Abstract
Women’s sexual decision making is a complex process balancing the potential rewards of conception and pleasure against the risks of possible low paternal care or sexually transmitted infection. Although neural processes underlying social decision making are suggested to overlap with those involved in economic decision making, the neural systems associated with women’s sexual decision making are unknown. Using fMRI, we measured the brain activation of 12 women while they viewed photos of men’s faces. Face stimuli were accompanied by information regarding each man’s potential risk as a sexual partner, indicated by a written description of the man’s number of previous sexual partners and frequency of condom use. Participants were asked to evaluate how likely they would be to have sex with the man depicted. Women reported that they would be more likely to have sex with low compared to high risk men. Stimuli depicting low risk men also elicited stronger activation in the anterior cingulate cortex (ACC), midbrain, and intraparietal sulcus, possibly reflecting an influence of sexual risk on women’s attraction, arousal, and attention during their sexual decision making. Activation in the ACC was positively correlated with women’s subjective evaluations of sex likelihood and response times during their evaluations of high, but not low, risk men. These findings provide evidence that neural systems involved in sexual decision making in women overlap with those described previously to underlie nonsexual decision making.
Keywords: mate choice, decision making, anterior cingulated, risk
Introduction
Sexual interactions carry both the potential for risks and rewards, including the transmission of sexually transmitted diseases and unintended pregnancy, as well as pleasure and social bonding. The decision of whether or not to engage in a sexual encounter, and specifically with whom to engage, requires an analysis of the costs and benefits of that potential sexual encounter and partner [21]. Thus, like other types of decision making, sexual decision making can be expected to engage a multitude of cognitive processes, including ones related to sensory processing and attention, and the evaluation of expected risks and rewards. While previous literature extensively reviews the neural substrates of nonsocial, and especially economic, decision making, it is unclear whether or not the same neural systems also underlie social, and particularly sexual, decision making.
Within the broader definition of decision making a distinction can be made between social and nonsocial decision making. Economic decision making is one of the most commonly investigated forms of nonsocial decision making. Social decision making involves the interaction with and assessment of other individuals and can be further broken into sexual and nonsexual decision making. Common to both social and nonsocial decision making is the assessment of costs, consequences, rewards, and the preference for an optimal behavioral outcome [13]. Considering the relevance of these processes to both types of decision making, one would predict the involvement of similar neural systems. Indeed, recent fMRI studies support an overlap in brain systems involved in both economic and social decision making, specifically in the anterior cingulate cortex, orbitofrontal cortex, insula, and striatum [15, 17, 32]. However, neural systems underlying sexual decision making, considered here a potentially distinct subset of social decision making, have not been specifically examined. Thus, it is as yet unknown whether neural systems involved in sexual decision making are the same as those involved in economic decision making, as recently has been shown to be the case for nonsexual forms of social decision making [15, 17, 18, 32].
The current study examined the neural systems associated with women’s sexual decision making. Brain activation was measured in women evaluating high and low risk potential sexual partners using fMRI. During this sexual decision making paradigm, we expected involvement of brain regions recruited during economic and other types of social decision making; including the anterior cingulate cortex, orbitofrontal cortex, ventral striatum, and amygdala [8, 15, 17, 18, 27, 31, 32]. Understanding women’s neural responses to potential sexual partners will not only further our understanding of women’s sexual decision making to inform health-related interventions; it may also further our understanding of the overlap in neural systems underlying social and nonsocial decision making.
Methods
Participants were 12 heterosexual women between ages 23 - 28 who were not using any form of hormonal birth control, not in a relationship, and did not have any contraindications for MRI. Participants were on average 25.2 years old (SD=1.9), 11 were Caucasian, one was African American, and all but one reported some previous sexual experience (Number of Lifetime Sexual Partners, Mean ± SD = 5.3 ± 3.7). None of the participants reported currently using medication or being under treatment for any psychological disorders, all reported regular periods 28 to 32 days, all women were single, and two women reported currently having a sexual partner (nonexclusive). Participants’ average (Mean ± SD) scores on psychosexual questionnaires (described in more detail below) were within the normal range: Sexual Inhibition Scale 1 = 34.25 ± 4.03; Sexual Inhibition Scale 2 = 32.83 ± 4.99; Sexual Excitation Scale = 51.33 ± 6.58; Sociosexual Orientation Inventory= 60. 92 ± 18.70.
To create the stimulus set, pictures of male faces were taken from public domain websites on the Internet, selected to be of generally the same age range as participants, depicting a neutral expression, and from a variety of ethnic backgrounds. All faces were edited to the same 640 × 480 pixel resolution with the same limited amount of background, and converted to black and white in Adobe Photoshop (Version 7.0.1, Adobe Systems Inc). Pictures with variable levels of attractiveness were selected based on pilot ratings from 15 women not involved in the fMRI testing. The face presentations were combined with information indicating whether the depicted man was of high or low sexual risk. Risk was varied using information about the men’s number of previous sexual partners and their typical use of condoms. Based on population estimates of the average number of sexual partners for women in this age group [22], low risk men had 2-5 previous sexual partners and usually used condoms. High risk men had 10-13 previous sexual partners and rarely used condoms. This written information was provided as a single number (2-13) and word (usually/rarely) in the top right corner of the stimulus screen. As part of another study, the male faces differed in facial masculinity on two dimensions. This 2 (sexual risk) by 2 (masculinity) design included, therefore, four presentations of the same male face (56 original). Specifically, two masculinized and two feminized exemplars of the same face were each presented as both a high and low risk sexual partner, for a total of 224 pictures. For the current study, we collapsed woman’s responses across the masculinity conditions before we compared responses based on sexual risk. Patterns of neural activation related to facial masculinity were analyzed separately and are presented elsewhere [25]. Stimuli were rear-projected using a Mitsubishi XL30U projector onto a frosted glass screen which was seen by the participant as a reflection in a mirror positioned in front of the participant’s line of view. Pictures were presented in eight blocks which were randomized for presentation order.
All women were tested during the late follicular phase of their menstrual cycles (days 10-12) to control for possible hormone influences [9, 12]. Before the first test session, participants completed a series of questionnaires regarding their psychosexual profiles, including the Sociosexual Orientation Inventory (SOI) [29] and the Sexual Inhibition/Sexual Excitation Scales (SIS/SES) [4, 16]. The SOI is a 7-item scale measuring an individual’s tendency to engage in short-term or uncommitted sexual encounters. The SIS/SES measures three factors using 45 four-point scale items: (a) propensity for sexual excitation (SES; range 20 to 80); (b) propensity for sexual inhibition due to “the threat of performance failure” (SIS1; range 14 to 56); and (c) propensity for sexual inhibition due to “the threat of performance consequences” (SIS2; range 11 to 44).
Imaging took place at the Indiana University Imaging Research Facility. Participants were screened and then comfortably positioned in an fMRI scanner where they performed the male evaluation task. Each face stimulus was presented for four seconds, during which participants were asked: “How likely would you be to have sex with this person?” (1=very unlikely, 2=unlikely, 3=likely, 4=very likely). Participants indicated their response on a button response box with a finger press. Participants were instructed to imagine themselves in a scenario in which they were open to a sexual encounter; to make their decision as if they were in that situation; and to base their decision on how attractive they found the man depicted and the supplementary information provided (partner number and condom use). All stimuli were presented using MATLAB 5.2 (MATHWORKS Inc., Natick, MA) on a Macintosh computer in a rapid variable ISI event-related design.
Imaging was carried out using a Siemens Magnetom Trio 3T whole body MRI and collected on an eight-channel phased-array head coil. Each MRI session took about an hour, during which functional and anatomical scans were acquired. First, a 3-plane scout was acquired to be used for choosing slice planes for the remaining scans (10 sec). Functional scans were Gradient-echo T2* EPI scans for BOLD-based functional neuroimaging (duration ~5 min, 8 scans/session, ~40 min). The fMRI pulse sequence had the following EPI parameters: TE = 25 ms, flip angle = 70°, FOV = 220 × 220 mm, matrix 64 × 64, in-plane resolution = 3.4 × 3.4 mm, slice thickness = 3.4 mm, gap thickness = 0 mm. A typical volume was 33 EPI slices acquired at a time of 60 ms per slice for a total volume acquisition time of 2 seconds (TR = 2). Slices were acquired parallel to the ACPC plane to efficiently cover the entire brain. Finally, a high resolution T1 3-D turbo-flash structural scan of the entire brain was acquired with 160 sagittal slices (FOV = 224 × 256; voxel size =1 × 1 × 1; T1=1,100 ms, TE=3.93ms, TR=14.375 ms, Flip Angle=12°; duration = 8 min).
Imaging data were preprocessed and analyzed using Brainvoyager™ software. Functional data preprocessing included 3-D motion correction, slice scan-time correction, spatial smoothing (3-D Gaussian FWHM 6mm), and linear trend removal. Functional slice data were co-registered to high-resolution structural volumes for each individual and normalized to Talaraich space. Imaging data were analyzed using Brainvoyager™ multi-study general linear model procedure. A random-effects whole-brain group average statistical parametric map (SPM) was calculated to examine the main effect of sexual risk (high minus low). This contrast, as shown in Figure 1, was thresholded at a voxel-wise probability of p<.001 with a cluster-size threshold of 10 voxels (393 mm3). The cluster threshold correction technique used here controls false positives, with a relative sparing of statistical power, which was important for studying the small effect sizes seen between our experimental conditions [10]. Corrections similar to this have been found to successfully manage the multiple testing problem [30].
Figure 1.
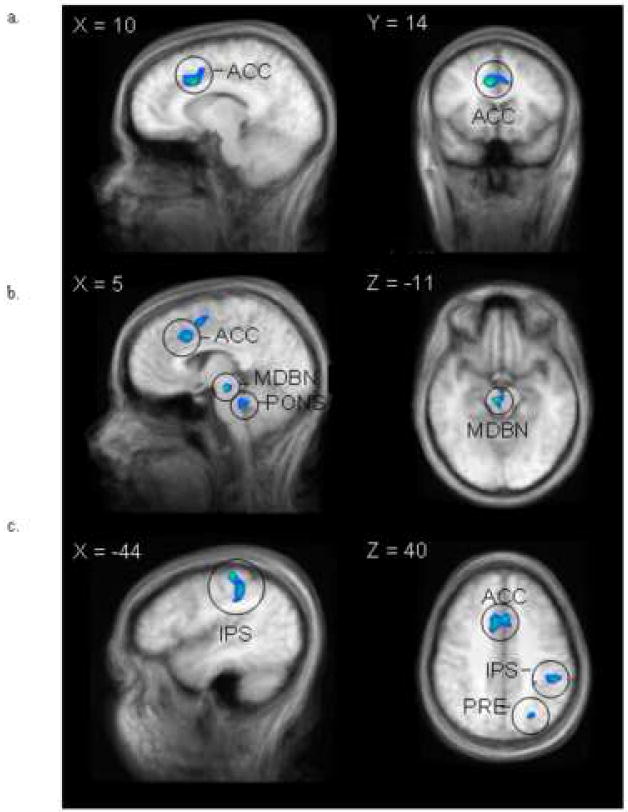
Areas of activation demonstrating increased activation to low versus high risk potential sexual partners. Brain regions included the bilateral anterior cingulate cortex (ACC), right midbrain (MDBN), pons (PONS), intraparietal sulcus (IPS), and precuneus (PRE).
Those brain regions determined from the whole-brain SPM analysis to differ in activation in response to high and low risk sexual partners became regions of interest (ROIs). Activation in these ROIs was further analyzed by extracting estimated timecourses using a deconvolution analysis on each individual. BOLD response averages (beta weights) between six to 10 seconds after stimulus onset (3-5 TR) were taken from within each ROI for each individual subject and averaged across the three data points. This average BOLD response for each ROI was then entered into SPSS (Version 16.0, SAS Institute Inc., Cary, NC) for correlation analyses (Spearman Bivariate two-tailed) with behavioral responses and psychosexual profiles (SES, SIS1, SIS2, SOI).
Results
Women indicated a higher likelihood of engaging in sex with low risk men (paired samples t-test, t11 = -5.3, p< .001; High, Mean ± SD = 1.41 ± .32; Low, Mean ± SD = 2.45 ± .59). Results of the correlation analyses with participants’ psychosexual variables (SES, SIS1, SIS2, SOI) and subjective ratings for high and low risk men demonstrated significant associations between women’s propensity for sexual inhibition (SIS) and their within-category ratings. Specifically, women’s subjective ratings of the likelihood of sex with the high risk men depicted were negatively associated with women’s scores of sexual inhibition due to the threat of performance consequences (SIS2, R10 =-.61, p=.04). Additionally, women’s subjective ratings of the likelihood of engaging in sex with men depicted as low risk were positively associated with their scores of sexual inhibition related to performance failure (SIS1, R10 =.58, p=.05). SES and SOI scores were not significantly associated with subjective ratings for high or low risk men.
Response times for making the subjective ratings did not significantly differ based on the risk of the man depicted (paired samples t-test, t11 = -1.94, p= .08; High, Mean secs ± SD = 1.58 ± .45; Low, Mean secs ± SD = 1.73 ± .42). Women’s subjective ratings were correlated with their response times during the evaluations of high (R10 =.60, p=.04), but not low, risk men. Response times were not associated with scores on any of our psychosocial variables.
An initial whole-brain group-average SPM comparing activation to high versus low risk men revealed that five general regions of the brain demonstrated more activation to low than high risk potential sexual partners (Figure 1); the bilateral dorsal anterior cingulate cortex (ACC; Right, Talaraich coordinates=8, 14, 34, max t-value = 7.25, uncorrected p-value = 0.00002; Left, Talaraich coordinates -3, 18, 38, max t-value = 6.58, uncorrected p-value = 0.00004), the right brainstem including the midbrain (Talaraich coordinates=6, -22, -11, max t-value = 7.58, uncorrected p-value = 0.00001) and pons (Talaraich coordinates=2, -34, -23, max t-value = 6.42, uncorrected p-value = 0.00005), and the left parietal cortex, including the intraparietal sulcus (IPS, Talaraich coordinates= -44, -33, 40, max t-value = 6.41, uncorrected p-value = 0.00005) and precuneus (Talaraich coordinates= -23, -65, 38, max t-value = 5.36; uncorrected p-value = 0.0002). Based on this contrast, these areas of differential responding to high versus low risk men, including the ACC, midbrain, pons, IPS, and precuneus, became ROIs for follow-up analyses.
Activation in two brain regions was significantly associated with women’s subjective ratings of high risk potential sexual partners (Table 1). Specifically, activation in the bilateral ACC was positively related to women’s subjective ratings of their likelihood of having sex with high risk men (right, R10=0.66, p=.02; left, R10=0.62, p=.03) and activation in the pons was inversely related to women’s rating of having sex with high risk men (R10=-0.57, p=.05). Together these correlations demonstrate an association between women’s self-rated higher likelihood of engaging in sex with high risk partners and increased activation in the ACC, as well as with decreased activation in the pons. We did not observe any brain regions from our ROIs in which activation predicted women’s subjective ratings of their likelihood of sex with low risk men.
TABLE 1.
Summary of relationships between neural activation in ROIs and women’s subjective ratings, response times, and psychosexual variables. Statistics presented are based on two-tailed Spearman bivariate correlations.
| Average Activation Response | Subj. Rating | R. Time | SIS1 | SIS2 | SES | SOI |
|---|---|---|---|---|---|---|
| Anterior Cingulate Cortex | ||||||
| Right | ||||||
| High Risk | 0.66 | 0.74 | -0.32 | -0.51 | -0.45 | 0.09 |
| Low Risk | -0.11 | 0.57 | -0.40 | -0.40 | -0.32 | -0.02 |
| Left | ||||||
| High Risk | 0.62 | 0.70 | -0.30 | -0.41 | -0.36 | -0.22 |
| Low Risk | -0.19 | 0.57 | -0.32 | -0.50 | -0.40 | -0.03 |
|
| ||||||
| Brainstem | ||||||
| Midbrain | ||||||
| High Risk | 0.35 | 0.83 | 0.33 | 0.06 | -0.54 | -0.12 |
| Low Risk | 0.32 | 0.65 | 0.03 | -0.04 | -0.31 | 0.01 |
| Pons | ||||||
| High Risk | -0.58 | -0.28 | -0.18 | 0.47 | 0.55 | -0.30 |
| Low Risk | 0.16 | 0.59 | 0.02 | 0.65 | 0.15 | -0.60 |
|
| ||||||
| Parietal Cortex | ||||||
| IPS | ||||||
| High Risk | 0.30 | 0.59 | 0.26 | 0.10 | -0.43 | -0.01 |
| Low Risk | 0.45 | 0.34 | 0.42 | 0.11 | -0.25 | 0.27 |
| Precuneus | ||||||
| High Risk | 0.11 | 0.08 | -0.03 | 0.18 | -0.40 | -0.17 |
| Low Risk | 0.07 | 0.04 | 0.06 | 0.23 | -0.27 | -0.14 |
Significant (p<.05) relationships are in bold.
Participants’ response times were strongly positively correlated with brain activation in many brain regions during the evaluation of the men (Table 1). Specifically, activation in the bilateral ACC was positively associated with response times during the evaluation of high (right ACC, R10=0.74, p=.006; left ACC, R10=0.70, p=.01), but not low, risk men. Activation in the midbrain was positively associated with women’s response times during the evaluation of all men (High, R10=0.83, p=.001; Low, R10=0.65, p=.02). For high, but not low, risk men, activation in the IPS was positively associated with women’s response times (R10=0.59, p=.05). In the pons, activation was positively associated with women’s response times during the evaluation of low (R10=0.59, p=.05), but not high, risk men. Activation in the precuneus was not related to response times for either type of male face stimuli.
Results from the correlation analyses demonstrated significant relationships between SES, SIS1, SIS2, and SOI and neural activation only in the pons (Table 1). Activation in the pons during the evaluation of low risk men was positively related to women’s scores of sexual inhibition due to performance consequences (SIS2, R10=0.65, p=.02) and negatively related to their scores of sociosexuality (SOI, R10=-0.60, p=.04).
Discussion
Women demonstrated different neural responses to male faces described to be of low or high risk as a potential sexual partner. Differences in activation were observed in the bilateral anterior cingulate cortex, midbrain, and parietal cortex. These brain regions are related to processes of attraction, attention, and arousal and have previously been reported to be differentially responsive to low versus high risk financial options [3, 5, 8, 26]. Because activation in the observed brain regions discriminated between low and high risk potential partners in a manner similar to patterns of neural activation previously observed in response to high and low risk financial options [8] and during social judgment [18], the current study’s findings support overlapping neural networks for financial, social, and sexual decision making.
The discrimination of ACC activation between high and low risk partners is of specific interest given extensive reports of its role in nonsexual decision making, for example during the evaluation of high or low risk financial rewards or of nonsexual social characteristics [3, 5, 8, 18, 23, 26, 27]. Activation in the ACC has been proposed to reflect perceived reward value, specifically related to the weighing of the costs, consequences, and benefits of possible outcomes to determine the optimal reward potential and appropriate behavior [1, 3, 14, 20, 24]. That activation in this region was positively correlated with women’s subjective ratings of sex likelihood and their response times suggests that activation in this region reflects increased processing effort, analysis, and positive appraisal of stimuli during decision making, as proposed previously for the ACC [3, 18, 23, 26]. Especially of interest is that these associations between behavior and ACC activation were observed only in response to high, but not low risk stimuli, consistent with previous work demonstrating increased activation in the ACC during the choice of a higher risk financial option [5, 11]. Together, results from the current study using a sexual decision making paradigm show a pattern of neural activation in the ACC that is remarkably consistent with previous work using other kinds of decision making paradigms.
Increased activation in the midbrain in response to men depicted as low risk sexual partners is consistent with previous work demonstrating increased midbrain activation in response to rewarding financial decisions [2, 8]. Furthermore, increased activation in the midbrain is consistent with work in humans and animal models demonstrating increased activation in the midbrain with positive reward prediction error [7, 28]. Activation in the midbrain was also positively associated with women’s response times, possibly reflecting the role of the midbrain dopaminergic system in learning and behavioral reinforcement [28]. Therefore, increased activation in the midbrain in response to low risk men may reflect increased dopamine signaling, reward prediction, and reward value for this preferred category of low risk men. Activation in the pons was higher to low risk men as well, but negatively related to women’s subjective ratings of high risk men, response times during the evaluation of low risk men, and related to a more inhibited psychosexual profile. The pons receives large projections from the cerebral cortex for projection to the cerebellum, and in turn receives large projections from the cerebellum for projection to the thalamus; therefore activation in this region may be an important relay between higher cortical areas and behavioral activation, specifically regarding physiological autonomic arousal. Finally, we observed increases in activation in the parietal cortex in response to low risk versus high risk men, including the IPS and precuneus. The observed involvement of IPS during women’s sexual decision making is consistent with previous literature demonstrating a role for the IPS in categorization [6], and is also consistent with a recent study of social evaluation that found parietal cortical recruitment during the judgment of famous faces [18]. Together, differences in activation observed in ACC, brainstem, and parietal cortex in response to high versus low risk sexual partners suggests that, consistent with nonsexual decision making, processes of attraction, arousal, and attention are integral to women’s sexual decision making.
Contrary to our hypotheses, we did not find activation in the orbitofrontal cortex, an area commonly observed to be activated during nonsexual decision making tasks [2], specifically in conjunction with activation in the ACC [5]. However, due to the ventral and anterior location, this region experiences significant signal dropout due to susceptibility artifacts, which may have prevented detection of changes in activation in this region. Also inconsistent with our hypotheses was the absence of an expected association between observed activation in the ACC and women’s psychosexual profiles. This was predicted based on previous research demonstrating that ACC activation during decision making varies with individual characteristics [3, 8, 17, 27]. Based on the relatively high r-values in the absence of statistical significance, however, we do not believe that the current study is conclusive regarding the influence of women’s psychosexual characteristics on neural activation during sexual decision making. To fully investigate the promising potential interaction between the two, future studies should use larger samples or use recruitment methods to increase the ability to detect potential individual differences.
In sum, this study provides further support for previous studies suggesting an overlap in brain systems underlying economic and social decision making [13, 15, 17, 32]. Together, the patterns of psychosexually mediated neural activation observed here in response to lower risk sexual partners may point to targets of cognitive interventions that would enhance women’s likelihood of safer sexual decisions and decrease their vulnerability to unplanned pregnancies or STDs. Specifically, given the difference in activation observed in the ACC and midbrain, regions integral to learning and behavioral reinforcement, cognitive and behavioral interventions should emphasize conditioning strategies pairing safer sexual decision, such as using condoms, with rewards. More generally, the current study supports a theoretical framework of shared neural networks underlying social, including sexual, and economic decision making.
Acknowledgments
We than Sunah Kim and Ryan Stevenson for programming, analysis, and technical help and NIH for funding (NICHHD-T32-HD-49339-0).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amiez C, Joseph JP, Procyk E. Anterior cingulate error-related activity is modulated by predicted reward. Eur J Neurosci. 2005;21:3447–3452. doi: 10.1111/j.1460-9568.2005.04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balleine BW. The neural basis of choice and decision making. J Neurosci. 2007;27:8159–8160. [Google Scholar]
- 3.Brown JW, Braver TS. Risk prediction and aversion by anterior cingulate cortex. Cognit Affect Behav Neurosci. 2007;7:266–277. doi: 10.3758/cabn.7.4.266. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter D, Janssen E, Graham C, Vorst H, Wicherts J. Women’s scores on the Sexual Excitation/Sexual Inhibition Scales (SIS/SES): Gender similarities and differences. J Sex Res. 2008;45:36–48. doi: 10.1080/00224490701808076. [DOI] [PubMed] [Google Scholar]
- 5.Cohen MX, Heller AS, Ranganath C. Functional connectivity with anterior cingulate and orbitofrontal cortices during decision-making. Cognitive Brain Res. 2005;23:61–70. doi: 10.1016/j.cogbrainres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Cohen-Kadosh R, Henik A, Rubinsten O, Mohr R, Dori H, van de Ven V, Zorzi M, Hendler T, Goebel R, Linden DE. Are numbers special? The comparison systems of the human brain investigated by fMRI. Neuropsychologia. 2005;43:1238–1248. doi: 10.1016/j.neuropsychologia.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 7.D’Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD response reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- 8.Doya K. Modulators of decision-making. Nat Neurosci. 2008;11:410–416. doi: 10.1038/nn2077. [DOI] [PubMed] [Google Scholar]
- 9.Dreher J, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Res Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 11.Fukui H, Murai T, Fukuyama H, Hayashi T, Hanakawa T. Functional activity related to risk anticipation during performance of the Iowa gambling task. NeuroImage. 2005;24:253–259. doi: 10.1016/j.neuroimage.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N. Hormonal cycle control modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayden BY, Parikh PC, Deaner RO, Platt ML. Economic principles motivating social attention in humans. Proc Natl Acad Sci B. 2007;274:1751–1756. doi: 10.1098/rspb.2007.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- 15.Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Janssen E, Vorst H, Finn P, Bancroft J. The sexual inhibition (SIS) and sexual excitation (SES) scales: I. Measuring sexual inhibition and excitation proneness in men. J Sex Res. 2002;39:114–126. doi: 10.1080/00224490209552130. [DOI] [PubMed] [Google Scholar]
- 17.Lee D. Game theory and neural basis of social decision making. Nat Neurosci. 2008;11:404–409. doi: 10.1038/nn2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindner M, Hundhammer T, Ciaramidaro A, Linden DEJ, Mussweiler T. The neural substrates of person comparison. NeuroImage. 2008;40:963–971. doi: 10.1016/j.neuroimage.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Marsh AA, Blair KS, Vythilingam M, Busis S, Blair RJR. Response options and expectations of reward in decision-making: The differential roles of dorsal and rostral anterior cingulate cortex. NeuroImage. 2007;35:979–988. doi: 10.1016/j.neuroimage.2006.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto K, Suzuki W, Tanaka K. Neuronal correlates of goal-based motor selection in the prefrontal cortex. Science. 2003;301:229–232. doi: 10.1126/science.1084204. [DOI] [PubMed] [Google Scholar]
- 21.Miller GF, Todd PM. Mate choice turns cognitive. Trends Cogn Sci. 1998;2:190–198. doi: 10.1016/s1364-6613(98)01169-3. [DOI] [PubMed] [Google Scholar]
- 22.Mosher WD, Chandra A, Jones J. Advance Data from Vital and Health Statistics. Vol. 362. National Center for Health Statistics; Hyattsville, MD: 2005. Sexual behavior and selected health measures: men and women 15-44 years of age, United States, 2002; p. 56. [PubMed] [Google Scholar]
- 23.Mulert C, Seifert C, Leicht G, Kirsch V, Ertl M, Karch S, Moosmann M, Lutz J, Moller H, Hegerl U, Pogarell O, Jager L. Single-trial coupling of EEG and fMRI reveals the involvement of early anterior cingulate cortex activation in effortful decision making. NeuroImage. 2008 doi: 10.1016/j.neuroimage.2008.04.236. [DOI] [PubMed] [Google Scholar]
- 24.Quilodran R, Rothe M, Procyk E. Behavioral shifts and action valuation in the anterior cingulate cortex. Neuron. 2008;57:314–325. doi: 10.1016/j.neuron.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 25.Rupp HA, James TW, Ketterson ED, Sengelaub DR, Janssen E, Heiman JR. Neural Activation in Women in Response to Masculinized Male Faces: Mediation by Hormones and Psychosexual Factors. Evol Hum Behav. doi: 10.1016/j.evolhumbehav.2008.08.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rushworth MFS, Behrens TEJ. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- 27.Rushworth MFS, Behrens TEJ, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci. 2007;11:168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Schultz W. The reward signal of midbrain dopamine neurons. News Physiol Sci. 1999;14:249–255. doi: 10.1152/physiologyonline.1999.14.6.249. [DOI] [PubMed] [Google Scholar]
- 29.Simpson JA, Gangestad SW. Individual differences in sociosexuality: evidence for convergent and discriminant validity. J Pers Soc Psychol. 1991;60:870–883. doi: 10.1037//0022-3514.60.6.870. [DOI] [PubMed] [Google Scholar]
- 30.Thirion B, Pinel P, Meriaux S, Roche A, Dehaene S, Poline J. Analysis of a large fMRI cohort: Statistical and methodological issues for group analyses. NeuroImage. 2007;35:105–120. doi: 10.1016/j.neuroimage.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 31.Winston JS, O’Doherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45:195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, Meyer-Lindenberg A. Know your place: Neural processing of social hierarchies in humans. Neuron. 2008;58:273–283. doi: 10.1016/j.neuron.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]


