Abstract
This review provides a summary of the contributions made by human functional neuroimaging studies to the understanding of neural correlates of saccadic control. The generation of simple visually-guided saccades (redirections of gaze to a visual stimulus or prosaccades) and more complex volitional saccades require similar basic neural circuitry with additional neural regions supporting requisite higher level processes. The saccadic system has been studied extensively in non-human primates (e.g. single unit recordings) and humans (e.g. lesions and neuroimaging). Considerable knowledge of this system’s functional neuroanatomy makes it useful for investigating models of cognitive control. The network involved in prosaccade generation (by definition exogenously-driven) includes subcortical (striatum, thalamus, superior colliculus, and cerebellar vermis) and cortical structures (primary visual, extrastriate, and parietal cortices, and frontal and supplementary eye fields). Activation in these regions is also observed during endogenously-driven voluntary saccades (e.g. antisaccades, ocular motor delayed response or memory saccades, predictive tracking tasks and anticipatory saccades, and saccade sequencing), all of which require complex cognitive processes like inhibition and working memory. These additional requirements are supported by changes in neural activity in basic saccade circuitry and by recruitment of additional neural regions (such as prefrontal and anterior cingulate cortices). Activity in visual cortex is modulated as a function of task demands and may predict the type of saccade to be generated, perhaps via top-down control mechanisms. Neuroimaging studies suggest two foci of activation within FEF - medial and lateral - which may correspond to volitional and reflexive demands, respectively. Future research on saccade control could usefully (i) delineate important anatomical subdivisions that underlie functional differences, (ii) evaluate functional connectivity of anatomical regions supporting saccade generation using methods such as ICA and structural equation modeling, (iii) investigate how context affects behavior and brain activity, and (iv) use multi-modal neuroimaging to maximize spatial and temporal resolution.
INTRODUCTION
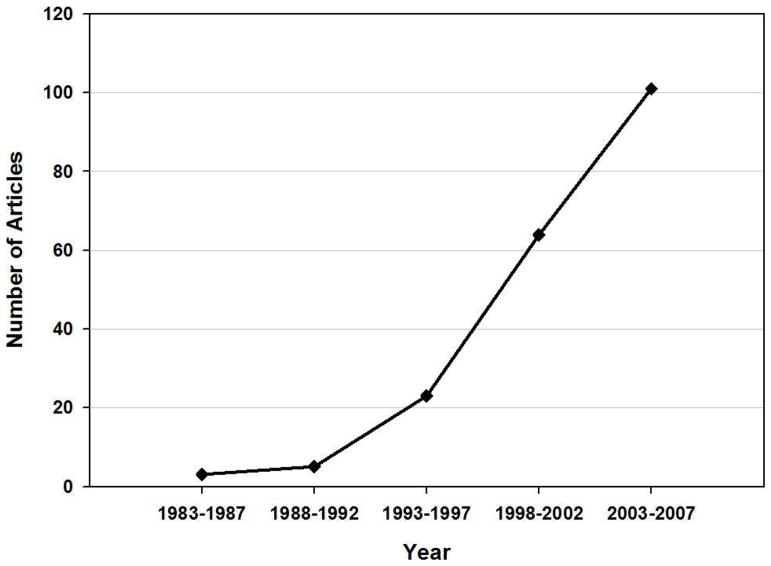
Multiple tools exist for studying the neural bases of saccade control, including human functional neuroimaging techniques. Functional neuroimaging is defined here to include techniques used for their excellent spatial resolution, such as positron emission tomography (PET) (see glossary) and functional magnetic resonance imaging (fMRI) (see glossary and text box 1), and techniques used for their excellent time resolution, such as multichannel electroencephalography (EEG) (see glossary and text box 2) and magnetoencephalography (MEG) (see glossary). The study of saccade control using functional neuroimaging began with EEG studies in the 1960s (e.g. Gaarder et al., 1964) and was followed by PET studies in the 1980s (e.g. Fox et al., 1985). Since then, the growth in published studies on human functional neuroimaging of saccades in the English language literature shows the citation list almost doubling every 4 years (see Figure 1). This review will provide a brief summary of the contributions made by human functional imaging studies to the understanding of neural correlates of saccadic control.
Figure 1.
Increase in number of published articles across years (1983–2007) using neuroimaging methods (PET, fMRI, EEG, MEG) to study saccadic eye movements. Data obtained from PubMed and PsycInfo databases and searched by keywords: Saccade and positron emission tomography/PET; Saccade and functional magnetic resonance imaging/fMRI; Saccade and electroencephalography/EEG; Saccade and magnetoencephalography/MEG. Results excluded literature reviews and were limited to those using human subjects and printed in English.
Exploration of the visual environment relies on two types of saccadic control (a distinction that may be more qualitative than quantitative; e.g. see Hutton, this issue). On the one hand, visually-guided saccades (also known as reflexive, refixation or pro-saccades) are generated to external cues and require simple and direct sensorimotor transformations (see glossary) for their successful implementation. In the following text, “prosaccades” is used in the context of tasks requiring visually-guided saccades to visual stimuli. On the other hand, volitional saccades are more cognitively complex responses that require higher order control processes such as inhibition, spatial memory, and analysis of contextual cues. The basic neural circuitry supporting the sensorimotor transformation part of saccade generation is similar for simpler and more complex saccadic responses (Leigh & Zee, 1999). As the factors determining saccadic response requirements become more complex, however, additional neural regions are recruited to support the requisite higher level processes (e.g., Munoz & Everling, 2004; Pierrot-Deseilligny et al., 2005; Sweeney et al., 2007).
A number of characteristics make the saccadic system extremely useful for investigating models of cognitive control. First, the system is particularly well understood based on an extensive literature that ranges from single unit recordings in primates (Johnston & Everling, this issue) to lesions studies in humans (Pierrot-Deseilligny et al., 2004). Second, there is good convergence between that literature and the human functional neuroimaging studies. Third, saccades can be measured precisely and with a number of reliable parameters (Smyrnis, this issue). As such, the study of cognitive control via saccadic system manipulations has applications across a diverse range of topics, extending from studies of basic motor function to normal cognitive neuroscience studies of executive control to investigations of behavioral and brain activity correlates of psychiatric conditions.
The following report on the neuroanatomy of the saccadic system in humans begins with a review of information on the neural circuitries known to support simple saccadic responses, such as pro- and express saccades. Second, we describe information on additional brain regions involved in supporting saccadic responses in more cognitively complex situations, such as during antisaccades (see Figure 2), ocular motor delayed response tasks, predictive saccadic tracking and saccade sequencing tasks. Basic neurophysiology, lesion, and neuropathology studies will be incorporated if they help clarify the functional neuroimaging data. Information gleaned from fMRI (quantified as blood oxygenation level dependent signal; BOLD) and PET studies will be supplemented with EEG and MEG studies to the extent that the latter yield at least some information about the brain regions involved in generating the signals measured at the sensors (e.g. via source analysis).
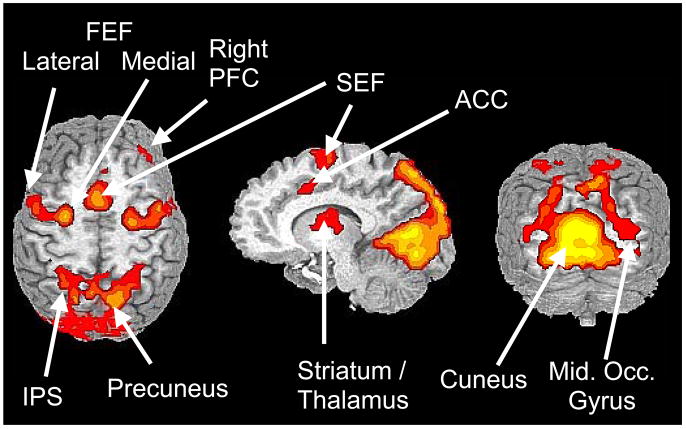
Figure 2.
Functional magnetic resonance imaging (fMRI) data showing basic neural circuitry associated with a volitional saccade (antisaccade) task. Data are shown on axial, sagittal and coronal slices with colors indicating increased signal associated with execution of a volitional saccade task. Data were acquired from 32 subjects on a 1.5T MRI system and are shown using radiological convention (right hemisphere on the left side). FEF, frontal eye field; SEF, supplementary eye field; PFC, prefrontal cortex; ACC, anterior cingulate cortex; IPS, intraparietal sulcus. Adapted from Dyckman et al, 2007.
PROSACCADE CIRCUITRY
Prosaccades
A prosaccade is a response that involves the simple redirection of gaze to a stimulus and typically is generated to align the fovea with visual targets of interest (see glossary). In conjunction with animal physiology (see Everling & Johnston, this issue) and human lesion studies (see Müri & Nyffeler, this issue), human functional neuroimaging studies have greatly contributed to our knowledge of the neural circuitry supporting saccadic eye movements. In the laboratory, prosaccades are made to a visual stimulus most often (although auditory stimuli are sometimes used; e.g. Russo & Bruce, 1994; Zambarbieri et al., 1982). Visual information enters through the retina and is sent via the optic tract to the lateral geniculate nucleus (see glossary) of the thalamus (see glossary) and then via the optic radiation to primary visual cortex. The visual stimulus is registered in primary visual cortex, with a strong contralateral bias, by 100–120ms after its presentation (Clementz et al., 2001, 2007; McDowell et al., 2005; Tendolkar et al., 2005; Tzelepi et al., 2004, Vanni et al., 2001). From primary visual cortex, information is sent to extrastriate cortical regions V2/V3 (in middle occipital gyrus; Brodmann’s areas 18/19). These brain regions are clearly involved in mapping relevant stimuli in visual space (Dyckman et al., 2007; Merriam et al., 2007), and are more strongly activated by stimuli in the contralateral visual field, regardless of whether the stimulus defines the location of an impending response (Clementz et al., 2007; McDowell et al., 2005). Visual cortex is also one of the few brain regions that has sometimes shown stronger activity during simple prosaccades than during more complex volitional saccades (Dyckman et al., 2007), although this pattern has not always been apparent (Mort et al 2003; Sweeney et al 1996).
Visual cortex (including striate and extrastriate visual regions) has access to the brainstem saccade generators through the superior colliculus (see glossary) (Collins et al., 2005; Lock et al., 2003). Given this direct connection it might be expected that when visual stimuli are presented, the response of neurons in striate and extrastriate regions could predict the subsequent saccadic responses. In fact, such a pattern has been demonstrated during sustained attention-related tasks. In such tasks changes in activity levels in specific neural ensembles precede presentation of relevant stimuli (Chawla et al., 1999; Driver & Frith, 2000; Pessoa et al., 2003) and influence subsequent behavioral responses.
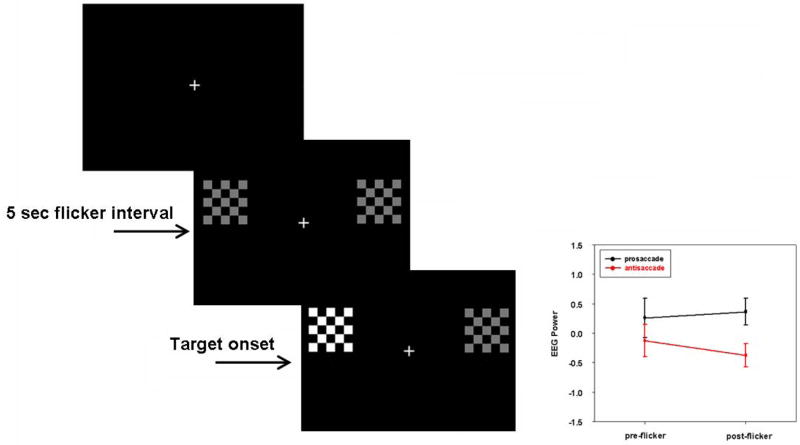
In a recent study (Clementz et al., 2008) we tested for a similar response in visual cortex by using variants of typical saccade paradigms that allowed the measurement of activity in relation to possible saccadic response locations prior to onset of an abruptly-appearing peripheral target. Subjects fixated on a central cross while peripheral checkerboards oscillated at frequencies that were measured with dense array EEG (see Figure 3). After approximately 5 sec, one of the oscillating checkerboards doubled in luminance, which cued participants to generate a saccade (either toward the brightened checkerboard for a pro-response or away from the brightened checkerboard for an anti-response). Figure 3 shows the activity at EEG sensors over visual cortex at the frequencies of the oscillating checkerboards. In preparation for pro-responses, the neural activity over visual cortex was maintained at baseline levels (and even tended to increase slightly), while prior to anti-responses activity during this same time interval decreased to levels significantly lower than those observed prior to pro-responses. These data indicate that early in the course of stimulus registration, activity in striate and extrastriate cortex is modulated as a function of task demands, perhaps via top-down control mechanisms (e.g., Clementz et al., 2007; Miller & Cohen, 2001; Moore & Armstrong, 2003; Ruff et al., 2007; Trappenberg et al., 2001).
Figure 3.
Participants initially fixated on the central cross. After a brief interval, two peripheral checkerboards were presented. Each checkerboard oscillated on and off at a different frequency for about 5 sec in order to invoke a steady state response at that frequency that could be measured with dense-array EEG. Following this interval, one of the peripheral checkerboards doubled in luminance. Brain activity over visual cortex was measured during the flicker interval before target onset. Activity (EEG power) on pro-and anti-trials deviated after onset of the flickering stimuli but not before (see the lower right plot). From Clementz et al., 2008.
From visual regions, position data (and other information relevant to subsequent motor output) travels via the dorsal stream to multiple parietal cortex regions, most prominently the superior parietal lobe (in Brodmann’s area 7) and parietal eye fields (Greenlee, 2000) (see glossary). These parietal cortex regions have (i) direct connections to the superior colliculus (Lynch et al., 1985; Pare & Wurtz, 2001), and (ii) reciprocal connections with frontal motor regions (e.g. frontal and supplementary eye fields; Barbas & Mesulam, 1981; Ferraina et al., 2002; Huerta et al., 1987; Tian & Lynch, 1996). There is considerable evidence from monkey neurophysiology (Andersen et al., 1990; Colby et al., 1996) and human lesion data (Braun et al., 1992; Gaymard et al., 1998; Pierrot-Deseilligny et al., 1991, 2002) that parietal cortex is critically important for various aspects of saccadic control. For instance, direct projections from parietal cortex to superior colliculus suggest a role in saccade triggering. This conclusion is supported by data showing that damage to parietal cortex increases prosaccade latencies (Heide & Kompf, 1998; Gaymard et al., 2003; Pierrot-Deseilligny et al., 1987) and that temporarily disrupting this region using transcranial magnetic stimulation (TMS) increases the latency of visually-guided saccades (Kapoula et al., 2001).
Since the earliest functional neuroimaging studies on saccades, parietal cortex activity has been observed across a variety of paradigms (Anderson et al., 1994; Bellebaum & Daum, 2006; Clementz et al., 2007; Dyckman et al., 2007; Heide et al., 2001; Matsuda et al., 2004; McDowell et al., 2002, 2005; O’Driscoll et al., 1995; Sweeney et al., 1996). Superior parietal cortex is known for supporting attention-associated functions (cf. Kastner et al., 1999; Simon et al., 2002). Specifically, parietal cortex is associated with visuo-spatial attention processes (e.g. Corbetta et al., 1998), including spatial updating (Heiser & Colby, 2006) and the transformation of sensory input into a motor command (Colby et al., 1996; Colby & Goldberg, 1999). As a result, most imaging studies show that parietal cortex activity is greater during volitional than reflexive saccades, an issue that will be discussed more completely in a later section.
An important, and as yet uncertain, issue in the functional neuroimaging literature, however, is what specific regions of parietal cortex support basic saccade generation. There has been some uncertainty regarding the location of a parietal cortex region involved in saccade generation. Many functional neuroimaging studies have provided data relevant to this issue, although with considerable variability between studies on the putative location of the human parietal eye field (PEF; e.g., Astafiev et al., 2003; Clementz et al., 2007; Connolly et al., 2003; Culham et al., 2006; Dyckman et al., 2007; Koyama et al., 2004; McDowell et al., 2005; Moon et al., 2007; Rushworth et al., 2001; Schluppeck et al., 2005; Simo et al., 2005). Nevertheless, a likely location of a parietal cortex region involved in saccade triggering appears to be in medial intraparietal sulcus (see glossary) (Koyama et al., 2004; see also Grefkes & Fink, 2005 for a comparison to the monkey literature). It is difficult to determine from blood-flow based neuroimaging methods alone whether this brain region is involved in saccade triggering, but data from two other studies, one using combined MEG/EEG (McDowell et al., 2005) and one using EEG alone (Clementz et al., 2007) are consistent in suggesting that this parietal cortex region may harbor the human homologue of the parietal eye field (PEF).
Frontal cortex is obviously important for motor control, eye movements included, with the frontal eye fields (FEF) (see glossary) and supplementary eye fields (SEF) (see glossary) having direct access to the brainstem saccade-generating circuitry (Huerta et al., 1986; Segraves, 1992; Shook et al., 1990; Yan et al., 2001). Increased activity in FEF during saccades is a consistent finding in functional neuroimaging studies (e.g. Anderson et al., 1994; Brown et al., 2006; Clementz et al., 2007; Connolly et al., 2000; Cornelissen et al., 2002; Darby et al., 1996; DeSouza et al., 2003; Dyckman et al., 2007; Kimmig et al., 2001; Matsuda et al., 2004; McDowell et al., 2002, 2005; Muri et al., 1998; O’Driscoll et al., 1995; Raemaekers et al., 2002; Sweeney et al., 1996). A significant contribution of functional brain imaging studies of saccades has been clarification of the location and distinct functions of FEF regions in humans. In contrast to monkeys, where FEF is located primarily in Brodmann’s area 8 (Bruce & Goldberg, 1985; Stanton et al., 1989; Tehovnik et al., 2000; this is still mistakenly identified as the location of human FEF in some cognitive neuroscience textbooks; e.g., Ward, 2006), FEF is more posterior in humans, in Brodmann’s area 6, just anterior to the primary hand representation (Amiez et al., 2006; Paus, 1996). In addition, there may be two distinct FEF regions, with the more lateral aspect being involved in generation of both reflexive and volitional saccades and the medial aspect being more important for the generation of saccades requiring a greater reliance on controlled processing with the aid of external visual cues (see, e.g., McDowell et al., 2005; Simo et al., 2005).
Consistent with its putative role in saccade initiation (e.g. Bruce & Goldberg, 1985; Pierrot-Deseilligny et al., 1991; Rivaud et al., 1994), activity in FEF is correlated with saccadic reaction time in both human fMRI studies (Connolly et al., 2005) and monkey neurophysiology (e.g. Dorris & Munoz, 1998; Everling & Munoz, 2000; Hanes & Schall, 1996). Using an event-related paradigm (see glossary), Connolly et al. (2005) found that BOLD activity in FEF during a preparatory period (a 2 sec gap before the target appeared) was correlated with saccadic reaction time. Greater FEF activity in the hemisphere contralateral to the direction of the eventual saccade was associated with faster reaction times. This is similar to monkey studies which have shown that the lower the pre-target activity in FEF, the longer it takes to boost the firing rate past the threshold for saccade triggering (Dorris & Munoz, 1998; Everling & Munoz, 2000; Hanes & Schall, 1996). In sum, the amount of activity in FEF when the target is presented may partially account for variations in reaction time within individuals and across different saccade paradigms.
Strong reciprocal connections exist between FEF and SEF which are located on the dorsomedial surface of each hemisphere, anterior to the supplementary motor area (Leigh & Zee, 1999; Pierrot-Deseilligny et al., 2002); they are thought to be the ocular motor extensions of the supplementary motor area (Schall, 2002). More specifically, Grosbas et al. (1999) defined the anatomic boundaries of the SEF using functional imaging as within the interhemispheric fissure in the area around the descending branch of the paracentral sulcus. As with FEF, microstimulation of SEF results in saccade generation in both non-human (Schlag & Schlag-Rey, 1987; Tanji, 1994) and human (Godoy et al., 1990) primates. Also similar to FEF, increased saccade-related activity in SEF has been observed in a number of functional neuroimaging studies (e.g. Berman et al., 1999; Brown et al., 2006; DeSouza et al., 2003; Dyckman et al., 2007; Gagnon et al., 2002; Kimmig et al., 2001; Matsuda et al., 2004; McDowell et al., 2002; Raemaekers et al., 2002). Functional neuroimaging during prosaccade tasks shows increased SEF activity associated with movement generation (e.g. Law et al., 1997; Luna et al., 1998; McDowell et al., 2005; Miller et al., 2005). Frequently, however, SEF activity is greater during cognitively complex saccades than during prosaccades (e.g. Clementz et al., 2007; Curtis & D’Esposito, 2003; DeSouza et al., 2003; Doricchi et al., 1997; Dyckman et al., 2007; Ford et al., 2005; Luna et al., 2001; McDowell et al., 2005; O’Driscoll et al., 1995; Raemaekers et al., 2006a, 2006b). This is consistent with human lesion and monkey neurophysiology data indicating that SEF is important for complex saccades, especially when motor sequences must be remembered for proper saccade generation and when stimuli are predictable (Gaymard et al., 1993; Kennard et al., 2005; Merriam et al., 2001; Parton et al., 2007; Simo et al., 2005; Stuphorn et al., 2000).
Cortical regions have direct reciprocal connections to multiple subcortical regions, implicating these regions in saccade generation (Lynch & Tian, 2005). Generally there are more issues complicating the study of subcortical structures with functional neuroimaging techniques; the specific limitations are dependent on the technique and the region. In general, subcortical regions are difficult to image because they are small and/or deep. Deep structures are difficult to evaluate with MEG and EEG because of signal drop-off from the source to the sensors (Nunez & Srinivasan, 2006). Deep structures also can pose a problem using blood-flow based methods, particularly for structures that are located close to vasculature, like the superior colliculus, making BOLD signal measurement more vulnerable to susceptibility artifacts (Petit & Beauchamp, 2003; Schneider & Kastner, 2005). While functional imaging of subcortical regions is not impossible, such challenges may explain why there are relatively few studies on the role of certain subcortical structures in saccade control.
The studies that do report on saccade-related activity in subcortical regions show activity in cerebellum (see glossary), striatum, thalamus, and superior colliculus (Ettinger et al., 2002; Matsuda et al., 2004; Optican, 2005; Sweeney et al., 1996). The cerebellum, specifically the vermis and fastigial nucleus, has been implicated in saccade accuracy by lesion studies in both humans and non-human primates (Bötzel et al., 1993; Noda et al., 1991; Takagi et al., 1998; Vahedi et al., 1995). The relative volume of the vermis was related to saccadic accuracy in humans (Ettinger et al. 2002). The striatum is a major input site for the basal ganglia (Alexander et al., 1986; Alexander & Crutcher 1990) (see glossary) and has been reported to play a role in both saccade initiation (Watanabe et al., 2003) and inhibition (Hikosaka et al., 2000). Functional neuroimaging studies on reflexive saccades generally do not indicate much saccade-related activity in striatum and/or thalamus (e.g. Brown et al., 2006, McDowell et al., 2002; Raemaekers et al., 2006), although there are some exceptions (Matsuda et al., 2004; Sweeney et al., 1996; Sylvester et al., 2005). The superior colliculus is clearly involved in the generation of reflexive saccades and has been the subject of many monkey neurophysiology studies (McPeek & Keller, 2004); increased saccade-related collicular activity has been reported using PET (Paus et al., 1995) and an event-related fMRI design (Petit & Beauchamp; 2003). Neggers et al. (2005) found that collicular activity was negatively correlated with saccade latency such that greater activity in superior colliculus was associated with faster saccades. They also observed differential activity in superior colliculus when the timing of fixation offset was manipulated: BOLD signal increased in superior colliculus during a 200 ms gap paradigm compared to a paradigm with no such gap. Neggers et al. (2005) suggested that this BOLD signal increase was caused by disinhibition of the entire motor map within superior colliculus in preparation for saccade generation.
Express Saccades
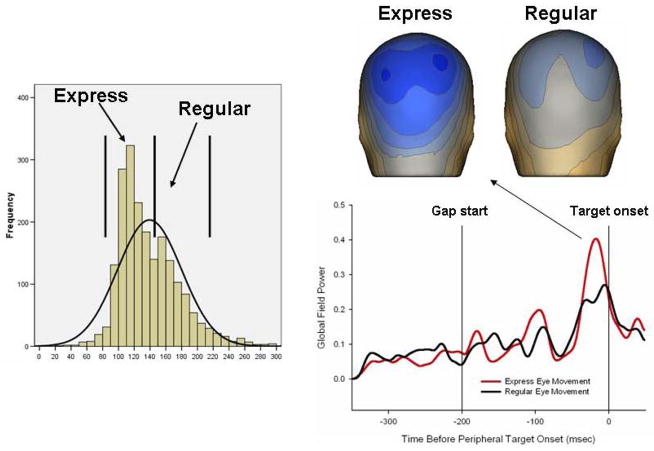
As mentioned above with regard to FEF and saccade triggering, saccadic reaction times can vary tremendously between trials for an individual participant. To study some putative causes of such wide variability in response times, reflexive saccade paradigms can be appropriately modified to increase the probability of eliciting fast latency saccades known as “express saccades” (Fischer & Ramsperger, 1984) (see glossary). Insertion of a “gap” (optimally 200ms; Clementz, 1996; Fischer & Weber, 1997; Weber & Fischer, 1995) between fixation point extinction and peripheral stimulus illumination typically results in a bimodal reaction time distribution with one peak around 110ms and another around 160ms (Fischer et al., 1993; see also Figure 4). The saccades around the first peak are called “express saccades”. Express saccades are considered to be reflex-like orienting movements (Fischer & Weber, 1997) mediated through the retino-cortical-tectal pathway and can be generated in the absence of the frontal eye fields (Schiller et al., 1987), but not after lesions of the superior colliculus (Schiller et al., 1997).
Figure 4.
The distribution on the right shows the typical bimodal reaction time distribution during a 200ms gap task. The plot at the lower right shows the global field power (over all EEG sensors) in relation to gap start and peripheral target onset. Note that during the gap interval, there are no visual stimuli on the screen. The topographies at the upper right show the pattern and strength (darker blue indicates stronger response) of EEG activity at the peak occurring 16ms before peripheral target onset for express and regular saccades.
A problem with using fMRI to study the neural correlates of express saccade generation is the limit on temporal resolution. To provide complementary data on the neural correlates of express saccade generation, we conducted the following study (Dyckman et al., manuscript in preparation). Participants generated about 150 refixation saccades during a typical 200ms gap paradigm while dense array (256 sensor) EEG data were collected. The reaction time distribution across all participants illustrates the typical express and regular peaks (e.g. Clementz, 1996; see Figure 4). In the EEG data, there was a distinct peak in the event-related potential (ERP) waveforms during the gap interval that preceded the peripheral target onset by 16 ms (Figure 4). This peak had an activity pattern that significantly predicted later express versus regular saccade generation. Although the topography of the neural activities did not differ between saccade types, the activity over extrastriate cortex was much stronger preceding subsequent express saccades even though the direction of movement was not yet known. Similar to what was posited by Everling et al. (1996), therefore, this finding suggests that express saccade generation is related to trial-to-trial variations in preparatory activities in extrastriate regions with direct input to the brainstem saccade-generating apparatus.
Prosaccade Summary
Functional neuroimaging studies in humans have greatly contributed to the identification and localization of regions comprising a network involved in prosaccade generation. At the subcortical level, this network includes the striatum, thalamus, and superior colliculus. Cortically, the network includes primary visual cortex and extrastriate cortex, regions of posterior parietal cortex, and FEF and SEF in frontal cortex. Activation in these same regions also has been observed during voluntary saccade tasks, which will be the focus of the next section.
VOLITIONAL SACCADE CIRCUITRY
Saccades intended to foveate a target of interest are one component of our ability to explore, and respond to, the visual environment. Such simple response capabilities, however, are not the only method for managing the visual environment. In order to attend to one aspect of the visual environment, responses to other parts of the visual world often must be suppressed. Tasks requiring inhibition, working memory and other processes that require attendance to contextual cues are considered volitional saccades. In the functional neuroimaging literature, the most frequently used volitional saccade paradigms are antisaccade and ocular motor delayed response (memory saccade) tasks, although data on predictive tracking and saccade sequencing tasks also have been reported. Below, we summarize data from each of these paradigms in turn.
Antisaccades
Antisaccades (Hallett, 1978) (see glossary) are voluntary saccades during which participants must inhibit the prepotent response towards a peripheral (usually visual) cue. During typical antisaccade trials subjects fixate on a central target and then the fixation point is extinguished and a peripheral cue is presented. Subjects are instructed to generate a saccade to the mirror location (same amplitude, opposite direction) of the peripheral cue as quickly and accurately as possible without looking at the cue itself. An initial glance towards the cue is an error and may be conceptualized as a failure of inhibition. Latencies for correct antisaccade responses are usually about 50 ms longer (Evdokimidis et al., 1996) than reflexive saccades, which may represent the additional processing necessary for the co-ordinate transformation process. Based on the extant literature, those additional processing requirements are supported by (i) changed activity levels in the basic saccade circuitry, and/or (ii) activity in newly recruited neural regions (for reviews, see: Everling & Fischer, 1998; Hutton & Ettinger, 2006; Munoz & Everling, 2004, Sweeney et al., 2007).
In terms of changed activity levels, visual cortex is unusual in that it may demonstrate stronger activity for prosaccades than for antisaccades, although this is not always the case (Raemaekers et al., 2007). In a direct comparison using fMRI between pro- and anti-saccades, increased activity related to prosaccades was seen only in middle occipital gyrus (Dyckman et al., 2007). In an EEG/MEG study, McDowell et al. (2005) observed greater activity for prosaccades relative to antisaccades in this region from the time of peripheral stimulus onset through 170ms post-stimulus. Clementz et al. (2008) found that visual cortex activity was actually significantly attenuated during a preparatory period preceding anti-saccade versus pro-saccade generation (see the “Prosaccade Circuitry” section above). This difference between pro- and anti-saccade activities during visual stimulus registration may reflect top-down influences from frontal and/or parietal cortices that decrease the probability that an error response could be generated to the cue on anti-trials.
Contrary to the pattern observed for visual cortex, greater activity for anti- than pro-saccades is commonly found in the other regions of saccade circuitry, particularly in parietal cortex, FEF and SEF (e.g. Curtis & D’Esposito, 2003; DeSouza et al., 2003; Doricchi et al., 1997; Dyckman et al., 2007; Ettinger et al., 2007; Ford et al., 2005; Luna et al., 2001; Moon et al., 2007; Medendorp et al., 2005; Raemaekers et al., 2006a, 2006b). For instance, increased activity in inferior parietal cortex has been observed in anti-compared with pro-saccades (Matsuda et al., 2004). A similar region showed activity during an inhibitory period preceding antisaccade generation (Ettinger et al., 2007), suggesting a possible inhibitory role of the region. Other evidence suggests regions in the area of intraparietal sulcus (within parietal cortex), may perform the vector inversion required to generate an antisaccade to the correct location (e.g. Clementz et al., 2007; Medendorp et al., 2005; Moon et al., 2007; Nyffeler et al., 2007; Zhang & Barash, 2000). Zhang and Barash (2000) recorded from neurons in LIP in nonhuman primates while they performed pro- and anti-saccade tasks. They found that population activity in this region switched from encoding the stimulus location to encoding the motor direction within 50 ms after signals reached LIP during a delayed version of an antisaccade task. Gottlieb and Goldberg (1999), however, reported that most LIP neurons coded for the cue location, not the response direction, although they did not use a delay period between stimulus onset and time of the response requirement. In an event-related fMRI study, Medendorp et al. (2005) reported contralateral hemisphere activity in response to visual stimuli in an area they called retinotopic intraparietal sulcus, which is situated similarly to the precuneus area identified in other studies (e.g. Dyckman et al., 2007). Once the instruction to make an antisaccade was given, however, activity shifted from the hemisphere contralateral to the visual stimulus to the hemisphere contralateral to the saccade goal during a delay period. A similar pattern was observed in intraparietal sulcus using MEG (Moon et al., 2007).
Greater FEF activity associated with anti- than pro-saccades is among the most consistent findings in the human functional neuroimaging literature on antisaccades (e.g. Clementz et al., 2007; Curtis & D’Esposito, 2003; DeSouza et al., 2003; Doricchi et al., 1997; Dyckman et al., 2007; Ford et al., 2005; Luna et al., 2001; Manoach et al., 2007; McDowell et al., 2005; Raemaekers et al., 2006a, 2006b). Importantly, increased signal in FEF on anti- compared to pro-saccade trials has been observed prior to saccade generation using both fMRI and EEG/MEG (e.g. Clementz et al., 2007; Connolly et al., 2002, 2005; DeSouza et al., 2003; Ford et al., 2005; Manoach et al., 2007; McDowell et al., 2005). These data have been interpreted by some as indicating a heightened level of inhibitory input to this region in preparation for an antisaccade (DeSouza et al., 2003; Manoach et al., 2007) and/or other inhibitory processes (Ettinger et al., 2007). This increase in FEF activity may be more specifically related to medial than to lateral FEF (Ettinger et al., 2007; McDowell et al., 2005; O’Driscoll et al., 1995; Tu et al., 2006), although there are insufficient data at this time to confidently draw this conclusion. Curtis and D’Esposito (2006) also found that FEF activity persisted after response selection until a saccade was made, suggesting that FEF are involved in prospective coding of the saccade goal.
Finally, increased antisaccade activity has been observed in SEF during human functional neuroimaging studies (e.g. Clementz et al., 2007; Curtis & D’Esposito, 2003; DeSouza et al., 2003; Doricchi et al., 1997; Dyckman et al., 2007; Ettinger et al., 2007; Ford et al., 2005; Luna et al., 2001; McDowell et al., 2005; O’Driscoll et al., 1995; Raemaekers et al., 2006a, 2006b). Similarly, single-unit recordings show that SEF neurons fired more before anti- than before pro-saccades (Amador et al., 2004; Schlag-Rey et al., 1997). Consistent with a competition model of saccade generation, such as the model proposed by Massen (2004), these data may indicate that the signal to generate a voluntary antisaccade competes with the signal to generate a prosaccade toward the cue, and that increased SEF activity prior to anti-responses offsets the tendency to glance toward the peripheral cue. In this way, SEF could slow the buildup of neuronal activity leading to a prosaccade (Boxer et al., 2006), allowing the initial saccade then to be the anti-response (Amador et al., 2004; Curtis & D’Esposito, 2003).
In addition to changes in levels of activity supporting basic prosaccade circuitry, there is evidence that additional brain regions often are recruited for the performance of more cognitively complex saccades. Neurophysiology and human lesion data indicate that activity in dorsolateral prefrontal cortex (DLPFC) (see glossary) may be associated with an inhibition function. DLPFC is located on the dorsolateral surface of frontal lobe, including the superior and middle frontal gyrus (BA 9 & 46; Petrides & Pandya, 1994), and this brain region clearly supports higher cognitive functions, such as attention, planning, spatial orientation, and behavioral restraint (Goldman-Rakic, 1995; Miller & Cohen, 2001). Lesions to DLPFC do not result in changes to prosaccade performance; however, patients with discrete lesions of the region make more antisaccade errors (Pierrot-Deseilligny et al., 1991, 2003).
Several human functional neuroimaging studies have reported DLPFC activity during antisaccades exclusively (e.g. Ettinger et al., 2007; DeSouza et al., 2003; Matsuda et al., 2004; McDowell et al., 2002; McDowell et al., 2005; Muri et al., 1998; Sweeney et al., 1996). Of importance, this inhibition-related DLPFC activity appears to precede response generation and is specific to correct anti-trials (DeSouza et al., 2003; Ford et al., 2005; Matthews et al., 2002; McDowell et al., 2005). DeSouza et al. (2003) used an event-related fMRI design to examine cortical activity that occurred in preparation for and during the execution of correct antisaccades. Right DLPFC showed significantly greater activity during the instruction phase prior to an antisaccade task than during the same period prior to a prosaccade task. This increase in activity took place before the cue to generate the antisaccade was given and may reflect top-down control signals to inhibit the unwanted reflexive saccade. Ford et al. (2005; using fMRI) and McDowell et al. (2005, using EEG/MEG) also found increased DLPFC activity prior to antisaccade generation, consistent with the putative role of DLPFC in the inhibition of an unwanted saccade toward the peripheral cue.
Not all studies have shown increased DLPFC activity during antisaccades relative to prosaccades (O’Driscoll et al., 1995; Paus et al., 1993; Raemaekers et al., 2002; Raemaekers et al., 2006a, 2006b); however, the context in which the tasks are performed has been shown to affect the underlying brain activity (Dyckman et al., 2007; Manoach et al., 2007). In an fMRI study, Dyckman et al. (2007) had participants perform pro- and anti-saccades in single saccade runs (i.e. only prosaccades or only antisaccades) and in a mixed saccade run (i.e. blocks of prosaccades alternating with blocks of antisaccades). Activity in DLPFC was greater for antisaccades than prosaccades when the single saccade runs were compared to one another, but when the tasks were presented together, there was no differential activity in DLPFC. One possible explanation for the result is that during the mixed saccade run, DLPFC may have tonic activity throughout the entire recording epoch due to the increased difficulty and more complex response selection requirements during the mixed task.
Findings from an EEG study by Clementz et al. (2007) are consistent with this interpretation. Participants completed prosaccade trials, antisaccade trials, and no-go trials in an interleaved design. The high temporal resolution of EEG showed temporally distinct peaks of activity that would not be evident using fMRI. Early right PFC activity (158ms post-stimulus presentation) was greater for anti- trials than for pro- or no-go trials, but later activity in this region (204ms post-stimulus) was the same for anti- and pro-trials. The lack of differences in PFC between pro- and anti-trials at the later time point is consistent with a lack of differential PFC activity observed in mixed saccade runs (Dyckman et al., 2007). Although the strength of the activity at this time point did not differ between pro- and anti-trials, there was a significant negative correlation between activity in right PFC and contralateral middle occipital gyrus activity only for anti-trials. Greater PFC activity was associated with reduced activity in middle occipital gyrus, perhaps exerting a top-down influence on visual cortex to suppress activity in order to avoid making a reflexive movement toward the stimulus.
Another region that is likely to be recruited for volitional, but not pro-, saccades is the anterior cingulate cortex (ACC) (see glossary), given its putative role in conflict monitoring (e.g. Braver et al., 2001; MacDonald et al., 2000; Miller & Cohen, 2001). Functional MRI studies have shown that ACC is involved in antisaccade performance (e.g. Brown et al., 2006; Doricchi et al., 1997; Gaymard et al., 1998; Matsuda et al., 2004; Milea et al., 2005; Polli et al., 2005), consistent with reports of increased ACC activity during other tasks requiring inhibitory control (e.g. Braver et al., 2001; Garavan et al., 2002). There are a number of theories regarding ACC function in the literature, including monitoring performance (Polli et al., 2005) and signaling the likelihood and actual occurrence of error responses (Brown & Braver, 2005; Endrass et al., 2007; Ford et al., 2005; Johnston et al., 2007; Nieuwenhuis et al., 2001). With regard to saccades, Ford et al. (2005) found increased activation of ACC for antisaccades, when an error is more likely, compared to prosaccades, when an error is less likely. Importantly, they also observed greater ACC activity for correct antisaccades as opposed to incorrect antisaccades in the period prior to stimulus onset. During the stimulus-response period, however, they observed greater ACC activity for incorrect antisaccades, suggesting that, in addition to signaling the likelihood of an error, ACC was also involved in error monitoring during the antisaccade task.
Differential patterns of activity have been observed between rostral (rACC) and dorsal (dACC) during antisaccades (Polli et al., 2005). In the early phase of an antisaccade trial, they found that deactivation of rACC was associated with correct performance. On the other hand, dACC showed no difference between correct and error trials during this same time period. Later, however, both rACC and dACC showed increased activity during error trials, consistent with the role of ACC in the evaluation of error responses and the results of Ford et al. (2005).
Subcortically, there are more reports of antisaccade-related than of prosaccade-related activity for striatum and thalamus (Brown et al., 2006; Dyckman et al., 2007; Ettinger et al., 2007; Sweeney et al., 1996; O’Driscoll et al., 1995). With the exception of Ettinger et al., 2007, much of the human functional neuroimaging research on the role of the striatum during voluntary saccades has been reported within the context of striatal dysfunction in schizophrenia. For instance, healthy subjects show increased antisaccade-related striatal activity compared with both subjects with schizophrenia and their relatives (Raemaekers et al., 2002, 2006, see also this issue). Increased antisaccade-related activity in the thalamus also has been reported both in the normal cognitive neuroscience literature (Dyckman et al., 2007; Ettinger et al., 2007; Matsuda et al., 2004; O’Driscoll et al., 1995) and within the context of being increased in healthy compared to schizophrenia subjects (Tu et al., 2006).
Ocular Motor Delayed Response (ODR) Tasks
ODR paradigms are voluntary saccade tasks that require both inhibitory and spatial working memory processes (e.g., Funahashi et al., 1989, 1993; Inoue et al., 2004; Sweeney et al., 1996). During ODR tasks, participants are instructed to remember the location of a peripherally presented visual target through a delay period (spatial working memory component) without making anticipatory saccades (inhibition component), and then to generate a saccade to that (unmarked) location after the delay period. Evidence from the human brain imaging literature demonstrates increased ODR-related activity in basic saccadic circuitry but with special emphasis on parietal and frontal regions (e.g. Berman & Colby, 2002; Brown et al., 2004; Camchong et al., 2006; Chafee & Goldman-Rakic, 2000; Curtis & D’Esposito, 2006; Geier et al., 2007; Inoue et al., 2004; Keedy et al., 2006; Luna & Sweeney, 1999; Ozyurt et al., 2006; Postle et al., 2000; Schluppeck et al., 2006; Srimal et al., 2007; Sweeney et al., 1996).
Regions in parietal cortex, specifically intraparietal sulcus (IPS; e.g. Brown et al., 2004; Curtis & D’Esposito, 2006; Geier et al., 2007; Schluppeck et al., 2006; Srimal & Curtis, 2007), and to a lesser extent supramarginal gyrus (SMG; e.g. Brown et al., 2004; Geier et al., 2007), have shown robust delay period activity during ODR tasks. Using a variable delay length (3–15 seconds in 1.5 second increments), Schluppeck et al. (2006) observed that the length of time the BOLD signal in IPS remained elevated corresponded to the length of the delay, clearly demonstrating sustained delay-period activity in IPS. A few studies have also indicated that ODR-related activity in IPS is lateralized, with stronger activity in IPS contralateral to the visual cue and subsequent saccade (Curtis & D’Esposito, 2006; Schluppeck et al., 2006).
Increased activity in FEF during memory-guided saccades has been a consistent finding in human functional neuroimaging studies (e.g. Brown et al., 2004; Camchong et al., 2006; Curtis & D’Esposito, 2006; Geier et al., 2007; Ozyurt et al., 2006; Sweeney et al., 1996; Srimal et al., 2008). Curtis and D’Esposito (2006) suggested that increased FEF activity during ODR tasks signals maintenance of the response requirement during the delay, as activity in this region persisted across the delay period. Srimal et al. (2008) investigated whether this delay period activity codes the location of the cue or prospectively codes the metric of the upcoming saccade, with their results supporting the former. Geier et al. (2007) examined the effect of short (2.5 sec) and long (10 sec) delays on activity in brain regions associated with ODR tasks. Participants did not know which trials would have short and which would have long delays. For left FEF, they observed sustained activity across both the short and long delay periods, suggesting that left FEF was involved in maintaining the cue location. Right FEF, however, showed sustained activity for the short delay, but the hemodynamic time course for the long delay showed two peaks. The first peak of activity was observed just prior to the time at which participants would make a response on short delay trials. When it became clear that it was not a short delay trial, activity decreased slightly then increased again prior to the time at which a response was required. This pattern in right FEF suggests that it may be involved in preparation and/or planning for a response, in addition to a possible role in maintenance.
There is considerable neurophysiology evidence that DLPFC supports ODR performance (e.g., Chafee & Goldman-Rakic, 1998; Chafee & Goldman-Rakic, 2000; Funahashi et al., 1989, 1993; Kojima & Goldman-Rakic, 1982; Takeda & Funahashi, 2002; Tsujimoto & Sawaguchi, 2004). In humans, lesions of the prefrontal cortex result in changes in saccade metrics and in an increased frequency of errors prior to central fixation offset (Pierrot-Deseilligny et al., 1991, 2003; Rivaud et al., 1994). Consistent with these data, human imaging studies, as well as both neurophysiological and imaging studies in monkeys, demonstrate increased signal in DLPFC associated with performance on delayed response tasks (Camchong et al., 2006; Chafee & Goldman-Rakic, 2000; Geier et al., 2007; Inoue et al., 2004; Keedy et al., 2006; Luna & Sweeney, 1999; O’Sullivan et al., 1995; Sweeney et al., 1996). It appears that DLPFC may be performing multiple functions during ODR tasks, including inhibition (e.g., Camchong et al., 2006, Ford et al., 2004, McDowell et al., 2002; Perlstein et al., 2003) and maintenance of spatial information over time to support memory-guided saccade performance (D’Esposito et al., 2000; Geier et al., 2007; Ploner et al., 1998, 2000; Postle et al., 2000). Whether these different functions are dependent on separate DLPFC regions, as one might expect, is at present uncertain.
Predictive Saccadic Tracking Tasks
Another volitional behavior of interest is predictive saccadic tracking (see glossary). During predictive tracking tasks, a stimulus typically alternates between fixed positions at a fixed time interval (McDowell et al., 1996; Ross & Ross, 1987). This can be considered a procedural learning task, as subjects comprehend the predictability of the target motion. Subjects soon generate reaction times around zero primarily due to the generation of saccades in anticipation of new target onset (Smit & VanGinsbergen, 1989). These so-called “anticipatory saccades” are hypothesized to be based on the memory trace of the sensory (visual) and/or motor signals generated during earlier trials. This dissociation between sensory-guided and volitional responses can be evaluated via human functional neuroimaging.
The expectation from the extant literature from other disciplines suggests a key role for FEF in predictive tracking (Everling & Munoz, 2000; Hanes & Schall 1995; Gaymard et al., 1999; Rivaud et al., 1994). Early PET studies (Law et al., 1997; O’Driscoll et al., 2000) showed predictive-related activity in medial and lateral FEF (as well as SEF, extrastriate, and medial occipital temporal cortex). In fMRI studies of predicable targets, more activity has been reported to predictable targets in FEF (as well as pre-SMA, ACC, pre-frontal and inferior parietal cortices, as well as dorsomedial thalamus and hippocampus). Gagnon et al. (2002) showed activity during predictive tasks in lateral and medial FEF that increased over time as the predictability became apparent. Simo et al. (2005) also showed a differentiation between activity in medial and lateral FEF; however, medial FEF was more associated with visuomotor responses depending on what they termed “externally-directed attentional states and sensorimotor transformations” (p.1987). It is suggested by Simo et al. (2005) that methodological differences may be responsible for the difference between their results and those of Gagnon et al. (2002), but more work is clearly needed to help clarify this issue as well as the functional differences between lateral and medial FEF. The relationship between FEF activity and predictive tracking might be a function of coordination with the cerebellum. Both FEFs and cerebellum are implicated in ocular motor preparation, with perhaps the cerebellum coordinating the timing between motor preparation and execution (O’Driscoll et al., 2000; Diener et al., 1989).
Saccade Sequence Tasks
The learning of saccade sequences is another volitional saccade task that invokes procedural learning, and involves, like predictive tracking, a change from more external sensory- to more internal volitional-driven circuitry. Subjects are provided with sequential targets and are instructed to generate saccades to the memorized positions in a learned order. Research has tended to focus on the role of SEF in these tasks because supplementary motor regions are clearly important for motor sequence learning (Isoda & Tanji, 2002, 2003; Mushiake et al., 1990) and lesions impair this ability by increasing errors (Gaymard et al., 1990, 1993) and decreasing final position accuracies (Parton et al., 2007).
The few studies using PET and/or fMRI to evaluate saccade sequence learning have provided additional evidence for the involvement of SEF in motor learning in healthy humans. A PET study (Petit et al., 1996) demonstrated that when learned saccade sequences were compared with self paced saccades, increased sequence-related activity was observed in the SEF, intraparietal cortex, superior frontal sulcus, and precuneus. A fMRI study (Grosbras et al., 2001), which was designed to emphasize the transition from sensory to memory-driven circuitry, juxtaposed well-practiced with newly acquired saccade sequences. The data showed greater activity in SEF and precuneus during newly learned saccade sequences. Grosbras et al. (2001) suggested that the area of greatest import in facilitating sequence acquisition may be the pre-SEF, an area more rostral and inferior to the traditional SEF region. Heide et al. (2001) suggest that pre-SEF may contribute more to planning and memorizing new sequence whereas SEF proper may contribute more to the execution of such memorized sequences. The functional distinctions between SEF and pre-SEF have yet to be fully understood (see, e.g., Merriam et al., 2001; Miller et al., 2005), so further research on their respective roles in saccadic control are warranted.
Besides SEF, other regions including PPC, FEF, and ACC also have been repeatedly implicated in saccade sequence learning (Baumann et al., 2006; Heide et al., 2001; Kawashima et al, 1998). In a triple-step version of the task, in which a sequence of three saccades was performed, Heide et al. (2001) reported bilateral activation of three areas of PPC – precuneus and two distinct areas of IPS. Of these regions, only the middle portion of the IPS showed significant difference in activation when compared with a visually-guided saccade baseline. This region may correspond to LIP in monkeys (Grefkes & Fink, 2005; Koyama et al., 2004) and may specifically contribute to the remapping of spatial representations of saccade targets to compensate for previous eye displacement (Heide et al., 2001), a notion supported by numerous other functional imaging studies (Dieterich et al., 1998; Goebel et al., 1998; Vallar et al., 1999; Harris et al., 2000).
Functional imaging studies also report FEF activation during saccadic sequences (Petit et al., 1996; Heide et al., 1995) which supports previous evidence for the role of FEF in the control of internally generated saccades in general (Pierrot-Deseilligny et al., 1995; Hanes & Schall, 1996; Heide & Kompf, 1998). Notably, FEF shows greater activation during the triple-step sequence paradigm compared to either pro- or memory saccades (Heide et al., 2001). Studies in monkeys report that FEF neurons may hold a saccade-related efference copy signal (Goldberg & Bruce, 1990; Umeno & Goldberg, 1997; Tian et al., 2000), so activation of this region could reflect the triggering of saccadic sequences and the spatial computations required for their accuracy.
Caudal anterior cingulate gyrus is also implicated in self-paced saccade sequences and may reflect the self-initiation and the control of intentional saccades (Heide et al., 2001). Lesion studies indicate that damage to the caudal cingulate impairs initiation and accuracy of internally generated intentional saccades (Gaymard et al., 1998). In comparison, the rostral portion of ACC may contribute to sustained attention and on-line monitoring of saccade sequence performance (Heide et al., 2001) as part of the cortical network for visuospatial attention (Carter et al., 1998; Nobre et al., 1997, 2000).
CONCLUSIONS
In reviewing the human functional neuroimaging literature on the transition from basic exogenously-driven sensorimotor (pro- and express saccades), to more endogenously-driven cognitively complex volitional saccade tasks (antisaccades, ocular motor delayed response (memory saccades), predictive saccadic tracking (anticipatory saccades), and saccade sequencing), there are three general conclusions. First, the basic saccadic circuitry supporting prosaccades is also activated by volitional tasks. Typically basic saccade circuitry that supports prosaccades is more active and additional cortical regions appear to be recruited to support volitional behavior (Connolly et al., 2000; Dyckman et al., 2007; Mort et al., 2003). The extant data indicate that prefrontal regions may be more active during antisaccade and other volitional saccade tasks that require inhibition and/or spatial memory (Camchong et al., 2006; Ford et al. 2005, Keedy et al., 2006; McDowell et al., 2002, 2005), although this effect may vary as a function of stimulus context (Clementz et al., 2007; Dyckman et al., 2007). While the majority of these studies used fMRI, a differential pattern showing increased activity preceding antisaccades as compared to prosaccades in medial FEF, SEF and prefrontal cortex also was demonstrated using EEG and MEG (Clementz et al., 2007; McDowell et al., 2005). Interestingly, this same pattern was observed during intracranial recordings of human SEF and FEF (Lachaux et al., 2006), suggesting that it is not simply an artifact of functional neuroimaging technologies.
Second, it appears as if activity in visual cortex is modulated as a function of task demands and may predict the type of saccade to be generated. For instance, in a high-density EEG study there was an increase in activity in visual cortex prior to prosaccades whereas the activity decreased prior to antisaccades (Clementz et al., 2008). These data indicate that activity in striate and extrastriate cortex early in the course of stimulus registration is modulated as a function of task demands, perhaps via top-down control mechanisms (e.g., Clementz et al., 2007; Miller & Cohen, 2001; Moore & Armstrong, 2003; Trappenberg et al., 2001).
Third, there is accumulating evidence from volitional saccade paradigms for two foci of activation within FEF: medial and lateral (Beauchamp et al., 2001; Berman et al., 1999; Ettinger et al., 2007; Lobel et al., 2001; Luna et al., 1998; Heide et al., 2001; Grosbras et al., 2001; McDowell et al., 2005; Mort et al., 2003; Paus, 1996; Perry & Zeki, 2000; Petit et al., 1996; Simo et al., 2005). The functional correlates of these different FEF regions have yet to be fully delimited, although one hypothesis is that they are differentially involved in sensorimotor versus volitional requirements. If medial FEF in humans are more associated with volitional demands, it would be closer in function to the monkey FEF and its use in more complex tasks (Koyama et al., 2004). If lateral FEF in humans is more closely associated with reflexive movements, it would be consistent with its placement in the pre-motor strip (Paus, 1996; Tehovnik et al., 2000).
Evidence supporting the division of FEF regions comes from various imaging technologies, including PET, fMRI, MEG/EEG and even diffusion tensor imaging (DTI), a MR-derived measure that allows for identification of fiber (white matter) tracts in the human brain. For instance, in a delayed antisaccade task designed to distinguish activity associated with either the inhibition or the generation of a saccade, Ettinger et al. (2007) reported that medial FEF activity was more associated with inhibition than generation. Similarly, an EEG/MEG study showed distinctly different patterns of activity between medial and lateral FEF (McDowell et al., 2005), with medial FEF activity being higher for anti- than pro-saccades throughout the response preparation period. Lateral FEF activity, however, initially increased for both pro- and anti-saccades, continued to increase on pro-trials, but subsequently decreased on anti-trials as the time for response generation neared, which was perhaps a neural correlate of an active inhibition process (McDowell et al., 2005). Finally, a recent DTI study in humans (Tomassini et al., 2007) reported that medial FEF and lateral FEF have different connections to parietal and frontal regions. Medial FEF projected to superior parietal lobe, dorsal prefrontal cortex and cingulate gyrus, regions most often associated with more volitional kinds of saccades. Medial FEF also were more closely associated with tasks that required nonstandard mapping (which required a motor response contingent upon a cue), whereas lateral FEF were more related to standard mapping tasks (such as reaching). At present, however, there is not enough scientific information to confidently summarize the functional differences between these two FEF regions in humans.
Future Directions
Based on the rapid growth in human functional neuroimaging studies of saccade performance and the advancement of technologies and analysis techniques used in the field, we have identified four issues that would be particularly helpful to consider when conducting and reporting future studies. First, it could be advantageous to clearly delineate important anatomical subdivisions that underlie functional differences, such as seen with the emerging distinction between lateral and medial FEF. Recently, Amiez et al. (2006) have shown that fMRI activity, which has been reported as showing individual variability in anatomic location (Darby et al., 1996), can be reliably related to morphological aspects of the sulci in the human brain at the individual subject level. This type of analysis could allow the assessment of the functional dissociation between brain regions that are very close to one another, such as regions within FEF, posterior parietal cortex, and cerebellum. Posterior parietal cortex supports numerous higher order functions, including various components of visuospatial and attentional processes. Parietal cortex activity in functional neuroimaging studies of saccadic performance is often widespread, including activity in precuneus, medial and lateral aspects of the intraparietal sulcus, supramarginal gyrus, and Brodmann’s area 7. In order to understand the unique, and collective, contributions of these regions, reports on posterior parietal cortex activity must be specific about the location as a function of task. In addition to consistent reporting of co-ordinates to allow for better precision of mapping relationships between anatomy and cognitive functions, another solution may be the use of cortical surface mapping which allows fine detail to be observed around sulci (e.g., see Konen et al., 2004).
Similarly, the cerebellum is clearly involved in saccade responses. The fastigial nucleus may be involved in amplitude modulation and possibly saccade triggering (Noda et al., 1991). The vermis also may be involved in determining saccadic accuracy (Robinson & Fuchs, 2001; Munoz, 2002; Vahedi et al., 1995). Imaging studies specifically have demonstrated increased activity in vermis during saccades (Law et al., 1997, Stephan et al., 2002) and a relationship between cerebellar volume and saccade accuracy (Ettinger et al., 2005). Despite the importance of cerebellum for motor functions, there are few functional neuroimaging reports focusing on cerebellum involvement in human saccadic responses (Dietrich et al., 2000; Law et al., 1997, Stephan et al., 2002). Perhaps this is partly the case for technical reasons. For example, with fMRI there are necessary compromises between tissue covered and acquisition speed (number of slices and the TR). With technological advancements such as ready access to 3T (or greater) MRI systems, this may be less of a limiting factor for studying cerebellar involvement in saccadic responses.
Second, evaluating functional connectivity of the anatomical regions that support saccade generation could be extremely useful. To the present, human brain mapping has been largely used to identify anatomical regions supporting specific saccadic task demands. Studying how these brain regions interact to regulate behavioral output under varying task demands could be particularly exciting (Babiloni et al., 2005; Ramnani et al., 2004). Functional connectivity analyses complement functional anatomy information by describing the flow of information between neural networks (circuitries). Although numerous analytical techniques are available to suit this purpose, we will discuss here two different classes of existing methods including (i) data driven methods and (ii) hypothesis driven models.
Statistical data driven methods that avoid the use of an a priori model include principal component analysis (PCA; e.g. Friston et al., 1993; Friston et al., 2000) and independent component analysis (ICA; e.g. Bell & Sejnowski, 1995; Calhoun et al., 2004; McKeown, 2000). For fMRI, these methods move from examining activity in individual voxels over time to segregating data into spatially and/or temporally distinct patterns (McKeown & Sjenowski, 1998) that account for shared variance in the neural activity data (sometimes referred to as latent variables). These methods are useful for extracting task-related signal, as well as signals from physiologically-related non-task components (movement and other artifacts) and various other transients (McKeown et al., 1998).
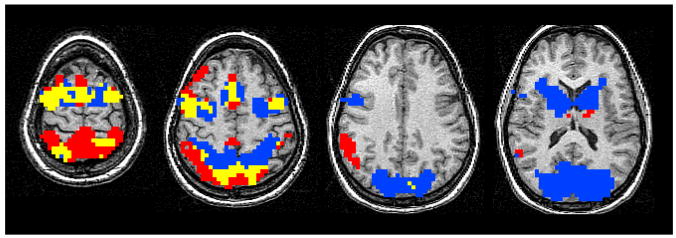
Recently a fMRI study from our laboratory (Dyckman et al., 2007) used spatial ICA to derive task-related waveforms and to eliminate noise in a block designed study of pro- and anti-saccade performance. Interestingly, ICA identified two task-related components with a slight temporal offset (a 3.8 sec shift). Regions where brain activities were significantly associated with both components were numerous, but there also were brain regions that showed differential activations as a function of component and task (Figure 5). For instance, the first component solely identified activity in striatum and visual areas, and the second component, the one shifted later in time by 3.8 sec, was associated with activations in higher-order parietal and frontal regions. Although the nature of the block design prohibits making strong inferences about timing, the distinction between the earlier component being associated with more basic visual and motor regions and the later component being associated with higher-order cognitive regions may be significant. Likewise, a fMRI study of memory saccades used PCA and reported distinct time patterns associated with regional variations in neural activations (Sugiura et al., 2004). These techniques also have been applied to EEG pro- and anti-saccade data in order to evaluate the time course of spatially related neural activations (e.g., Clementz et al., 2007; Klein & Feige, 2005; Richards, 2003).
Figure 5.
Images displaying antisaccade activity from 4 illustrative axial slices (from superior to inferior z=64, 48, 32, 16). Colors show antisaccade-related activity that is correlated with model-free reference waves identified using ICA. The blue areas show activity associated with component 1, the red areas show activity associated with component 2, and the yellow areas show activity associated with both components. Figures are shown using radiological convention (right hemisphere on the left side). Adapted from Dyckman et al, 2007.
A second example of the various approaches to functional connectivity analysis are hypothesis driven models that are based on known, suspected, and/or observed functional anatomy. The basis is that functionally related anatomical regions will have mutually influential patterns of activity. The goal is to make statements about causal effects among tasks and regions where inference is restricted to networks comprised of pre-selected regions of interest. Examples of such approaches (see, e.g., Penny et al., 2004, for a more detailed description) include structural equation modeling (SEM), which is the most widely used model for connectivity analyses in functional neuroimaging (Ramnani et al., 2004; Goncalves & Hall, 2003), dynamic causal modeling, which was developed specifically for fMRI data (Friston et al., 2003), and cortical connectivity analyses using event-related fMRI (Miller et al., 2005) or multimodal imaging data (Babiloni et al., 2005). A particularly interesting study (Buchel et al., 1999) used path analysis to identify individual learning-related performance of an object-location association task that was associated with connectivity between distinct cortical systems specialized for spatial and object processing. Given that circuitry supporting saccade performance is extremely well-defined, connectivity analyses may be particularly well-suited for this research area.
Third, the above summary generally reported on human functional neuroimaging data showing activity being related to one type of saccade task and that condition of interest was compared to a baseline task, often either prosaccades or a fixation condition. It is apparent, however, that the comparison task is critically important for drawing inferences about the brain regions that support higher order cognitive operations. There is clear evidence that the context in which saccade tasks are presented affects behavior and brain activity based on how tasks alternate within blocks (Dyckman et al., 2007) and these effects can be discerned even on a trial-by-trial basis (Cherkasova et al., 2002; Manoach et al., 2007). As described above, we presented subjects with a blocked fMRI study of antisaccades that were presented in runs of either a single saccade type (blocks of antisaccades versus blocks of fixation) or mixed saccade types (blocks of antisaccades compared with blocks of prosaccades). In the mixed runs only precuneus, SEF and FEF showed increased anti-saccade-related activity, suggesting the importance of these regions in supporting more complex saccade responses. Other brain regions, such as DLPFC, however, showed anti-saccade-related activity only during the single task comparison, suggesting that they are involved with more general cognitive process, such as executive control and/or context updating in addition to possible roles in complex saccade generation. Furthermore, within the mixed runs more errors were generated in the first trial after a switch (e.g. from pro- to anti-saccade block), as had been reported by others (Cherkasova et al., 2002). This trial by trial performance difference also has been shown to be related to brain activity. Manoach et al., (2007) used event-related fMRI to demonstrate that a preceding antisaccade resulted in longer reaction times and decreased activity in saccade-related brain regions. Thus, the context in which saccadic trials are presented is clearly important and likely under-evaluated in functional neuroimaging studies. Optimally, this issue should be addressed in the discussion section of relevant functional neuroimaging reports. At the very least detailed information should be reported in methods sections concerning the types, number and order of trials presented.
The fourth and final goal for future human functional neuroimaging studies should be the use of multi-modal imaging as a means for developing the most sophisticated spatial and temporal models of neural activations that support saccadic control (see, e.g., Babiloni et al., 2005). The integration of human functional neuroimaging modalities with high spatial resolution (PET and fMRI), and those offering excellent temporal resolution (EEG and MEG) promises to “reveal the temporal and topographical bases of behavioral control” (Garavan et al., 2002; p. 1828). In the future we can expect multimodal imaging studies of saccadic system functioning to a) provide an improved understanding of the functional interactions between anatomical regions, and b) determine how those interactions mediate cognitive control in healthy humans, as well as in those with psychiatric and neurological disorders.
FMRI
Functional magnetic resonance imaging (fMRI) is a non-invasive neuroimaging technique conducted using a MRI system similar to those found in hospital settings. This technique allows for the investigation of brain changes associated with perceptual, motor and cognitive processes. Typically such processes are supported by increased activity levels in task-related neural circuits, in which case increased neural activity is associated with a cascade of events that may include increased metabolic rate, vasodialation, and increased blood volume and blood flow. The resulting influx of oxygenated hemoglobin surpasses the local neuronal metabolic need so there is an increased ratio of oxygenated to deoxygenated hemoglobin. Because deoxygenated hemoglobin is paramagnetic (disrupts local magnetic signals), greater oxygenation results in a corresponding decrease in magnetic signal disruption (i.e. effectively a signal increase). Thus, fMRI is typically based on the “blood oxygen level dependent” (BOLD) signal, a measure of local hemodynamic changes that are indirectly associated with changes in neural activity.
BOLD data are often displayed on high resolution structural brain images as a map of colored voxels indicating areas of signal change. Regions of signal change can be described in three dimensions using standard normalized brain space (e.g. Talairach Atlas or Montreal Neurologic Institute Atlas co-ordinates).
An advantage of fMRI when compared to other neuroimaging techniques is its excellent spatial resolution (i.e. detection of “where” in the brain changes occur). In whole brain fMRI studies the spatial resolution is typically on the order of a few millimeters. A disadvantage, however, is the relatively poor temporal resolution (i.e. detection of changes in the brain over time). Because of the hemodynamic basis of fMRI, the temporal resolution has a limit on the order of seconds, much slower than neuronal firing rates.
EEG
Electroencephalography (EEG) is a non-invasive neuroimaging technique for recording neural activity using sensors affixed to the scalp (numbering from only a few to as many as 256). The neural signals recorded by EEG sensors are thought to be primarily graded postsynaptic field potentials from the apical dendrites of thousands of synchronously-firing pyramidal cells in localized regions of superficial cerebral cortex. When EEG signals are extracted from the ongoing EEG in relation to specific stimuli (e.g., the onset of a visual target prior to saccade generation), cognitive events, or the onset of a particular motor response (e.g., generation of an antisaccade), the resulting waveforms, typically averaged over many trials of the same type, are called event-related potentials (ERPs). ERPs are derived by averaging the ongoing EEG signals in relation to the onset of an event of interest (e.g., from 200 ms before to 800 ms after stimulus onset). After averaging, the voltage deviations specifically associated with processing the event(s) can be seen more clearly because the random neural activity not related to stimulus processing are “averaged out” (those signals are not time-locked to event processing).
ERP data historically have been displayed as single sensor voltage variations over time, showing peaks and valleys (positive and negative voltage variations) in the ERP signal in relation to event onset. It is also common to show topographic maps at an individual time point of interest (e.g., the P100, with ‘P’ indicating the voltage at the top of the head, historically at Cz, the most superior, or vertex, sensor position, and ‘100’ indicating that the neural activity occurred 100 ms after event onset). Topographic maps are 2D or 3D displays of isopotential contour lines (much like an elevation graph for mountainous terrain) that are useful for superficially inferring the brain regions that were most active at that time point. To more accurately infer the neural sources generating the signals recorded at the scalp, however, it is necessary to use sophisticated mathematical algorithms that transform the data from “sensor” to “source” space, with the source space solutions then being displayed on 3D reconstructions of the brain (which can end up looking much like fMRI data).
An advantage of EEG/ERPs when compared to fMRI is the excellent temporal resolution (detecting “when” changes in neural activity occurred in relation to an event of interest) on a millisecond time scale. A disadvantage of this technology, however, is uncertain spatial resolution (identifying where changes in neural activity occurred) because signals recorded at the scalp must be used to estimate from where in the brain the signals of interest originated.
Acknowledgments
The authors would like to thank Dr. Jazmin Camchong, Michael Amlung and Friederike Meyer for their contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE, Crutcher MD. Neural representations of the target (goal) of visually guided arm movements in three motor areas of the monkey. J Neurophysiol. 1990;64(1):164–178. doi: 10.1152/jn.1990.64.1.164. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Amador N, Schlag-Rey M, Schlag J. Primate antisaccade. II. Supplementary eye field neuronal activity predicts correct performance. J Neurophysiol. 2004;91(4):1672–1689. doi: 10.1152/jn.00138.2003. [DOI] [PubMed] [Google Scholar]
- Amiez C, Kostopoulos P, Champod AS, Petrides M. Local morphology predicts functional organization of the dorsal premotor region in the human brain. J Neurosci. 2006;26(10):2724–2731. doi: 10.1523/JNEUROSCI.4739-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson TJ, Jenkins IH, Brooks DJ, Hawken MB, Frackowiak RS, Kennard C. Cortical control of saccades and fixation in man. A PET study. Brain. 1994;117(Pt 5):1073–1084. doi: 10.1093/brain/117.5.1073. [DOI] [PubMed] [Google Scholar]
- Armstrong IT, Chan F, Riopelle RJ, Munoz DP. Control of saccades in Parkinson’s disease. Brain Cogn. 2002;49(2):198–201. [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23(11):4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni F, Cincotti F, Babiloni C, Carducci F, Mattia D, Astolfi L, et al. Estimation of the cortical functional connectivity with the multimodal integration of high-resolution EEG and fMRI data by directed transfer function. Neuroimage. 2005;24(1):118–131. doi: 10.1016/j.neuroimage.2004.09.036. [DOI] [PubMed] [Google Scholar]
- Band GP, van Boxtel GJ. Inhibitory motor control in stop paradigms: review and reinterpretation of neural mechanisms. Acta Psychol (Amst) 1999;101(2–3):179–211. doi: 10.1016/s0001-6918(99)00005-0. [DOI] [PubMed] [Google Scholar]
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. II. Spatial properties. J Neurophysiol. 1991;66(3):1109–1124. doi: 10.1152/jn.1991.66.3.1109. [DOI] [PubMed] [Google Scholar]
- Barbas H, Mesulam MM. Organization of afferent input to subdivisions of area 8 in the rhesus monkey. J Comp Neurol. 1981;200(3):407–431. doi: 10.1002/cne.902000309. [DOI] [PubMed] [Google Scholar]
- Basso G, Nichelli P, Wharton CM, Peterson M, Grafman J. Distributed neural systems for temporal production: a functional MRI study. Brain Res Bull. 2003;59(5):405–411. doi: 10.1016/s0361-9230(02)00941-3. [DOI] [PubMed] [Google Scholar]
- Baumann O, Frank G, Rutschmann RM, Greenlee MW. Cortical activation during sequences of memory-guided saccades: a functional MRI study. Neuroreport. 2007;18(5):451–455. doi: 10.1097/WNR.0b013e32805868ba. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Petit L, Ellmore TM, Ingeholm J, Haxby JV. A parametric fMRI study of overt and covert shifts of visuospatial attention. Neuroimage. 2001;14(2):310–321. doi: 10.1006/nimg.2001.0788. [DOI] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7(6):1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Daum I. Time course of cross-hemispheric spatial updating in the human parietal cortex. Behav Brain Res. 2006;169(1):150–161. doi: 10.1016/j.bbr.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Berman RA, Colby CL. Spatial working memory in human extrastriate cortex. Physiol Behav. 2002;77(4–5):621–627. doi: 10.1016/s0031-9384(02)00897-1. [DOI] [PubMed] [Google Scholar]
- Berman RA, Colby CL, Genovese CR, Voyvodic JT, Luna B, Thulborn KR, et al. Cortical networks subserving pursuit and saccadic eye movements in humans: an FMRI study. Hum Brain Mapp. 1999;8(4):209–225. doi: 10.1002/(SICI)1097-0193(1999)8:4<209::AID-HBM5>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoz A. The role of inhibition in the hierarchical gating of executed and imagined movements. Brain Res Cogn Brain Res. 1996;3(2):101–113. doi: 10.1016/0926-6410(95)00035-6. [DOI] [PubMed] [Google Scholar]
- Bizzi E. Discharge of frontal eye field neurons during eye movements in unanesthetized monkeys. Science. 1967;157(796):1588–1590. doi: 10.1126/science.157.3796.1588. [DOI] [PubMed] [Google Scholar]
- Blekher TM, Yee RD, Kirkwood SC, Hake AM, Stout JC, Weaver MR, et al. Oculomotor control in asymptomatic and recently diagnosed individuals with the genetic marker for Huntington’s disease. Vision Res. 2004;44(23):2729–2736. doi: 10.1016/j.visres.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Boch RA, Goldberg ME. Participation of prefrontal neurons in the preparation of visually guided eye movements in the rhesus monkey. J Neurophysiol. 1989;61(5):1064–1084. doi: 10.1152/jn.1989.61.5.1064. [DOI] [PubMed] [Google Scholar]
- Bodner M, Kroger J, Fuster JM. Auditory memory cells in dorsolateral prefrontal cortex. Neuroreport. 1996;7(12):1905–1908. doi: 10.1097/00001756-199608120-00006. [DOI] [PubMed] [Google Scholar]
- Botzel K, Rottach K, Buttner U. Normal and pathological saccadic dysmetria. Brain. 1993;116(Pt 2):337–353. doi: 10.1093/brain/116.2.337. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Garbutt S, Rankin KP, Hellmuth J, Neuhaus J, Miller BL, et al. Medial versus lateral frontal lobe contributions to voluntary saccade control as revealed by the study of patients with frontal lobe degeneration. J Neurosci. 2006;26(23):6354–6363. doi: 10.1523/JNEUROSCI.0549-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer AL, Garbutt S, Rankin KP, Hellmuth J, Neuhaus J, Miller BL, et al. Medial versus lateral frontal lobe contributions to voluntary saccade control as revealed by the study of patients with frontal lobe degeneration. J Neurosci. 2006;26(23):6354–6363. doi: 10.1523/JNEUROSCI.0549-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun D, Weber H, Mergner T, Schulte-Monting J. Saccadic reaction times in patients with frontal and parietal lesions. Brain. 1992;115(Pt 5):1359–1386. doi: 10.1093/brain/115.5.1359. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11(9):825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307(5712):1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Brown MR, DeSouza JF, Goltz HC, Ford K, Menon RS, Goodale MA, et al. Comparison of memory- and visually guided saccades using event-related fMRI. J Neurophysiol. 2004;91(2):873–889. doi: 10.1152/jn.00382.2003. [DOI] [PubMed] [Google Scholar]
- Brown MR, Goltz HC, Vilis T, Ford KA, Everling S. Inhibition and generation of saccades: rapid event-related fMRI of prosaccades, antisaccades, and nogo trials. Neuroimage. 2006;33(2):644–659. doi: 10.1016/j.neuroimage.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985;53(3):603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Buchel C, Coull JT, Friston KJ. The predictive value of changes in effective connectivity for human learning. Science. 1999;283(5407):1538–1541. doi: 10.1126/science.283.5407.1538. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pekar JJ. A method for comparing group fMRI data using independent component analysis: application to visual, motor and visuomotor tasks. Magn Reson Imaging. 2004;22(9):1181–1191. doi: 10.1016/j.mri.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Camchong J, Dyckman KA, Chapman CE, Yanasak NE, McDowell JE. Basal ganglia-thalamocortical circuitry disruptions in schizophrenia during delayed response tasks. Biol Psychiatry. 2006;60(3):235–241. doi: 10.1016/j.biopsych.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol. 1998;79(6):2919–2940. doi: 10.1152/jn.1998.79.6.2919. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Inactivation of parietal and prefrontal cortex reveals interdependence of neural activity during memory-guided saccades. J Neurophysiol. 2000;83(3):1550–1566. doi: 10.1152/jn.2000.83.3.1550. [DOI] [PubMed] [Google Scholar]
- Chawla D, Rees G, Friston KJ. The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci. 1999;2(7):671–676. doi: 10.1038/10230. [DOI] [PubMed] [Google Scholar]
- Cherkasova MV, Manoach DS, Intriligator JM, Barton JJ. Antisaccades and task-switching: interactions in controlled processing. Exp Brain Res. 2002;144(4):528–537. doi: 10.1007/s00221-002-1075-z. [DOI] [PubMed] [Google Scholar]
- Clementz BA. The ability to produce express saccades as a function of gap interval among schizophrenia patients. Exp Brain Res. 1996;111(1):121–130. doi: 10.1007/BF00229561. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Brahmbhatt SB, McDowell JE, Brown R, Sweeney JA. When does the brain inform the eyes whether and where to move? An EEG study in humans. Cereb Cortex. 2007;17(11):2634–2643. doi: 10.1093/cercor/bhl171. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Gao Y, McDowell JE, Moratti S, Keedy SK, Sweeney J. Modulation of neural investment in peripheral target locations preceding pro-and anti-saccades. Manuscript under review 2008 [Google Scholar]
- Clementz BA, McDowell JE, Stewart SE. Timing and magnitude of frontal activity differentiates refixation and anti-saccade performance. Neuroreport. 2001;12(9):1863–1868. doi: 10.1097/00001756-200107030-00020. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol. 1996;76(5):2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Collins CE, Lyon DC, Kaas JH. Distribution across cortical areas of neurons projecting to the superior colliculus in new world monkeys. Anat Rec A Discov Mol Cell Evol Biol. 2005;285(1):619–627. doi: 10.1002/ar.a.20207. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Andersen RA, Goodale MA. FMRI evidence for a ‘parietal reach region’ in the human brain. Exp Brain Res. 2003;153(2):140–145. doi: 10.1007/s00221-003-1587-1. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Desouza JF, Menon RS, Vilis T. A comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing. J Neurophysiol. 2000;84(3):1645–1655. doi: 10.1152/jn.2000.84.3.1645. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Goltz HC, Munoz DP. fMRI activation in the human frontal eye field is correlated with saccadic reaction time. J Neurophysiol. 2005;94(1):605–611. doi: 10.1152/jn.00830.2004. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, et al. A common network of functional areas for attention and eye movements. Neuron. 1998;21(4):761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Cornelissen FW, Kimmig H, Schira M, Rutschmann RM, Maguire RP, Broerse A, et al. Event-related fMRI responses in the human frontal eye fields in a randomized pro- and antisaccade task. Exp Brain Res. 2002;145(2):270–274. doi: 10.1007/s00221-002-1136-3. [DOI] [PubMed] [Google Scholar]
- Culham JC, Valyear KF. Human parietal cortex in action. Curr Opin Neurobiol. 2006;16(2):205–212. doi: 10.1016/j.conb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Success and failure suppressing reflexive behavior. J Cogn Neurosci. 2003;15(3):409–418. doi: 10.1162/089892903321593126. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Selection and maintenance of saccade goals in the human frontal eye fields. J Neurophysiol. 2006;95(6):3923–3927. doi: 10.1152/jn.01120.2005. [DOI] [PubMed] [Google Scholar]
- Darby DG, Nobre AC, Thangaraj V, Edelman R, Mesulam MM, Warach S. Cortical activation in the human brain during lateral saccades using EPISTAR functional magnetic resonance imaging. Neuroimage. 1996;3(1):53–62. doi: 10.1006/nimg.1996.0006. [DOI] [PubMed] [Google Scholar]
- DeSouza JF, Menon RS, Everling S. Preparatory set associated with pro-saccades and anti-saccades in humans investigated with event-related FMRI. J Neurophysiol. 2003;89(2):1016–1023. doi: 10.1152/jn.00562.2002. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Ballard D, Zarahn E, Aguirre GK. The role of prefrontal cortex in sensory memory and motor preparation: an event-related fMRI study. Neuroimage. 2000;11(5 Pt 1):400–408. doi: 10.1006/nimg.2000.0571. [DOI] [PubMed] [Google Scholar]
- Diener HC, Dichgans J, Guschlbauer B, Bacher M, Langenbach P. Disturbances of motor preparation in basal ganglia and cerebellar disorders. Prog Brain Res. 1989;80:481–488. doi: 10.1016/s0079-6123(08)62247-5. discussion 479–480. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Bucher SF, Seelos KC, Brandt T. Cerebellar activation during optokinetic stimulation and saccades. Neurology. 2000;54(1):148–155. doi: 10.1212/wnl.54.1.148. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Perani D, Incoccia C, Grassi F, Cappa SF, Bettinardi V, et al. Neural control of fast-regular saccades and antisaccades: an investigation using positron emission tomography. Exp Brain Res. 1997;116(1):50–62. doi: 10.1007/pl00005744. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Munoz DP. Saccadic probability influences motor preparation signals and time to saccadic initiation. J Neurosci. 1998;18(17):7015–7026. doi: 10.1523/JNEUROSCI.18-17-07015.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J, Frith C. Shifting baselines in attention research. Nat Rev Neurosci. 2000;1(2):147–148. doi: 10.1038/35039083. [DOI] [PubMed] [Google Scholar]
- Dyckman KA, Camchong J, Clementz BA, McDowell JE. An effect of context on saccade-related behavior and brain activity. Neuroimage. 2007;36(3):774–784. doi: 10.1016/j.neuroimage.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Endrass T, Reuter B, Kathmann N. ERP correlates of conscious error recognition: aware and unaware errors in an antisaccade task. Eur J Neurosci. 2007;26(6):1714–1720. doi: 10.1111/j.1460-9568.2007.05785.x. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Antonova E, Crawford TJ, Mitterschiffthaler MT, Goswani S, Sharma T, et al. Structural neural correlates of prosaccade and antisaccade eye movements in healthy humans. Neuroimage. 2005;24(2):487–494. doi: 10.1016/j.neuroimage.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Ffytche DH, Kumari V, Kathmann N, Reuter B, Zelaya F, et al. Decomposing the neural correlates of antisaccade eye movements using event-related FMRI. Cereb Cortex. 2008;18(5):1148–1159. doi: 10.1093/cercor/bhm147. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Kumari V, Chitnis XA, Corr PJ, Sumich AL, Rabe-Hesketh S, et al. Relationship between brain structure and saccadic eye movements in healthy humans. Neurosci Lett. 2002;328(3):225–228. doi: 10.1016/s0304-3940(02)00517-7. [DOI] [PubMed] [Google Scholar]
- Evdokimidis I, Smyrnis N, Constantinidis TS, Stefanis NC, Avramopoulos D, Paximadis C, et al. The antisaccade task in a sample of 2,006 young men. I. Normal population characteristics. Exp Brain Res. 2002;147(1):45–52. doi: 10.1007/s00221-002-1208-4. [DOI] [PubMed] [Google Scholar]
- Everling S, Fischer B. The antisaccade: a review of basic research and clinical studies. Neuropsychologia. 1998;36(9):885–899. doi: 10.1016/s0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Everling S, Krappmann P, Preuss S, Brand A, Flohr H. Hypometric primary saccades of schizophrenics in a delayed-response task. Exp Brain Res. 1996;111(2):289–295. doi: 10.1007/BF00227306. [DOI] [PubMed] [Google Scholar]
- Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci. 2000;20(1):387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraina S, Pare M, Wurtz RH. Comparison of cortico-cortical and cortico-collicular signals for the generation of saccadic eye movements. J Neurophysiol. 2002;87(2):845–858. doi: 10.1152/jn.00317.2001. [DOI] [PubMed] [Google Scholar]
- Fischer B, Ramsperger E. Human express saccades: extremely short reaction times of goal directed eye movements. Exp Brain Res. 1984;57(1):191–195. doi: 10.1007/BF00231145. [DOI] [PubMed] [Google Scholar]
- Fischer B, Weber H. Effects of stimulus conditions on the performance of antisaccades in man. Exp Brain Res. 1997;116(2):191–200. doi: 10.1007/pl00005749. [DOI] [PubMed] [Google Scholar]
- Fischer B, Weber H, Biscaldi M, Aiple F, Otto P, Stuhr V. Separate populations of visually guided saccades in humans: reaction times and amplitudes. Exp Brain Res. 1993;92(3):528–541. doi: 10.1007/BF00229043. [DOI] [PubMed] [Google Scholar]
- Ford KA, Goltz HC, Brown MR, Everling S. Neural processes associated with antisaccade task performance investigated with event-related FMRI. J Neurophysiol. 2005;94(1):429–440. doi: 10.1152/jn.00471.2004. [DOI] [PubMed] [Google Scholar]
- Fox PT, Fox JM, Raichle ME, Burde RM. The role of cerebral cortex in the generation of voluntary saccades: a positron emission tomographic study. J Neurophysiol. 1985;54(2):348–369. doi: 10.1152/jn.1985.54.2.348. [DOI] [PubMed] [Google Scholar]
- Friston K, Phillips J, Chawla D, Buchel C. Nonlinear PCA: characterizing interactions between modes of brain activity. Philos Trans R Soc Lond B Biol Sci. 2000;355(1393):135–146. doi: 10.1098/rstb.2000.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS. Principal component analysis learning algorithms: a neurobiological analysis. Proc Biol Sci. 1993;254(1339):47–54. doi: 10.1098/rspb.1993.0125. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19(4):1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61(2):331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365(6448):753–756. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- Gaarder K, Krauskopf J, Graf V, Kropfl W, Armington JC. Averaged Brain Activity Following Saccadic Eye Movement. Science. 1964;146:1481–1483. doi: 10.1126/science.146.3650.1481. [DOI] [PubMed] [Google Scholar]
- Gagnon D, O’Driscoll GA, Petrides M, Pike GB. The effect of spatial and temporal information on saccades and neural activity in oculomotor structures. Brain. 2002;125(Pt 1):123–139. doi: 10.1093/brain/awf005. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17(4):1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96(14):8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard B, Lynch J, Ploner CJ, Condy C, Rivaud-Pechoux S. The parieto-collicular pathway: anatomical location and contribution to saccade generation. Eur J Neurosci. 2003;17(7):1518–1526. doi: 10.1046/j.1460-9568.2003.02570.x. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Pierrot-Deseilligny C, Rivaud S. Impairment of sequences of memory-guided saccades after supplementary motor area lesions. Ann Neurol. 1990;28(5):622–626. doi: 10.1002/ana.410280504. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Ploner CJ, Rivaud S, Vermersch AI, Pierrot-Deseilligny C. Cortical control of saccades. Exp Brain Res. 1998;123(1–2):159–163. doi: 10.1007/s002210050557. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Ploner CJ, Rivaud-Pechoux S, Pierrot-Deseilligny C. The frontal eye field is involved in spatial short-term memory but not in reflexive saccade inhibition. Exp Brain Res. 1999;129(2):288–301. doi: 10.1007/s002210050899. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Rivaud S, Pierrot-Deseilligny C. Role of the left and right supplementary motor areas in memory-guided saccade sequences. Ann Neurol. 1993;34(3):404–406. doi: 10.1002/ana.410340317. [DOI] [PubMed] [Google Scholar]
- Geier CF, Garver KE, Luna B. Circuitry underlying temporally extended spatial working memory. Neuroimage. 2007;35(2):904–915. doi: 10.1016/j.neuroimage.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy J, Luders H, Dinner DS, Morris HH, Wyllie E. Versive eye movements elicited by cortical stimulation of the human brain. Neurology. 1990;40(2):296–299. doi: 10.1212/wnl.40.2.296. [DOI] [PubMed] [Google Scholar]
- Goebel R, Khorram-Sefat D, Muckli L, Hacker H, Singer W. The constructive nature of vision: direct evidence from functional magnetic resonance imaging studies of apparent motion and motion imagery. Eur J Neurosci. 1998;10(5):1563–1573. doi: 10.1046/j.1460-9568.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Bruce CJ. Primate frontal eye fields. III. Maintenance of a spatially accurate saccade signal. J Neurophysiol. 1990;64(2):489–508. doi: 10.1152/jn.1990.64.2.489. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Architecture of the prefrontal cortex and the central executive. Ann N Y Acad Sci. 1995;769:71–83. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- Goncalves MS, Hall DA. Connectivity analysis with structural equation modelling: an example of the effects of voxel selection. Neuroimage. 2003;20(3):1455–1467. doi: 10.1016/s1053-8119(03)00394-x. [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Goldberg ME. Activity of neurons in the lateral intraparietal area of the monkey during an antisaccade task. Nat Neurosci. 1999;2(10):906–912. doi: 10.1038/13209. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265(5180):1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- Greenlee MW. Human cortical areas underlying the perception of optic flow: brain imaging studies. Int Rev Neurobiol. 2000;44:269–292. doi: 10.1016/s0074-7742(08)60746-1. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. The functional organization of the intraparietal sulcus in humans and monkeys. J Anat. 2005;207(1):3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosbras MH, Leonards U, Lobel E, Poline JB, LeBihan D, Berthoz A. Human cortical networks for new and familiar sequences of saccades. Cereb Cortex. 2001;11(10):936–945. doi: 10.1093/cercor/11.10.936. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Lobel E, Van de Moortele PF, LeBihan D, Berthoz A. An anatomical landmark for the supplementary eye fields in human revealed with functional magnetic resonance imaging. Cereb Cortex. 1999;9(7):705–711. doi: 10.1093/cercor/9.7.705. [DOI] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18(10):1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274(5286):427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Harris IM, Egan GF, Sonkkila C, Tochon-Danguy HJ, Paxinos G, Watson JD. Selective right parietal lobe activation during mental rotation: a parametric PET study. Brain. 2000;123(Pt 1):65–73. doi: 10.1093/brain/123.1.65. [DOI] [PubMed] [Google Scholar]
- Heide W, Binkofski F, Seitz RJ, Posse S, Nitschke MF, Freund HJ, et al. Activation of frontoparietal cortices during memorized triple-step sequences of saccadic eye movements: an fMRI study. Eur J Neurosci. 2001;13(6):1177–1189. doi: 10.1046/j.0953-816x.2001.01472.x. [DOI] [PubMed] [Google Scholar]
- Heide W, Blankenburg M, Zimmermann E, Kompf D. Cortical control of double-step saccades: implications for spatial orientation. Ann Neurol. 1995;38(5):739–748. doi: 10.1002/ana.410380508. [DOI] [PubMed] [Google Scholar]
- Heide W, Kompf D. Combined deficits of saccades and visuo-spatial orientation after cortical lesions. Exp Brain Res. 1998;123(1–2):164–171. doi: 10.1007/s002210050558. [DOI] [PubMed] [Google Scholar]
- Heiser LM, Colby CL. Spatial updating in area LIP is independent of saccade direction. J Neurophysiol. 2006;95(5):2751–2767. doi: 10.1152/jn.00054.2005. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80(3):953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. II. Cortical connections. J Comp Neurol. 1987;265(3):332–361. doi: 10.1002/cne.902650304. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology. 2006;43(3):302–313. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- Inoue M, Mikami A, Ando I, Tsukada H. Functional brain mapping of the macaque related to spatial working memory as revealed by PET. Cereb Cortex. 2004;14(1):106–119. doi: 10.1093/cercor/bhg109. [DOI] [PubMed] [Google Scholar]
- Isoda M, Tanji J. Cellular activity in the supplementary eye field during sequential performance of multiple saccades. J Neurophysiol. 2002;88(6):3541–3545. doi: 10.1152/jn.00299.2002. [DOI] [PubMed] [Google Scholar]
- Isoda M, Tanji J. Contrasting neuronal activity in the supplementary and frontal eye fields during temporal organization of multiple saccades. J Neurophysiol. 2003;90(5):3054–3065. doi: 10.1152/jn.00367.2003. [DOI] [PubMed] [Google Scholar]
- Johnston K, Levin HM, Koval MJ, Everling S. Top-down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron. 2007;53(3):453–462. doi: 10.1016/j.neuron.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Kapoula Z, Isotalo E, Muri RM, Bucci MP, Rivaud-Pechoux S. Effects of transcranial magnetic stimulation of the posterior parietal cortex on saccades and vergence. Neuroreport. 2001;12(18):4041–4046. doi: 10.1097/00001756-200112210-00037. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22(4):751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Tanji J, Okada K, Sugiura M, Sato K, Kinomura S, et al. Oculomotor sequence learning: a positron emission tomography study. Exp Brain Res. 1998;122(1):1–8. doi: 10.1007/s002210050485. [DOI] [PubMed] [Google Scholar]
- Keedy SK, Ebens CL, Keshavan MS, Sweeney JA. Functional magnetic resonance imaging studies of eye movements in first episode schizophrenia: smooth pursuit, visually guided saccades and the oculomotor delayed response task. Psychiatry Res. 2006;146(3):199–211. doi: 10.1016/j.pscychresns.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Kennard C, Mannan SK, Nachev P, Parton A, Mort DJ, Rees G, et al. Cognitive processes in saccade generation. Ann N Y Acad Sci. 2005;1039:176–183. doi: 10.1196/annals.1325.017. [DOI] [PubMed] [Google Scholar]
- Kimmig H, Greenlee MW, Gondan M, Schira M, Kassubek J, Mergner T. Relationship between saccadic eye movements and cortical activity as measured by fMRI: quantitative and qualitative aspects. Exp Brain Res. 2001;141(2):184–194. doi: 10.1007/s002210100844. [DOI] [PubMed] [Google Scholar]
- Klein C, Feige B. An independent components analysis (ICA) approach to the study of developmental differences in the saccadic contingent negative variation. Biol Psychol. 2005;70(2):105–114. doi: 10.1016/j.biopsycho.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Kojima S, Goldman-Rakic PS. Delay-related activity of prefrontal neurons in rhesus monkeys performing delayed response. Brain Res. 1982;248(1):43–49. doi: 10.1016/0006-8993(82)91145-3. [DOI] [PubMed] [Google Scholar]
- Konen CS, Kleiser R, Wittsack HJ, Bremmer F, Seitz RJ. The encoding of saccadic eye movements within human posterior parietal cortex. Neuroimage. 2004;22(1):304–314. doi: 10.1016/j.neuroimage.2003.12.039. [DOI] [PubMed] [Google Scholar]
- Koyama M, Hasegawa I, Osada T, Adachi Y, Nakahara K, Miyashita Y. Functional magnetic resonance imaging of macaque monkeys performing visually guided saccade tasks: comparison of cortical eye fields with humans. Neuron. 2004;41(5):795–807. doi: 10.1016/s0896-6273(04)00047-9. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Hoffmann D, Minotti L, Berthoz A, Kahane P. Intracerebral dynamics of saccade generation in the human frontal eye field and supplementary eye field. Neuroimage. 2006;30(4):1302–1312. doi: 10.1016/j.neuroimage.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Law I, Svarer C, Holm S, Paulson OB. The activation pattern in normal humans during suppression, imagination and performance of saccadic eye movements. Acta Physiol Scand. 1997;161(3):419–434. doi: 10.1046/j.1365-201X.1997.00207.x. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The neurology of eye movements. 3. New York: Oxford University Press; 1999. [Google Scholar]
- Lobel E, Kahane P, Leonards U, Grosbras M, Lehericy S, Le Bihan D, et al. Localization of human frontal eye fields: anatomical and functional findings of functional magnetic resonance imaging and intracerebral electrical stimulation. J Neurosurg. 2001;95(5):804–815. doi: 10.3171/jns.2001.95.5.0804. [DOI] [PubMed] [Google Scholar]
- Lock TM, Baizer JS, Bender DB. Distribution of corticotectal cells in macaque. Exp Brain Res. 2003;151(4):455–470. doi: 10.1007/s00221-003-1500-y. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. Cognitive functional magnetic resonance imaging at very-high-field: eye movement control. Top Magn Reson Imaging. 1999;10(1):3–15. doi: 10.1097/00002142-199902000-00002. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Strojwas MH, McCurtain BJ, Berman RA, Genovese CR, et al. Dorsal cortical regions subserving visually guided saccades in humans: an fMRI study. Cereb Cortex. 1998;8(1):40–47. doi: 10.1093/cercor/8.1.40. [DOI] [PubMed] [Google Scholar]
- Lynch JC, Graybiel AM, Lobeck LJ. The differential projection of two cytoarchitectonic subregions of the inferior parietal lobule of macaque upon the deep layers of the superior colliculus. J Comp Neurol. 1985;235(2):241–254. doi: 10.1002/cne.902350207. [DOI] [PubMed] [Google Scholar]
- Lynch JC, Tian JR. Cortico-cortical networks and cortico-subcortical loops for the higher control of eye movements. Prog Brain Res. 2005;151:461–501. doi: 10.1016/S0079-6123(05)51015-X. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Thakkar KN, Cain MS, Polli FE, Edelman JA, Fischl B, et al. Neural activity is modulated by trial history: a functional magnetic resonance imaging study of the effects of a previous antisaccade. J Neurosci. 2007;27(7):1791–1798. doi: 10.1523/JNEUROSCI.3662-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massen C. Parallel programming of exogenous and endogenous components in the antisaccade task. Q J Exp Psychol A. 2004;57(3):475–498. doi: 10.1080/02724980343000341. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Matsuura M, Ohkubo T, Ohkubo H, Matsushima E, Inoue K, et al. Functional MRI mapping of brain activation during visually guided saccades and antisaccades: cortical and subcortical networks. Psychiatry Res. 2004;131(2):147–155. doi: 10.1016/j.pscychresns.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Matthews A, Flohr H, Everling S. Cortical activation associated with midtrial change of instruction in a saccade task. Exp Brain Res. 2002;143(4):488–498. doi: 10.1007/s00221-002-1003-2. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Brown GG, Paulus M, Martinez A, Stewart SE, Dubowitz DJ, et al. Neural correlates of refixation saccades and antisaccades in normal and schizophrenia subjects. Biol Psychiatry. 2002;51(3):216–223. doi: 10.1016/s0006-3223(01)01204-5. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Clementz BA, Wixted JT. Timing and amplitude of saccades during predictive saccadic tracking in schizophrenia. Psychophysiology. 1996;33(1):93–101. doi: 10.1111/j.1469-8986.1996.tb02112.x. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Kissler JM, Berg P, Dyckman KA, Gao Y, Rockstroh B, et al. Electroencephalography/magnetoencephalography study of cortical activities preceding prosaccades and antisaccades. Neuroreport. 2005;16(7):663–668. doi: 10.1097/00001756-200505120-00002. [DOI] [PubMed] [Google Scholar]
- McKeown MJ. Detection of consistently task-related activations in fMRI data with hybrid independent component analysis. Neuroimage. 2000;11(1):24–35. doi: 10.1006/nimg.1999.0518. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Jung TP, Makeig S, Brown G, Kindermann SS, Lee TW, et al. Spatially independent activity patterns in functional MRI data during the stroop color-naming task. Proc Natl Acad Sci U S A. 1998;95(3):803–810. doi: 10.1073/pnas.95.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown MJ, Sejnowski TJ. Independent component analysis of fMRI data: examining the assumptions. Hum Brain Mapp. 1998;6(5–6):368–372. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<368::AID-HBM7>3.0.CO;2-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci. 2004;7(7):757–763. doi: 10.1038/nn1269. [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Goltz HC, Vilis T. Remapping the remembered target location for anti-saccades in human posterior parietal cortex. J Neurophysiol. 2005;94(1):734–740. doi: 10.1152/jn.01331.2004. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Colby CL, Thulborn KR, Luna B, Olson CR, Sweeney JA. Stimulus-response incompatibility activates cortex proximate to three eye fields. Neuroimage. 2001;13(5):794–800. doi: 10.1006/nimg.2000.0742. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Genovese CR, Colby CL. Remapping in human visual cortex. J Neurophysiol. 2007;97(2):1738–1755. doi: 10.1152/jn.00189.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milea D, Lobel E, Lehericy S, Pierrot-Deseilligny C, Berthoz A. Cortical mechanisms of saccade generation from execution to decision. Ann N Y Acad Sci. 2005;1039:232–238. doi: 10.1196/annals.1325.022. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller LM, Sun FT, Curtis CE, D’Esposito M. Functional interactions between oculomotor regions during prosaccades and antisaccades. Hum Brain Mapp. 2005;26(2):119–127. doi: 10.1002/hbm.20146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SY, Barton JJ, Mikulski S, Polli FE, Cain MS, Vangel M, et al. Where left becomes right: a magnetoencephalographic study of sensorimotor transformation for antisaccades. Neuroimage. 2007;36(4):1313–1323. doi: 10.1016/j.neuroimage.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421(6921):370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Mort DJ, Perry RJ, Mannan SK, Hodgson TL, Anderson E, Quest R, et al. Differential cortical activation during voluntary and reflexive saccades in man. Neuroimage. 2003;18(2):231–246. doi: 10.1016/s1053-8119(02)00028-9. [DOI] [PubMed] [Google Scholar]
- Munoz DP. Commentary: saccadic eye movements: overview of neural circuitry. Prog Brain Res. 2002;140:89–96. doi: 10.1016/S0079-6123(02)40044-1. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5(3):218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Muri RM, Heid O, Nirkko AC, Ozdoba C, Felblinger J, Schroth G, et al. Functional organisation of saccades and antisaccades in the frontal lobe in humans: a study with echo planar functional magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 1998;65(3):374–377. doi: 10.1136/jnnp.65.3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushiake H, Inase M, Tanji J. Selective coding of motor sequence in the supplementary motor area of the monkey cerebral cortex. Exp Brain Res. 1990;82(1):208–210. doi: 10.1007/BF00230853. [DOI] [PubMed] [Google Scholar]
- Neggers SF, Raemaekers MA, Lampmann EE, Postma A, Ramsey NF. Cortical and subcortical contributions to saccade latency in the human brain. Eur J Neurosci. 2005;21(10):2853–2863. doi: 10.1111/j.1460-9568.2005.04129.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001;38(5):752–760. [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM, Frackowiak RS, Frith CD. Functional localization of the system for visuospatial attention using positron emission tomography. Brain. 1997;120(Pt 3):515–533. doi: 10.1093/brain/120.3.515. [DOI] [PubMed] [Google Scholar]
- Noda H. Cerebellar control of saccadic eye movements: its neural mechanisms and pathways. Jpn J Physiol. 1991;41(3):351–368. doi: 10.2170/jjphysiol.41.351. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric fields of the brain: the neurophysics of EEG. 2. Oxford: Oxford University Press; 2006. [Google Scholar]
- Nyffeler T, Rivaud-Pechoux S, Pierrot-Deseilligny C, Diallo R, Gaymard B. Visual vector inversion in the posterior parietal cortex. Neuroreport. 2007;18(9):917–920. doi: 10.1097/WNR.0b013e32811d6cf5. [DOI] [PubMed] [Google Scholar]
- O’Driscoll GA, Alpert NM, Matthysse SW, Levy DL, Rauch SL, Holzman PS. Functional neuroanatomy of antisaccade eye movements investigated with positron emission tomography. Proc Natl Acad Sci U S A. 1995;92(3):925–929. doi: 10.1073/pnas.92.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll GA, Wolff AL, Benkelfat C, Florencio PS, Lal S, Evans AC. Functional neuroanatomy of smooth pursuit and predictive saccades. Neuroreport. 2000;11(6):1335–1340. doi: 10.1097/00001756-200004270-00037. [DOI] [PubMed] [Google Scholar]
- Optican LM. Sensorimotor transformation for visually guided saccades. Ann N Y Acad Sci. 2005;1039:132–148. doi: 10.1196/annals.1325.013. [DOI] [PubMed] [Google Scholar]
- O’Sullivan EP, Jenkins IH, Henderson L, Kennard C, Brooks DJ. The functional anatomy of remembered saccades: a PET study. Neuroreport. 1995;6(16):2141–2144. doi: 10.1097/00001756-199511000-00011. [DOI] [PubMed] [Google Scholar]
- Ozyurt J, Rutschmann RM, Greenlee MW. Cortical activation during memory-guided saccades. Neuroreport. 2006;17(10):1005–1009. doi: 10.1097/01.wnr.0000224765.00078.4e. [DOI] [PubMed] [Google Scholar]
- Pare M, Wurtz RH. Progression in neuronal processing for saccadic eye movements from parietal cortex area lip to superior colliculus. J Neurophysiol. 2001;85(6):2545–2562. doi: 10.1152/jn.2001.85.6.2545. [DOI] [PubMed] [Google Scholar]
- Parton A, Nachev P, Hodgson TL, Mort D, Thomas D, Ordidge R, et al. Role of the human supplementary eye field in the control of saccadic eye movements. Neuropsychologia. 2007;45(5):997–1008. doi: 10.1016/j.neuropsychologia.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Location and function of the human frontal eye-field: a selective review. Neuropsychologia. 1996;34(6):475–483. doi: 10.1016/0028-3932(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Paus T, Marrett S, Worsley KJ, Evans AC. Extraretinal modulation of cerebral blood flow in the human visual cortex: implications for saccadic suppression. J Neurophysiol. 1995;74(5):2179–2183. doi: 10.1152/jn.1995.74.5.2179. [DOI] [PubMed] [Google Scholar]
- Paus T, Petrides M, Evans AC, Meyer E. Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J Neurophysiol. 1993;70(2):453–469. doi: 10.1152/jn.1993.70.2.453. [DOI] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ. Comparing dynamic causal models. Neuroimage. 2004;22(3):1157–1172. doi: 10.1016/j.neuroimage.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Cole MA, Larson M, Kelly K, Seignourel P, Keil A. Steady-state visual evoked potentials reveal frontally-mediated working memory activity in humans. Neurosci Lett. 2003;342(3):191–195. doi: 10.1016/s0304-3940(03)00226-x. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Zeki S. The neurology of saccades and covert shifts in spatial attention: an event-related fMRI study. Brain. 2000;123(Pt 11):2273–2288. doi: 10.1093/brain/123.11.2273. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: from modulation of sensory processing to top-down control. J Neurosci. 2003;23(10):3990–3998. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit L, Beauchamp MS. Neural basis of visually guided head movements studied with fMRI. J Neurophysiol. 2003;89(5):2516–2527. doi: 10.1152/jn.00988.2002. [DOI] [PubMed] [Google Scholar]
- Petit L, Orssaud C, Tzourio N, Crivello F, Berthoz A, Mazoyer B. Functional anatomy of a prelearned sequence of horizontal saccades in humans. J Neurosci. 1996;16(11):3714–3726. doi: 10.1523/JNEUROSCI.16-11-03714.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative architectonic analysis of the human and the macaque frontal cortex. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Vol. 9. Amsterdam: Elsevier; 1994. pp. 17–58. [Google Scholar]
- Pierrot-Deseilligny C, Milea D, Muri RM. Eye movement control by the cerebral cortex. Curr Opin Neurol. 2004;17(1):17–25. doi: 10.1097/00019052-200402000-00005. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Muri RM, Nyffeler T, Milea D. The role of the human dorsolateral prefrontal cortex in ocular motor behavior. Ann N Y Acad Sci. 2005;1039:239–251. doi: 10.1196/annals.1325.023. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Muri RM, Ploner CJ, Gaymard B, Demeret S, Rivaud-Pechoux S. Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain. 2003;126(Pt 6):1460–1473. doi: 10.1093/brain/awg148. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Muri RM, Ploner CJ, Gaymard B, Rivaud-Pechoux S. Cortical control of ocular saccades in humans: a model for motricity. Prog Brain Res. 2003;142:3–17. doi: 10.1016/S0079-6123(03)42003-7. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Ploner CJ, Muri RM, Gaymard B, Rivaud-Pechoux S. Effects of cortical lesions on saccadic: eye movements in humans. Ann N Y Acad Sci. 2002;956:216–229. doi: 10.1111/j.1749-6632.2002.tb02821.x. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Rivaud S, Gaymard B, Agid Y. Cortical control of reflexive visually-guided saccades. Brain. 1991;114(Pt 3):1473–1485. doi: 10.1093/brain/114.3.1473. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Rivaud S, Gaymard B, Muri R, Vermersch AI. Cortical control of saccades. Ann Neurol. 1995;37(5):557–567. doi: 10.1002/ana.410370504. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Rivaud S, Penet C, Rigolet MH. Latencies of visually guided saccades in unilateral hemispheric cerebral lesions. Ann Neurol. 1987;21(2):138–148. doi: 10.1002/ana.410210206. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Rosa A, Masmoudi K, Rivaud S, Gaymard B. Saccade deficits after a unilateral lesion affecting the superior colliculus. J Neurol Neurosurg Psychiatry. 1991;54(12):1106–1109. doi: 10.1136/jnnp.54.12.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploner CJ, Gaymard B, Rivaud S, Agid Y, Pierrot-Deseilligny C. Temporal limits of spatial working memory in humans. Eur J Neurosci. 1998;10(2):794–797. doi: 10.1046/j.1460-9568.1998.00101.x. [DOI] [PubMed] [Google Scholar]
- Ploner CJ, Gaymard BM, Rivaud-Pechoux S, Baulac M, Clemenceau S, Samson S, et al. Lesions affecting the parahippocampal cortex yield spatial memory deficits in humans. Cereb Cortex. 2000;10(12):1211–1216. doi: 10.1093/cercor/10.12.1211. [DOI] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Cain MS, Thakkar KN, Rauch SL, Manoach DS. Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission. Proc Natl Acad Sci U S A. 2005;102(43):15700–15705. doi: 10.1073/pnas.0503657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Berger JS, Taich AM, D’Esposito M. Activity in human frontal cortex associated with spatial working memory and saccadic behavior. J Cogn Neurosci. 2000;12(Suppl 2):2–14. doi: 10.1162/089892900564028. [DOI] [PubMed] [Google Scholar]
- Raemaekers M, Jansma JM, Cahn W, Van der Geest JN, van der Linden JA, Kahn RS, et al. Neuronal substrate of the saccadic inhibition deficit in schizophrenia investigated with 3-dimensional event-related functional magnetic resonance imaging. Arch Gen Psychiatry. 2002;59(4):313–320. doi: 10.1001/archpsyc.59.4.313. [DOI] [PubMed] [Google Scholar]
- Raemaekers M, Ramsey NF, Vink M, van den Heuvel MP, Kahn RS. Brain activation during antisaccades in unaffected relatives of schizophrenic patients. Biol Psychiatry. 2006;59(6):530–535. doi: 10.1016/j.biopsych.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Raemaekers M, Vink M, van den Heuvel MP, Kahn RS, Ramsey NF. Effects of aging on BOLD fMRI during prosaccades and antisaccades. J Cogn Neurosci. 2006;18(4):594–603. doi: 10.1162/jocn.2006.18.4.594. [DOI] [PubMed] [Google Scholar]
- Raemaekers M, Vink M, Zandbelt B, van Wezel RJ, Kahn RS, Ramsey NF. Test-retest reliability of fMRI activation during prosaccades and antisaccades. Neuroimage. 2007;36(3):532–542. doi: 10.1016/j.neuroimage.2007.03.061. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Behrens TE, Penny W, Matthews PM. New approaches for exploring anatomical and functional connectivity in the human brain. Biol Psychiatry. 2004;56(9):613–619. doi: 10.1016/j.biopsych.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Richards JE. Cortical sources of event-related potentials in the prosaccade and antisaccade task. Psychophysiology. 2003;40(6):878–894. doi: 10.1111/1469-8986.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivaud S, Muri RM, Gaymard B, Vermersch AI, Pierrot-Deseilligny C. Eye movement disorders after frontal eye field lesions in humans. Exp Brain Res. 1994;102(1):110–120. doi: 10.1007/BF00232443. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Fuchs AF. The role of the cerebellum in voluntary eye movements. Annu Rev Neurosci. 2001;24:981–1004. doi: 10.1146/annurev.neuro.24.1.981. [DOI] [PubMed] [Google Scholar]
- Ross SM, Ross LE. Children’s and adults’ predictive saccades to square-wave targets. Vision Res. 1987;27(12):2177–2180. doi: 10.1016/0042-6989(87)90131-3. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Bestmann S, Blankenburg F, Bjoertomt O, Josephs O, Weiskopf N, et al. Distinct causal influences of parietal versus frontal areas on human visual cortex: evidence from concurrent TMS-fMRI. Cereb Cortex. 2008;18(4):817–827. doi: 10.1093/cercor/bhm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Paus T, Sipila PK. Attention systems and the organization of the human parietal cortex. J Neurosci. 2001;21(14):5262–5271. doi: 10.1523/JNEUROSCI.21-14-05262.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo GS, Bruce CJ. Frontal eye field activity preceding aurally guided saccades. J Neurophysiol. 1994;71(3):1250–1253. doi: 10.1152/jn.1994.71.3.1250. [DOI] [PubMed] [Google Scholar]
- Schall JD. The neural selection and control of saccades by the frontal eye field. Philos Trans R Soc Lond B Biol Sci. 2002;357(1424):1073–1082. doi: 10.1098/rstb.2002.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PH, Sandell JH. Interactions between visually and electrically elicited saccades before and after superior colliculus and frontal eye field ablations in the rhesus monkey. Exp Brain Res. 1983;49(3):381–392. doi: 10.1007/BF00238780. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Sandell JH, Maunsell JH. The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. J Neurophysiol. 1987;57(4):1033–1049. doi: 10.1152/jn.1987.57.4.1033. [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M. Evidence for a supplementary eye field. J Neurophysiol. 1987;57(1):179–200. doi: 10.1152/jn.1987.57.1.179. [DOI] [PubMed] [Google Scholar]
- Schlag-Rey M, Amador N, Sanchez H, Schlag J. Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature. 1997;390(6658):398–401. doi: 10.1038/37114. [DOI] [PubMed] [Google Scholar]
- Schluppeck D, Glimcher P, Heeger DJ. Topographic organization for delayed saccades in human posterior parietal cortex. J Neurophysiol. 2005;94(2):1372–1384. doi: 10.1152/jn.01290.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider KA, Kastner S. Visual responses of the human superior colliculus: a high-resolution functional magnetic resonance imaging study. J Neurophysiol. 2005;94(4):2491–2503. doi: 10.1152/jn.00288.2005. [DOI] [PubMed] [Google Scholar]
- Segraves MA. Activity of monkey frontal eye field neurons projecting to oculomotor regions of the pons. J Neurophysiol. 1992;68(6):1967–1985. doi: 10.1152/jn.1992.68.6.1967. [DOI] [PubMed] [Google Scholar]
- Shook BL, Schlag-Rey M, Schlag J. Primate supplementary eye field: I. Comparative aspects of mesencephalic and pontine connections. J Comp Neurol. 1990;301(4):618–642. doi: 10.1002/cne.903010410. [DOI] [PubMed] [Google Scholar]
- Simo LS, Krisky CM, Sweeney JA. Functional neuroanatomy of anticipatory behavior: dissociation between sensory-driven and memory-driven systems. Cereb Cortex. 2005;15(12):1982–1991. doi: 10.1093/cercor/bhi073. [DOI] [PubMed] [Google Scholar]
- Simon O, Mangin JF, Cohen L, Le Bihan D, Dehaene S. Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe. Neuron. 2002;33(3):475–487. doi: 10.1016/s0896-6273(02)00575-5. [DOI] [PubMed] [Google Scholar]
- Smit AC, Van Gisbergen JA. A short-latency transition in saccade dynamics during square-wave tracking and its significance for the differentiation of visually-guided and predictive saccades. Exp Brain Res. 1989;76(1):64–74. doi: 10.1007/BF00253624. [DOI] [PubMed] [Google Scholar]
- Srimal R, Curtis CE. Persistent neural activity during the maintenance of spatial position in working memory. Neuroimage. 2008;39(1):455–468. doi: 10.1016/j.neuroimage.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton GB, Deng SY, Goldberg ME, McMullen NT. Cytoarchitectural characteristic of the frontal eye fields in macaque monkeys. J Comp Neurol. 1989;282(3):415–427. doi: 10.1002/cne.902820308. [DOI] [PubMed] [Google Scholar]
- Stephan T, Mascolo A, Yousry TA, Bense S, Brandt T, Dieterich M. Changes in cerebellar activation pattern during two successive sequences of saccades. Hum Brain Mapp. 2002;16(2):63–70. doi: 10.1002/hbm.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature. 2000;408(6814):857–860. doi: 10.1038/35048576. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Watanabe J, Maeda Y, Matsue Y, Fukuda H, Kawashima R. Different roles of the frontal and parietal regions in memory-guided saccade: a PCA approach on time course of BOLD signal changes. Hum Brain Mapp. 2004;23(3):129–139. doi: 10.1002/hbm.20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney JA, Luna B, Keedy SK, McDowell JE, Clementz BA. fMRI studies of eye movement control: investigating the interaction of cognitive and sensorimotor brain systems. Neuroimage. 2007;36(Suppl 2):T54–60. doi: 10.1016/j.neuroimage.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, et al. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. J Neurophysiol. 1996;75(1):454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- Sylvester R, Haynes JD, Rees G. Saccades differentially modulate human LGN and V1 responses in the presence and absence of visual stimulation. Curr Biol. 2005;15(1):37–41. doi: 10.1016/j.cub.2004.12.061. [DOI] [PubMed] [Google Scholar]
- Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol. 1998;80(4):1911–1931. doi: 10.1152/jn.1998.80.4.1911. [DOI] [PubMed] [Google Scholar]
- Takeda K, Funahashi S. Prefrontal task-related activity representing visual cue location or saccade direction in spatial working memory tasks. J Neurophysiol. 2002;87(1):567–588. doi: 10.1152/jn.00249.2001. [DOI] [PubMed] [Google Scholar]
- Tanji J. The supplementary motor area in the cerebral cortex. Neurosci Res. 1994;19(3):251–268. doi: 10.1016/0168-0102(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA, Chou IH, Slocum WM, Schiller PH. Eye fields in the frontal lobes of primates. Brain Res Brain Res Rev. 2000;32(2–3):413–448. doi: 10.1016/s0165-0173(99)00092-2. [DOI] [PubMed] [Google Scholar]
- Tendolkar I, Ruhrmann S, Brockhaus-Dumke A, Pauli M, Mueller R, Pukrop R, et al. Neural correlates of visuo-spatial attention during an antisaccade task in schizophrenia: an ERP study. Int J Neurosci. 2005;115(5):681–698. doi: 10.1080/00207450590887475. [DOI] [PubMed] [Google Scholar]
- Tian J, Schlag J, Schlag-Rey M. Testing quasi-visual neurons in the monkey’s frontal eye field with the triple-step paradigm. Exp Brain Res. 2000;130(4):433–440. doi: 10.1007/s002219900282. [DOI] [PubMed] [Google Scholar]
- Tian JR, Lynch JC. Corticocortical input to the smooth and saccadic eye movement subregions of the frontal eye field in Cebus monkeys. J Neurophysiol. 1996;76(4):2754–2771. doi: 10.1152/jn.1996.76.4.2754. [DOI] [PubMed] [Google Scholar]
- Tomassini V, Jbabdi S, Klein JC, Behrens TE, Pozzilli C, Matthews PM, et al. Diffusion-weighted imaging tractography-based parcellation of the human lateral premotor cortex identifies dorsal and ventral subregions with anatomical and functional specializations. J Neurosci. 2007;27(38):10259–10269. doi: 10.1523/JNEUROSCI.2144-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappenberg TP, Dorris MC, Munoz DP, Klein RM. A model of saccade initiation based on the competitive integration of exogenous and endogenous signals in the superior colliculus. J Cogn Neurosci. 2001;13(2):256–271. doi: 10.1162/089892901564306. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S, Sawaguchi T. Neuronal representation of response-outcome in the primate prefrontal cortex. Cereb Cortex. 2004;14(1):47–55. doi: 10.1093/cercor/bhg090. [DOI] [PubMed] [Google Scholar]
- Tu PC, Yang TH, Kuo WJ, Hsieh JC, Su TP. Neural correlates of antisaccade deficits in schizophrenia, an fMRI study. J Psychiatr Res. 2006;40(7):606–612. doi: 10.1016/j.jpsychires.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Tzelepi A, Lutz A, Kapoula Z. EEG activity related to preparation and suppression of eye movements in three-dimensional space. Exp Brain Res. 2004;155(4):439–449. doi: 10.1007/s00221-003-1741-9. [DOI] [PubMed] [Google Scholar]
- Umeno MM, Goldberg ME. Spatial processing in the monkey frontal eye field. I. Predictive visual responses. J Neurophysiol. 1997;78(3):1373–1383. doi: 10.1152/jn.1997.78.3.1373. [DOI] [PubMed] [Google Scholar]
- Vahedi K, Rivaud S, Amarenco P, Pierrot-Deseilligny C. Horizontal eye movement disorders after posterior vermis infarctions. J Neurol Neurosurg Psychiatry. 1995;58(1):91–94. doi: 10.1136/jnnp.58.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar G, Lobel E, Galati G, Berthoz A, Pizzamiglio L, Le Bihan D. A fronto-parietal system for computing the egocentric spatial frame of reference in humans. Exp Brain Res. 1999;124(3):281–286. doi: 10.1007/s002210050624. [DOI] [PubMed] [Google Scholar]
- Vanni S, Tanskanen T, Seppa M, Uutela K, Hari R. Coinciding early activation of the human primary visual cortex and anteromedial cuneus. Proc Natl Acad Sci U S A. 2001;98(5):2776–2780. doi: 10.1073/pnas.041600898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. The student’s guide to cognitive neuroscience. Hove: Psychology Press; 2006. [Google Scholar]
- Watanabe K, Lauwereyns J, Hikosaka O. Neural correlates of rewarded and unrewarded eye movements in the primate caudate nucleus. J Neurosci. 2003;23(31):10052–10057. doi: 10.1523/JNEUROSCI.23-31-10052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Fischer B. Gap duration and location of attention focus modulate the occurrence of left/right asymmetries in the saccadic reaction times of human subjects. Vision Res. 1995;35(7):987–998. doi: 10.1016/0042-6989(94)00186-p. [DOI] [PubMed] [Google Scholar]
- Yan YJ, Cui DM, Lynch JC. Overlap of saccadic and pursuit eye movement systems in the brain stem reticular formation. J Neurophysiol. 2001;86(6):3056–3060. doi: 10.1152/jn.2001.86.6.3056. [DOI] [PubMed] [Google Scholar]
- Zambarbieri D, Schmid R, Magenes G, Prablanc C. Saccadic responses evoked by presentation of visual and auditory targets. Exp Brain Res. 1982;47(3):417–427. doi: 10.1007/BF00239359. [DOI] [PubMed] [Google Scholar]
- Zhang M, Barash S. Neuronal switching of sensorimotor transformations for antisaccades. Nature. 2000;408(6815):971–975. doi: 10.1038/35050097. [DOI] [PubMed] [Google Scholar]