Abstract
Human plasmacytoid dendritic cells (PDCs) can drive naïve, allogeneic CD4+CD25− T cells to differentiate into CD4+CD25+Foxp3+ regulatory T cells (Tregs). However, the intracellular mechanism(s) underlying PDC-induced Treg generation is unknown. Here we show human PDCs express high levels of indoleamine 2,3-dioxygenase (IDO), an intracellular enzyme that catabolizes tryptophan degradation. Triggering of Toll-like receptor 9 with CpG oligodeoxynucleotides activates PDCs to upregulate surface expression of B7 ligands and HLA-DR antigen, but also significantly increases the expression of IDO, and results in the generation of inducible Tregs from CD4+CD25− T cells with potent suppressor cell function. Blocking IDO activity with a pharmacologic inhibitor 1-methyl-D-tryptophan (1MT) significantly abrogates PDC-driven inducible Treg generation and suppressor cell function. Adding kynurenine (KYN), the immediate downstream metabolite of tryptophan, bypasses the 1MT effect, and restores PDC-driven Treg generation. Our results demonstrate that IDO pathway is essential for PDC-driven Treg generation from CD4+CD25− T cells, and implicates the generation of KYN-pathway metabolites as the critical ediator of this process.
Keywords: Plasmacytoid dendritic cells; Regulatory T cells; Indoleamine 2,3-dioxygenase; Toll-like receptor; CpG oligodeoxynucleotides; Immune tolerance
Introduction
Plasmacytoid dendritic cells (PDCs) are a unique DC subset that plays a critical role in regulating innate and adaptive immune responses (1–3). PDCs sense the microbial pathogen components via Toll-like receptor (TLR) recognition, rapidly produce large amounts of type I interferons (IFN-α/β), and activate diverse cell types such as NK cells, macrophages, and CD11c+ DCs to mount immune responses against microbial infections. In addition to stimulating immune responses, increasing evidence suggests that PDCs may also represent a naturally-occurring regulatory DC subset (1–11). Under certain circumstances PDCs appear to be able to induce the differentiation of murine CD4+CD25+FoxP3+ regulatory T cells (Tregs) that downregulate immune responses (5). In humans, PDCs can prime allogeneic naïve CD8+ T cells to differentiate into CD8+ suppressor T cells (4, 9). Recently, we have shown that human PDCs activated by CpG oligodeoxynucleotide (CpG ODN)-mediated TLR9 ligation can induce the generation of CD4+CD25+FoxP3+ Tregs from CD4+CD25− T cells (11). These Tregs strongly inhibited autologous or allogeneic T cell proliferation in vitro. However, the mechanism underlying PDC-induced human Treg generation remains unknown.
One candidate is the enzyme indoleamine 2,3-dioxygenase (IDO). IDO is the first and rate-limiting step in the degradation of tryptophan (Tryp) to the downstream metabolite kynurenine (KYN) and subsequent metabolites along the KYN pathway (12, 13). In mice, IDO is expressed in certain DC subsets, including PDCs, that have been linked to immunosuppression and tolerance induction (14–20). However, the relationship between IDO expression by CpG-activated PDCs and generation of human Tregs has not been previously investigated. In this study, we show that human PDCs express IDO and significantly increase IDO expression in response to TLR9 ligation. IDO expression is necessary for human PDC-induced Treg generation from naïve CD4+CD25− T cells, and that this effect can be blocked by the addition of its pharmacologic inhibitor 1MT or pharmacologically reproduced by the addition of its downstream metabolite KYN.
Materials And Methods
PDC, B, and T cell isolation
Human PBMC were isolated under IRB-approved protocols from apheresis products of healthy blood donors (Memorial Blood Centers of Minnesota, Minneapolis, MN) by Ficoll-Paque density gradient centrifugation. PDCs were isolated from PBMC using BDCA-4 cell isolation kits and the MACS system, followed by staining and sorting to collect purified Lin−CD11c−CD123+ PDCs, as reported previously (11). CD4+CD45RA+ naïve T cells were isolated from PBMC using CD4 T cell isolation kits followed by positive selection with CD45RA microbeads. The purity of naïve CD4+ T cells was greater than 95% for CD4+CD45RA+ expression and less than 0.5% for CD25+ expression. B cells were isolated from PBMC with CD19 microbeads and the MACS system to greater than 98% purity of CD19+ B cells. All cell isolations kits and microbeads were from Miltenyi Biotec (Bergisch Gladbach, Germany).
Assays for assessing the role of TLR9 signaling and IDO pathway in PDC-induced CD4+ Treg generation
For assessment of CpG effects on PDC surface expression of CD80, CD86, and HLA-DR, sorted PDCs were cultured in RPMI 1640 medium supplemented with 10% human AB serum in the presence of either phosphorothioate-modified type-A CpG ODN 2216: ggGGGACGATCGTCgggggG or type-B CpG ODN 2006: tcgtcgttttgtcgttttgtcgtT, purchased from Integrated DNA Technologies (Coralville, IA), at a final concentration of 1 μg/ml and plated at 2 × 105 PDCs per well in 48-well plates. Flow cytometry of PDCs cultured with either CpG ODNs was performed at 48 h after CpG ODN exposure. To assess the potential effects of CpG ODNs on PDC-induced Treg generation from naïve CD4+ T cells, CD4+CD45RA+ naive T cells were primed with allogeneic PDCs or irradiated B cells (30 Gy) at a 10:1 ratio (e.g., 2 × 106 naive CD4+ T cells plus 2 × 105 PDCs per well in 24-well plates, or 4 × 105 naive CD4+ T cells plus 4 × 104 PDCs per well in 96-well plates) in RPMI 1640 medium supplemented with 10% human AB serum with or without the addition of CpG ODNs at a final concentration of 1 μg/ml. In some experiments, blocking mAbs against CD80, CD86, HLA-DR or the control IgG Ab (R&D Systems, Minneapolis, MN) were added to CpG-PDC-mediated naïve CD4+ T cell priming cultures at a final Ab concentration ranging from 0.1 to 10 μg/ml. After 7 d, primed T cells in cultures were harvested, assessed for their surface phenotype, intracellular Foxp3 expression, and their function in MLR assays. The frequency of CD4+CD25+Foxp3+ Treg cells generated from each priming culture condition was determined by flow cytometry. The absolute number of CD4+CD25+Foxp3+ Treg cells generated from each well of a specific priming culture condition was calculated by multiplying total cell count with the percentage of CD4+CD25+Foxp3+ T cells. To determine the extent to which the IDO pathway was required for CpG facilitation of Treg generation, 1-methyl-D-tryptophan (1MT, Sigma-Aldrich) was added to CpG-PDC-T cell and CpG-B cell-T cell priming cultures at a final concentration of 250 μM. Alternatively or in addition to 1MT, KYN obtained from Sigma-Aldrich was added to the PDC-T cell priming cultures at a final concentration of 50 μM as indicated.
Proliferation and suppression assays
The suppressor cell potency of PDC-primed CD4+ T cells on d 7 of culture was determined by plating the primed T cells at graded doses as responders to irradiated allogeneic PBMC in MLR cultures or as third-party T cells into MLR cultures where freshly purified autologous naïve CD4+ T cells were stimulated with irradiated allogeneic PBMC. In some experiments, CD4+CD25+FoxP3+ and CD4+CD25−FoxP3− T cell populations on d 7 of primed CD4+ T cell cultures were sorted and used in functional assays. In all T cell proliferation assays, plates were incubated at 37°C for 5 d and pulsed with 1 μCi of 3H-thymidine per well for the last 18 h before harvesting. All determinations were carried out in triplicate and 3H-thymidine incorporation (cpm) was determined and presented as mean ±SD.
Flow Cytometry
Fluorescent mAbs against human CD3, CD4, CD11c, CD19, CD25, CD40, CD45, CD45RA, CD45RO, CD80, CD86, CD123, HLA-DR, lineage (Lin) markers, and isotype control Abs were from BD Biosciences (San Diego, CA). PE-conjugated anti-human Foxp3 staining set (PCH101) was from eBiosciences (San Diego, CA) and used per manufacture’s instruction. Mean fluorescence intensity (MFI) and positive cell percentages of stained cells were determined by flow cytometry.
RT-PCR for IDO expression
Total RNA was extracted from 3 × 105 purified PDCs or B cells with or without culturing for 48 h in medium containing ODN 2216 (3 μg/ml), ODN 2216 + 1MT(250 μM), ODN 2006 (3 μg/ml), ODN 2006 + 1MT (250 μM), respectively; or from 3 × 105 PDCs cultured in medium containing either ODN 2216 (3 μg/ml) or ODN 2006 (3 μg/ml) with or without in addition of mAbs against CD80/CD86, HLA-DR, or the control IgG Ab (10 μg/ml). The RNA was reverse transcribted to cDNA, IDO gene expression was analyzed by SYBR® Green PCR Master Mix (invitrogen, CA) using a Perkin Elmer ABI Prism 7700 Sequence Detection System (Applied Biosystems, Piscataway, NJ). β-actin was used as internal control. PCR parameters: 50°C 2 min, 95°C 2 min; 40 cycles: 95°C 15 s, 60°C 30 s. The sequences of real-time PCR primers for IDO: Forward primer: 5′-AGA AGT GGG CTT TGC TCT GC -3′, Reverse primer: 5′-TGG CAA GAC CTT ACG GAC ATC TC-3′. Primers for β-actin: Forward primer: 5′-TAC CTC ATG AAG ATC CTC A -3′, Reverse primer: 5′-TTC GTG GAT GCC ACA GGA C -3′.
Western blots for IDO expression
Protein lysates were prepared from 5 × 105 fresh or cultured PDCs or B cells using RIPA buffer (50mM Tris-HCl pH 7.4, 150mM NaCl, 1mM PMSF, 1mM EDTA, 5 μg/ml Aprotinin, 5 μg/ml Leupeptin, 1% Triton x-100, 1% Sodium deoxycholate, 0.1% SDS). Protein lysate was separated on 4–12% NuPAGE® Novex Bis-Tris Gels (Invitorgen, Carlsbad, CA), and transferred to a nitrocellulose membrane. Western blot was performed with a polyclonal rabbit anti-human IDO antibody (IDO H-110:sc25808) and anti-rabbit IgG -horseradish peroxidase (HRP) from Santa Cruz Biotechnology, Inc., (Santa Cruz, CA), and visualized by enhanced chemiluminescence (Pierce Biotechnology, Inc, Rockford, IL.). Membranes were stripped and re-blotted with an antibody specific for β-actin as a loading control. IDO protein expression levels were quantified using an Alpha Innotech Fluorochem 8000 imaging system (Alpha Innotech Corp. San Leandro, CA).
HPLC measurement of tryptophan and kynurenine
Culture supernatants collected from d 7 CpG-PDC-T cell priming cultures with or without the addition of 1MT were measured for Tryp and KYN by HPLC analysis as reported previously (21). Briefly, supernatants were extracted with HPLC grade methanol, the precipitate was removed by centrifugation, and the supernatant was dried under speed-vacuum at low and medium heat. Samples were resuspended in HPLC grade water, injected onto a C18 column (Luna C-18; 250 × 4.6 mm, 5 μm; Phenomenex, Torrance, CA), and eluted with a linear gradient of acetonitrile in water (0–80% over 20 min). Absorbance was measured at 225 nm wavelength using a System Gold 166 Detector (Beckman Coulter, Fullerton, CA) and compared against standard curves for Tryp and KYN.
Data analysis
Data from experiments are expressed as the mean ± SD. Statistical analysis of the results between groups was performed by student’s t test. Values of p < 0.05 were considered significant.
Results
Inducible Treg generation from CD4+CD25− T cells requires B7 ligand and HLA-DR expression on PDCs
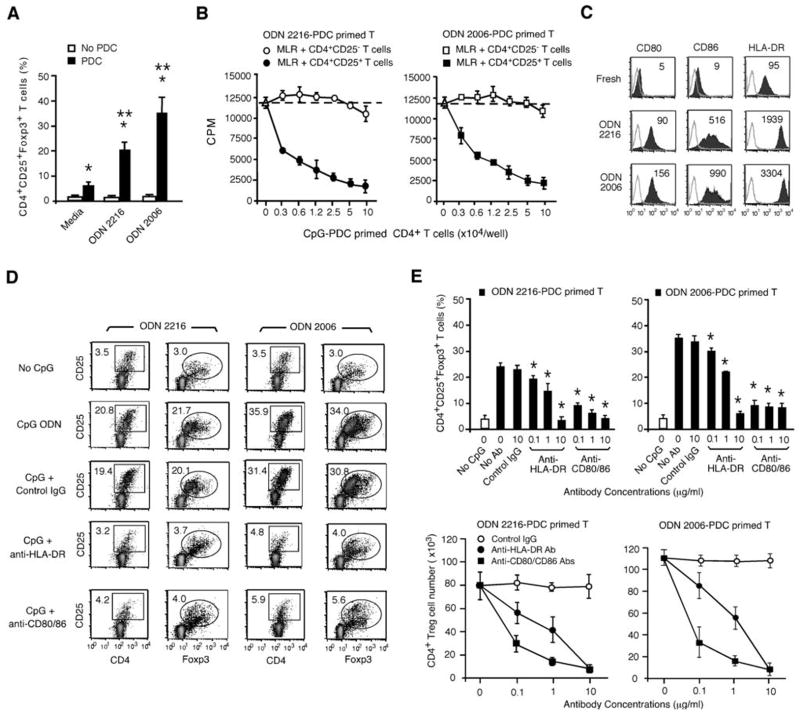
Previously we have shown that TLR9 signaling by CpG ODNs significantly promoted PDC-mediated priming of allogeneic naïve human CD4+CD25− T cells to differentiate into CD4+CD25+Foxp3+ Tregs (11). The addition of either the type-A CpG ODN 2216 or the type-B CpG ODN 2006 to PDC cultures significantly increased the frequency of CD4+CD25+Foxp3+ Tregs from 5.7±3.1% to 21.6±5.2% or 34.2±7.8%, respectively, at d 7 of cultures (Fig. 1A). Noticeably, culturing freshly isolated naïve CD4+CD25− T cells with either CpG ODN in the absence of PDCs did not induce the generation of CD4+CD25+Foxp3+ Tregs (Fig 1A), confirming our previous finding that CpG ODNs alone had no direct effect on the induction of CD4+CD25+Foxp3+ Tregs from CD4+CD25− naïve CD4+ T cells (11). The CD4+CD25+Foxp3+ Tregs induced by CpG-activated PDCs (CpG-PDCs) strongly suppressed naïve CD4+ T cell proliferation to alloantigens in MLR assays. The suppression mediated by CpG-PDC-primed T cells in MLR cultures was CD4+CD25+Foxp3+ T cell dose-dependent (Fig. 1B). Because Treg induction by CpG-PDCs required cell-cell contact (11), studies were performed to determine which cell surface molecules may be required for this effect. Given the dependence of CD28/B7 and TCR signaling on Treg generation, we focused upon the expression of CD80, CD86 molecules and HLA-DR antigens. Freshly isolated human blood PDCs expressed HLA-DR antigens but very low levels of the T-cell costimulatory molecules CD80 and CD86 (Fig. 1C). Triggering of TLR9 on PDCs by ODN 2216 or ODN 2006 rapidly upregulated cell surface expression of CD80, CD86, and HLA-DR antigens and facilitated PDCs survival, in contrast to non-CpG control cultures (Fig. 1C; data not shown). To determine whether the upregulation of these antigens on CpG-PDCs was required for Treg generation, graded concentrations of mAbs against CD80/CD86 or HLA-DR were added into the priming cultures of PDCs and allogeneic naïve CD4+CD25− T cells. Both mAbs against CD80/CD86 or HLA-DR antigens effectively abrogated the capability of CpG-PDCs to induce CD4+CD25+Foxp3+ Tregs, whereas control IgG Ab had no significant effect on CpG-PDC-induced CD4+ Tregs (Fig. 1D & 1E). The blocking effects of anti-CD80/CD86 or anti-HLA-DR mAbs on the frequency and number of CpG-PDC-induced Tregs were mAb dose-dependent with a particularly striking inhibitory effect of low concentrations of anti-B7 ligand mAbs on inducible CD4+ Treg generation (Fig. 1E). These findings demonstrate that PDC-mediated allogeneic CD4+ Treg generation is dependent upon CD4+ T cell signals delivered by both HLA-DR antigens and B7 ligands.
Figure 1. PDC-driven Treg generation from CD4+CD25− T cells is dependent upon HLA-DR and B7 ligand (CD80, CD86) ligation.
(A) Purified naïve CD4+ T cells were cultured in CM with or without freshly isolated allogeneic PDCs in the presence or absence of CpG ODN. CD4+CD25+Foxp3+ Tregs generated in CD4+ T cell cultures with or without PDCs and/or CpG ODN were determined at d 7. The data presented are aggregate results from five experiments from individual donors and are expressed as the mean ±SD. *, p < 0.01, compared PDC with no PDC cultures. **, p < 0.01, compared PDC with CpG-PDC cultures. (B) CD4+CD25+FoxP3+ and CD4+CD25−FoxP3− T cells isolated from CpG-PDC-CD4+ T cell priming cultures (donor A naive CD4+ T cells vs. donor B PDCs) were plated at graded doses into MLR cultures where freshly purified autologous (donor A) naïve CD4+ T cells were stimulated with irradiated allogeneic PBMCs from donor B. T cell proliferation was assessed in a 5-day MLR assays and measured by 3H-Thymidine incorporation. (C) Surface expression of CD80, CD86, HLA-DR on PDCs before or after CpG stimulation for 48 h was assessed by flow cytometry using specific fluorescent (filled) or isotype control mAbs (unfilled). MFI is indicated. (D) Anti-CD80/CD86, HLA-DR, and control IgG mAbs (10 μg/ml) were added to PDC-naïve CD4+ T cell priming cultures in the presence of CpG ODN. The percentages of CD4+CD25+Foxp3+ Tregs generated in cultures were determined at d 7. The data shown in B, C, and D are representative results from one of three independent and reproducible experiments. (E) Anti-CD80/CD86 or HLA-DR Abs (0.1–10 μg/ml), and control IgG Ab (10 μg/ml) were added to PDCs and naïve CD4+ T cell priming cultures. At d 7, the frequencies of CD4+CD25+Foxp3+ Tregs generated in the priming cultures were identified by flow cytometry (upper panels). The absolute number of CD4+CD25+Foxp3+ Tregs generated per well from the priming cultures in 96-well plates was determined and analyzed (lower panels). The data presented are aggregate results from three experiments and are expressed as the mean ±SD. *, p < 0.01, compared the frequency of CD4+CD25+Foxp3+ Tregs generated in priming cultures with or without the addition of blocking antibody.
Human PDCs express IDO and upregulate IDO in response to TLR9 ligation
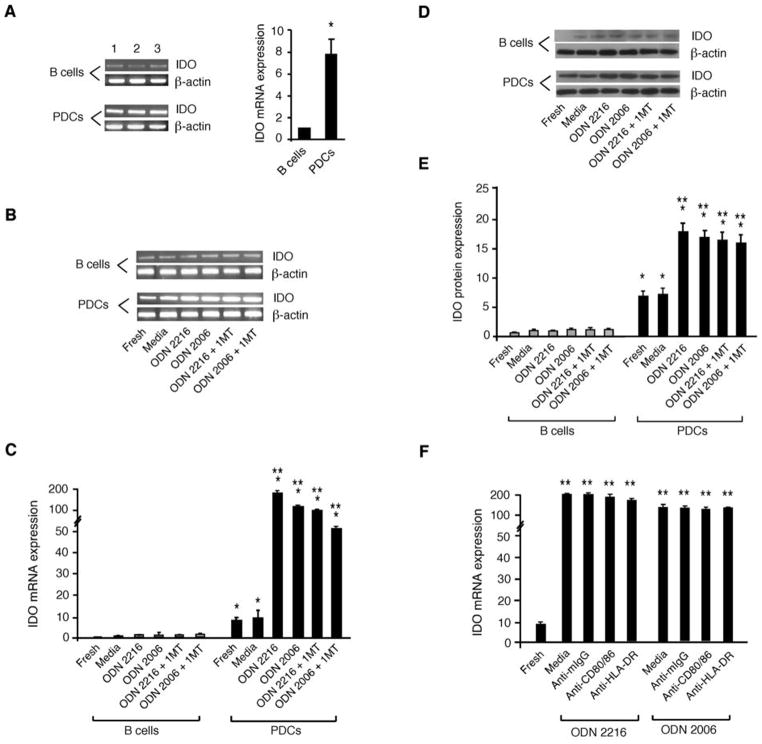
The IDO pathway has been implicated as mechanism for tolerance and immunosuppression (12, 13). TLR9 ligation of murine PDCs has been shown to induce IDO expression (16) and IDO expressing DCs induce Treg generation (22, 23). However, it was not known whether CpG-activated human PDCs expressed IDO mRNA or protein or used the IDO enzymatic pathway for generating Tregs. To determine whether human PDCs expressed and upregulated IDO in response to TLR9 ligation, RNA was prepared from fresh or cultured PDCs and RT-PCR was performed to quantify IDO expression before and after exposure to CpG ODNs. Our data show that freshly isolated human PDCs expressed IDO mRNA and protein (Fig 2). The IDO mRNA expression level in fresh human PDCs is about 7.7±1.4 fold higher than freshly isolated human B cells (Fig. 2A). TLR9 signaling with ODN 2216 or ODN 2006 not only activated PDCs and sustained the survival of PDCs in culture but also significantly increased their IDO mRNA expression during culture, compared with uncultured PDCs or PDCs cultured in media alone (Fig. 2B & 2C). Although the majority of PDCs cultured in media alone became apoptotic and do not survive long-term, the small number of PDCs capable of surviving in the media only group had a comparable level to the uncultured PDCs. In contrast, both fresh and CpG-stimulated human B cells expressed barely detectable levels of IDO mRNA (Fig. 2B & 2C). To determine whether IDO protein was expressed in CpG-stimulated PDCs or B cells, Western blots were performed using a human IDO-specific antibody and the IDO protein expression levels in both fresh and cultured PDCs and B cells were determined and quantified. The data showed the lack of IDO protein expression in fresh B cells and barely detectable levels of IDO protein in CpG-stimulated human B cells (Fig. 2D & 2E). These Western blots data confirmed that freshly isolated human PDCs expressed readily detectable levels of IDO protein at levels much higher than B cells (Fig. 2D & 2E). TLR9 signaling by CpG ligation significantly increased IDO protein expression in PDCs to a far greater extent than in B cells (Fig. 2E). These findings demonstrate that human PDCs express IDO and upregulate IDO in response to TLR9 ligation. To determine whether the blockade of B7 and HLA-DR with specific Abs in CpG-PDC-cultures would reduce IDO expression in CpG-activated PDCs, we examined if anti-CD80/86 mAbs, anti-HLA-DR mAb, or control IgG added to CpG-PDC cultures affected IDO expression in CpG-PDCs. The results confirmed that blockade of either B7 ligand binding or HLA-DR did not cause down-regulation of IDO mRNA expression in CpG-activated PDCs (Fig. 2F).
Figure 2. PDCs expression of IDO is upregulated by TLR9 signaling and unaffected by 1MT exposure or mAbs against either B7 ligand or HLA-DR.
(A) Real-time RT-PCR data of IDO mRNA expression in freshly purified PDCs and B cells from three representative donors are shown in the left panel and are densitometrically assessed and presented as mean ± SD for statistic analysis in the adjacent diagram of the right panel. *, p < 0.01; compared the levels of IDO mRNA relative expression in PDCs with B cells. (B) RT-PCR results of IDO expression and loading control β-actin in fresh or cultured PDCs and B cells with or without CpG ODN, or CpG ODN + 1MT treatment for 48 h. Data shown are representative results from one of three individual donors. (C) Real-time RT-PCR results of IDO mRNA expression in purified PDCs and B cells were compared with that of cultured PDCs and B cells with or without CpG ODN, or CpG ODN + 1MT treatment for 48 h. Data were normalized to β-actin and expressed as signal densitometry per 2 ×105 cells. (D) IDO and β-actin protein expression in fresh or cultured PDCs and B cells with or without CpG ODN, or CpG ODN + 1MT for 48 h was determined by Western blot. Data shown are representative results from one of three individual donors. (E) IDO and β-actin protein expression in fresh or cultured PDCs and B cells with or without CpG ODN, or CpG ODN + 1MT for 48 h was determined by Western blot and quantified using an Alpha Innotech Fluorochem 8000 imaging system. Data were normalized to β-actin and expressed as signal densitometry per 5 ×105 cells. The data in C and E are aggregate results from 3 experiments and are presented as mean ± SD. *, p < 0.01; compared the levels of IDO mRNA or protein expression in PDCs with B cells. **, p < 0.01; compared the levels of IDO mRNA or protein expression in CpG-stimulated PDCs with fresh PDCs. (F) Real-time RT-PCR results of IDO mRNA expression in fresh or cultured CpG-stimulated PDCs with or without the addition of anti-CD80/CD86 Abs, anti-HLA-DR Ab, or control IgG Ab (10 μg/ml) for 48 h. Data shown are accumulative results from two experiments and are presented as mean ± SD. **, p < 0.01; compared the levels of IDO mRNA expression in CpG-stimulated PDCs in the presence or absence of blocking or control Abs with fresh PDCs.
PDCs employ the IDO pathway to drive Tregs generation from CD4+CD25− T cells
We have previously shown that naïve CD4+ T cells primed in cultures containing CpG-activated PDCs acquire characteristics of Tregs, being hyporesponsive to secondary alloantigen stimulation and strongly inhibiting the proliferation of autologous or allogeneic CD4+ T cells in MLR cultures (11). To test the hypothesis that IDO was mechanistically involved in PDC-mediated Treg generation, 1MT, a pharmacologic inhibitor of IDO enzymatic activity, was added to the priming cultures containing CpG ODN, PDCs and naïve allogeneic CD4+CD25− T cells. Although RT-PCR results suggested a small difference in IDO mRNA expression levels between CpG vs. CpG/1MT-treated PDCs (Fig. 2C), there was no significant difference of IDO protein expression between the CpG vs. CpG/1MT-treated PDCs by Western blot (Fig. 2E). The addition of 1MT to CpG-stimulated B cell cultures had no detectable effect on their IDO expression at either the mRNA or protein level (Fig. 2B–2E).
Although the addition of 1MT did not inhibit the upregulation of B7 ligands and HLA-DR by either type-A or type-B CpG ODN-activated PDCs (Fig. 3A), 1MT added to CpG-PDC- T cell priming cultures significantly reduced the generation of CD4+CD25+Foxp3+ cells by CpG-PDCs (Fig. 3B). Consistent with the lower frequency of inducible Tregs generated from CD4+CD25− T cells, 1MT also reduced the suppressor cell functional activity of T cells generated at the end of the CpG-PDC supported T cell priming culture, the latter assessed by the capacity of primed T cells to suppress autologous CD4+CD25− T cell proliferation in a primary MLR culture (Fig. 3C). When 1MT was added to CpG-PDC-T cell priming cultures, primed CD4+ T cells became alloreactive rather than suppressive (Fig. 4A). The potency of primed T cell-mediated suppression of autologous naïve CD4+ T cell proliferation was assessed by adding in graded numbers of CpG-PDC primed T cells to the MLR culture. As shown in Fig 4B, CpG-PDC primed T cells were potent suppressors such that a 50% inhibition of primary MLR proliferation was seen when adding primed T cells at 0.6 × 104 cells/well, a ratio of 1:333 to autologous CD4+ T cell responders in MLR culture, a ratio that substantially exceeded the typical ratio needed for non-induced Tregs to suppress MLR responses. The presence of 1MT in the priming culture markedly reduced the development of Tregs and the suppressor activity, measured as the inability of PDC-primed T cells to inhibit proliferation of autologous naïve CD4+ T cells in MLR cultures (Fig 4B).
Figure 3. IDO plays an important role in CpG-activated PDC-driven Treg generation from CD4+CD25− T cells.
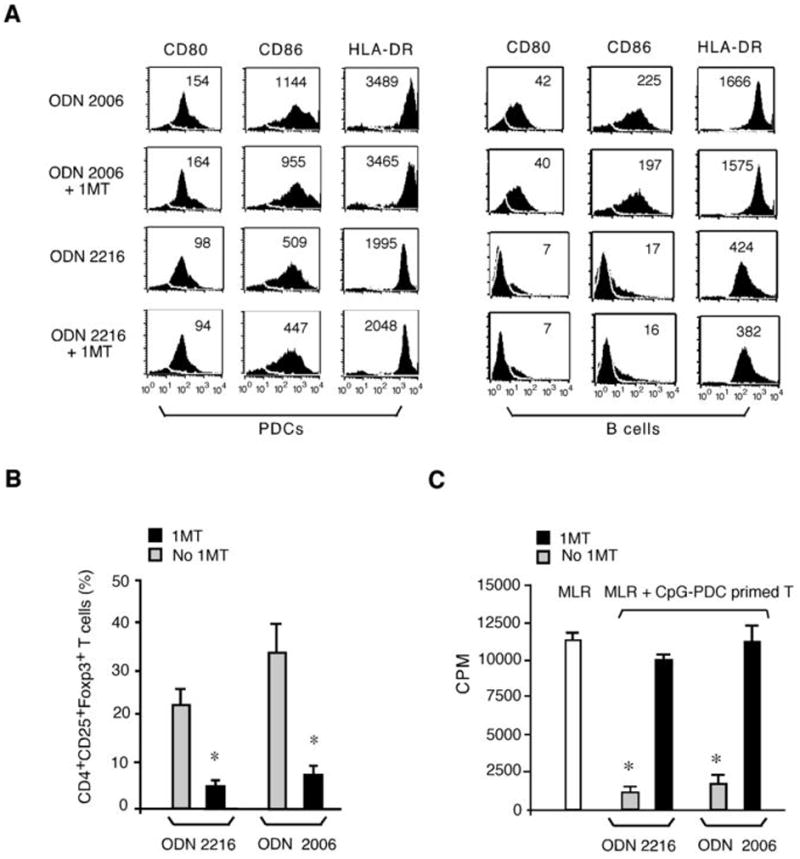
(A) Surface expression of CD80, CD86, HLA-DR on PDCs (left panels) or B cells (right panels) cultured with or without CpG ODN ± 1MT for 48 h. The data shown are from one representative experiment with indicated MFIs. (B) The percentages of CD4+CD25+Foxp3+ Tregs generated in CpG-PDC and naïve CD4+ T cell priming cultures with or without 1MT were determined at d 7. The data shown are aggregated results from three experiments of different donors and are expressed as the mean ±SD. *, p < 0.01; compared 1MT with no 1MT cultures. (C) CpG-PDC primed CD4+ T cells from cultures with or without 1MT were plated into an MLR assay where freshly isolated autologous naïve CD4+ T cells were stimulated with irradiated allogeneic PBMC. The data shown are representative results from one of three experiments. The open bar is T cell proliferation in primary MLR culture without the addition of third party T cells. *, p < 0.01; compared the suppressor activity on T cell proliferation in MLR culture by CpG-PDC primed CD4+ T cells derived from 1MT or no 1MT cultures.
Figure 4. 1MT abrogates the generation of functional suppressor cells and hyporesponsiveness of CpG-activated allogeneic PDC-primed CD4+CD25− T cells.
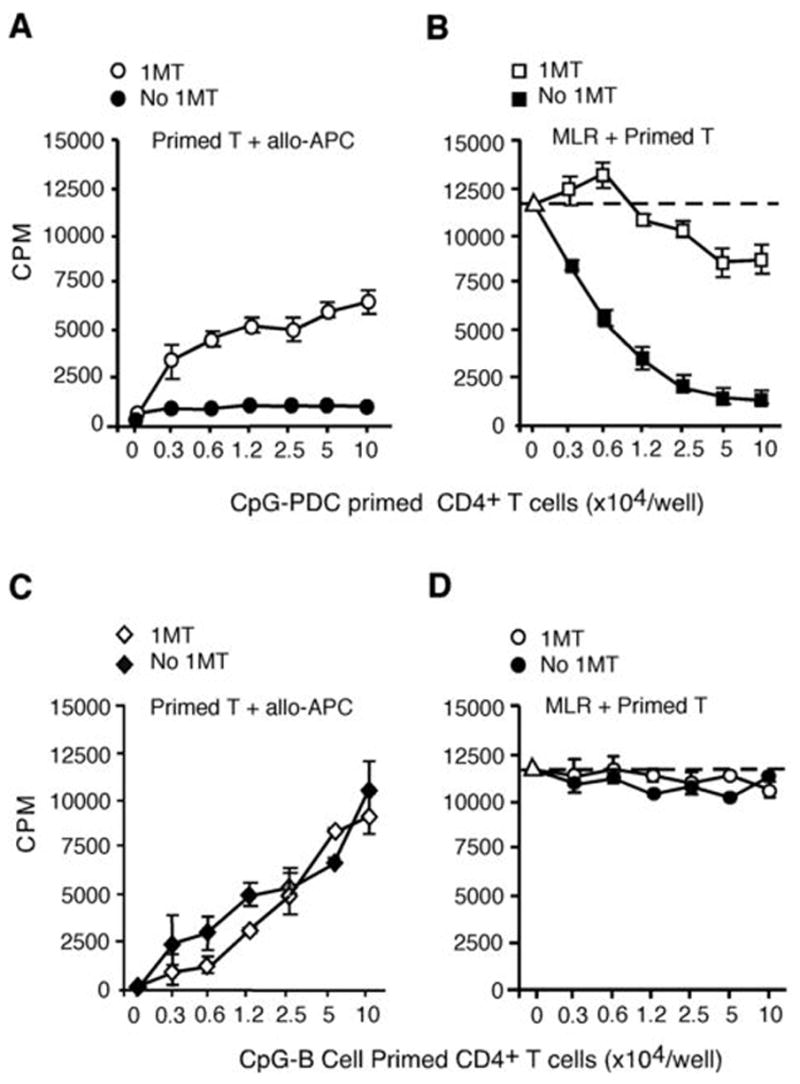
(A) CD4+CD25− T cells primed by ODN 2006-PDCs with or without 1MT were plated at graded doses as responders to irradiated allogeneic PBMC from the priming donor in an MLR assay. (B) ODN 2006-PDC primed CD4+ T cells with or without 1MT were added at graded doses into MLR assays where freshly purified autologous naïve CD4+CD25− T cells were stimulated with irradiated allogeneic PBMC from the priming donor. (C) CD4+CD25− T cells primed with ODN 2006-activated B cells, with or without 1MT present during the priming MLR culture were used as responders in a secondary MLR culture, using irradiated PBMC from the priming donor as stimulators. (D) CD4+CD25− T cells primed with ODN 2006-treated B cells with or without 1MT were plated at graded doses into an MLR assay where freshly purified autologous naïve CD4+ T cells were stimulated with irradiated allogeneic PBMC from the priming donor. Data in A–D are representative of at least separate 5 experiments from 5 different donors.
Because PDCs have the capacity to express IDO, we hypothesized that IDO competent APCs are poised to induce Tregs following the exposure to proinflammatory signals such as TLR9. To determine whether other APC types that can be activated by CpG ODNs to upregulate B7 ligands and HLA-DR are similarly capable of inducing Tregs, freshly isolated B cells were treated with either a type-B CpG ODN 2006, which potently activates human B cells, or a type-A CpG ODN 2216, which does not activate B cells. ODN 2006 but not ODN 2216-activated B cells upregulated cell surface expression of B7 ligands and HLA-DR antigens (Fig. 3A, right panels). Similar to PDCs, the addition of 1MT to ODN 2006-activated B cells did not prevent the upregulation of these cell surface antigens (Fig. 3A, right panels). In contrast to PDCs, primed T cells obtained from ODN 2006-activated B cell priming cultures retained their alloreactivity upon re-stimulation and this occurred independent of the presence of 1MT during the initial T cell priming phase (Fig. 4C). Consistent with the lack of T cell hyporesponsiveness seen in CpG-activated B cell cultures, no demonstrable suppressor was seen when these primed T cells from CpG-activated B cell cultures with or without 1MT exposure were added to a primary MLR culture initiated autologous CD4+CD25− T cell responses to PBMC stimulators obtained from the same donor sources used for allogeneic B cells in the priming culture (Fig. 4D). Thus, whereas Tregs could be generated from CD4+25− T cell cultures containing CpG-activated, IDO-expressing PDCs, Tregs could not be generated from cultures containing CpG-activated, IDO-expressing PDCs and 1MT or CpG-activated B cells, excluding a direct effect of CpG ODNs on CD4+25− T cells in Treg generation.
A downstream Tryp metabolite is sufficient to bypass the need for IDO enzyme activity in the generation of inducible Tregs from CD4+CD25− T cells
IDO degrades the essential amino acid Tryp to KYN, which is then metabolized by other enzymes to subsequent metabolites along the KYN pathway (18, 24–27). We hypothesized that if KYN-pathway metabolites were mechanistically responsible for the generation of Tregs, then adding exogenous KYN to priming MLR cultures should be able to bypass the inhibitory effect of 1MT on IDO enzymatic activity and restore Treg generation in CpG-PDC-T cell priming cultures (Fig. 5A). Analysis of Tryp and KYN levels in culture supernatants from media alone, naïve CD4+ T cells cultured with or without CpG-PDCs showed that significant Tryp depletion (Fig. 5B) and KYN production (Fig. 5C) were detected in CpG-PDC-T cell priming cultures, which were completely blocked by the addition of 1MT. Importantly, the addition of KYN to the CpG-PDC-T cell priming culture indeed bypassed the 1MT blocking effect and restored CD4+CD25+Foxp3+ Treg generation in CpG-activated, allogeneic PDC-driven priming MLR cultures (Fig 5D). As would be anticipated from flow cytometry findings, the presence of CpG-PDCs in the priming culture resulted in T cell hyporesponsiveness to alloantigenic restimuation, reflective of the high proportion of CD4+CD25+ T cells that co-expressed FoxP3 (Fig. 5E). 1MT added to the priming culture reversed this hyporesponsiveness, which could be restored by supplementing 1MT-treated cultures with KYN (Fig 5E). T cells obtained from priming cultures in the absence of 1MT or KYN suppressed naïve, autologous CD4+CD25− T cell responses to irradiated allogeneic stimulators, which was blocked by the addition of 1MT and retored by the presence of KYN in the priming MLR cultures (Fig. 5F). These findings thus demonstrate that the effect of IDO on adaptive Treg generation could be reproduced by exogenous KYN when endogenous IDO was blocked, and implicate KYN-pathway metabolites as the mechanism of IDO-induced Treg generation (Fig. 5G).
Figure 5. Tryptophan metabolites of IDO pathway are critical for CD4+ Treg induction.
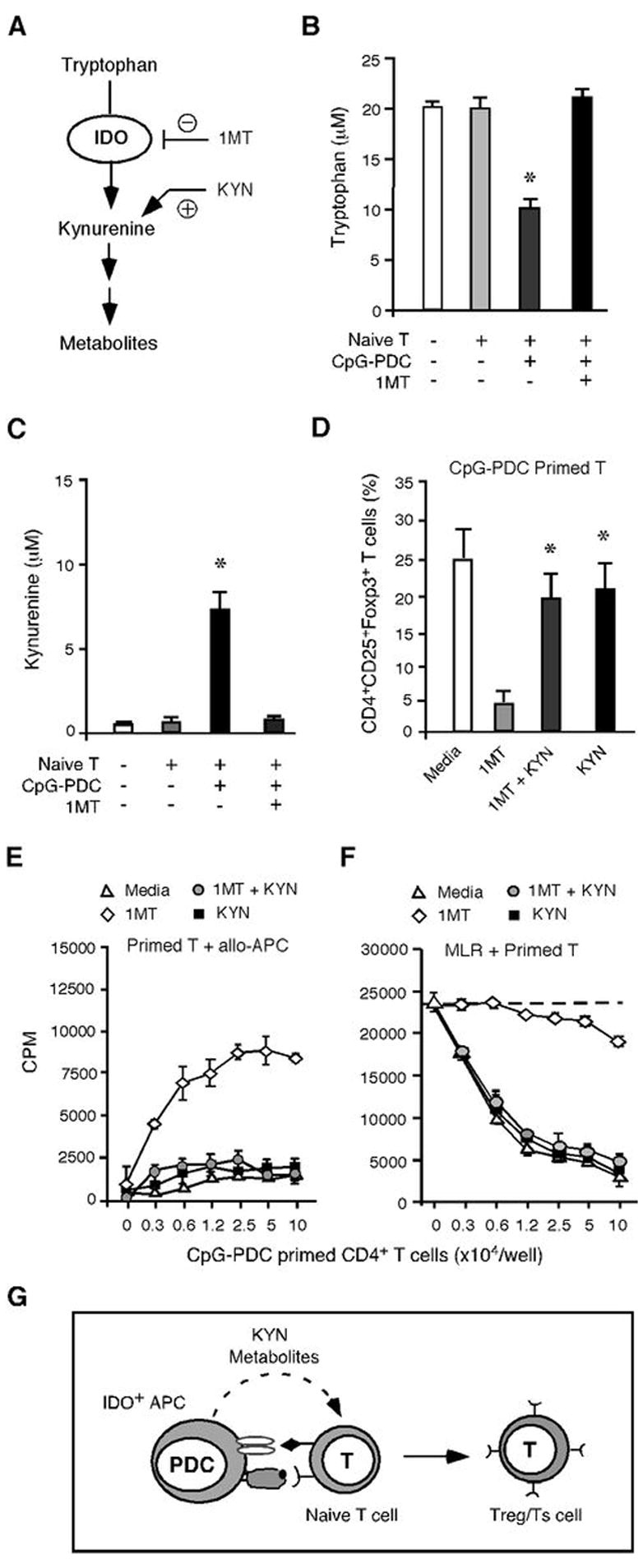
(A) Schematic representation of IDO pathway and Tryp catabolism. (B), (C) Culture supernatants collected at d 7 from media alone, naïve CD4+ T cells cultured with or without ODN 2006-PDCs and 1MT were measured for Tryp and KYN by HPLC analysis. The data shown are results from two experiments of different donors and are expressed as the mean ±SD. *, p < 0.01 (compared CpG-PDC-T cell cultures with or without 1MT). (D) The percentage of CD4+CD25+Foxp3+ Tregs generated in ODN 2216-PDC primed allogeneic naïve CD4+ T cell cultures with or without 1MT and/or KYN was determined at d 7. The data shown are aggregated results from three experiments of different donors and are expressed as the mean ±SD. *, p < 0.01 (compared KYN, KYN/IMT with 1MT cultures). (E) Naïve CD4+CD25− T cells primed with ODN 2216-PDCs with or without 1MT and/or KYN were plated at graded doses as responders to irradiated PBMC from the PDC donor in an MLR assay. (F) Naïve CD4+ T cells primed with ODN 2216-PDCs with or without 1MT and/or KYN were plated into an MLR assays where freshly isolated autologous naïve CD4+ T cells were stimulated with irradiated allogeneic PBMC. (G) Schematic representation of KYN-pathway metabolites as a critical signaling event employed by PDCs to promote Treg generation.
Discussion
This study provides the first evidence that human PDCs express high levels of IDO mRNA and protein in response to TLR9 ligation, and employ the IDO pathway to induce the differentiation of CD4+CD25+Foxp3+ Tregs from CD4+CD25− T cells. Our findings further implicate IDO-mediated production of metabolites in the KYN pathway as the mechanism of Treg generation by PDCs, adding to a growing number of studies indicating that IDO+ APCs, and in particular PDCs of rodent or human origin, are immunosuppressive in vitro and in vivo (12, 13, 17–20, 26, 28–33).
Our previous report indicated that CpG-activated human PDCs could induce the generation of Tregs from naïve CD4+ T cells in an allogeneic MLR culture (11). Our results indicate that the induction of human Tregs from CD4+CD25− T cells required PDCs to express B7 ligands and HLA-DR antigen, which was upregulated by CpG exposure. The upregulation of B7 ligands and HLA-DR on CpG-activated B cells by itself proved insufficient for inducible Treg generation although both were required for CpG-activated PDCs Treg induction. These data suggested a difference in the downstream effect of B7 engagement on PDCs vs. B cells accounted for the induction of Tregs.
Anti-inflammatory cytokines have been linked to Treg induction. Although the induction of CD4+ T cells with both anergic and suppressor cell properties from MLR cultures in rodents and humans has been observed under conditions of high IL-10 and/or TGFβ exposure (34–40). CpG-activated PDCs do not produce high levels of either cytokine (11). In fact, we have reported that CpG ODNs induced proinflammatory cytokines (TNFα, IL-6 and IFNα), rather than anti-inflammatory cytokines (11), consistent with other investigators who have shown that TLR9 ligation of PDCs results in the release of IFNα along with causing immune suppression and increasing Tryp catabolism (11, 16, 41–45). In animal studies, It has been demonstrated that engagement of B7 ligand expressing DCs by a soluble form of CTLA-4 (CTLA-4-Ig) or by CTLA-4 expressed on naturally occurring murine Tregs induced Tryp catabolism in B7 ligand expressing DCs, providing a direct link between Tryp catabolism, DCs and Treg suppression (25, 46). Our data demonstrated the requirement for B7 ligand expression on PDCs for human Treg induction. A recent study demonstrated that human PDCs express high levels of inducible costimulator ligand (ICOS-L), which plays an important role in the ability of PDCs to induce the differentiation of naïve CD4+ T cells to differentiate into IL-10-producing Tregs (47). Therefore, multiple ligands and cytokines preferentially expressed by PDCs are likely contributing to the regulatory function of this unique DC subset.
Importantly, the capacity of PDCs to induce Tregs was not dependent upon an immature maturation stage of CpG-activated PDCs that resulted in tolerance, but rather to some intrinsic property of PDCs that differed from B cells since both APCs were stimulated by CpG 2006 to upregulate B7 ligands and HLA-DR yet only PDCs were capable of inducing Tregs. Our results directly point to IDO as a critical downstream regulator of inducible human Treg generation by CpG-activated PDCs. First, IDO mRNA and protein is induced in CpG-PDCs but not in CpG-B cells. Exposure of naïve CD4+CD25− T-cells to high IDO-expressing CpG-PDCs or CpG-B cells that express little IDO, only CpG-PDCs induced the differentiation of Tregs, implicating IDO in Treg induction. Second, Tregs were not induced in priming culture containing 1MT and Treg generation was restored by adding exogenous KYN in the presence of 1MT. Exogenous KYN can be spontaneously taken up by cells (48) and metabolized to a variety of compounds along the KYN pathway, depending on the pattern of enzymes expressed by the cells (49). Several studies have shown that downstream Tryp metabolites such as KYN appear to have a direct suppressive effect on murine T cell responses, causing inhibition of proliferation and apoptosis of T cells (12, 18, 22, 25, 27, 44, 50–52). Recently, the metabolite 3-hydroxyanthranilic acid and a synthetic structural analog N-(3,4,-Dimethoxycinnamoyl) anthranilic acid (3,4-DAA), has been shown to inhibit inflammatory cytokine production by auto-reactive T cells, and reverse paralysis in a mouse model of experimental autoimmune encephalomyelitis (27). Our data indicate that KYN or its metabolites as participating in the induction of human Tregs by CpG-activated PDCs, extending recent reports indicating that Tryp catabolites can generate Tregs in rodents from CD4+CD25− T cells (22, 52).
We favor the explanation that Tregs were induced from CD4+CD25− cells rather than from CD4+CD25+FoxP3+ cells. CD4+CD25+FoxP3+ cells were present at a frequency of ≤0.5% in the initial culture. For the marked increase in the proportion of these cells at the end of CpG-PDC priming cultures, CD4+CD25+FoxP3+ cells would need to proliferate or survive to a far greater extent than CD4+CD25−FoxP3− cells which were the overwhelmingly dominant population at the time of MLR initiation. Since the inducible Tregs present at the end of priming culture were hyporesponsive to alloantigen stimulation and suppressed naïve autologous CD4+CD25− to irradiated stimulators, a preferential expansion or survival or CD4+CD25+FoxP3+ vs. CD4+CD25− cells in the initial priming MLR culture seems highly unlikely. Consistent with the prior studies (11), our data showed that only the CD4+CD25+ cells isolated from CpG-PDC priming cultures had suppressor cell function, indicating a role for the induction of CD4+CD25+FoxP3+ as mediators of suppressor cell function. Moreover, the induced Tregs could be titered to very low Treg to CD4+CD25− T cell ratios (e.g. 1:333 for 50% inhibition) suggesting that these inducible Tregs may be biologically more potent than is typically achieved with purified CD4+CD25+ Tregs activated and expanded ex vivo. In aggregate, these data support the hypothesis that CpG-PDCs induce Tregs from CD4+CD25− cells.
Our findings clearly demonstrate that CpG-PDC-mediated allogeneic CD4+ Treg generation is dependent upon both the CD4+ T cell activation signals delivered by HLA-DR antigens and B7 ligands, and also the presence of IDO. Both type-A (2216) and type-B (2006) CpG ODN can effectively activate PDCs, up-regulate IDO expression in PDCs, and lead to the enhanced induction of CD4+ Tregs. We do not know of the threshold amounts of IDO functional activity required to induce Tregs. Once these threshold amounts are achieved, further IDO upregulation might not contribute to Treg generation. In these in vitro cultures, Treg generation occurred with both CpG ODN types. Therefore, the higher level of Treg generation seen in the experiments with ODN 2006 may relate to the combined effects of “sufficient” IDO enzyme activity to cause tryptophan depletion and the generation of metabolites along with higher upregulation of B7 ligands and HLA-DR. Responding CD4+CD25− T cells in ODN 2006 cultures may be more effectively driven into a Treg phenotype as might be expected with APCs expressing higher B7 ligand levels, consistent with the findings that B7 ligands are required to optimally support in vivo rodent Treg generation and in vitro human Treg proliferation. A previous study showed that ligation of B7-1/B7-2 (CD80/CD86) on human IDO+ monocyte-derived DCs was required for induction of functional IDO enzymatic activity by IFNγ or by activated T cells (21). In our current manuscript, we showed that the addition of anti-CD80/86 Abs to CpG ODN-stimulated PDCs did not affect IDO upregulation by PDCs. Thus, the induction of IDO by CpG ODN in PDCs does not appear dependent on the B7 pathway. Therefore, the obligatory role of B7-1/B7-2 and HLA-DR in CpG-PDC-induced Tregs generation appears to reflect their requirement for activating the CD4+ T cells in allo-MLR, not in the induction of IDO itself. Significant Tryp depletion and KYN production were detected in the IDO+ DC-mediated T cell priming cultures, which could be fully blocked by the addition of 1MT. Future studies to determine the threshold levels of tryptophan and kynurenine present in the supernatants at various time points of the priming cultures may ultimately lead to define the “optimal” level, timing and duration of tryptophan depletion and kynurenine accumulation that is prerequisite for human Treg induction.
In summary, we have shown that CpG-activated human PDCs upregulate B7 ligands, HLA-DR and IDO enzymatic activity each of which was required to drive the generation of CD4+CD25+FoxP3+ T cells with potent suppressor cell function from CD4+CD25− T cells. In contrast, B cells exposed to CpG express little IDO protein and were not capable of generating Tregs from CD4+CD25− T cells. Whereas 1MT blocked CpG-PDC induced generation of Tregs, the addition of Tryp catabolites could overcome the 1MT effect. Collectively, these data suggest novel strategies for the use of PDCs as a means to induce CD4+ Tregs for tolerance induction, which may offer new opportunities in autoimmunity and transplantation (53–55).
Footnotes
This work was supported by research grants from the Randy Shaver Cancer Research Community Fund and Children’s Cancer Research Fund (to W.C.); National Institutes of Health R01AI34495, 2R37HL56067, P01 AI056299 grants (to B.R.B.) and R01CA103320, R01CA096651, R01CA112431 grants (to D.H.M.).
Abbreviations used in this paper: 1MT, 1-methyl-D-tryptophan, Foxp3, forkhead box p3 transcriptional repressor; KYN, kynurenine; ODN, oligodeoxynucleotides; PDC, plasmacytoid dendritic cell; Treg, regulatory T cell; Tryp, tryptophan.
Publisher's Disclaimer: “This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The Jl). The American Association of Immunologists, Inc. (AAI), publisher of The Jl, holds the copyright to this manuscript. This manuscript has not yet been copyedited or subjected to editorial proofreading by The Jl; hence it may differ from the final version published in The Jl (online and in print). AAI (The Jl) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the United States National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.”
Disclosures
D.H.M has intellectual property interests in the therapeutic use of IDO and IDO inhibitors, and receive consulting income from NewLink Genetics, Inc., which holds a license to develop the technology for clinical trials.
References
- 1.Liu YJ. IPC: Professional Type 1 Interferon-Producing Cells and Plasmacytoid Dendritic Cell Precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 2.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 3.Chen W. The role of plasmacytoid dendritic cells in immunity and tolerance. Curr Opin Organ Transplant. 2005;10:181–185. [Google Scholar]
- 4.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin P, Del Hoyo GM, Anjuere F, Arias CF, Vargas HH, Fernandez LA, Parrillas V, Ardavin C. Characterization of a new subpopulation of mouse CD8alpha+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential. Blood. 2002;100:383–390. doi: 10.1182/blood.v100.2.383. [DOI] [PubMed] [Google Scholar]
- 6.Kuwana M. Induction of anergic and regulatory T cells by plasmacytoid dendritic cells and other dendritic cell subsets. Hum Immunol. 2002;63:1156–1163. doi: 10.1016/s0198-8859(02)00754-1. [DOI] [PubMed] [Google Scholar]
- 7.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 8.Bilsborough J, George TC, Norment A, Viney JL. Mucosal CD8alpha+ DC, with a plasmacytoid phenotype, induce differentiation and support function of T cells with regulatory properties. Immunology. 2003;108:481–492. doi: 10.1046/j.1365-2567.2003.01606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, Curiel T, Lange A, Zou W. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65:5020–5026. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 10.Gilliet M, Liu YJ. Human plasmacytoid-derived dendritic cells and the induction of T-regulatory cells. Hum Immunol. 2002;63:1149–1155. doi: 10.1016/s0198-8859(02)00753-x. [DOI] [PubMed] [Google Scholar]
- 11.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, Blazar BR, Chen W. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 12.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 13.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24:242–248. doi: 10.1016/s1471-4906(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 14.Grohmann U, Fallarino F, Bianchi R, Belladonna ML, Vacca C, Orabona C, Uyttenhove C, Fioretti MC, Puccetti P. IL-6 inhibits the tolerogenic function of CD8 alpha+ dendritic cells expressing indoleamine 2,3-dioxygenase. J Immunol. 2001;167:708–714. doi: 10.4049/jimmunol.167.2.708. [DOI] [PubMed] [Google Scholar]
- 15.Mellor AL, Baban B, Chandler P, Marshall B, Jhaver K, Hansen A, Koni PA, Iwashima M, Munn DH. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J Immunol. 2003;171:1652–1655. doi: 10.4049/jimmunol.171.4.1652. [DOI] [PubMed] [Google Scholar]
- 16.Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol. 2005;175:5601–5605. doi: 10.4049/jimmunol.175.9.5601. [DOI] [PubMed] [Google Scholar]
- 17.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fallarino F, Vacca C, Orabona C, Belladonna ML, Bianchi R, Marshall B, Keskin DB, Mellor AL, Fioretti MC, Grohmann U, Puccetti P. Functional expression of indoleamine 2,3-dioxygenase by murine CD8 alpha(+) dendritic cells. Int Immunol. 2002;14:65–68. doi: 10.1093/intimm/14.1.65. [DOI] [PubMed] [Google Scholar]
- 19.Fallarino F, Asselin-Paturel C, Vacca C, Bianchi R, Gizzi S, Fioretti MC, Trinchieri G, Grohmann U, Puccetti P. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J Immunol. 2004;173:3748–3754. doi: 10.4049/jimmunol.173.6.3748. [DOI] [PubMed] [Google Scholar]
- 20.Fallarino F, Orabona C, Vacca C, Bianchi R, Gizzi S, Asselin-Paturel C, Fioretti MC, Trinchieri G, Grohmann U, Puccetti P. Ligand and cytokine dependence of the immunosuppressive pathway of tryptophan catabolism in plasmacytoid dendritic cells. Int Immunol. 2005;17:1429–1438. doi: 10.1093/intimm/dxh321. [DOI] [PubMed] [Google Scholar]
- 21.Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 22.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 23.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 25.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 26.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 Kinase in T Cells Mediates Proliferative Arrest and Anergy Induction in Response to Indoleamine 2,3-Dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Platten M, Ho PP, Youssef S, Fontoura P, Garren H, Hur EM, Gupta R, Lee LY, Kidd BA, Robinson WH, Sobel RA, Selley ML, Steinman L. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310:850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- 28.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL, Jr, Mellor AL. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 29.Mellor AL, Keskin DB, Johnson T, Chandler P, Munn DH. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J Immunol. 2002;168:3771–3776. doi: 10.4049/jimmunol.168.8.3771. [DOI] [PubMed] [Google Scholar]
- 30.Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596–3599. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 31.Belladonna ML, Grohmann U, Guidetti P, Volpi C, Bianchi R, Fioretti MC, Schwarcz R, Fallarino F, Puccetti P. Kynurenine pathway enzymes in dendritic cells initiate tolerogenesis in the absence of functional IDO. J Immunol. 2006;177:130–137. doi: 10.4049/jimmunol.177.1.130. [DOI] [PubMed] [Google Scholar]
- 32.Tas SW, Vervoordeldonk MJ, Hajji N, Schuitemaker JH, van der Sluijs KF, May MJ, Ghosh S, Kapsenberg ML, Tak PP, de Jong EC. Noncanonical NF-kappaB signaling in dendritic cells is required for indoleamine 2,3-dioxygenase (IDO) induction and immune regulation. Blood. 2007;110:1540–1549. doi: 10.1182/blood-2006-11-056010. [DOI] [PubMed] [Google Scholar]
- 33.Boasso A, Herbeuval JP, Hardy AW, Anderson SA, Dolan MJ, Fuchs D, Shearer GM. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–3359. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeller JC, Panoskaltsis-Mortari A, Murphy WJ, Ruscetti FW, Narula S, Roncarolo MG, Blazar BR. Induction of CD4+ T cell alloantigen-specific hyporesponsiveness by IL-10 and TGF-beta. J Immunol. 1999;163:3684–3691. [PubMed] [Google Scholar]
- 35.Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 37.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25- precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 38.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166:7282–7289. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 39.Zheng SG, Wang JH, Koss MN, Quismorio F, Jr, Gray JD, Horwitz DA. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J Immunol. 2004;172:1531–1539. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- 40.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 41.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeshita F, Leifer CA, Gursel I, Ishii KJ, Takeshita S, Gursel M, Klinman DM. Cutting edge: Role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J Immunol. 2001;167:3555–3558. doi: 10.4049/jimmunol.167.7.3555. [DOI] [PubMed] [Google Scholar]
- 43.Vollmer J, Weeratna R, Payette P, Jurk M, Schetter C, Laucht M, Wader T, Tluk S, Liu M, Davis HL, Krieg AM. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur J Immunol. 2004;34:251–262. doi: 10.1002/eji.200324032. [DOI] [PubMed] [Google Scholar]
- 44.Fallarino F, Puccetti P. Toll-like receptor 9-mediated induction of the immunosuppressive pathway of tryptophan catabolism. Eur J Immunol. 2006;36:8–11. doi: 10.1002/eji.200535667. [DOI] [PubMed] [Google Scholar]
- 45.Romani L, Bistoni F, Perruccio K, Montagnoli C, Gaziano R, Bozza S, Bonifazi P, Bistoni G, Rasi G, Velardi A, Fallarino F, Garaci E, Puccetti P. Thymosin alpha1 activates dendritic cell tryptophan catabolism and establishes a regulatory environment for balance of inflammation and tolerance. Blood. 2006;108:2265–2274. doi: 10.1182/blood-2006-02-004762. [DOI] [PubMed] [Google Scholar]
- 46.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 47.Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ, Gilliet M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moffett JR, Els T, Espey MG, Walter SA, Streit WJ, Namboodiri MA. Quinolinate immunoreactivity in experimental rat brain tumors is present in macrophages but not in astrocytes. Exp Neurol. 1997;144:287–301. doi: 10.1006/exnr.1996.6365. [DOI] [PubMed] [Google Scholar]
- 49.Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Wachter H. Characteristics of interferon induced tryptophan metabolism in human cells in vitro. Biochim Biophys Acta. 1989;1012:140–147. doi: 10.1016/0167-4889(89)90087-6. [DOI] [PubMed] [Google Scholar]
- 50.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Fioretti MC, Puccetti P. Tryptophan catabolism generates autoimmune-preventive regulatory T cells. Transpl Immunol. 2006;17:58–60. doi: 10.1016/j.trim.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 53.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 54.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bluestone JA. Regulatory T-cell therapy: is it ready for the clinic? Nat Rev Immunol. 2005;5:343–349. doi: 10.1038/nri1574. [DOI] [PubMed] [Google Scholar]