Abstract
BACKGROUND
The efficacy of influenza vaccines may decline during years when the circulating viruses have antigenically drifted from those included in the vaccine.
METHODS
We carried out a randomized, double-blind, placebo-controlled trial of inactivated and live attenuated influenza vaccines in healthy adults during the 2004–2005 influenza season and estimated both absolute and relative efficacies.
RESULTS
A total of 1247 persons were vaccinated between October and December 2004. Influenza activity in Michigan began in January 2005 with the circulation of an antigenically drifted type A (H3N2) virus, the A/California/07/2004-like strain, and of type B viruses from two lineages. The absolute efficacy of the inactivated vaccine against both types of virus was 77% (95% confidence interval [CI], 37 to 92) as measured by isolating the virus in cell culture, 75% (95% CI, 42 to 90) as measured by either isolating the virus in cell culture or identifying it through real-time polymerase chain reaction, and 67% (95% CI, 16 to 87) as measured by either isolating the virus or observing a rise in the serum antibody titer. The absolute efficacies of the live attenuated vaccine were 57% (95% CI, −3 to 82), 48% (95% CI, −7 to 74), and 30% (95% CI, −57 to 67), respectively. The difference in efficacy between the two vaccines appeared to be related mainly to reduced protection of the live attenuated vaccine against type B viruses.
CONCLUSIONS
In the 2004–2005 season, in which most circulating viruses were dissimilar to those included in the vaccine, the inactivated vaccine was efficacious in preventing laboratory-confirmed symptomatic illnesses from influenza in healthy adults. The live attenuated vaccine also prevented influenza illnesses but was less efficacious. (ClinicalTrials.gov number, NCT00133523.)
For many years, placebo-controlled trials of the inactivated influenza vaccine used in the military found that it was 70 to 90% efficacious in preventing infection with influenza as identified by a rise in serum antibody titer, as long as the virus strain used in the vaccine resembled the strain in circulation.1 Questions have been raised as to how well the vaccine provides protection against infection when the circulating virus has antigenically drifted and differs to some extent from the strain used in the vaccine. 2 The validity of using serologic confirmation of infection, rather than isolation and identification of the virus, to determine efficacy has also been questioned.3
The live attenuated influenza vaccine has been developed more recently. It has been shown to be efficacious in young children in cases in which virus isolation has been used to confirm that the illness was caused by influenza.4 In both child and adult populations, the vaccine was shown to be protective even during years in which the circulating virus had antigenically drifted from the virus included in the vaccine.5,6 However, the key efficacy study of the trivalent live attenuated vaccine in adults did not include laboratory confirmation of influenza.6
We carried out a clinical trial to determine the efficacy, as measured by laboratory confirmation of influenza, of both the inactivated and live attenuated influenza vaccines in the healthy adult population for whom both vaccines are currently licensed for use. The study was conducted in Michigan during the winter of 2004–2005, when antigenically drifted type A viruses, the A/California/07/2004-like strain of H3N2, were circulating, as were type B viruses of two lineages.
METHODS
STUDY DESIGN AND OBJECTIVES
The study was a randomized, double-blind, placebo-controlled, community-based trial. Our primary objective was to evaluate the absolute efficacies, as compared with placebo, of the inactivated and live attenuated influenza vaccines in preventing laboratory-confirmed symptomatic influenza caused by circulating strains (whether they were antigenically similar or dissimilar to the strains included in the vaccines). Secondary objectives included evaluating the relative efficacy of one vaccine as compared with the other. MedImmune provided the live attenuated vaccine and Sanofi Pasteur provided the antigens used in the serologic tests; these companies had no role in the design, analysis, interpretation, or reporting of the study. The study was designed and carried out by the authors, who also analyzed the data; the authors take full responsibility for the data, the analysis, and the completeness and accuracy of this article.
PARTICIPANT ENROLLMENT, RANDOMIZATION, AND FOLLOW-UP
Eligible participants were healthy men and women 18 to 46 years of age, recruited at four study sites (two university sites and two community sites) in Michigan, who had not yet received an influenza vaccine for the 2004–2005 season. Persons with any health condition for which the inactivated vaccine was recommended, and persons for whom either vaccine was contraindicated, were excluded.7 The study was approved by the institutional review board at the University of Michigan Medical School.
At enrollment, written informed consent was obtained from potential participants, and study eligibility was determined. Preintervention blood specimens were collected from eligible participants, who were then randomly assigned to receive one intervention: the inactivated influenza vaccine or the matching placebo (physiologic saline) by intramuscular injection or the live attenuated influenza vaccine or matching placebo (physiologic saline) by intranasal spray, in ratios of 5:1 and 5:1, respectively. Four site-specific randomization schedules, generated with the use of a random permuted block design with a block size of 12, were used to assign participants sequentially to receive a vaccine or a placebo as they enrolled. Since the trial was double-blind, participants and the nurses who administered the study vaccine or placebo were unaware of whether the participant was receiving vaccine or placebo but were aware of the route of administration.
Participants recorded data on local and systemic reactions to vaccine or placebo on diary cards each day for 7 days after the intervention. They returned to the study sites 3 to 5 weeks after the intervention for collection of the diary cards and for collection of postintervention (preseason) blood specimens.
Influenza surveillance was conducted from November 2004 through April 2005. Participants were contacted twice monthly by e-mail or telephone and were instructed to contact study staff in the event of illness with at least two respiratory or systemic signs or symptoms. Throat-swab specimens were collected for the isolation and identification of influenza virus, and participants were followed for collection of data on illness characteristics. During the period from April through May 2005, participants returned to the study sites for collection of postseason blood specimens.
VACCINES AND PLACEBOS
Both the inactivated trivalent vaccine (Fluzone, Sanofi Pasteur) and the live attenuated trivalent vaccine (FluMist, MedImmune) were licensed for use in the 2004–2005 influenza season. Each 0.5-ml dose of Fluzone was formulated to contain 15 µg of hemagglutinin from each of the following strains: A/New Caledonia/20/99 (H1N1), A/Wyoming/3/2003 (H3N2, A/Fujian/411/2002-like strain), and B/Jiangsu/10/2003 (B/Shanghai/361/2002-like strain [Yamagata lineage]). Each 0.5-ml dose of FluMist was formulated to contain a 106.5–7.5 median tissue-culture infective dose of live attenuated influenza virus reassortants of the following strains: A/New Caledonia/20/99 (H1N1), A/Wyoming/3/2003 (H3N2 A/Fujian/411/2002-like strain), and B/Jilin/20/2003 (B/Shanghai/361/2002-like strain [Yamagata lineage]). Identical syringes were filled on-site with the inactivated vaccine or matching placebo (physiologic saline) by study nurses who were aware of the intervention assignments. The live attenuated influenza vaccine and matching placebo (physiologic saline) were preloaded in identical nasal spray devices by the manufacturer.
EFFICACY MEASUREMENTS
Symptomatic influenza was defined as illness characterized by at least one respiratory symptom (cough or nasal congestion) and at least one systemic symptom (fever or feverishness or chills or body aches).8 To qualify as a case of symptomatic influenza, the illness also must have occurred during the period of surveillance-defined influenza activity and at least 2 weeks after receipt of vaccine or placebo. The primary end point was a case of symptomatic influenza type A or B that was laboratory-confirmed, either by isolation of the influenza virus in cell culture or by a rise by a factor of four or more in the serum antibody titer against a circulating influenza strain on hemagglutination–inhibition testing (serologic determination). Additional end points included illness confirmed through isolation of the virus only, through either isolation of the virus or identification of the virus through real-time polymerase chain reaction (PCR), through real-time PCR only, and through serologic determination only.
LABORATORY ASSAYS
Isolation of influenza in cell culture, type identification (of influenza A or B) using the fluorescence antibody assay, and serologic assays using the hemagglutination-inhibition test were performed in the influenza laboratory at the University of Michigan School of Public Health.9–11 All throat swabs collected during the surveillance period were cultured to identify participants with culture-positive influenza and to define the period of local influenza activity. All isolates were typed according to strain and evaluated for antigenic relatedness to vaccine strains by the Influenza Branch at the Centers for Disease Control and Prevention (CDC). In addition, all throat-swab specimens obtained from participants with symptomatic influenza were tested at the University of Michigan by means of real-time PCR assays using the Taqman system (Applied Biosystems); primers and probes used in this assay were developed by the CDC Influenza Branch and were designed for universal detection of influenza A and B viruses. All collected serum samples were tested with the hemagglutination-inhibition assay, with the virus strains present in the vaccines used as antigens. In addition, serum samples from participants with symptomatic illness were tested against the circulating type A (H3N2) (A/California/07/2004-like) virus and the circulating type B (B/Hawaii/33/2004-like) virus, representing the Victoria lineage not included in the vaccine.
STATISTICAL ANALYSIS
In efficacy analyses, we considered both placebo groups (participants receiving physiologic saline through either injection or intranasal spray) to be equivalent and combined them. Absolute efficacy was estimated by calculating the relative risk of laboratory-confirmed symptomatic influenza in each vaccine group as compared with the placebo group; relative efficacy was estimated by calculating the relative risk of laboratory-confirmed symptomatic influenza in one vaccine group as compared with the other vaccine group. The relative risk was calculated by comparing the cumulative incidence (the observed proportion) of cases in the vaccine group and the cumulative incidence of cases in the placebo group (or in the other vaccine group) and determining the exact confidence intervals. Point estimates of vaccine efficacy were calculated as (1 − the relative risk) × 100. Differences in the proportions of reported postintervention reactions between each vaccine group and the matching placebo group were analyzed with an appropriate chi-square test or, when necessary, Fisher’s exact test. Statistical analyses were conducted with the use of SAS software (release 8.2, SAS Institute) and StatXact software (version 7, Cytel).
The intention-to-treat analysis involved all enrolled participants who were randomly assigned to a vaccine or placebo group and received a vaccine or a placebo; this population was used in the analysis of influenza infection confirmed through isolation of the virus in cell culture or identification of the virus through real-time PCR. Per-protocol analyses were limited to participants who provided all three annual blood specimens according to the timing specified in the protocol — in particular, having the postintervention (pre-season) blood specimen collected at least 3 weeks after receipt of a vaccine or a placebo and at least 2 weeks before the beginning of local influenza activity. This limited population was used in the analysis of influenza cases that were serologically determined.
Assuming absolute vaccine efficacies of 80%, our study was planned to have a statistical power sufficient to estimate efficacy with a two-sided 95% confidence interval (CI) with a positive lower bound. Enrollment was then planned on the basis of the total number of end points required to achieve this power (8 end points in either vaccine group and 15 in the placebo group); given a conservative attack rate for community influenza of 5%, we estimated that we would need to enroll 1800 subjects. A P value of less than 0.05, or a positive lower bound of the 95% CI for vaccine efficacy, was considered to indicate statistical significance.
RESULTS
PARTICIPANTS
Enrollment of subjects began in mid-October 2004 and continued through mid-December 2004. A total of 1253 subjects were eligible, and 1247 participants provided a preintervention blood specimen and then received a vaccine or a placebo (Table 1). The mean age of participants was 26.9 years, and 628 participants (50.4%) reported having received influenza vaccine previously. Participant characteristics were similar across the inactivated vaccine group, the live attenuated vaccine group, and the placebo group.
Table 1.
Baseline Characteristics of the 1247 Study Participants during the 2004–2005 Influenza Season in Michigan.*
| Live Attenuated Vaccine Group | Inactivated Vaccine Group | Placebo Group | Total | |
|---|---|---|---|---|
| Characteristic | (N = 519) | (N = 522) | (N = 206) | (N = 1247) |
| Percentage of participants | 41.6 | 41.9 | 16.5 | 100 |
| Mean age — yr | 26.3±9.0 | 27.2±9.4 | 27.8±9.9 | 26.9±9.3 |
| Age category — no. (%) | ||||
| 18–19 yr | 162 (31.2) | 156 (29.9) | 64 (31.1) | 382 (30.6) |
| 20–24 yr | 151 (29.1) | 129 (24.7) | 49 (23.8) | 329 (26.4) |
| 25–34 yr | 83 (16.0) | 96 (18.4) | 30 (14.6) | 209 (16.8) |
| 35–46 yr | 123 (23.7) | 141 (27.0) | 63 (30.6) | 327 (26.2) |
| Sex — no. (%) | ||||
| Women | 313 (60.3) | 334 (64.0) | 128 (62.1) | 775 (62.1) |
| Men | 206 (39.7) | 188 (36.0) | 78 (37.9) | 472 (37.9) |
| Race or ethnic group — no. (%)† | ||||
| White | 444 (85.5) | 452 (86.6) | 179 (86.9) | 1075 (86.2) |
| Nonwhite | 75 (14.5) | 70 (13.4) | 27 (13.1) | 172 (13.8) |
| Prior receipt of influenza vaccine — no. (%) | 265 (51.1) | 263 (50.4) | 100 (48.5) | 628 (50.4) |
Plus–minus values are means ±SD. Placebo was physiologic saline administered as either an intramuscular injection (103 participants) or an intranasal spray (103 participants). For the purposes of efficacy analyses, both placebos were considered equivalent and were combined.
Race or ethnic group was self-reported. “Nonwhite” included black, Asian, Hispanic, and other or mixed.
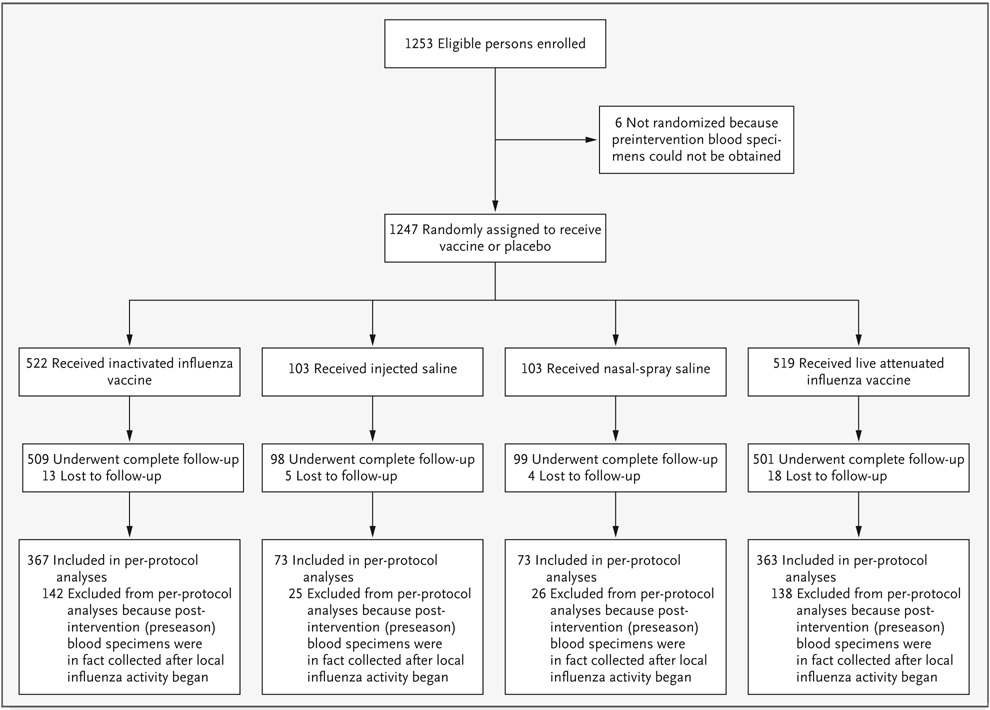
Forty participants (3.2%) did not complete all scheduled visits; loss to follow-up did not differ significantly among the three groups (P = 0.39) (Fig. 1). A total of 331 additional participants were excluded from per-protocol analyses because their postintervention (preseason) blood specimens were in fact collected after local influenza activity began. As a result, 876 (70.2%) participants were included in per-protocol analyses; the distribution of these participants was similar among the three groups (P = 0.97).
Figure 1.
Enrollment and Follow-up of Study Participants during the 2004–2005 Influenza Season in Michigan.
REPORTED REACTOGENICITY
Among the local and systemic reactions reported on diary cards, only arm soreness was significantly more likely to be reported by recipients of inactivated vaccine than by recipients of the matching placebo (Table 2). Runny nose or congestion, cough, headache, and muscle aches were all significantly more likely to be reported by recipients of live attenuated vaccine than by recipients of the matching placebo. The reporting of symptoms as moderate or severe, although common, did not result in any participant’s withdrawal from the study.
Table 2.
Local and Systemic Reactions to Vaccine or Placebo Occurring within 7 Days, as Reported by 1205 Participants (96.6%).*
| Reported Reaction | Inactivated Vaccine (N = 501) | Placebo (Intramuscular Injection) (N = 99) | Difference in Risk | P Value | Live Attenuated Vaccine (N = 506) | Placebo (Intranasal Spray) (N = 99) | Difference in Risk | P Value |
|---|---|---|---|---|---|---|---|---|
| no. of participants (%) | % | no. of participants (%) | % | |||||
| Fever | 37 (7.4) | 5 (5.1) | 2.3 | 0.41 | 32 (6.3) | 7 (7.1) | −0.8 | 0.78 |
| Chills | 40 (8.0) | 5 (5.1) | 2.9 | 0.31 | 27 (5.3) | 5 (5.1) | 0.2 | 0.91 |
| Runny nose or congestion | 150 (29.9) | 20 (20.2) | 9.7 | 0.05 | 247 (48.8) | 30 (30.3) | 18.5 | 0.001 |
| Cough | 80 (16.0) | 16 (16.2) | −0.2 | 0.96 | 92 (18.2) | 8 (8.1) | 10.1 | 0.01 |
| Sore throat | 93 (18.6) | 12 (12.1) | 6.5 | 0.12 | 127 (25.1) | 16 (16.2) | 8.9 | 0.06 |
| Headache | 171 (34.1) | 25 (25.3) | 8.8 | 0.09 | 192 (37.9) | 25 (25.3) | 12.6 | 0.02 |
| Muscle aches | 74 (14.8) | 13 (13.1) | 1.7 | 0.67 | 67 (13.2) | 5 (5.1) | 8.1 | 0.02 |
| Weakness | 102 (20.4) | 14 (14.1) | 6.3 | 0.15 | 117 (23.1) | 17 (17.2) | 5.9 | 0.19 |
| Abdominal pain | 28 (5.6) | 3 (3.0) | 2.6 | 0.29 | 20 (4.0) | 2 (2.0) | 2.0 | 0.56 |
| Trouble breathing | 10 (2.0) | 2 (2.0) | 0.0 | 1.00 | 17 (3.4) | 1 (1.0) | 2.4 | 0.33 |
| Red eyes | 15 (3.0) | 1 (1.0) | 2.0 | 0.49 | 10 (2.0) | 2 (2.0) | 0.0 | 1.00 |
| Arm soreness | 270 (53.9) | 20 (20.2) | 33.7 | <0.001 | 15 (3.0) | 4 (4.0) | −1.0 | 0.53 |
| Arm redness | 29 (5.8) | 2 (2.0) | 3.8 | 0.12 | 6 (1.2) | 1 (1.0) | 0.2 | 1.00 |
| Other* | 32 (6.4) | 6 (6.1) | 0.3 | 0.90 | 39 (7.7) | 6 (6.1) | 1.6 | 0.57 |
Other reported problems that occurred in at least 5 participants were nausea (14 participants), sneezing (8 participants), diarrhea (7 participants), dizziness (7 participants), and nosebleed (6 participants); none were significantly more likely to be reported by participants who received a vaccine as compared with those who received the matching placebo.
SERIOUS ADVERSE EVENTS
Four serious adverse events occurred among participants within 30 days of receipt of vaccine or placebo. Only one — hospitalization for acute pericarditis with moderate effusion after receipt of the live attenuated vaccine — was considered to be possibly related to the study intervention. Comprehensive study of the serum samples collected immediately before administration of the vaccine and 4 weeks later did not indicate an infectious cause of the pericarditis; the participant recovered completely. The other three serious adverse events — participants hospitalized for mononucleosis, for exacerbated hypertension and cardiomyopathy, and for injuries resulting from a car accident — were considered to be unrelated to the study intervention. Additional information on all serious adverse events occurring during followup is presented in Table 3.
Table 3.
Serious Adverse Events Reported by Participants within Approximately 6 Months after Receipt of a Vaccine or a Placebo.*
| Reported Serious Adverse Event | Intervention | No. of Days from Receipt of Vaccine or Placebo to Onset of Event | Relation of Event to Intervention |
|---|---|---|---|
| Acute pericarditis | Live attenuated vaccine | 17 | Possibly associated |
| Injuries from car accident | Live attenuated vaccine | 9 | Definitely not associated |
| Mononucleosis | Inactivated vaccine | 14 | Probably not associated |
| Exacerbated hypertension and cardiomyopathy | Inactivated vaccine | 17 | Probably not associated |
| Repair of anterior cruciate ligament | Placebo | 33 | Definitely not associated |
| Uterine myomectomy | Inactivated vaccine | 74 | Definitely not associated |
| Repair of three partially amputated fingers | Inactivated vaccine | 89 | Definitely not associated |
| Ovarian cyst | Inactivated vaccine | 90 | Definitely not associated |
| Orchiectomy for testicular cancer | Inactivated vaccine | 91 | Definitely not associated |
| Rotator cuff repair | Live attenuated vaccine | 161 | Definitely not associated |
Placebo was physiologic saline administered as either an intramuscular injection or intranasal spray. The relation of the event to the intervention was determined by the independent safety monitor for this trial.
IMMUNE RESPONSE TO VACCINE
Hemagglutination-inhibition assays showed that the serum antibody titer for the influenza A H3 component of the vaccines increased by a factor of four or more from preintervention levels in 110 recipients of live attenuated vaccine (21.2%) and 348 recipients of inactivated vaccine (66.7%) (P<0.001), as did the serum antibody titer for the influenza B component of the vaccines in 70 recipients of live attenuated vaccine (13.5%) and 445 recipients of inactivated vaccine (85.2%) (P<0.001) and the serum antibody titer for the influenza A H1 component of the vaccines in 44 recipients of live attenuated vaccine (8.5%) and 367 recipients of inactivated vaccine (70.3%) (P<0.001).
LABORATORY-CONFIRMED INFLUENZA
Thirty-two participants (2.6%) had culture-confirmed influenza (Table 4), including 14 infected with influenza A (H3N2) and 18 infected with influenza B. All influenza A (H3N2) isolates were A/California/07/2004-like; this strain was nationally predominant during the 2004–2005 season and was considered to be antigenically drifted from the H3N2 component present in the vaccines. 12,13 Influenza B isolates represented the two influenza B lineages that circulated nationally during that season; 7 of the infected participants had an isolate identified as B/Shanghai/361/2002-like (Yamagata lineage), and 11 of those infected had an isolate identified as B/Hawaii/33/2004-like (Victoria lineage).12,14
Table 4.
Estimated Absolute and Relative Efficacies of the Inactivated Influenza Vaccine and the Live Attenuated Influenza Vaccine during the 2004–2005 Influenza Season in Michigan.*
| Laboratory-Confirmed Symptomatic Influenza | Cumulative Incidence of Influenza | Relative Risk (95% CI) | Percent Relative Reduction (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Inactivated Vaccine (N = 522) | Live Attenuated Vaccine (N = 519) | Placebo (N = 206) | Inactivated Vaccine vs. Placebo | Live Attenuated Vaccine vs. Placebo | Inactivated vs. Live Attenuated Vaccine | Inactivated Vaccine vs. Placebo | Live Attenuated Vaccine vs. Placebo | Inactivated vs. Live Attenuated Vaccine | |
| no. of participants (%) | |||||||||
| Culture positive | 7 (1.3) | 13 (2.5) | 12 (5.8) | 0.23 (0.08 to 0.63) | 0.43 (0.18 to 1.03) | 0.54 (0.18 to 1.44) | 77 (37 to 92) | 57 (−3 to 82) | 46 (−44 to 82) |
| Real-time PCR positive | 10 (1.9) | 18 (3.5) | 15 (7.3) | 0.26 (0.11 to 0.63) | 0.48 (0.23 to 1.02) | 0.55 (0.23 to 1.26) | 74 (37 to 89) | 52 (−2 to 77) | 45 (−26 to 77) |
| Culture or real-time PCR positive | 10 (1.9) | 21 (4.0) | 16 (7.8) | 0.25 (0.10 to 0.58) | 0.52 (0.26 to 1.07) | 0.47 (0.20 to 1.05) | 75 (42 to 90) | 48 (−7 to 74) | 53 (−5 to 80) |
| Serologic positive† | 6 (1.6) | 20 (5.5) | 11 (7.5) | 0.22 (0.07 to 0.63) | 0.72 (0.33 to 1.67) | 0.30 (0.10 to 0.77) | 78 (37 to 93) | 28 (−67 to 67) | 70 (23 to 90) |
| Culture or serologic positive† | 10 (2.7) | 21 (5.8) | 12 (8.2) | 0.33 (0.13 to 0.84) | 0.70 (0.33 to 1.57) | 0.47 (0.20 to 1.04) | 67 (16 to 87) | 30 (−57 to 67) | 53 (−4 to 80) |
Relative reduction in vaccine efficacy was defined as (1 − relative risk) × 100. CI denotes confidence interval, and PCR polymerase chain reaction.
These cases were reported for the per-protocol population, defined as the participants who provided all three annual blood specimens according to the timing specified in the protocol. In this population, 367 participants received the inactivated vaccine, 363 the live attenuated vaccine, and 146 placebo.
Forty-three participants (3.4%) had PCR-confirmed influenza, including 28 participants with influenza A, 14 participants with influenza B, and 1 participant with both influenza A and influenza B (two different episodes of illness). Forty-seven participants (3.8%) were infected with influenza as confirmed through either isolation of the virus in cell culture or identification of the virus through real-time PCR (Table 4).
Thirty-seven participants included in per-protocol analyses (4.2%) had serologic evidence of influenza infection. Of these participants, 20 were infected with influenza A (H3N2) and 17 with influenza B. Forty-three participants included in per-protocol analyses (4.9%) were infected with influenza, as confirmed by either cell culture or serologic testing (the primary end point) (Table 4).
ABSOLUTE AND RELATIVE ESTIMATES OF VACCINE EFFICACY
Absolute vaccine efficacy (as compared with placebo), as estimated for culture-confirmed cases only, was 77% (95% CI, 37 to 92) for the inactivated vaccine and 57% (95% CI, −3 to 82) for the live attenuated vaccine (Table 4). There was a 46% relative reduction (95% CI, −44 to 82) in culture-confirmed influenza among recipients of inactivated vaccine as compared with recipients of live attenuated vaccine.
Absolute vaccine efficacy, as estimated for cases confirmed through cell culture or PCR, was 75% (95% CI, 42 to 90) for the inactivated vaccine and 48% (95% CI, −7 to 74) for the live attenuated vaccine (Table 4). There was a 53% relative reduction (95% CI, −5 to 80) in these cases of influenza among recipients of the inactivated vaccine as compared with recipients of the live attenuated vaccine.
Absolute vaccine efficacy, as estimated for culture-confirmed or serologically determined cases of influenza (the primary end point in the per-protocol population), was 67% (95% CI, 16 to 87) for the inactivated vaccine and 30% (95% CI, −57 to 67) for the live attenuated vaccine (Table 4). There was a 53% (95% CI, −4 to 80) relative reduction in these cases among recipients of the inactivated vaccine as compared with recipients of the live attenuated vaccine.
Vaccine efficacy, as determined with the use of cell culture alone or combined cell culture and PCR, was also estimated for cases of type A influenza and type B influenza separately (Table 5). Absolute vaccine efficacy against culture-confirmed influenza A was 74% (95% CI, −11 to 95) for the inactivated vaccine and 74% (95% CI, −12 to 95) for the live attenuated vaccine. When PCR results were also considered, the absolute efficacy was similar for the inactivated vaccine but was decreased for the live attenuated vaccine. For type B influenza, the absolute efficacy against culture-confirmed illness was 80% (95% CI, 8 to 97) for the inactivated vaccine but only 40% (95% CI, −103 to 81) for the live attenuated vaccine.
Table 5.
Estimated Absolute and Relative Efficacies of the Inactivated Influenza Vaccine and the Live Attenuated Influenza Vaccine against Influenza A and Influenza B during the 2004–2005 Influenza Season in Michigan.*
| Laboratory-Confirmed Symptomatic Influenza | Cumulative Incidence of Influenza | Relative Risk (95% CI) | Percent Relative Reduction (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Inactivated Vaccine (N = 522) | Live Attenuated Vaccine (N = 519) | Placebo (N = 206) | Inactivated Vaccine vs. Placebo | Live Attenuated Vaccine vs. Placebo | Inactivated vs. Live Attenuated Vaccine | Inactivated Vaccine vs. Placebo | Live Attenuated Vaccine vs. Placebo | Inactivated vs. Live Attenuated Vaccine | |
| no. of participants (%) | |||||||||
| Influenza A | |||||||||
| Culture positive | 4 (0.8) | 4 (0.8) | 6 (2.9) | 0.26 (0.05 to 1.11) | 0.26 (0.05 to 1.12) | 0.99 (0.19 to 5.34) | 74 (−11 to 95) | 74 (−12 to 95) | 1 (−434 to 81) |
| Culture or real-time PCR positive | 7 (1.3) | 12 (2.3) | 9 (4.4) | 0.31 (0.10 to 0.93) | 0.53 (0.20 to 1.42) | 0.58 (0.19 to 1.60) | 69 (7 to 90) | 47 (−42 to 80) | 42 (−60 to 81) |
| Influenza B | |||||||||
| Culture positive | 3 (0.6) | 9 (1.7) | 6 (2.9) | 0.20 (0.03 to 0.92) | 0.60 (0.19 to 2.03) | 0.33 (0.06 to 1.33) | 80 (8 to 97) | 40 (−103 to 81) | 67 (−33 to 94) |
| Culture or real-time PCR positive | 3 (0.6) | 9 (1.7) | 7 (3.4) | 0.17 (0.03 to 0.74) | 0.51 (0.17 to 1.61) | 0.33 (0.06 to 1.33) | 83 (26 to 97) | 49 (−61 to 83) | 67 (−33 to 94) |
Relative reduction in vaccine efficacy was defined as (1 − relative risk) × 100. CI denotes confidence interval, and PCR polymerase chain reaction.
DISCUSSION
In February each year, the influenza virus strains to be included in the next season’s vaccine are selected.15 The subsequent influenza outbreak is most often caused by a virus or viruses similar or identical to those in the vaccine. However, when a circulating virus has changed, or antigenically drifted, from the strain in the vaccine, the efficacy of the inactivated vaccine is believed to decline.2,7,16 In the 2004–2005 season, the type A virus had antigenically drifted to A/California/07/2004-like virus, leading to concern that the efficacy of the inactivated vaccine would be low, given the genetic differences between the two viruses; in hemagglutination-inhibition tests using serum from ferrets inoculated with virus in the vaccine, inhibition of the new virus was lower than that of the virus in the vaccine by a factor of 8.12,13 Two markedly different lineages of type B viruses, Yamagata and Victoria, had been circulating globally for a number of years.14 One or the other had typically predominated, but in the winter of 2004–2005, when a Yamagata type B virus was selected for use in the vaccine, viruses of both lineages were in circulation.12
In this year, when antigenically drifted type A (H3N2) influenza and both vaccine-like and variant type B viruses were circulating, the inactivated vaccine worked well. This result was somewhat unexpected, given problems reported in past years when antigenically drifted viruses were circulating.2,7,16 It is reassuring that our results for the inactivated vaccine were consistent across the methods used to confirm influenza. The lower absolute efficacy of the live attenuated vaccine, as compared with the inactivated vaccine, was also not anticipated, given previous reports of efficacy in years when antigenically drifted strains circulated.5,6 The live attenuated vaccine still appeared to be protective, particularly against type A influenza, although absolute efficacy estimates were not significant. The estimation of relative efficacy did not indicate a significant advantage of the inactivated vaccine over the live attenuated vaccine.
How can we explain our results among adults for the 2004–2005 influenza season? The use of antibody titer to confirm infection with influenza may lead to overestimation of the efficacy of the inactivated vaccine and underestimation of the efficacy of the live attenuated vaccine.17 Among cases of influenza that were confirmed by isolating the virus in cell culture or identifying it through real-time PCR, the inactivated vaccine provided good protection against both type A and type B viruses, but the live attenuated vaccine appeared to protect reasonably well against type A viruses but protected poorly against type B viruses. However, our study did not have the statistical power to draw conclusions from analyses of individual types of influenza. There were differences in the exact B viruses included in the two vaccines, but both were B/Shanghai/361/2002-like viruses and were considered to be antigenically equivalent to each other by the Food and Drug Administration. Low protection against type B influenza, which has been report ed previously for the live attenuated vaccine, has been attributed to a poor match between the circulating strain and the vaccine strain.18
Could these findings be generalized to other years, when the circulating viruses are either closely matched to vaccine strains or different from them? One previous head-to-head comparison of an experimental bivalent live attenuated vaccine and an inactivated vaccine suggested that the two were equally protective against some but not all end points.3 In children, the live attenuated vaccine has been consistently shown to be efficacious, even against antigenically drifted strains,4,5 and a recent report19 suggests that it is more efficacious than the recommended two-dose inactivated vaccine. However, in our study, the low antibody response to the live attenuated vaccine in hemagglutination-inhibition assays, which has also been observed in previous studies,17,20 suggests that some adults may not become infected by the vaccine viruses because of past infection with influenza. In contrast, there appear to be high rates of seroconversion in seronegative children. 4,21,22 However, protection provided by the live attenuated vaccine has been observed in spite of the lack of an immune response in hemagglutination- inhibition assays, perhaps owing to the production of secretory IgA antibody.17,20 More information is needed on the efficacy of the live attenuated vaccine in adults in other years. Even if it is not as efficacious as the inactivated vaccine in adults, its intranasal route of administration might still be an advantage as the United States moves toward a recommendation of universal use of influenza vaccines. The live attenuated vaccine could also be useful in a pandemic, given that the population would have no preexisting antibodies for the virus, and one dose of the vaccine would be expected to protect against it.
Acknowledgments
Supported by a grant from the National Institute of Allergy and Infectious Diseases (UO1 AI057853).
Dr. Victor reports receiving consulting fees from Wyeth, and Dr. Monto reports receiving consulting fees from GlaxoSmithKline, MedImmune, Solvay, and Novartis. No other potential conflict of interest relevant to this article was reported.
We thank Sarah Campbell, Director of Health Services, and the study staff at Central Michigan University for their substantial contributions to the success of the study; Dr. Janet Gilsdorf, University of Michigan Medical School, Department of Pediatrics and Communicable Diseases, for serving as the independent safety monitor; and the staff of the Influenza Division, Centers for Disease Control and Prevention, for identifying the strains of viruses isolated and for sharing their real-time PCR protocol.
Footnotes
FULL TEXT OF ALL JOURNAL ARTICLES ON THE WORLD WIDE WEB
Access to the complete text of the Journal on the Internet is free to all subscribers. To use this Web site, subscribers should go to the Journal’s home page (www.nejm.org) and register by entering their names and subscriber numbers as they appear on their mailing labels. After this one-time registration, subscribers can use their passwords to log on for electronic access to the entire Journal from any computer that is connected to the Internet. Features include a library of all issues since January 1993 and abstracts since January 1975, a full-text search capacity, and a personal archive for saving articles and search results of interest. All articles can be printed in a format that is virtually identical to that of the typeset pages. Beginning 6 months after publication, the full text of all Original Articles and Special Articles is available free to nonsubscribers who have completed a brief registration.
REFERENCES
- 1.Davenport FM. Control of influenza. Med J Aust. 1973 Suppl:33–38. doi: 10.5694/j.1326-5377.1973.tb111174.x. [DOI] [PubMed] [Google Scholar]
- 2.Bridges CB, Thompson WW, Meltzer MI, et al. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: a randomized controlled trial. JAMA. 2000;284:1655–1663. doi: 10.1001/jama.284.13.1655. [DOI] [PubMed] [Google Scholar]
- 3.Edwards KM, Dupont WD, Westrich MK, Plummer WD, Jr, Palmer PS, Wright PF. Randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J Infect Dis. 1994;169:68–76. doi: 10.1093/infdis/169.1.68. [DOI] [PubMed] [Google Scholar]
- 4.Belshe RB, Mendelman PM, Treanor J, et al. Efficacy of live attenuated, cold-adapted, trivalent, intranasal influenza vaccine in children. N Engl J Med. 1998;338:1405–1412. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 5.Belshe RB, Gruber WC, Mendelman PM, et al. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intra-nasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J Pediatr. 2000;136:168–175. doi: 10.1016/s0022-3476(00)70097-7. [DOI] [PubMed] [Google Scholar]
- 6.Nichol KL, Mendelman PM, Mallon KP, et al. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA. 1999;282:137–144. doi: 10.1001/jama.282.2.137. [DOI] [PubMed] [Google Scholar]
- 7.Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2004;53(RR6):1–40. [Erratum, MMWR Morb Mortal Wkly Rep 2004;53:743.] [PubMed] [Google Scholar]
- 8.Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160:3243–3247. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- 9.Department of Communicable Disease Surveillance and Response. [Accessed November 16, 2006];Geneva: World Health Organization; WHO manual on animal influenza: diagnosis and surveillance. 2002 (WHO/CDS/CSR/NCS/2002.5 Rev1.), at http://www.who.int/csr/resources/publications/influenza/whocdscsrncs20025rev.pdf.
- 10.Zambon M. Laboratory diagnosis of influenza. In: Nicholson KG, Webster RG, Hay AJ, editors. Textbook of influenza. Malden, MA: Blackwell Science; 1998. pp. 291–313. [Google Scholar]
- 11.Weinberg GA, Erdman DD, Edwards KM, et al. Superiority of reverse-transcription polymerase chain reaction to conventional viral culture in the diagnosis of acute respiratory tract infections in children. J Infect Dis. 2004;189:706–710. doi: 10.1086/381456. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. [Accessed November 16, 2006];2004–05 U.S. influenza season summary. at http://www.cdc.gov/flu/weekly/weeklyarchives2004-2005/04-05summary.htm.
- 13.Recommended composition of influenza virus vaccines for use in the 2005–2006 influenza season. Wkly Epidemiol Rec. 2005;80:71–75. [PubMed] [Google Scholar]
- 14.Rota PA, Wallis TR, Harmon MW, Rota JS, Kendal AP, Nerome K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology. 1990;175:59–68. doi: 10.1016/0042-6822(90)90186-u. [DOI] [PubMed] [Google Scholar]
- 15.Recommended composition of influenza virus vaccines for use in the 2004–2005 influenza season. Wkly Epidemiol Rec. 2004;79:88–92. [PubMed] [Google Scholar]
- 16.Update: influenza activity — United States, 1997–98 season. MMWR Morb Mortal Wkly Rep. 1998;47:196–200. [PubMed] [Google Scholar]
- 17.Treanor JJ, Kotloff K, Betts RF, et al. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) inf luenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine. 1999;18:899–906. doi: 10.1016/s0264-410x(99)00334-5. [DOI] [PubMed] [Google Scholar]
- 18.Stoddard J, Lee M, Walker R, Kemble G, Mendelman P. Cross-protection against antigenic variants by live-attenuated influenza vaccine in children. Proceedings of the Pediatric Academic Societies’ 2005 Annual Meeting; May 14–17, 2005; Washington, DC. abstract. [Google Scholar]
- 19.Belshe R. Comparison of efficacy and safety of cold-adapted influenza vaccine, trivalent (CAIV-T) with trivalent inactivated influenza vaccine (TIV) in children 6–59 months of age. Presented at the Pediatric Academic Society Meeting; April 29–May 2, 2006; San Francisco. abstract. [Google Scholar]
- 20.Belshe RB, Gruber WC, Mendelman PM, et al. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J Infect Dis. 2000;181:1133–1137. doi: 10.1086/315323. [DOI] [PubMed] [Google Scholar]
- 21.King JC, Jr, Lagos R, Bernstein DI, et al. Safety and immunogenicity of low and high doses of trivalent live cold-adapted influenza vaccine administered intranasally as drops or spray to healthy children. J Infect Dis. 1998;177:1394–1397. doi: 10.1086/517822. [DOI] [PubMed] [Google Scholar]
- 22.Gruber WC, Belshe RB, King JC, et al. Evaluation of live attenuated influenza vaccines in children 6–18 months of age: safety, immunogenicity, and efficacy. J Infect Dis. 1996;173:1313–1319. doi: 10.1093/infdis/173.6.1313. [DOI] [PubMed] [Google Scholar]