurred directly before the behavioral test by placing individual mice into a clear Plexiglas tube with a flat bottom and curved (semi-circle shaped) siding. In all cases, mice were moved to a testing room under low lit conditions, placed in the restraint tubing and set back in their home cages for 15 min. Following restraint, animals were removed from the tubes and allowed to freely move about the cage for 15 min, as based on previous studies where 30 min of restraint reduced maternal aggression (Gammie and Stevenson 2006). Prior to the dam being placed in restraint, pups were removed from the home cage and placed under a warming light until they were reunited with the dam following the maternal aggression test. All mice (n=36) were either exposed to restraint stress for 15 min or not restrained as a control on postpartum Days 5 and/or 6. For example, if a mouse was restrained on Day 5, it was not restrained on Day 6 and if a mouse was not restrained on Day 5 it was restrained on Day 6, thus each animal received every treatment in a counterbalanced order. The effect of day on behavior did not significantly affect the results. When evaluated with a two way ANOVA analysis there was no effect of day on any measure of maternal defense, including time to first bite, number of attacks or mean number of attacks (data not shown). Thus, day was not included in a further analysis and allowed restraint vs. non-restraint to be compared on postpartum Days 5 and 6. Separation from pups for long periods (> 5 hours) prior to testing decreases maternal aggression, but separation for a short time does not affect maternal aggression in rats or mice (Stern and Kolunie, 1993; Svare and Gandelman, 1973). Still, to rule out any subtle effects that 30 min pup separation might have on maternal aggression, unrestrained mice were also separated from their pups 30 min prior to testing. All mice used in this study were first tested on postpartum Day 4 without restraint to provide a baseline level of maternal aggression. All mice were age matched, housed together in the same room, and tested within a 10 day window. All restraint treatments were counterbalanced such that for Days 5 and 6, an equal number of animals of each genotype were restrained or not restrained on each of the days and the testing of individuals from the two genotypes was interspersed.
Elevated plus maze
The plus maze apparatus was made of black Plexiglas and consisted of two open arms (35 x 5 cm) and two closed arms of the same dimensions with walls 15 cm high. The maze was elevated 70 cm off the ground. The arms were connected in the middle by a square (5 cm x 5 cm). Indirect lighting was used in an otherwise dark room. All testing was conducted on postpartum Day 7. Mice were tested individually in 3 min sessions. Each mouse had its pups removed and was either restrained (WT, n= 8; KO, n= 9) or unrestrained (WT, n=9; KO, n= 10) as previously described. Mice from both groups were placed on the center platform facing an open arm to initiate the test. Behaviors scored were the number of closed and open arm entries and time spent in the center, closed and open arms of the maze. Arm entries were defined as entry of all four paws into the arm. Each test session was recorded on videotape and subsequently analyzed off-line by individuals blind to testing conditions.
Immunohistochemistry for c-Fos
One day following the last behavioral test (postpartum Day 8) mice were either restrained or unrestrained for 15 minutes, returned to their home cage with their pups and brains were collected 105 minutes later (±5 min). No behavioral tests were performed. To analyze how restraint affected c-Fos activity, 31 mice were randomly assigned to 2 groups: unrestrained (WT, n= 6; KO, n= 8) and restrained (WT, n= 8; KO, n= 9). Following isoflurane anesthesia, mice were sacrificed and the brains removed. Brains were post-fixed overnight in 6% acrolein in phosphate buffered saline (PBS) and cryoprotected in 30% sucrose in PBS for two days. Brains were frozen on a platform and cut into 40 micron thick sections using a sliding microtome (Leica, Microsystems, Heidelberg, Germany) and stored in a cryoprotectant solution at –20 degrees C until processing for immunohistochemistry with antibodies specific to recognizing c-Fos. All sections underwent previously described immunohistochemical processes, which included incubating the sections for two days at 4 degrees C with rabbit anti-Fos antibodies (1:15,000; Calbiochem, San Diego, CA, catalog # PC38) (Gammie, Negron et al. 2004; D’Anna, Stevenson et al. 2005). The sections were then mounted, dehydrated in a series of ethyl alcohols and xylenes, and coverslipped. Brain sections from all animals were processed simultaneously in one batch.
Analysis of c-Fos immunoreactivity (Fos-IR)
For all sections, previously described bright field microscopy was used for counting c-Fos-positive cells (Gammie, Negron et al. 2004; D’Anna, Stevenson et al. 2005). Dimensions and locations of boxes used to count cells in specific regions are those that have been previously used by other studies (Gammie, Negron et al. 2004; D’Anna, Stevenson et al. 2005; D’Anna and Gammie 2006; Gammie and Stevenson 2006; Lee and Gammie 2006).
Data analysis
Data were analyzed with the assistance of the statistical software, SigmaStat 3.0 (SPSS Inc, Chicago), in which the normality of the data were determined and the correct parametric or non-parametric test utilized. Maternal aggression and other maternal behavior testing variables were analyzed using a one way analysis of variance (ANOVA). In the cases where the data were not normally distributed, (i.e. nest-building) a non-parametric Kruskal-Wallis one way ANOVA on Ranks test was performed. In the case of latency to first attack, if an animal was non-aggressive, a time of 300 s was assigned (the maximum time of the maternal aggression test). Likewise, if an animal did not retrieve at least 4 pups or any pups at all, a time of 120 seconds was assigned as previously performed (Gammie, Negron et al. 2004; D’Anna, Stevenson et al. 2005).
For maternal defense analysis, a two way RM ANOVA was used to examine effects of restraint type and genotype interaction. In the previous analysis mice with varying levels of aggression were included. However, to decrease variability and uncover more robust differences in aggression, further analysis included only mice with 10 sec of more of maternal aggression. The rationale for the 10 sec cutoff is that if one is testing the hypothesis that stress decreases aggression, then mice with low aggression cannot be properly be evaluated due to a floor effect. Maternal aggression data were then analyzed by establishing differences between unrestrained and restrained conditions for each mouse to further incorporate individual variability into the statistical model. The standard p-value cutoff of 0.05 was used to evaluate the significance of the behavioral data. Non-normal maternal defense data from postpartum Day 4 were either transformed with natural log (ln) or square root to allow for the performance of parametric tests.
For maternal behavior analysis, a two way RM ANOVA was used to examine effects of restraint type and genotype interaction on all measures. For elevated plus maze analysis, a two-way ANOVA was used to examine restraint type and genotype interaction on the number of closed and open-arm entries and the time spent in the center, closed and open arms of the maze.
For c-Fos analysis, a two way ANOVA examined restraint type and genotype interaction. Separate planned comparisons (one way ANOVAs) were used to test the effect of restraint stress and non- restraint on the number of c-Fos positive cells in 44 separate brain regions. In cases where the data were not normally distributed, a non-parametric Kruskal-Wallis ANOVA on Ranks test was used. For all other measures, parametric tests were employed. Areas evaluated include those previously implicated in either maternal aggression and enriched with CRF1 and/or CRF2 receptors.
In order to correct for multiple comparisons the False Discovery Rate method of Storey (2002) was applied using the open source software Qvalue (Dabney and Storey 2002). This method is used to estimate the p-value cutoff to use for each test that will yield a global, experiment-wide, false discovery rate of 5% and has been used previously to correct for multiple comparisons (Gammie, Negron et al. 2004; D’Anna, Stevenson et al. 2005; Rhodes, Ryabinin et al. 2005). To examine the number of c-Fos positive cells in all brain regions, applying the pvalue standard cut-off of 0.05 would only yield a false discovery rate of 4%, therefore, it remained appropriate to apply the pvalue 0.05 to the data set.
In addition, all brain regions with significant changes in c-Fos expression were examined with the most recent restraint treatment (restraint vs. non-restraint) received the day before brain collection as a covariate to examine if previous experience altered final c-Fos expression. There was not an effect of previous restraint treatment on c-Fos expression (see Results).
Acknowledgments
This work was supported by NIH Grant R01 MH066086 to S.C.G, American Psychological Association Diversity Program in Neuroscience Fellowship to K.L.D, and the Ford Foundation Disseration Fellowship to K.L.D. The authors wish to thank Emily Bethea, Kelly Clinkenbeard, Mike Foley, Jessica Hubbard, Allen Irgens, Patrick Kleven, Rachel Leisemann, Caleigh Mandel-Brehm, Derek Powell, Sharon Stevenson, Grace Lee, and Nina Hasen for technical assistance and Kate Skogen and Jeff Alexander for animal care.
Abbreviations
- cPAG
caudal periaqueductal gray
- CRF2
corticotropin releasing factor receptor 2
- Ucn
urocortin
- CRF1
corticotropin releasing factor receptor 1
- BNSTd
the bed nucleus of the stria terminalis dorsal
- DG
dentate gyrus
- IL
infralimbic cortex
- NuAbC
nucleus accumbens core
- LPO
lateral preoptic area
- PVN
paraventricular hypothalamic nucleus
- AHA
anterior hypothalamic area anterior
- VMH
ventromedial hypothalamus
- AHP
anterior hypothalamic area posterior
- DM
dorsomedial hypothalamus
- PeF
perifornical nucleus
- SCN
suprachiasmatic nucleus
- AOM
anterior olfactory nucleus medial
- AOP
anterior olfactory nucleus posterior
- VTT
ventral tenia tecta
- MFC
medial frontal cortex
- LSV1
lateral septum ventral
- PVA
paraventricular thalamic nucleus anterior
- PH
posterior hypothalamic area
- VTA
ventral tegmental area
- DRD
dorsal raphe nucleus dorsal
- NuASh
nucleus accumbens shell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anisman H, Zacharko R. Depression: the predisposing influences of stress. Behav Brain Sci. 1982;5:89–137. [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–4. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. Increased depression-like behaviors in corticotropin-releasing factor receptor-2 deficient mice: Sexually dichotomous responses. J Neurosci. 2003;23:5295–5301. doi: 10.1523/JNEUROSCI.23-12-05295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers D, Lovenberg T, De Souza E. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: Comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- da Costa AP, Kampa RJ, Windle RJ, Ingram CD, Lightman SL. Region-specific immediate-early gene expression following the administration of corticotropin-releasing hormone in virgin and lactating rats. Brain Res. 1997;770:151–62. doi: 10.1016/s0006-8993(97)00764-6. [DOI] [PubMed] [Google Scholar]
- da Costa AP, Wood S, Ingram CD, Lightman SL. Region-specific reduction in stress-induced c-fos mRNA expression during pregnancy and lactation. Brain Res. 1996;742:177–84. doi: 10.1016/s0006-8993(96)00962-6. [DOI] [PubMed] [Google Scholar]
- Dabney A, Storey JD. QVALUE [Computer software] 2002 Retrieved from http://facultywashingtonedu/~jstorey/qvalue/
- D’Anna K, Stevenson SA, Gammie S. Urocortin 1 and 3 impair maternal defense behavior in mice. Behav Neurosci. 2005;119:1061–71. doi: 10.1037/0735-7044.119.4.1061. [DOI] [PubMed] [Google Scholar]
- D’Anna KL, Gammie SC. Hypocretin-1 dose-dependently modulates behaviour in mice. J Neuroendocrinol. 2006;18:553–566. doi: 10.1111/j.1365-2826.2006.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–5. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Farrokhi C, Blanchard DC, Griebel G, Yang M, Gonzales C, Markham C, Blanchard RJ. Effects of the CRF1 antagonist SSR125543A on aggressive behaviors in hamsters. Pharmacol Biochem Behav. 2004;77:465–469. doi: 10.1016/j.pbb.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Gammie S, Bethea E, Stevenson S. Altered maternal profiles in corticotropin-releasing factor receptor 1 deficient mice. BMC Neurosci. 2007;8:1–15. doi: 10.1186/1471-2202-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie S, Garland T, Stevenson SA. Artificial selection for increased maternal defense behavior in mice. Behav Genet. 2006;36:713–722. doi: 10.1007/s10519-006-9071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie S, Nelson RJ. Maternal aggression is reduced in neuronal nitric oxide synthase-deficient mice. Hormones & Behavior. 1999;38:13–20. doi: 10.1006/hbeh.2000.1595. [DOI] [PubMed] [Google Scholar]
- Gammie SC. Current Models and Future Directions for Understanding the Neural Circuitries of Maternal Behaviors in Rodents. Behav Cogn Neurosci Rev. 2005;4:119–135. doi: 10.1177/1534582305281086. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Hasen NS, Stevenson SA, Bale TL, D’Anna KL. Elevated stress sensitivity in corticotropin-releasing factor 2 mice decreases maternal, but not intermale aggression. Behav Brain Res. 2005;160:681–695. doi: 10.1016/j.bbr.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Huang PL, Nelson RJ. Maternal aggression in endothelial nitric oxide synthase-deficient mice. Hormones & Behavior. 2000;38:13–20. doi: 10.1006/hbeh.2000.1595. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Negron A, Newman SM, Rhodes JS. Corticotropin-releasing factor inhibits maternal aggression in mice. Behav Neurosci. 2004;118:805–814. doi: 10.1037/0735-7044.118.4.805. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Stevenson SA. Effects of daily and acute restraint stress during lactation on maternal aggression and behavior in mice. Stress. 2006;9:171–180. doi: 10.1080/10253890600969106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, Stevenson SA. Intermale aggression in corticotropin-releasing factor receptor 1 deficient mice. Behav Brain Res. 2006;171:63–69. doi: 10.1016/j.bbr.2006.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilov Y, Perekrest S, Novikova N, Korneva E. Stress-induced changes in cellular responses in hypothalamic structures to administration of an antigen (lipopolysaccharide) (in terms of c-Fos protein expression) Neurosci Behav Physiol. 2008;38:189–194. doi: 10.1007/s11055-008-0028-9. [DOI] [PubMed] [Google Scholar]
- Givalois L, Arancibia S, Tapia-Arancibia L. Concomitant changes in CRH mRNA levels in rat hippocampus and hypothalamus following immobilization stress. Brain Research Molecular Brain Research. 2000;75:166–171. doi: 10.1016/s0169-328x(99)00290-9. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid M, Lopresti M, Watkins L, Maier S. The role of corticotropin releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci. 2001;22:1020–1026. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. Low doses of corticotropin-releasing hormone injected in to the dorsal raphe nucleus block the behavioral consequences of uncontrollable stress. Behav Brain Res. 2003;147:55–64. doi: 10.1016/s0166-4328(03)00133-5. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Schmid M, LoPresti M, Der-Avakian A, Pelleymounter M, Foster A, Watkins L, Maier S. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Zoeller RT, McGivern RF. Changes in vasoactive intestinal peptide and arginine vasopressin expression in the suprachiasmatic nucleus of the rat brain following footshock stress. Neurosci Lett. 2007;425:99–104. doi: 10.1016/j.neulet.2007.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley S, McBlane J, Critchley M, Njung’e K. Multiple serotonin mechanisms in animal models of anxiety: environmental, emotional and cognitive factors. Behav Brain Res. 1993;58:203–210. doi: 10.1016/0166-4328(93)90104-x. [DOI] [PubMed] [Google Scholar]
- Hasen N, Gammie SC. Maternal aggression: New insights from Egr-1. Brain Res. 2006;1108:147–156. doi: 10.1016/j.brainres.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Hasen NH, Gammie SC. Differential fos activation in virgin and lactating mice in response to an intruder. Physiol Behav. 2005;84:681–695. doi: 10.1016/j.physbeh.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Hauger R, Grigoriadis D, Dallman M, Plotsky P, Vale W, Dautzenberg F. International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev. 2003;55:21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- Henry B, Vale WW, Markou A. The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. J Neurosci. 2006;26:9142–9152. doi: 10.1523/JNEUROSCI.1494-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg S. A review of the validity and variability of the Elevated Plus-Maze as an animal model of anxiety. Pharmacology Biochemistry and Behavior Anxiety, Stress and Depression. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Lee G, Gammie SC. Chlordiazepoxide enhancement of maternal aggression is associated with decreased c-Fos activity in lateral septum and caudal periaqueductal gray. Pharmacol Biochem Behav. 2006;86:176–187. [Google Scholar]
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev. 2002;26:869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Makino S, Hashimoto K, Gold PW. Multiple feedback mechanisms activating corticotropin-releasing hormone system in the brain during stress. Pharmacol Biochem Behav. 2002;73:147–158. doi: 10.1016/s0091-3057(02)00791-8. [DOI] [PubMed] [Google Scholar]
- O’Hara M, Schlechte J, Lewis D, Varner M. Controlled prospective study of postpartum mood disorders: psychological, environmental and hormonal variables. J Abnorm Psychol. 1991;100:63–73. doi: 10.1037//0021-843x.100.1.63. [DOI] [PubMed] [Google Scholar]
- Olivier B, Pattij T, Wood S, Oosting R, Sarnyai Z, Toth M. The 5-HT(1A) receptor knockout mouse and anxiety. Behavioral Pharmacology. 2001;12:439–450. doi: 10.1097/00008877-200111000-00004. [DOI] [PubMed] [Google Scholar]
- Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain pituitary. Proc Natl Acad Sci U S A. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Ryabinin A, Crabbe J. Patterns of brain activation associated with contextual conditioning to methamphetamine in mice. Behav Neurosci. 2005;119:759–771. doi: 10.1037/0735-7044.119.3.759. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Tocco G, Shors TJ, Thompson R. Activation of immediate early genes after acute stress. Neuroreport. 1991;2:17–20. doi: 10.1097/00001756-199101000-00004. [DOI] [PubMed] [Google Scholar]
- Sibille E, Pavlides C, Benke D, Toth M. Genetic inactivation of the Serotonin(1A) receptor in mice results in downregulation of major GABA(A) receptor alpha subunits, reduction of GABA(A) receptor binding, and benzodiazepine-resistant anxiety. J Neurosci. 2000;20:2758–65. doi: 10.1523/JNEUROSCI.20-08-02758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens-Simon C, Kelly L, Wallis J. The timing of norplant insertion and postpartum depression in teenagers. J Adolesc Health. 2000;26:408–413. doi: 10.1016/s1054-139x(99)00091-9. [DOI] [PubMed] [Google Scholar]
- Toth M. 5-HT1A receptor knockout mouse as a genetic model of anxiety. Eur J Pharmacol. 2003;463:177–184. doi: 10.1016/s0014-2999(03)01280-9. [DOI] [PubMed] [Google Scholar]
- Van Bogaert M, Oosting R, Toth M, Groenink L, van Oorschot R, Olivier B. Effects of genetic background and null mutation of 5-HT1A receptors on basal and stress-induced body temperature: Modulation by serotonergic and GABAA-ergic drugs. Eur J Pharmacol. 2006;550:84–90. doi: 10.1016/j.ejphar.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Yokoyama C, Sasaki K. Regional expressions of Fos-like immunoreactivity in rat cerebral cortex after stress; restraint and intraperitoneal lipopolysaccharide. Brain Res. 1999;816:267–275. doi: 10.1016/s0006-8993(98)00927-5. [DOI] [PubMed] [Google Scholar]