Abstract
Prostagladin (PG) E2, a major product of activated macrophages, has been implicated in atherosclerosis and plaque rupture. The PGE2 receptors, EP2 and EP4, are expressed in atherosclerotic lesions and are known to inhibit apoptosis in cancer cells. To examine the roles of macrophage EP4 and EP2 in apoptosis and early atherosclerosis, fetal liver cell transplantation was used to generate LDLR−/− mice chimeric for EP2−/− or EP4−/− hematopoietic cells. After 8-weeks on a Western diet, EP4−/− → LDLR−/− mice, but not EP2−/− → LDLR−/− mice, had significantly reduced aortic atherosclerosis with increased apoptotic cells in the lesions. EP4−/− peritoneal macrophages had increased sensitivity to pro-apoptotic stimuli, including palmitic acid and free cholesterol loading, which was accompanied by suppression of activity of p-Akt, p-Bad and NF-kB-regulated genes. Thus, EP4 deficiency inhibits the PI3K/Akt and NF-kB pathways compromising macrophage survival and suppressing early atherosclerosis, identifying macrophage EP4 signaling pathways as molecular targets for modulating the development of atherosclerosis.
Keywords: Atherosclerosis, Cyclooxygenase, EP4 signaling, NF-κB, Macrophages, Apoptosis
Introduction
Atherosclerosis, the underlying cause of coronary heart disease and stroke, is a complex degenerative process including an important inflammatory component(Ross, 1999). All of the major cell types involved in atherogenesis produce eicosanoids, and mounting evidence supports critical roles for the prostaglandins and their specific receptors in atherogenesis and plaque stability. Much attention has been given to the roles of prostacyclin (PGI2) and thromboxane A2 (TXA2) and their receptors in atherothrombosis(Grosser et al., 2006). Genetic deletion of the PGI2 receptor accelerates the neointimal proliferation in response to vascular injury(Cheng et al., 2002), whereas deletion of the TXA2 receptor reduces atherosclerosis in apoE null mice(Kobayashi et al., 2004). Although eicosanoids have been implicated in atherogenesis and plaque stability(Egan et al., 2005; Linton and Fazio, 2008) the roles of PGE2 and its specific prostanoid receptors in atherogenesis have not been examined directly.
Macrophages are the major cell type of early atherosclerotic lesions and their activation by a variety of stimuli results in abundant COX-mediated production of PGE2. Acting in an autocrine and paracrine manner, PGE2 modulates inflammatory responses via a family of four membrane-spanning G protein-coupled receptors termed EP1, EP2, EP3 and EP4(Breyer et al., 2001). Human atherosclerotic plaque cells have been reported to express EP4 and EP2(Gómez-Hernández et al., 2006). Interestingly, platelet EP3 expression has recently been shown to promote thrombosis in response to vascular PGE2 production induced by injury of carotid arteries in apoE deficient mice (Gross et al., 2007). Deletion of EP2 promotes salt-sensitive hypertension in mice fed a very high sodium diet (Kennedy et al., 1999) whereas EP4 has been shown to be the primary mediator of anti-inflammatory effects of PGE2 and a regulator of chemokine production in vitro(Takayama et al., 2002; Takayama et al., 2006). The EP4 receptor has been reported to be responsible for the PGE2-mediated increase in matrix metalloproteinases (MMPs) in macrophages (Pavlovic et al., 2006) that may initiate unstable plaques. Together, these data suggest that macrophage EP2 and EP4 receptor activity may play important roles in the pathogenesis of atherosclerosis.
Interestingly, PGE2 modulates apoptosis acting predominantly through the EP2 and EP4 receptors(Chun et al., 2007; Tessner et al., 2004; Vassiliou et al., 2004), yet the role of PGE2-mediated apoptosis in arterial macrophages has not been defined. Though EP2 and EP4 may have redundant functions, they employ different signaling pathways. Both EP2 and EP4 mediate signaling via cAMP-dependent protein kinase (PKA), leading to the phosphorylation of the cAMP-responsive element binding protein, which is known to regulate Bcl-2(Regan, 2003), glycogen synthase kinase, and Bad(Fujino et al., 2003; Lizcano et al., 2000). In addition, EP4 has been reported to activate a phosphoinositide-3-kinase (PI3K) that in turn activates Akt, also known as protein kinase B (PKB). Akt inhibits cell death pathways by directly phosphorylating and inactivating proteins involved in apoptosis, including Bad, procaspase 9, and members of the Forkhead pro-inflammatory family(Brunet et al., 1999; Cardone et al., 1998; Datta et al., 1997). Akt also regulates the NF-κB pathway changing its anti-apoptotic and pro-inflammatory functions(Madrid et al., 2000; Ozes et al., 1999; Romashkova and Makarov, 1999). Recently, PGE2 has been reported to modulate LPS induced activation of NFkB by a novel EP4 receptor associated protein (Minami et al., 2008). Thus, activation of EP4 may modulate signaling pathways regulating macrophage survival and inflammatory functions in atherosclerosis that are distinct from those of EP2.
The goal of the current study was to examine the roles of macrophage EP2 and EP4 receptors in apoptosis and atherogenesis in vivo. Given the fact that mice with targeted deletion of EP4 gene die soon after birth(Nguyen et al., 1997; Schneider et al., 2004; Segi et al., 1998), we used the approach of fetal liver cell transplantation(Babaev et al., 1999) to generate LDLR−/− mice chimeric for expression of EP2 and EP4 by hematopoietic cells. Our data demonstrate that the absence of EP4 in hematopoietic cells promotes macrophage apoptosis through down-regulation of PI3K and NF-κB signaling pathways and this is associated with significantly suppressed early atherosclerosis in LDLR−/− mice.
Results
Generation of mice with EP4 or EP2 null hematopoietic cells
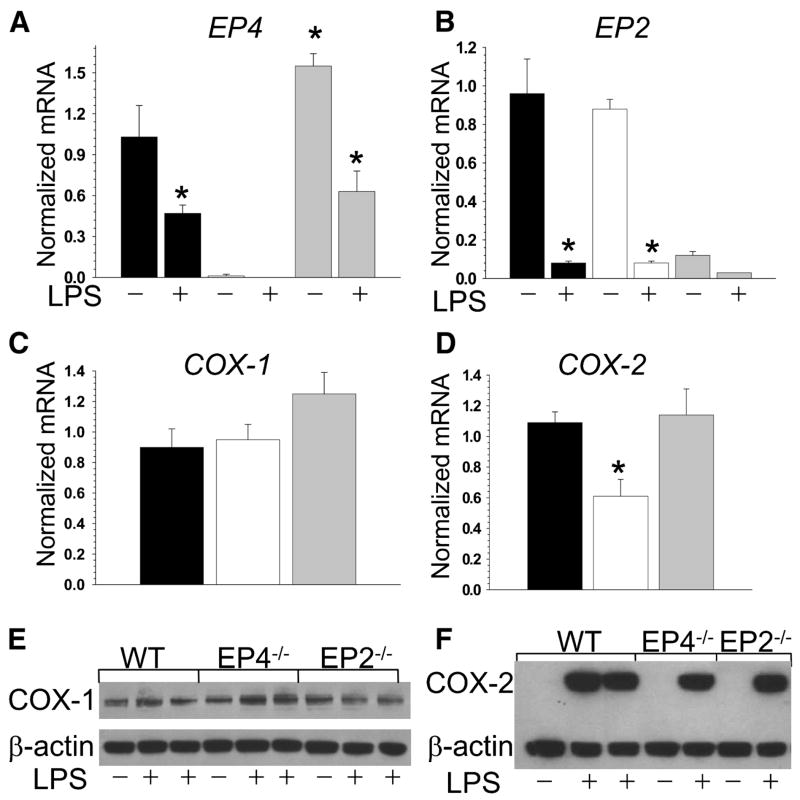
Because EP4 null mice die soon after birth(Nguyen et al., 1997; Schneider et al., 2004; Segi et al., 1998), we used fetal liver cell (FLC) transplantation to generate mice chimeric for gene deletions of EP2 or EP4 in their hematopoietic cells(Babaev et al., 1999). Eight-week-old female LDLR−/− mice were lethally irradiated and transplanted with female wild type (WT; n=12), EP4−/−(n=13) or EP2−/−(n=13) FLC. Twelve weeks post-transplantation, peritoneal macrophages were isolated from recipient mice, and EP4 and EP2 gene expression were analyzed by real-time PCR. The results indicate a complete change in the genotype to the donor types with a compensatory increase of EP4 mRNA expression levels in EP2−/− cells and no changes in EP2 gene expression in EP4−/− macrophages (Fig. 1A,B). Interestingly, LPS treatment for 5 hours significantly suppressed both EP4 and EP2 gene expression levels in all types of macrophages (Fig. 1A,B).
Figure 1. EP2 deficiency causes a compensatory increases in EP4 gene expression and EP4 deficiency suppress COX-2 production in peritoneal macrophages.
(A–D) EP4(A), EP2(B), COX-1(C) and COX-2(D) gene expression levels in peritoneal macrophages isolated from LDLR−/− mice reconstituted with WT(▪), EP4−/−(□) or EP2−/−(
 ) FLC and fed with the Western diet for 8 weeks. Macrophages were treated with media alone (control) or with LPS (50ng/ml) for 5 hours. The gene expression levels were measured by real-time PCR. Graphs represent data (Mean ± SEM) with the same number (n=3) of mice per group (*p<0.05 between control and treated with LPS cells of the same group, and between WT and EP4−/− cells by One Way ANOVA analysis).
) FLC and fed with the Western diet for 8 weeks. Macrophages were treated with media alone (control) or with LPS (50ng/ml) for 5 hours. The gene expression levels were measured by real-time PCR. Graphs represent data (Mean ± SEM) with the same number (n=3) of mice per group (*p<0.05 between control and treated with LPS cells of the same group, and between WT and EP4−/− cells by One Way ANOVA analysis).
(E,F) COX-1 (E) and COX-2 (F) protein levels in peritoneal macrophages. Macrophages were treated with media alone or with LPS for 5 hours. Cell extract (20μg/line) was resolved on 10% Bis-Tris gel and analyzed by Western blot.
Since EP2 or EP4 receptor activity may affect COX production(Fujino et al., 2003), we measured COX-1 and COX-2 expression in the peritoneal macrophages from LDLR−/− mice reconstituted with EP2−/−, EP4−/−, or WT macrophages. COX-1 gene and protein expression levels were similar in all three types of cells (Fig. 1C and E). In contrast, EP4−/− macrophages had significantly lower levels of LPS-induced COX-2 gene expression than WT or EP2−/− macrophages (Fig. 3D). COX-2 protein expression was detected only in LPS treated cells and was slightly lower (17%) in EP4−/−macrophages compared to WT and EP2−/− cells (Fig. 1F).
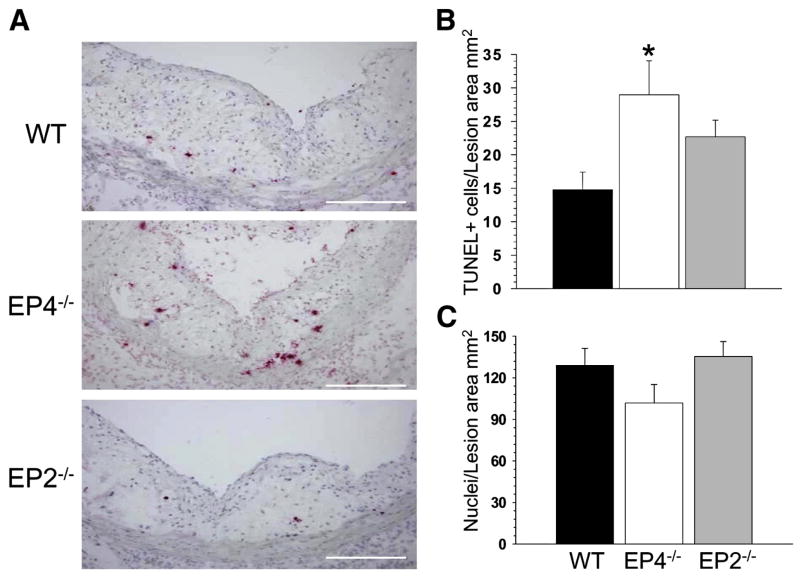
Figure 3. EP4 deficiency in hematopoietic cells increases apoptosis in atherosclerotic lesions.
(A) Distribution of TUNEL+ cells in the proximal aorta of mice reconstituted with different FLC and fed with the Western diet for 8 weeks (20x magnification). The scale bars represent 20μm.
(B,C) Percent of TUNEL+ cells (B) and numbers of DAPI-stained nucleus cells/MOMA-2+ area (C) in atherosclerotic lesions of LDLR−/− mice reconstituted with WT(▪), EP4−/−(□) or EP2−/−(
 ) FLC and fed with the Western diet for 8 weeks. Graphs represent data (Mean ± SEM) with different number (n=12, 13 and 13, respectively) mice per group (*p<0.05 between mice reconstituted with WT and EP4−/− cells by One Way ANOVA analysis).
) FLC and fed with the Western diet for 8 weeks. Graphs represent data (Mean ± SEM) with different number (n=12, 13 and 13, respectively) mice per group (*p<0.05 between mice reconstituted with WT and EP4−/− cells by One Way ANOVA analysis).
EP4 deficiency in hematopoietic cells suppresses early atherosclerosis and increases apoptosis in atherosclerotic lesions
Four weeks post-transplantation, the recipient mice were challenged with the Western diet for 8 weeks. There were no statistically significant differences in serum total cholesterol and triglyceride levels between the 3 groups of recipient mice reconstituted with WT, EP4−/− or EP2−/−FLC on the chow or atherogenic diets (Supplemental data; Table 1). Analysis of the serum samples by size exclusion chromatography revealed an accumulation of cholesterol in VLDL, LDL, and IDL fractions with no differences between mice reconstituted with WT, EP4−/− or EP2−/− FLC (Fig. 2A).
Figure 2. EP4 deficiency in hematopoietic cells does not affect plasma lipid levels but significantly suppresses early atherosclerosis.
(A) FPLC profiles in LDLR−/− mice reconstituted WT(•), EP4−/−(○), or EP2−/−(
 ) FLCs. The data are represented as the average of total cholesterol of mice (n=3 per group) reconstituted different FLC and fed with the Western diet for 8 weeks. Fractions 14–17 contain VLDL; fractions 18–24 are IDL/LDL; and fractions 25–30 contain HDL.
) FLCs. The data are represented as the average of total cholesterol of mice (n=3 per group) reconstituted different FLC and fed with the Western diet for 8 weeks. Fractions 14–17 contain VLDL; fractions 18–24 are IDL/LDL; and fractions 25–30 contain HDL.
(B–D) Atherosclerotic lesions in aorta en face (B) and extent of atherosclerotic lesion area in the distal (C) and proximal (D) aortas of LDLR−/− mice reconstituted WT(•), EP4−/−(○), or EP2−/−(
 ) FLCs. Graphs represent data (Mean ± SEM) with different number (n=12, 13 and 13, respectively) mice of each genotype (p<0.05 between control and reconstituted with EP4−/− FLC groups by One Way ANOVA analysis, the Tukey test).
) FLCs. Graphs represent data (Mean ± SEM) with different number (n=12, 13 and 13, respectively) mice of each genotype (p<0.05 between control and reconstituted with EP4−/− FLC groups by One Way ANOVA analysis, the Tukey test).
To evaluate the extent of atherosclerotic lesions, we utilized two alternative methods: analysis of atherosclerotic lesions en face in whole aortas and cross sections of the aortic sinus. En face analysis demonstrated that LDLR−/− mice reconstituted with EP4−/− FLC had significantly smaller (57%) lesions compared to mice transplanted with WT or EP2−/− FLCs (1.5±0.2% vs. 2.6±0.6 and 2.5+0.5%, respectively; Fig. 2B,C). Similarly, mice reconstituted with EP4−/− FLCs had smaller atherosclerotic lesions in the proximal aortas than mice transplanted with WT or EP2−/− FLC (322461+34555μm2 vs. 452708+14407μm2 and 358179+35133μm2, respectively; p < 0.05 vs. WT; Fig. 2D). Although there appeared to be a trend for a reduction in atherosclerosis in the EP2−/− → LDLR−/− mice, the difference was not statistically significant vs. WT → LDLR−/− mice or EP4−/− → LDLR−/− mice.
Since PGE2-dependent EP4 activation has been reported to mediate cell survival effects in dendritic cells and epithelial cells(Chun, 2007 #12; Tessner, 2004 #52; Vassiliou, 2004 #55), we examined whether EP4 might modulate apoptosis in atherosclerotic lesions. Therefore, we stained serial sections (n=6/aorta) from the proximal aorta with a terminal deoxynucleotidyl transferase-mediated dUTP nick-end-labeling (TUNEL). Mice reconstituted with EP4−/− FLC had an increased (160%) percentage of TUNEL-positive (TUNEL+) cells in atherosclerotic lesions compared to mice transplanted with WT FLC (Fig. 3A,B). This was accompanied by a strong trend for a decrease in the number of nuclei in the atherosclerotic lesions(p=0.06) (Fig. 3C) stained with macrophage-specific antibody MOMA-2 in combination with DAPI as described (Babaev et al., 2005). Mice reconstituted with EP2−/− FLC had a similar trend for increased numbers of TUNEL+ cells that was not statistically significant (by a One Way ANOVA). Thus, EP4 deficiency in hematopoietic cells does not affect plasma lipid levels but significantly suppresses early atherosclerosis and increases apoptosis in atherosclerotic lesions.
Macrophage EP4 deficiency increases sensitivity to apoptotic stimuli
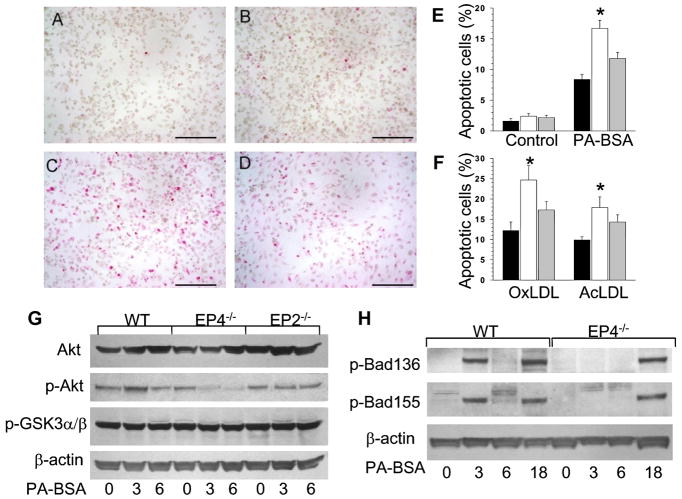
Next we examined in vitro the impact of deficiency of EP2 or EP4 on macrophage sensitivity to apoptosis. To generate cells for these studies, we lethally irradiated and transplanted C57BL6 mice with WT (n=16), EP4−/− (n=14) and EP2−/−(n=12) FLCs. Ten weeks post-transplantation, thioglycollate-elicited peritoneal macrophages were isolated from these mice and treated with 0.5 mM palmitic acid (PA), a stress-mediated lipotoxic factor that rapidly increases saturated lipid content in the rough endoplasmic reticulum (ER) inducing ER stress which triggers apoptotic cell death(Borradaile et al., 2006). PA complexed with BSA treatment increased TUNEL+ cell numbers in all cell types (Fig. 4B-D) with a significantly higher percentage in EP4−/− macrophages than in EP2−/− and WT cells (Fig. 4E). Remarkably, compared to WT and EP2−/− cells, EP4−/− macrophages also showed enhanced sensitivity to different stimuli including free cholesterol loading with acetylated LDL in combination with an ACAT inhibitor (Yao and Tabas, 2001), or oxidized LDL (Fig. 4F) or a specific inhibitor of IκB phosphorylation, BAY 11-7082 (20μM) that decreases expression of NF-κB (Supplemental data, Fig A1). Thus, EP4 deficiency in macrophages significantly increases their sensitivity to apoptosis.
Figure 4. EP4 deficiency in macrophages increases susceptibility to apoptosis and suppresses Akt and Bad phosphorylation.
(A–D) Detection of TUNEL+ cells in untreated WT(A) and treated with PA-BSA (0.5 mM for 18 hours) WT(B), EP4−/−(C) or EP2−/−(D) macrophages (x20).
(E, F) Percent of TUNEL+ cells in WT(▪), EP4−/−(□) or EP2−/−(
 ) macrophages untreated or treated with PA-BSA (E), oxLDL (100μg/ml) or AcLDL (100μg/ml) plus an ACAT inhibitor, Sandoz 58035 (10μg/ml) (F) for 24 hours. Graphs represent data (Mean ± SEM) with the same number (n=3) of mice per group (*p<0.05 between WT and EP4−/− cells by One Way ANOVA analysis)
) macrophages untreated or treated with PA-BSA (E), oxLDL (100μg/ml) or AcLDL (100μg/ml) plus an ACAT inhibitor, Sandoz 58035 (10μg/ml) (F) for 24 hours. Graphs represent data (Mean ± SEM) with the same number (n=3) of mice per group (*p<0.05 between WT and EP4−/− cells by One Way ANOVA analysis)
(G,H) Expression of Akt, p-Akt(Ser473), and p-GSK3α/β(H), p-Bad(Ser136 and 155) and β-actin (J) in WT, EP4−/− or EP2−/− peritoneal macrophages untreated and treated with PA-BSA (0.5mM) for 3,6 or 18 hours. Cell extract (100μg/line) was resolved and analyzed by Western blot using antibodies against the proteins as indicated.
PI3K/Akt signaling pathway is affected in EP4 null macrophages
To define the molecular mechanisms responsible for EP4-mediated macrophage apoptosis, we examined whether sensitivity to apoptosis in EP4−/− macrophages is associated with changes in signal transduction pathways. EP4−/−, EP2−/− and WT peritoneal macrophages were treated with PA-BSA for 3, 6 or 18 hours, and the activity of Akt and Bad signal proteins, as indicated by phosphorylation, were analyzed by western blot. EP4−/−macrophages had significantly decreased levels of basal and stimulated p-Akt, whereas EP2−/− cells had suppression only of stimulated p-Akt (Fig. 4G). In contrast, Akt levels varied insignificantly, and p-GSK3α/β and β-actin protein levels were similar in the three cell types (Fig. 4G). In addition, compared to WT cells, EP4−/− macrophages had completely abolished phosphorylation of Bad (Serine 136 and 155) after 3 hours of PA-BSA treatment (Fig 3H). Bad phosphorylation was seen after 18 hours of treatment. This suppression of p-Akt and p-Bad activity in EP4−/− macrophages indicates that the PI3K/Akt pathway may be responsible for the increase in apoptosis.
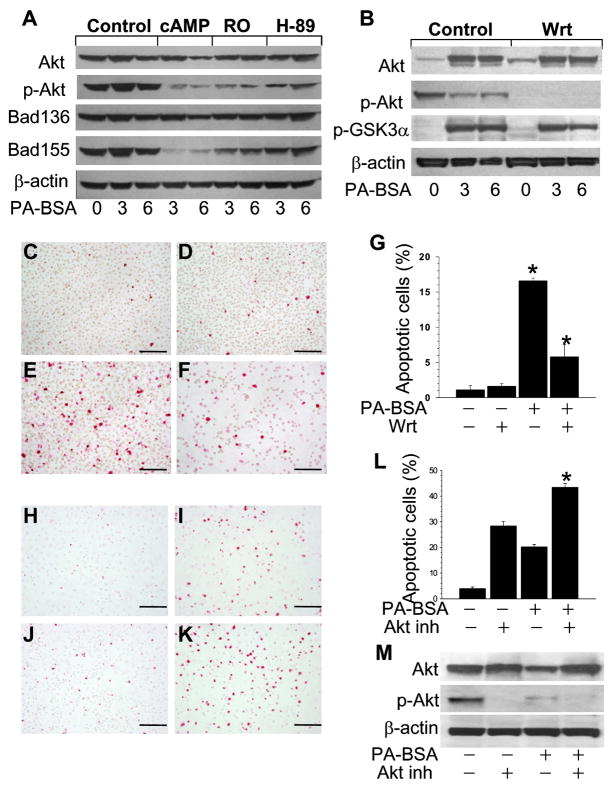
To test this hypothesis, we treated WT macrophages with PA-BSA alone or with different cAMP agonists including dibutyryl cAMP or a type IV phosphodiesterase inhibitor, RO-20-1724 for 3 and 6 hours. Stimulation of the cAMP pathway with dibutyryl cAMP and, to a lesser extent, with RO-20-1724 significantly suppressed phosphorylation of Akt (Fig 5A). The dibutyryl cAMP also suppressed the phosphorylation of Bad155. Treatment with a PKA inhibitor, H89, had only minor effects on phosphorylation of Akt and Bad (Fig 5A).
Figure 5. Inhibition of the PI3K/Akt signaling pathway in WT macrophages accelerates apoptosis.
(A) Expression of Akt, p-Akt(Ser 473), Bad(Ser 136 and 155) and β-actin proteins in WT macrophages treated with PA-BSA (0.5mM) alone or with dibutyryl cAMP (1mM), RO-20-1724 (100μM) or, PKA inhibitor, H89 (10μM), for 3 and 6 hours.
(B) Expression of Akt, p-Akt, GKS3α/β and β-actin proteins in WT macrophages treated with PA-BSA alone or with wortmannin (Wrt, 100nM) for 3 or 6 hours. Extracted proteins (100μg/lane) were resolved and analyzed by Western blot using antibodies against the proteins as indicated.
(C–F) Apoptosis in WT macrophages untreated (C) or treated with Wrt(50nM; D) or PA-BSA(E) alone or in combination with Wrt(F) macrophages for 18 hours (x20). The scale bars represent 20 μm
(G) Percent of apoptotic macrophages untreated and treated with Wrt (50nM; D), PA-BSA alone or in combination with Wrt for 18 hours. Graphs represent data (Mean ± SEM) with the same number (n=3) of mice per group (*p<0.05 between untreated cells and treated with PA-BSA alone and with Wrt by One Way ANOVA analysis).
(H–K) Apoptosis in WT macrophages untreated(H) or treated with the Akt inhibitor IV (10μM; I) or PA-BSA(J) alone or in combination with the Akt inhibitor IV(K) for 24 hours (x20). The scale bars represent 20 μm
(L) Percent of apoptotic macrophages untreated and treated with the Akt inhibitor IV (10μM), PA-BSA alone or in combination with the Akt inhibitor IV for 24 hours (*p<0.05 between cells treated with PA-BSA alone and with the Akt inhibitor by One Way ANOVA analysis, the Tukey test).
(M) Expression of Akt, p-Akt, and β-actin in WT macrophages treated with Akt inhibitor IV or PABSA alone or incombination for 6 hours. Extracted proteins (100μg/lane) were resolved and analyzed by Western blot using antibodies against the proteins as indicated.
In contrast, treatment of WT macrophages with a potent PI3K inhibitor, wortmannin (Wrt, 100nM), alone or in combination with PA-BSA for 3 and 6 hours, completely abolished p-Akt activation (Fig 5B). Interestingly, more prolonged treatment with PA-BSA together with Wrt (50nM) for 18 hours increased the number of apoptotic cells (Fig 5C) producing a dramatic cell loss (~70%) possibly due to the role of the PI3K/Akt pathway in cell attachment and migration (Shiojima, 2002 #83). This may explain the decrease in the percentage of TUNEL+ cells in the group treated with PA-BSA together and Wrt compared to the treatment with PA-BSA alone (Fig 4D).
Finally, to examine whether decreased Akt activation is responsible for enhanced apoptosis in macrophages, we treated WT cells with PA-BSA with or without a cell-permeable Akt inhibitor IV which does not affect PI3K. This inhibitor acted as a pro-apoptotic factor and significantly (>2 fold) increased apoptotic cell numbers when combined with PA-BSA compared to PA-BSA alone (Fig 5I). The analysis of the signaling pathway demonstrated that the Akt inhibitor alone or in combination with PA-BSA completely eliminated Akt phosphorylation without affecting Akt and β-actin (Fig 5H). Together these results demonstrate that the PI3K/Akt signaling pathway is suppressed in EP4 null macrophages leading to increased apoptosis.
EP4 deficiency suppresses the NF-κB-pathway
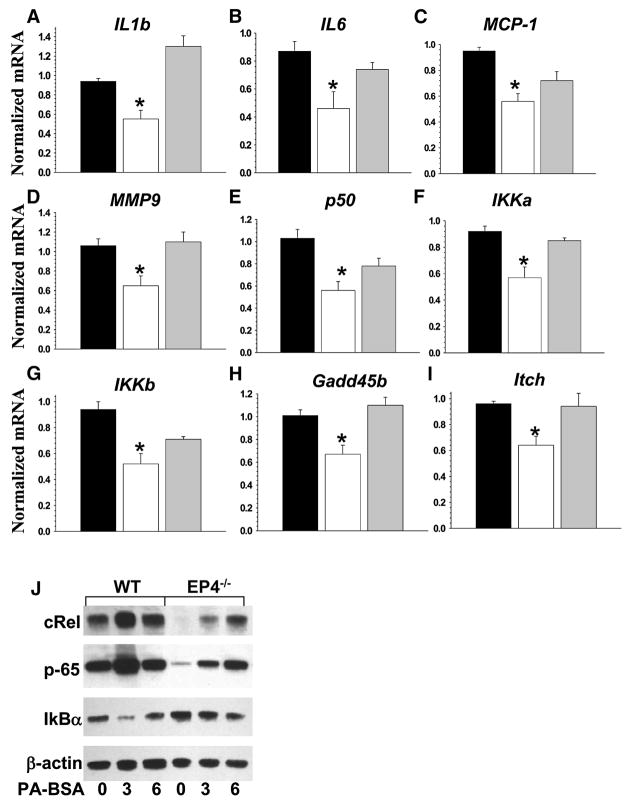
Since Akt directly regulates the activity of the transcription factor NF-κB(Madrid et al., 2000; Ozes et al., 1999; Romashkova and Makarov, 1999), we examined whether macrophage EP4 deficiency has an impact on inflammatory and anti-apoptotic genes. Peritoneal macrophages were stimulated with LPS (50ng/ml) for 5 hours and the expression pattern of the genes was analyzed by real-time PCR. There were no differences in the expression levels of TNFα, Gro1 and MMP-2 genes between WT, EP4−/− and EP2−/− macrophages. However, EP4−/−macrophages had significantly decreased expression levels of inflammatory (IL1β, IL6, MCP-1 and MMP9), NF-κB-related (p50, IKKα and IKKβ) and anti-apoptotic (Gadd45 and Itch) genes compared to WT and EP2−/− cells (Fig. 6A–I). In addition, two NF-κB-related proteins, cRel and p65, but not IκBα, were suppressed in EP4−/− macrophages compared to WT cells (Fig. 6JK). Thus, the NF-κB pathway in EP4 null macrophages is significantly suppressed.
Figure 6.
EP4 deficiency in macrophages suppresses NF-kB-related gene and protein expression levels. (A–I). Expression levels of inflammatory (IL1β, IL6, MCP1, MMP9), NF-κB-related (p-50, IKKα, IKKβ) and anti-apoptotic (Gadd45β and Itch) genes in WT(▪), EP4−/−(□) or EP2−/−(
 ) macrophages. The cells were treated with LPS (50nM) for 5 hours and gene expression levels were measured by real-time PCR. Graphs represent data (Mean ± SEM) of two experiments with the same number (n=3) mice per group (*p<0.05 between WT and EP4−/− cells by One Way ANOVA analysis).
) macrophages. The cells were treated with LPS (50nM) for 5 hours and gene expression levels were measured by real-time PCR. Graphs represent data (Mean ± SEM) of two experiments with the same number (n=3) mice per group (*p<0.05 between WT and EP4−/− cells by One Way ANOVA analysis).
(J). Protein expression levels in WT and EP4−/− macrophages treated with PA-BSA (500 μM) for 3 and 6 hours. Cell extract (100μg/line) was resolved and analyzed by Western blot. The graph represents the data of two different experiments.
Discussion
PGs are critical mediators of inflammation and PGE2 is a major product of activated macrophages, regulating immune responses, cytokine production, and apoptosis(Chun et al., 2007; Nataraj et al., 2001; Takayama et al., 2002; Vassiliou et al., 2004). PGE2 has been implicated in the pathogenesis of atherosclerosis, and two of its four receptors, EP2 and EP4, are expressed in atherosclerotic lesions(Gómez-Hernández et al., 2006). However, the roles of macrophage EP2 and EP4 have not been previously examined in vivo. Because EP4 deficiency is lethal in mice, we used FLC transplantation to generate LDLR−/− mice chimeric for EP2 or EP4 gene deletions in hematopoietic cells. Here we demonstrate that LDLR−/− mice reconstituted with EP4−/− hematopoietic cells had significant decreases in atherosclerosis of the proximal and distal aortas (59 and 71%, respectively) compared to mice transplanted with WT FLC. EP2 deficiency in hematopoietic cells showed a trend for similar, but modest, effects on atherosclerosis that were not statistically significant. Since serum lipid and lipoprotein profiles did not differ between the groups, these data indicate that EP4 deficiency in macrophages is responsible for reducing early atherosclerosis.
Although COX-2 mediated expression of PGE2 has been shown to promote cell growth and suppress apoptosis in a variety of tumors(Backlund et al., 2005; George et al., 2007; Kern et al., 2006), the roles of PGE2 and its receptors in modulating macrophage apoptosis in atherogenesis have not been previously examined. Recently, PGE2 has been reported to mediate strong pro-survival effects via EP4 and EP2 in epithelial and dendritic cells(Tessner et al., 2004; Vassiliou et al., 2004). In our study, mice reconstituted with EP4−/− FLC had a striking increase in the number of apoptotic cells in atherosclerotic lesions compared to the recipients with WT hematopoietic cells. A similar trend for an affect of macrophage EP2 on apoptosis in atherosclerosis was observed but did not achieve statistical significance. Our results demonstrate that deficiency of EP4 promotes apoptosis in atherosclerotic lesions in vivo. Because the gene-targeted mice used in these experiments were derived from embryonic stem cells of the 129 strain and then crossed onto the C57BL/6 background, it is possible that passenger genes from the 129 strain may have contributed to the observed phenotype(Lusis et al., 2007). As pointed out by Lusis, a contribution of passenger genes is less likely when, as in our study, the mice have been backcrossed into C57BL/6 strain for 10 or more generations and when the results test a prior hypothesis(Lusis et al., 2007). Furthermore, our current data are consistent with previous studies indicating that PGE2 mediates strong pro-survival effects via EP4 and EP2 in epithelial and dendritic cells(Tessner et al., 2004; Vassiliou et al., 2004) and suppressed apoptosis in a variety of tumors(Backlund et al., 2005; George et al., 2007; Kern et al., 2006).
Apoptosis plays an important role in the development of atherosclerotic lesions and plaque instability(Nhan et al., 2005; Tabas, 2005). In contrast to advanced atherosclerotic lesions characterized by an abundance of apoptotic cells due possibly to defective phagocytic clearance(Kockx, 1998; Tabas, 2005), macrophage apoptosis in the early stages of atherosclerosis can reduce plaque cellularity and volume. For example, deletion of the pro-apoptotic factor Bax in hematopoietic cells significantly accelerates early atherosclerosis in LDLR−/− mice(Liu et al., 2005). In agreement with these findings, when the apoptotic inhibitor AIM (Spα /Api6 gene) is deleted, LDLR−/− mice have significantly increased macrophage apoptosis and suppressed early atherosclerosis(Arai et al., 2005). In addition, mice heterozygous for macrophage colony-stimulating factor have a dramatic (<1% of controls) reduction of atherosclerosis(Rajavashisth et al., 1998). A recent report demonstrated that macrophage phospholipase Cβ 3 deficiency is associated with increased apoptosis in atherosclerotic lesions and suppression of atherosclerosis(Wang et al., 2008). Our present study further extends this association between apoptosis and lesion formation by demonstrating that EP4 deficiency in hematopoietic cells significantly increases macrophage apoptosis and suppresses early atherosclerosis. Given that in advanced atherosclerotic lesions apoptosis has been implicated in promoting necrotic cell death and a greater level of plaque instability(Han et al., 2006; Thorp et al., 2008), it is reasonable to speculate that macrophage EP-4 expression may modulate plaque stability in advanced lesions. Thus, EP4 and its downstream signaling pathways may provide new targets for modulating atherogenesis and plaque stability.
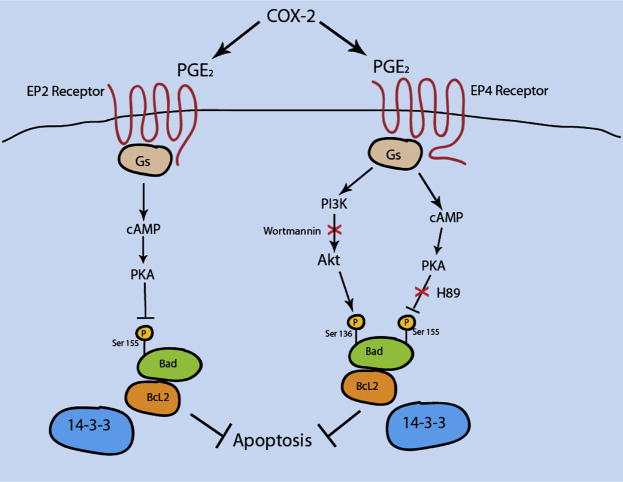
To examine the hypothesis that macrophage EP4 deficiency promotes apoptosis in vitro, peritoneal macrophages were challenged with PA-BSA, which induces ER stress and apoptosis(Borradaile et al., 2006) or free cholesterol loading (Yao and Tabas, 2001), treatment with oxidized LDL or with the inhibitor of IkB phosphorylation. All these stimuli induced apoptosis at higher levels in EP4−/− macrophages compared to WT and EP2−/− macrophages. This indicates the importance of EP4 in macrophage survival signaling. Figure 7 demonstrates that both EP2 and EP4 receptors may signal through the cAMP/PKA pathway inducing phosphorylation of a pro-apoptotic member of the Bcl-2 protein family, Bad at Ser155(Regan, 2003). In addition, EP4 activate the PI3K Akt pathway by phosphorylating Bad at two serine residues (Ser112 or Ser136) (Fujino et al., 2003). In its dephosphorylated state, Bad induces apoptosis by binding anti-apoptotic Bcl-2 family members, such as Bcl-xl, thereby allowing two other proapoptotic members, Bak and Bax, to form complexes, leading to the release of cytochrome c, caspase activation, and finally apoptosis(Wei et al., 2001). In contrast, phosphorylated Bad releases Bcl-xl and binds to the cytoplasmic anchorage protein, 14-3-3, undergoing cytoplasmic sequestration; this protects cells from apoptotic induction from different stimuli(Bergmann, 2002; Datta et al., 1999). Here we demonstrate that EP4−/− macrophages had significantly diminished levels of Akt phosphorylation (Figure 4H). In addition, Akt completely abolishes the activity of Bad at an early stage (3 hrs) of PA-BSA treatment. Thus, genetic deficiency of EP4 in macrophages suppresses the PI3K/Akt signaling pathway, reducing activation of Bad, which results in increased apoptosis.
Figure 7.
A schematic presentation of the EP2 and EP4 receptor signaling pathways specific for mouse peritoneal macrophages in relevance to apoptosis.
Next we undertook pharmacological experiments to confirm the relevance of the PI3K/Akt signaling pathway in mediating the impact of EP4 deficiency on macrophage apoptosis. The results strongly support the concept that EP4 deficiency suppresses macrophage survival, as treatment with cAMP agonists, but not with PKA inhibitor H89, reduces phosphorylation of Akt (Figure 4A). Similarly, only cAMP agonists suppressed phosphorylation of Bad155, which is specific for the cAMP/PKA pathway(Regan, 2003). In contrast, both the PI3K and the Akt inhibitors completely abolished p-Akt activation (Fig. 5B, M) and dramatically increased apoptosis in macrophages (Fig. 5C, L). Our data are supported by recent reports demonstrating that macrophages constitutively express active Akt(Liu et al., 2001) and that Akt null macrophages are more susceptible to apoptosis(Fernández-Hernando et al., 2007). Correspondingly, the inhibition of the PI3K pathway significantly accelerates macrophage apoptosis(Liu et al., 2001) and the deletion of the PI3K p110gamma gene significantly inhibits murine atherosclerosis(Chang et al., 2007). Together, these experiments indicate that activation of the PI3K/Akt pathway is important for macrophage survival. The suppression of this pathway in EP4 null macrophages promotes apoptosis and attenuates the development of early atherosclerosis.
In addition to an impact on apoptosis, PGE2 may impact the development and fate of atherosclerotic plaques through a variety of mechanisms. For example, the PI3K/Akt pathway has been shown to be crucial in the regulation of macrophage chemotaxis and migration(Curnock et al., 2002; Hirsch et al., 2000). EP4 has been proposed to mediate PGE2-induced production of MMP-9 in macrophages(Pavlovic et al., 2006) and given that apoE−/− mice deficient for MMP-9 gene expression have reduced atherosclerosis with decreased macrophage infiltration and collagen deposition(Luttun et al., 2004), all these may promote plaque destabilization. Furthermore, PGE2 has been proposed to impact atherosclerosis through EP4-mediated modulation of inflammation. Pretreatment of bone marrow-derived macrophages with PGE2 prior to activation with LPS, results in a transitory attenuation of the early phase of production of chemokines and inflammatory cytokines(Minami et al., 2008). Interestingly, this anti-inflammatory effect is mediated by a novel EP4 receptor associated protein that inhibits LPS induced activation of NFkB(Minami et al., 2008). In the absence of pre-treatment with PGE2, we report that activation of EP4−/− macrophages with LPS results in significant suppression of NF-κB-responsive genes and proteins, and suggesting that macrophage EP-4 may impact atherosclerosis by its effects on inflammatory cytokines such as IL-6 and MCP-1 (Fig. 6). This data is consistent with evidence that activation of Akt can stimulate several signaling pathways including NF-κB(Madrid et al., 2000; Ozes et al., 1999; Romashkova and Makarov, 1999). The NF-κB activation may induce transcription of anti-apoptotic genes (including FLIP, the caspase inhibitors cIAP1/2 and Bcl2 member activation) changing the balance to pro-survival stimuli(Karin and Lin, 2002) and increasing the cells resistance to apoptosis(Verma and Mehta, 2007). Thus, the suppression of both the PI3K/Akt and NF-κB anti-apoptotic pathways may synergistically contribute to the increased apoptosis in EP4−/− macrophages.
Selective inhibition of COX-2 and non-selective inhibition of COX by NSAIDs have been reported to increase the risk for cardiovascular events(Bresalier et al., 2005; Grosser et al., 2006; Solomon et al., 2005). Macrophage apoptosis has been proposed to promote the formation of unstable plaques and to contribute to plaque rupture(Tabas, 2005). Given that COX-2 inhibition has been shown to promote apoptosis in a variety of tumors (Backlund et al., 2005; Kern et al., 2006), it is reasonable to speculate, based on our current findings, that that COX-2 inhibition might increase apoptosis in atherosclerotic plaques by inhibiting PGE2 production and its EP4-mediated pro-survival pathways in macrophages, providing a potential mechanism for plaque rupture and increased cardiovascular events.
In conclusion, EP4 disruption in macrophages promotes apoptosis and significantly suppresses early atherosclerosis in vivo. The genetic and pharmacological inhibition of EP4 in macrophages suppresses the PI3K/Akt signaling pathway, reducing activation of Bad, thereby promoting apoptosis. These data indicate that macrophage EP4 expression plays a crucial role in mediating pro-survival signaling. Therefore, the PGE2/EP4 pathway may provide therapeutic opportunities to modulate the development of atherosclerosis and plaque stability.
Experimental Procedures
Animal Procedures
Mice heterozygous for EP4(Schneider et al., 2004) and EP2 gene(Kennedy et al., 1999) were on the C57BL/6 background (10th backcross). Recipient LDLR−/− (on C57BL/6 background) and C57BL/6 mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Mice were maintained in microisolator cages on a rodent chow diet containing 4.5% fat (PMI 5010, St. Louis, MO) or a Western type diet containing 21% milk fat and 0.15% cholesterol Teklad, Madison, WI). Animal care and experimental procedures were performed according to the regulations of Vanderbilt University's Animal Care Committee.
Fetal liver cell (FLC) isolation, genotyping and transplantation
Female EP4+/− (or EP2+/−) mice were intercrossed with male EP4+/− (or respectively EP2+/−) mice and pregnancy was determined by the presence of a vaginal plug. On day 14–16 of gestation, embryos were isolated and their gender was determined by PCR as described(Babaev et al., 1999). To identify the genotype of EP4−/− FLC, a set of primers were generated (AGC GAG TCC TTA GGC TTT TAA GT, GGA GTC ACT TTC CCT TGA GAA G and AAC GAG CCA TTT ACC ACT TGC) and used for PCR analysis (Supplemental data, Figure A2a). FLCs were isolated and transplanted (3x106) into lethally irradiated (9Gy) recipient LDLR−/− or C57BL6 mice as described(Babaev et al., 1999). FLC transplantation reconstitutes all hematopoietic cell lineages including macrophages, T and B cells (Forrester et al., 1991).
Serum Lipids and Lipoprotein Distribution Analysis
The serum total cholesterol and triglyceride levels were determined on samples obtained from mice fasted for 4 hours as described(Fazio et al., 1997). Fast performance liquid chromatography (FPLC) was performed on an HPLC system model 600 (Waters, Milford, MA) using a Superose 6 column (Pharmacia, Piscataway, NJ).
Analysis of Aortic Lesions
Aortas were flushed through the left ventricle and the entire aorta was dissected for en face analysis as described(Babaev et al., 2005). Cryosections of the proximal aorta were analyzed using an Imaging system KS 300 (Kontron Electronik GmbH.).
Peritoneal macrophage isolation and treatment
Thioglycollate- or concanavalin-A-elicited peritoneal macrophages were isolated as described(Han et al., 2006)from mice reconstituted with wild type, EP4−/−and EP2−/− bone marrow. Two days later, macrophages were treated with palmitic acid complexed to BSA prepared as described(Borradaile et al., 2006). Cells were treated with PA-BSA (500 μM) alone or combined with dibutyryl cAMP, RO-20-1724 (100μM), H89 (10mM) or wartmannin (all from Sigma), or Akt inhibitor (EMD, Calbiochem).
Apoptosis assessment
Serial 5-micron cryosections from the proximal aorta were fixed in 4% paraformaldehyde in PBS, treated with 3% citric acid and apoptotic cells were detected by the TUNEL (TdT-mediated dUTP nick end labeling) technique using the in situ cell death detection kit (Roche Applied Science). After treatment with Fast Red TR/Naphthol AS-NX substrate (Sigma), TUNEL-positive (TUNEL+) cells were counted in 6 different sections from each aorta. Peritoneal macrophages were cultured in Laboratory-Tek chamber slides (Nalge Nunc International). Cells were fixed, treated with 3% citric acid and TUNEL+ cells were detected by the In Situ Cell Death detection kit.
RNA Isolation and real-time PCR
Total RNA was isolated and relative quantitation of the target mRNA was performed as described(Babaev et al., 2007). The gene expression assays (Applied Biosystems, Foster City, CA) were normalized with 18S ribosomal RNA as an endogenous control.
Western blotting
Cells were lysed on ice with a lysis buffer (Cell Signaling Technology, Danvers, MA) containing a protease (Sigma) and phosphatase (Pierce) inhibitor cocktails. Protein concentrations were determined with the DC Protein assay kit (Bio-Rad Laboratories). Lysates (20 or 100 μg/lane) were resolved by NuPAGE Bis-Tris elecrophoresis (Invitrogen) and transferred onto polyvinylidene difluoride nitrocellulose membranes (Amersham Bioscience). Blots were probed with rabbit antibodies to Akt, p-Akt, pGSK3α/β, p-Bad136, p-Bad155 (all from Cell signaling), c-Rel, NF-kB p65 and IkBα (all from Santa Cruz Biotechnology), β-actin antibody (Abcam, Inc. Cambridge, MA) and goat anti-rabbit horseradish peroxidase-conjugated secondary antibodies (Upstate Cell Signaling, Lake Placid, NY). Proteins were visualized with ECL western blotting detection reagents (GE healthcare) on X-ray films. Protein levels were quantified by densitometry normalizing to β-actin.
Statistical Analysis
The statistical differences in mean serum lipids and aortic lesion areas between the groups were determined by a One Way ANOVA test using SigmaStat V.2 (SPSS Inc., Chicago, IL).
Supplementary Material
Supplemental data include one table and two Figures.
Acknowledgments
We thank Youmin Zhang and Robert P. Runner for excellent technical expertise. This work was supported by National Institutes of Health grants HL53989, HL65405, HL57986, HL65709, GM15431and DK59637 (Lipid, Lipoprotein and Atherosclerosis Core of the Vanderbilt Mouse Metabolic Phenotype Centers). The EP2−/− and EP4−/− mice were developed with the support of DK37097 to RMB and MB.
Footnotes
None of the authors of this paper have a financial interest related to these studies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL, Mak PA, Edwards PA, Mangelsdorf DJ, Tontonoz P, Miyazaki T. A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metabolism. 2005;1:201–213. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Babaev VR, Fazio S, Gleaves LA, Carter KJ, Semenkovich CF, Linton MF. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in vivo. J Clin Invest. 1999;103:1697–1705. doi: 10.1172/JCI6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaev VR, Ishiguro H, Ding L, Yancey PG, Dove DE, Kovacs WJ, Semenkovich CF, Fazio S, Linton MF. Macrophage expression of peroxisome proliferator-activated receptor-alpha reduces atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;116:1404–1412. doi: 10.1161/CIRCULATIONAHA.106.684704. [DOI] [PubMed] [Google Scholar]
- Babaev VR, Yancey PG, Ryzhov SV, Kon V, Breyer MD, Magnuson MA, Fazio S, Linton MF. Conditional knockout of macrophage PPAR{gamma} increases atherosclerosis in C57BL/6 and low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:1647–1653. doi: 10.1161/01.ATV.0000173413.31789.1a. [DOI] [PubMed] [Google Scholar]
- Backlund MG, Mann JR, Dubois RN. Mechanisms for the prevention of gastrointestinal cancer: the role of prostaglandin E2. Oncology. 2005;1:28–32. doi: 10.1159/000086629. [DOI] [PubMed] [Google Scholar]
- Bergmann A. Survival signaling goes BAD. Developmental Cell. 2002;3:607–608. doi: 10.1016/s1534-5807(02)00328-3. [DOI] [PubMed] [Google Scholar]
- Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, et al. Cardiovascular Events Associated with Rofecoxib in a Colorectal Adenoma Chemoprevention Trial. N Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: Subtypes and signaling. Ann Revof Pharm Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Chang JD, Sukhova GK, Libby P, Schvartz E, Lichtenstein AH, Field SJ, Kennedy C, Madhavarapu S, Luo J, Wu D, Cantley LC. Deletion of the phosphoinositide 3-kinase p110{gamma} gene attenuates murine atherosclerosis. Proc Natl Acad Sci. 2007;104:8077–8082. doi: 10.1073/pnas.0702663104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Austin SC, Rocca B, Koller BH, Coffman TM, Grosser T, Lawson JA, FitzGerald GA. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science. 2002;296:539–541. doi: 10.1126/science.1068711. [DOI] [PubMed] [Google Scholar]
- Chun KS, Akunda JK, Langenbach R. Cyclooxygenase-2 Inhibits UVB-Induced Apoptosis in Mouse Skin by Activating the Prostaglandin E2 Receptors, EP2 and EP4. Cancer Res. 2007;67:2015–2021. doi: 10.1158/0008-5472.CAN-06-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnock AP, Logan MK, Ward SG. Chemokine signalling: pivoting around multiple phosphoinositide 3-kinases. Immunology. 2002;105:125–136. doi: 10.1046/j.1365-2567.2002.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Egan KM, Wang M, Lucitt MB, Zukas AM, Pure E, Lawson JA, FitzGerald GA. Cyclooxygenases, Thromboxane, and Atherosclerosis: Plaque Destabilization by Cyclooxygenase-2 Inhibition Combined With Thromboxane Receptor Antagonism. Circulation. 2005;111:334–342. doi: 10.1161/01.CIR.0000153386.95356.78. [DOI] [PubMed] [Google Scholar]
- Fazio S, Babaev VR, Murray AB, Hasty AH, Carter KJ, Gleaves LA, Atkinson JB, Linton MF. Increased atherosclerosis in mice reconstituted with apolipoprotein E null macrophages. Proc Natl Acad Sci USA. 1997;94:4647–4652. doi: 10.1073/pnas.94.9.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Hernando C, Ackah E, Jun Yu J, Suárez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ, Sessa WS. Loss of Akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metabolism. 2007;6:446–457. doi: 10.1016/j.cmet.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester LM, Bernstein A, Rossant J, Nagy A. Long-term reconstitution of the mouse hematopoietic system by embryonic stem cell-derived fetal liver. Proc Natl Acad Sci USA. 1991;88:7514–7517. doi: 10.1073/pnas.88.17.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino H, Xu W, Regan JW. Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-Kinase and extracellular signal-regulated kinases. J Biol Chem. 2003;278:12151–12156. doi: 10.1074/jbc.M212665200. [DOI] [PubMed] [Google Scholar]
- George RJ, Sturmoski MA, Anant S, Houchen CW. EP4 mediates PGE2 dependent cell survival through the PI3 kinase/AKT pathway. Prostaglandins & Other Lipid Mediators. 2007;83:112–120. doi: 10.1016/j.prostaglandins.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Hernández A, Martín-Ventura JL, Sánchez-Galán E, Vidal C, Ortego M, Blanco-Colio LM, Ortega L, Tuñón J, Egido J. Overexpression of COX-2, Prostaglandin E Synthase-1 and Prostaglandin E Receptors in blood mononuclear cells and plaque of patients with carotid atherosclerosis: Regulation by nuclear factor-[kappa]B. Atherosclerosis. 2006;187:139. doi: 10.1016/j.atherosclerosis.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Gross S, Tilly P, Hentsch D, Vonesch JL, Fabre JE. Vascular wall-produced prostaglandin E2 exacerbates arterial thrombosis and atherothrombosis through platelet EP3 receptors. J Exp Med. 2007;204:311–320. doi: 10.1084/jem.20061617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Kennedy CR, Zhang Y, Brandon S, Guan Y, Coffee K, Funk CD, Magnuson MA, Oates JA, Breyer MD, Breyer RM. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nature Medicine. 1999;5:217–220. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- Kern MA, Haugg AM, Koch AF, Schilling T, Breuhahn K, Walczak H, Fleischer B, Trautwein C, Michalski C, Schulze-Bergkamen H, et al. Cyclooxygenase-2 inhibition induces apoptosis signaling via death receptors and mitochondria in hepatocellular carcinoma. Cancer Res. 2006;66:7059–7066. doi: 10.1158/0008-5472.CAN-06-0325. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Tahara Y, Matsumoto M, Iguchi M, Sano H, Murayama T, Arai H, Oida H, Yurugi-Kobayashi T, Yamashita JK, et al. Roles of thromboxane A(2) and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J Clin Invest. 2004;114:784–794. doi: 10.1172/JCI21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kockx MM. Apoptosis in the atherosclerotic plaque: quantitative and qualitative aspects. Arterioscler Thromb Vasc Biol. 1998;18:1519–1522. doi: 10.1161/01.atv.18.10.1519. [DOI] [PubMed] [Google Scholar]
- Linton MF, Fazio S. Cyclooxygenase products and atherosclerosis. Drug Discovery Today: Therapeutic Strategies. 2008 doi: 10.1016/j.ddstr.2008.05.006. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Perlman H, Pagliari LJ, Pope RM. Constitutively Activated Akt-1 Is Vital for the Survival of Human Monocyte-differentiated Macrophages: Role of Mcl-1, Independent of Nuclear Factor (NF)-{kappa}B, Bad, or Caspase Activation. J Exp Med. 2001;194:113–126. doi: 10.1084/jem.194.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Thewke DP, Su YR, Linton MF, Fazio S, Sinensky MS. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol. 2005;25:174–179. doi: 10.1161/01.ATV.0000148548.47755.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizcano JM, Morrice N, Cohen P. Regulation of BAD by cAMP-dependent protein kinase is mediated via phosphorylation of a novel site, Ser155. Biochem J. 2000;349:547–557. doi: 10.1042/0264-6021:3490547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusis AJ, Yu J, Wang SS. The Problem of Passenger Genes in Transgenic Mice. Arterioscler Thromb Vasc Biol. 2007;27:2100–2103. doi: 10.1161/ATVBAHA.107.147918. [DOI] [PubMed] [Google Scholar]
- Luttun A, Lutgens E, Manderveld A, Maris K, Collen D, Carmeliet P, Moons L. Loss of Matrix Metalloproteinase-9 or Matrix Metalloproteinase-12 Protects Apolipoprotein E-Deficient Mice Against Atherosclerotic Media Destruction but Differentially Affects Plaque Growth. Circulation. 2004;109:1408–1414. doi: 10.1161/01.CIR.0000121728.14930.DE. [DOI] [PubMed] [Google Scholar]
- Madrid LV, Wang CY, Guttridge DC, Schottelius AJG, Baldwin AS, Jr, Mayo MW. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappa B. Mol Cell Biol. 2000;20:1626–1638. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami M, Shimizu K, Okamoto Y, Folco E, Ilasaca ML, Feinberg MW, Aikawa M, Libby P. Prostaglandin E Receptor Type 4-associated Protein Interacts Directly with NF-{kappa}B1 and Attenuates Macrophage Activation. J Biol Chem. 2008;283:9692–9703. doi: 10.1074/jbc.M709663200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataraj C, Thomas DW, Tilley SL, Nguyen M, Mannon R, Koller BH, Coffman TM. Receptors for prostaglandin E2 that regulate cellular immune responses in the mouse. J Clin Invest. 2001;108:1229–1235. doi: 10.1172/JCI13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, Camenisch T, Snouwaert JN, Hicks E, Coffman TM, Anderson PA, Malouf NN, Koller BH. The prostaglandin receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature. 1997;390:78–81. doi: 10.1038/36342. [DOI] [PubMed] [Google Scholar]
- Nhan TQ, Liles WC, Schwartz SM. Role of Caspases in Death and Survival of the Plaque Macrophage. Arterioscler Thromb Vasc Biol. 2005;25:895–903. doi: 10.1161/01.ATV.0000159519.07181.33. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Pavlovic S, Du B, Sakamoto K, Khan KMF, Natarajan C, Breyer RM, Dannenberg AJ, Falcone DJ. Targeting prostaglandin E2 receptors as an alternative strategy to block cyclooxygenase-2-dependent extracellular matrix-induced matrix metalloproteinase-9 expression by macrophages. J Biol Chem. 2006;281:3321–3328. doi: 10.1074/jbc.M506846200. [DOI] [PubMed] [Google Scholar]
- Rajavashisth T, Qiao JH, Tripathi S, Tripathi J, Mishra N, Hua M, Wang XP, Loussararian A, Clinton S, Libby P, Lusis A. Heterozygous osteopetrotic (op) mutation reduces atherosclerosis in LDL receptor- deficient mice. J Clin Invest. 1998;101:2702–2710. doi: 10.1172/JCI119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sciences. 2003;74:143–153. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Romashkova JA, Makarov SS. NF-[kappa]B is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis--an inflammatory disease. New Eng J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Schneider A, Guan Y, Zhang Y, Magnuson MA, Pettepher C, Loftin CD, Langenbach R, Breyer RM, Breyer MD. Generation of a conditional allele of the mouse prostaglandin EP4 receptor. Genesis: J Genetics Dev. 2004;40:7–14. doi: 10.1002/gene.20048. [DOI] [PubMed] [Google Scholar]
- Segi E, Sugimoto Y, Yamasaki A, Aze Y, Oida H, Nishimura T, Murata T, Matsuoka T, Ushikubi F, Hirose M, et al. Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochem Biophys Res Commun. 1998;246:7–12. doi: 10.1006/bbrc.1998.8461. [DOI] [PubMed] [Google Scholar]
- Solomon SD, McMurray JJV, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M the Adenoma Prevention with Celecoxib Study I. Cardiovascular Risk Associated with Celecoxib in a Clinical Trial for Colorectal Adenoma Prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- Tabas I. Consequences and Therapeutic Implications of Macrophage Apoptosis in Atherosclerosis: The Importance of Lesion Stage and Phagocytic Efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- Takayama K, Garcia-Cardena G, Sukhova GK, Comander J, Gimbrone MA, Jr, Libby P. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J Biol Chem. 2002;277:44147–44154. doi: 10.1074/jbc.M204810200. [DOI] [PubMed] [Google Scholar]
- Takayama K, Sukhova GK, Chin MT, Libby P. A novel prostaglandin E receptor 4-associated protein participates in antiinflammatory signaling. Circ Res. 2006;98:499–504. doi: 10.1161/01.RES.0000204451.88147.96. [DOI] [PubMed] [Google Scholar]
- Tessner TG, Muhale F, Riehl TE, Anant S, Stenson WF. Prostaglandin E2 reduces radiation-induced epithelial apoptosis through a mechanism involving AKT activation and bax translocation. J Clin Invest. 2004;114:1676–1685. doi: 10.1172/JCI22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk Receptor Mutation Reduces Efferocytosis Efficiency and Promotes Apoptotic Cell Accumulation and Plaque Necrosis in Atherosclerotic Lesions of Apoe−/− Mice. Arterioscler Thromb Vasc Biol. 2008;28:1421–1428. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassiliou E, Sharma V, Jing H, Sheibanie F, Ganea D. Prostaglandin E2 Promotes the Survival of Bone Marrow-Derived Dendritic Cells. J Immunol. 2004;173:6955–6964. doi: 10.4049/jimmunol.173.11.6955. [DOI] [PubMed] [Google Scholar]
- Verma A, Mehta K. Transglutaminase-mediated activation of nuclear transcription factor-kappaB in cancer cells: a new therapeutic opportunity. Current Cancer Drug Targets. 2007;7:559–565. doi: 10.2174/156800907781662275. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu B, Wang P, Dong X, Fernandez-Hernando C, Li Z, Hla T, Li Z, Claffey K, Smith JD, Wu D. Phospholipase C beta3 deficiency leads to macrophage hypersensitivity to apoptotic induction and reduction of atherosclerosis in mice. J Clin Invest. 2008;118:195–204. doi: 10.1172/JCI33139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EHY, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao PM, Tabas I. Free Cholesterol Loading of Macrophages Is Associated with Widespread Mitochondrial Dysfunction and Activation of the Mitochondrial Apoptosis Pathway. J Biol Chem. 2001;276:42468–42476. doi: 10.1074/jbc.M101419200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental data include one table and two Figures.