Abstract
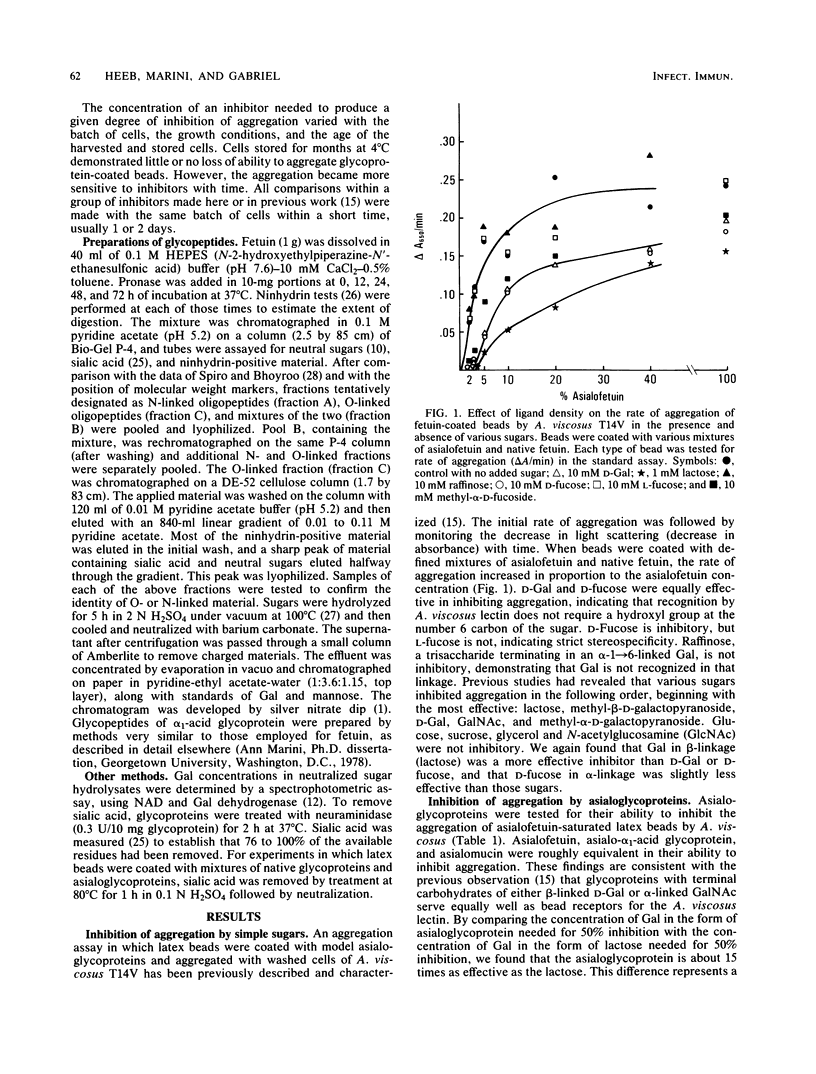
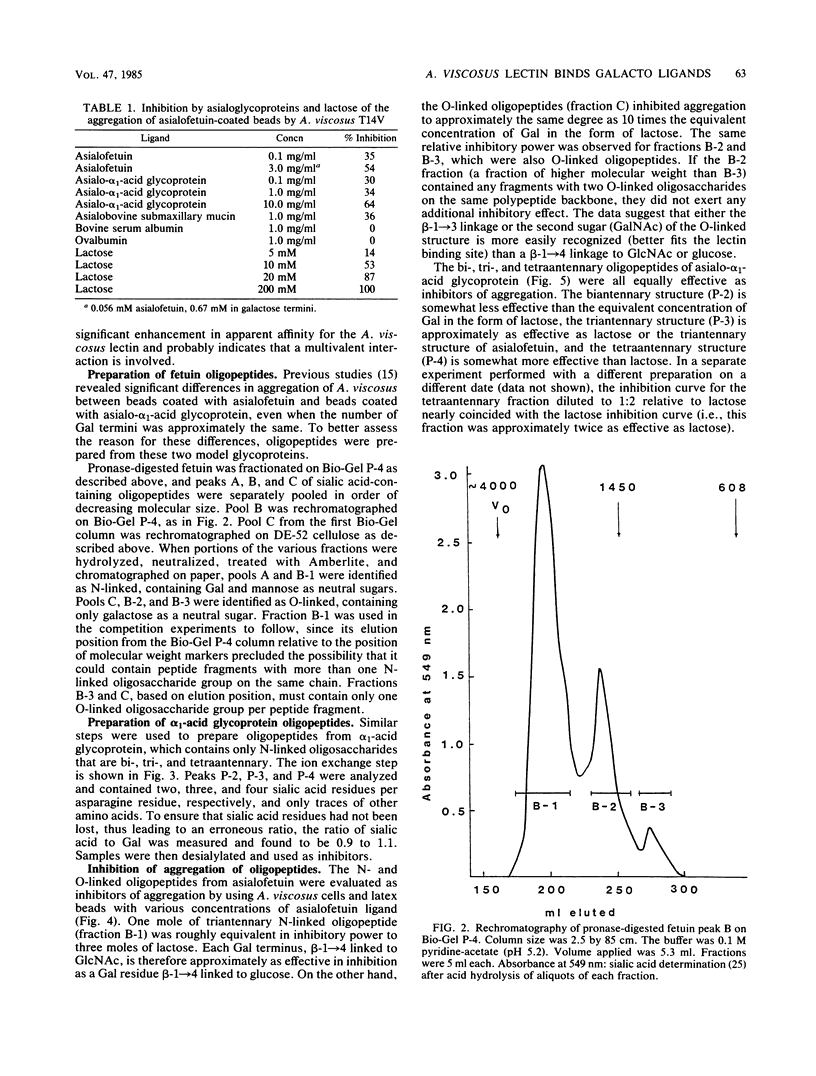
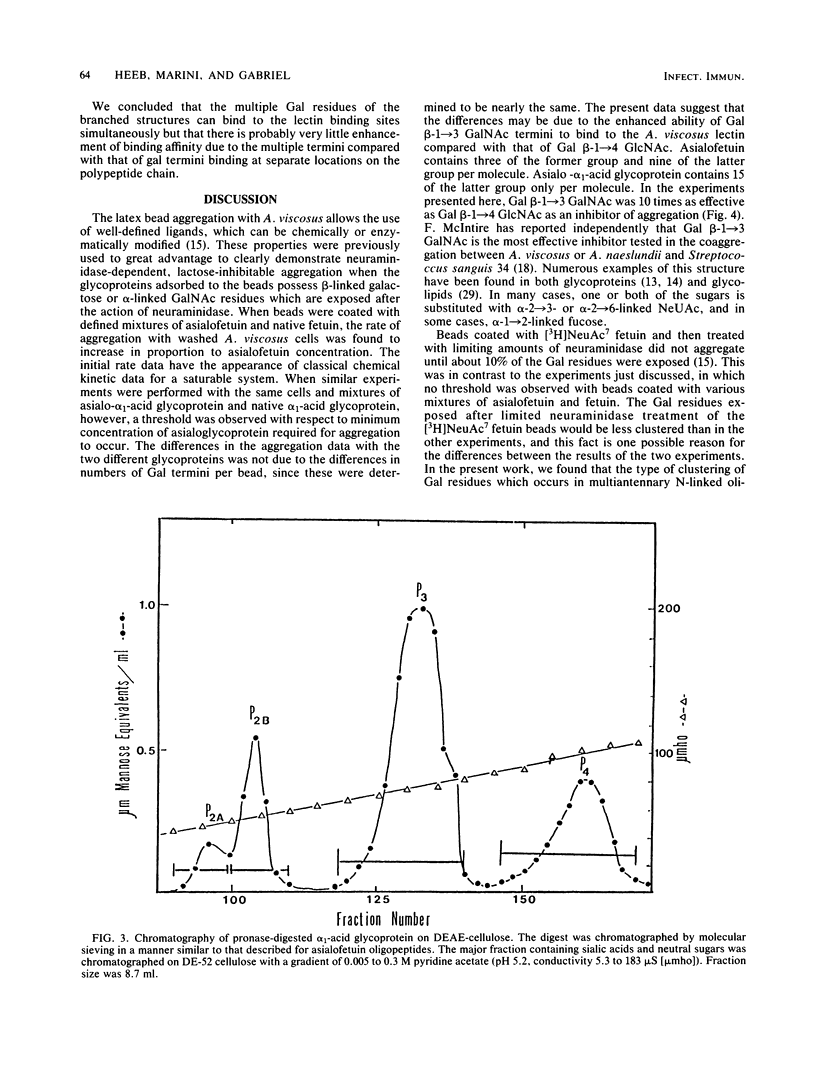
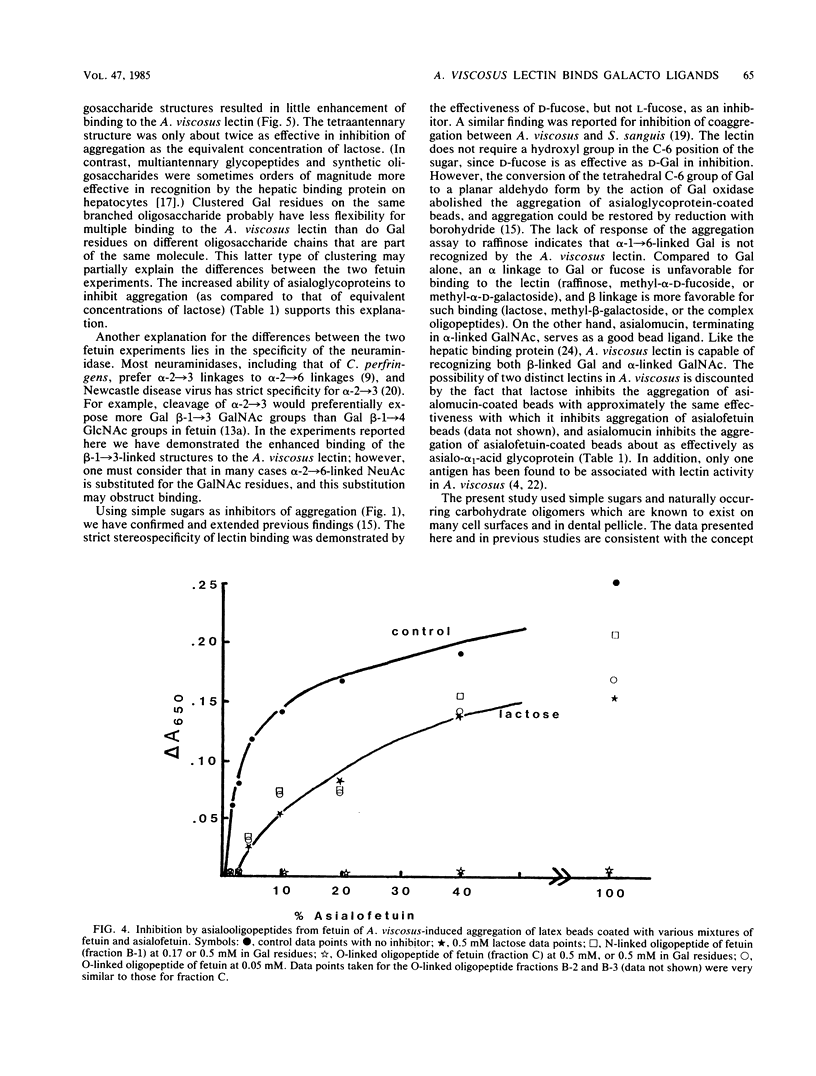
The specificity requirements for the binding of Actinomyces viscosus T14V were examined by testing simple sugars, oligopeptides, and glycoproteins as inhibitors of the aggregation of glycoprotein-coated latex beads and washed A. viscosus cells. Lactose was the most inhibitory simple sugar; D-fucose and D-galactose were equally inhibitory, methyl-alpha-D-fucoside was slightly less inhibitory, and L-fucose and raffinose were not inhibitory. The concentration of galactose residues required for 50% inhibition of aggregation was 15 times higher in the form of lactose than in the form of asialoglycoprotein, suggesting an enhancement of lectin binding when galactose residues are clustered. However, when the inhibitory power of bi-, tri-, and tetraantennary asialooligopeptides of alpha 1-acid glycoprotein was compared with that of equivalent concentrations of galactose in the form of lactose, the biantennary form was slightly less effective than lactose, the triantennary form was approximately as effective as lactose, and the tetraantennary form was slightly more effective than lactose. Steric interference may prevent this type of clustering from enhancing lectin binding. The O-linked asialooligopeptides of asialofetuin were 10 times more inhibitory than an equivalent concentration of galactose in the form of N-linked asialooligopeptides. Thus, galactose beta-1----3 linked to N-acetylgalactosamine exhibits greater specificity for the A. viscosus lectin than does galactose beta-1----4 linked to N-acetylglucosamine. These results, taken together with previously reported data, are consistent with a lectin of low affinity, binding enhanced by multivalency, and specificity for beta-linked galactose.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowden G. H., Hardie J. M. Commensal and pathogenic Actinomyces species in man. Soc Appl Bacteriol Symp Ser. 1973 Jan;2:277–299. [PubMed] [Google Scholar]
- CHAPPELL J. B., GREVILLE G. D. Effect of silver ions on mitochondrial adenosine triphosphatase. Nature. 1954 Nov 13;174(4437):930–931. doi: 10.1038/174930b0. [DOI] [PubMed] [Google Scholar]
- Cisar J. O., Barsumian E. L., Curl S. H., Vatter A. E., Sandberg A. L., Siraganian R. P. Detection and localization of a lectin on Actinomyces viscosus T14V by monoclonal antibodies. J Immunol. 1981 Oct;127(4):1318–1322. [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Webb E. L., Wheeler T. T., Fischlschweiger W., Birdsell D. C., Mansheim B. J. Role of surface fimbriae (fibrils) in the adsorption of Actinomyces species to saliva-treated hydroxyapatite surfaces. Infect Immun. 1981 Sep;33(3):908–917. doi: 10.1128/iai.33.3.908-917.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Wheeler T. T., Cisar J. O. Specific inhibition of adsorption of Actinomyces viscosus T14V to saliva-treated hydroxyapatite by antibody against type 1 fimbriae. Infect Immun. 1984 Feb;43(2):497–501. doi: 10.1128/iai.43.2.497-501.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello A. H., Cisar J. O., Kolenbrander P. E., Gabriel O. Neuraminidase-dependent hamagglutination of human erythrocytes by human strains of Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1979 Nov;26(2):563–572. doi: 10.1128/iai.26.2.563-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzeniek R. Substrate specificity of neuraminidases. Histochem J. 1973 May;5(3):271–290. doi: 10.1007/BF01004994. [DOI] [PubMed] [Google Scholar]
- Ellen R. P., Walker D. L., Chan K. H. Association of long surface appendages with adherence-related functions of the gram-positive species Actinomyces naeslundii. J Bacteriol. 1978 Jun;134(3):1171–1175. doi: 10.1128/jb.134.3.1171-1175.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finne J. Structure of the O-glycosidically linked carbohydrate units of rat brain glycoproteins. Biochim Biophys Acta. 1975 Dec 15;412(2):317–325. doi: 10.1016/0005-2795(75)90046-x. [DOI] [PubMed] [Google Scholar]
- Glöckner W. M., Newman R. A., Dahr W., Uhlenbruck G. Alkali-labile oligosaccharides from glycoproteins of different erythrocyte and milk fat globule membranes. Biochim Biophys Acta. 1976 Sep 7;443(3):402–413. doi: 10.1016/0005-2736(76)90460-0. [DOI] [PubMed] [Google Scholar]
- Heeb M. J., Costello A. H., Gabriel O. Characterization of a galactose-specific lectin from Actinomyces viscosus by a model aggregation system. Infect Immun. 1982 Dec;38(3):993–1002. doi: 10.1128/iai.38.3.993-1002.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan H. V., Hammond B. F. Filamentous bacteria isolated from human root surface caries. Arch Oral Biol. 1972 Sep;17(9):1333–1342. doi: 10.1016/0003-9969(72)90166-5. [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Townsend R. R., Hardy M. R., Lönngren J., Arnarp J., Haraldsson M., Lönn H. Binding of synthetic oligosaccharides to the hepatic Gal/GalNAc lectin. Dependence on fine structural features. J Biol Chem. 1983 Jan 10;258(1):199–202. [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Barlow J. J., Matta K. L. Structural preferences of beta-galactoside-reactive lectins on Actinomyces viscosus T14V and Actinomyces naeslundii WVU45. Infect Immun. 1983 Aug;41(2):848–850. doi: 10.1128/iai.41.2.848-850.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Vatter A. E. Inhibitors of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34: beta-galactosides, related sugars, and anionic amphipathic compounds. Infect Immun. 1982 Apr;36(1):371–378. doi: 10.1128/iai.36.1.371-378.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson J. C., Weinstein J., Dorland L., van Halbeek H., Vliegenthart J. F. Newcastle disease virus contains a linkage-specific glycoprotein sialidase. Application to the localization of sialic acid residues in N-linked oligosaccharides of alpha 1-acid glycoprotein. J Biol Chem. 1982 Nov 10;257(21):12734–12738. [PubMed] [Google Scholar]
- Qureshi J. V., Gibbons R. J. Differences in the adsorptive behavior of human strains of Actinomyces viscosus and Actinomyces naeslundii to saliva-treated hydroxyapatite surfaces. Infect Immun. 1981 Jan;31(1):261–266. doi: 10.1128/iai.31.1.261-266.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revis G. J., Vatter A. E., Crowle A. J., Cisar J. O. Antibodies against the Ag2 fimbriae of Actinomyces viscosus T14V inhibit lactose-sensitive bacterial adherence. Infect Immun. 1982 Jun;36(3):1217–1222. doi: 10.1128/iai.36.3.1217-1222.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar M., Liao J., Kabat E. A., Tanabe T., Ashwell G. The binding site of rabbit hepatic lectin. J Biol Chem. 1979 May 10;254(9):3170–3174. [PubMed] [Google Scholar]
- Skoza L., Mohos S. Stable thiobarbituric acid chromophore with dimethyl sulphoxide. Application to sialic acid assay in analytical de-O-acetylation. Biochem J. 1976 Dec 1;159(3):457–462. doi: 10.1042/bj1590457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro R. G., Bhoyroo V. D. Structure of the O-glycosidically linked carbohydrate units of fetuin. J Biol Chem. 1974 Sep 25;249(18):5704–5717. [PubMed] [Google Scholar]
- Suzuki A., Ishizuka I., Yamakawa T. Isolation and characterization of a ganglioside containing fucose from boar testis. J Biochem. 1975 Nov;78(5):947–954. doi: 10.1093/oxfordjournals.jbchem.a131001. [DOI] [PubMed] [Google Scholar]


