Abstract
Normal aging is often accompanied by impairments in forming new memories, and studies of aging rodents have revealed structural and functional changes to the hippocampus that might point to the mechanisms behind such memory loss. In this article, we synthesize recent neurobiological and neurophysiological findings into a model of the information-processing circuit of the aging hippocampus. The key point of the model is that small concurrent changes during aging strengthen the auto-associative network of the CA3 subregion at the cost of processing new information coming in from the entorhinal cortex. As a result of such reorganization in aged memory-impaired individuals, information that is already stored would become the dominant pattern of the hippocampus to the detriment of the ability to encode new information.
Introduction
Aging is often accompanied by cognitive decline across multiple performance domains [1]. For many individuals, cognitive aging is particularly associated with impairments in remembering recent events and in learning complex associations [2,3]. Intriguingly, whereas the ability to form memories of new events and associations declines with age, memories of older events are often rigidly retained [4]. Could this paradox of cognitive aging provide a clue as to why new memories fail in aging?
In this paper, we highlight three key characteristics of deficient episodic memories that are associated with normal aging: loss of contextual details, susceptibility to interference, and an attenuated response to novelty. We will then present recent findings from animal models of cognitive aging that suggest a neurobiological and neurophysiological basis for these impairments. We will review evidence that age-related memory loss is identified with anatomically specific and subtle changes in brain connectivity, leading to structural and functional changes within the hippocampus that might account for cognitive aging.
Age-related difficulties with contexts, interference and novelty
Young people not only remember critical events from the past, but also can recall details about where and when an event occurred or the source of information obtained. Fewer of these contextual or source details are included in recently acquired memories of older adults [1]. For example, in formal testing of verbal memory, the memories of aged individuals often lack details such as the color, case or font of words presented visually, whether the speakers of words were male or female, and even whether items were presented visually or aurally [1]. One crucial component of context is the spatial location where items were experienced. Aged adults are impaired in multiple aspects of spatial memory, from recalling where an item was located [5,6] to navigating through a recently learned environment [7,8]. In addition, memories of aged individuals are more adversely affected by increased similarity between contexts or objects [9,10]. These observations suggest that aging is associated with a decrease in the amount of information stored in memory that can be brought to bear in distinguishing experiences that share common elements of contextual information.
In addition, the elderly are disproportionately affected by interference from irrelevant information [11]. Aged individuals often have more difficulty in forgetting stored information that becomes irrelevant [11]. Consequently, memories of the elderly are particularly vulnerable to proactive interference, which occurs when previously stored memories conflict with the retrieval of new memories [12]. Conversely, in verbal learning, aged individuals are helped more by a reduction of interference between target words and lures [10]. Thus, an age-associated impairment in distinguishing experiences might also arise, in part, as a consequence of failures to update or inhibit previously stored information.
Older individuals also have an attenuated response to novelty. In young adults, an unexpected novel sound among repeated sounds causes an increase in neuronal synchronization ∼300 ms later, named the P300 event-related potential [13]. Normal aging is associated with a decreased amplitude and a longer latency of the P300 [14,15], and the deficit can be greater in cognitively impaired individuals [16]. Similarly, a sudden occasional sound results in smaller physical startle reactions and event-related potentials in elderly adults [17,18]. Furthermore, aging is associated with an attenuated electrophysiological response to new items, and with impairments in determining whether items are new or have been seen previously [14]. Weakened processing of novelty might extend the training required for aged individuals to learn a novel environment [19].
Recent evidence from animal models suggests that the cognitive deficits associated with normal aging might arise from a common neurobiological source. Findings in amnesic patients and studies of animals with experimentally induced hippocampal damage have long highlighted the crucial role of the hippocampal region in learning and remembering events and spatial relationships [20]. Recent studies of neural encoding in hippocampal subregions have supported a specialized role for this system in episodic memory, whereby experiences that share common elements of contextual information can be distinguished [21-23]. At the same time, evidence has accumulated that hippocampal dysfunction is likely to be a key contributor to age-associated memory impairment [2,3]. Here, we will suggest that a subtle reorganization of hippocampal circuits during aging might have a profound impact on memory processing. In particular, the following sections will present a neurobiological model of the aged hippocampus that offers an explanation of how the encoding of new contextual details is compromised by interference from previously stored memories.
Neurobiology of the aging hippocampus
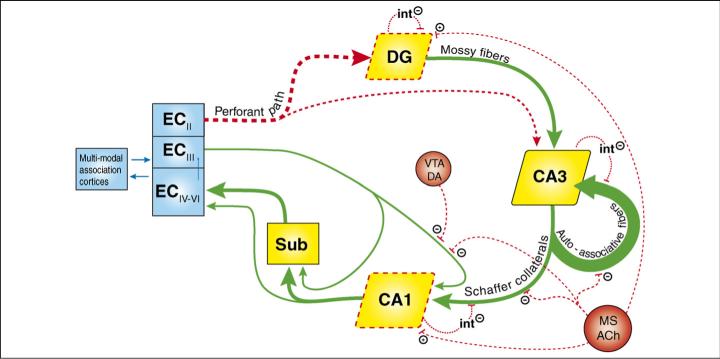
In young animals the hippocampus receives highly processed multi-modal information from widespread cortical association areas [24]. Sensory information enters the hippocampus largely via the perforant path, which projects from the entorhinal cortex (EC) to the dentate gyrus (DG), area CA3 and area CA1 (Figure 1). Outputs of the principle neurons in the DG project forward to CA3, then outputs of CA3 neurons project to the CA1 subregion, and thence to the subiculum. Both CA1 and the subiculum are the origins of a major return pathway to the EC. Three features of the circuit have especially important bearing in the context of neurocognitive aging. First, the DG is composed of roughly four times as many projection cells as the EC or CA3 [25]. This divergence and subsequent convergence of information has led to the idea that the DG might reduce the similarity of input patterns (pattern separation) [26,27]. Second, CA3 is unique within the hippocampus for containing recurrent connections: greater than 95% of input to each CA3 cell comes from CA3 collaterals. These so-called auto-associative fibers might facilitate the completion of incomplete input patterns (called pattern completion) [28]. Third, each subregion of the hippocampus receives inputs from the EC directly, in addition to its preceding subregion. This might enable comparison of the original cortical inputs with hippocampus-processed inputs, and this comparator function is hypothesized to be particularly important in CA1 [29]. The following sections will identify neurobiological changes in the aging hippocampus, and these will be described, as far as the data allow, in a subregion-specific manner.
Figure 1.
A neurobiological model of the aged hippocampus. Thick arrows represent the classic trisynaptic pathway, including the CA1 outputs to the entorhinal cortex (EC) via the subiculum (Sub). See main text for further description of this pathway. Age-related deteriorations in functional connectivity dealt with in this article are denoted by red broken lines, whereas connections depicted with green arrows remain intact (or have not yet been tested). With aging, the EC provides less input to the dentate gyrus (DG) and CA3, the medial septum (MS) provides less ACh-mediated modulation, interneuron (int) activity is decreased, the ventral tegmental area (VTA) provides less dopamine-mediated (DA) modulation, and the DG and CA1 are less excitable (depicted with red broken boxes). These changes might increase the activity of CA3 recurrent collaterals (auto-associative fibers, thickest arrow).
Reduced connections from the EC to the hippocampus
Although hippocampal volume is reduced in elderly humans [30], there is no evidence that the normal aged hippocampus contains fewer neurons [31]. This suggests that the volume loss might arise from synapse loss [2,32]. However, there is no evidence for gross differences in synaptic markers between aged rats that have memory impairments, aged rats that have intact memory abilities, and young rats, suggesting that the aged hippocampus as a whole does not undergo a widespread loss of connectional integrity [33]. Instead, synapse loss is highly specific, affecting only the cortical inputs to the hippocampus. In aged rats, the DG receives approximately one-third fewer synaptic contacts from the EC than in young rats [34,35]. The loss of input affects not only the connections of layer II EC neurons onto DG granule cells but also collaterals that feed forward onto CA3 pyramidal neurons [35]. Moreover, the extent of synaptic reduction correlates with the degree of spatial memory impairment in aged rats [35]. Corresponding to the loss of synaptic markers, a loss of cortical input has been detected electrophysiologically in studies showing that stimulation of the perforant path generates less excitation of the DG in aged rats than in young ones [31].
In contrast to the loss of EC inputs to CA3 cells, synapses onto CA3 cells from their own recurrent collaterals are not reduced in number during aging [35]. Furthermore, even among those aged rats with the greatest learning impairments, the CA1 subregion does not suffer any detectible loss in numbers of synapses, either in the stratum radiatum (CA3 inputs) [35,36] or in the stratum lacunosum-moleculare (EC inputs) [35].
Recent findings in elderly humans have demonstrated that aged subjects who have memory impairments possess a remarkably similar pattern of synapse loss in the perforant path. Scheff et al. [37] reported that the number of synapses receiving EC input in the outer molecular layer of the DG was reduced in elderly subjects who had mild cognitive impairment compared with cognitively intact elderly subjects, and the extent of synapse reduction correlated with memory ability. Furthermore, the use of diffusion-tensor imaging, a new magnetic-resonance imaging (MRI) technique, has revealed a marked atrophy in the perforant path of aged memory-impaired humans compared with young ones [38].
Thus, for aged memory-impaired animals and humans, the primary cortical extrinsic input to the early processing stages of the hippocampus is substantially reduced. Subsequent intrinsic auto-associative connections of CA3 and its outputs to CA1 are, however, predominantly spared.
Reduced modulation by the cholinergic system
The degree of modulation of the hippocampus mediated by ACh is attenuated in aged individuals, and the extent of this loss correlates with the degree of memory impairment [39-41]. The decrease in ACh-mediated modulation is reflected in reductions in the levels of ACh-processing enzymes in the medial septum, which provides the major cholinergic innervation to all three subregions of the hippocampus (see Figure 1 for modulation details) [42-46]. Furthermore, there is a blunted response in aged animals in all three hippocampal subregions to electrophysiological stimulation of cholinergic input fibers [47]. Direct application of muscarinic ACh receptor agonists also results in a blunted response in aged animals in tissue samples taken from the whole hippocampus [39,40] and, when measured at higher anatomical resolution, in CA3 and CA1 but not in the DG [48]. In humans, ACh-mediated modulation is also clearly reduced with normal aging: for example, levels of ACh-processing enzymes are decreased in the hippocampus [49]. In young individuals, modulation by ACh is believed to be crucial for switching between modes of recall and storage within the hippocampus [29], and impaired ACh function in aging might reduce the relative influence of new information in the hippocampus [50,51].
Reduced activity of inhibitory interneurons
Compounding the dysfunction of ACh-mediated modulation is a further loss of modulation from inhibitory interneurons within the hippocampus. Markers for GABAergic cells are reduced in aged rats for a wide variety of interneuron types and across the three hippocampal subregions [52,53]. Specific losses have also been found for interneurons in the hilus that express neuropeptide Y [54], and for GABAergic interneurons of CA1 but not those of DG or CA3 [55]. These interneuron losses might disturb the delicate balance between excitation and inhibition within the aged hippocampus.
Reduced modulation by the dopaminergic system
The hippocampal CA1 subregion receives dopaminergic projections from the ventral tegmental area (and other midbrain nuclei), but with normal aging the ventral tegmental area loses neurons [56] and dopamine-transport capabilities [57]. Furthermore, dopamine levels are reduced in the dorsal hippocampus of aged rats [58], and the extent of these losses correlates with memory ability [56,58]. In aged humans too, modulation by dopamine is weakened: dopamine receptors are reduced in number in the aged CA1 hippocampus [59,60]. In young individuals, modulation by dopamine has a critical role in facilitating plasticity in CA1 hippocampus [61], and the age-related reduction in dopamine function might contribute to impairments in synaptic plasticity.
Weakened synaptic plasticity
For neuronal networks to encode new information, some connections must be rapidly strengthened while others are weakened. In the hippocampus of aged rats, the ability of neurons to undergo synaptic plasticity is compromised, as measured by long-term potentiation (LTP) and long-term depression (LTD) (for excellent reviews, see Refs [31,62-64]). In the DG, the perforant path connection in aged rats is less excitable [65] and requires greater stimulation to induce LTP, suggesting an increased threshold [66]. Furthermore, potentiation in the DG decays faster in aged rats than in young rats [67]. In CA3, LTP at the perforant path connection also decays faster in aged rats [68] (the potentiation threshold has not yet been determined in CA3). In CA1, there is no evidence for a higher induction threshold at the CA3 input, but LTP does not reach the same level of potentiation in aged animals as in young animals [31]. In all subregions, enhanced levels of synaptic plasticity can be reached in aged memory-impaired animals, suggesting that, although it is weakened in subregion-specific ways, the fundamental capacity for LTP induction remains.
LTD in aged rats has been investigated at the connection between CA3 Schaffer collaterals and CA1 neurons. As would be expected based on the deficits in ACh signaling, in aged memory-impaired rats plasticity (LTD) is not induced by an ACh receptor agonist, whereas LTD is induced by this method to similar levels in unimpaired aged and young rats [69]. Substantial alteration of NMDA-receptor-mediated LTD has also been observed. In one study, NMDA-receptor-dependent LTD was enhanced in aged CA1 [70], whereas another recent study reported a pronounced reduction [69]. These discrepant results might be due to differences in strains of animals or methodological differences.
Several recent studies point to potential mechanisms underlying the weakened synaptic plasticity at the CA3–CA1 connection in aged rats. First, a reduction in the size of perforated synapses, and therewith AMPA receptors, located in the CA1 stratum radiatum might render those synapses less effective [71]. Indeed, a reduction in the excitability of CA3–CA1 synapses has been observed electrophysiologically in vitro [65,72] (but see Ref. [69]). Second, reduced cholinergic input decreases the excitability of CA1 neurons [73]. Third, the excitability at CA3–CA1 connections is attenuated by a prolongation of the afterhyperpolarization, and this furthermore does not decrease with learning in aged memory-impaired rats [63]. Finally, the regulation of intracellular Ca2+ levels in CA1 pyramidal neurons is dysfunctional in aged rats, and this could impede synaptic plasticity [62]. In summary, memory impairment in aging is associated with alterations in the extent and form of plasticity in the connectivity of hippocampal networks.
Summary of the neurobiology of the aging hippocampus
Small changes within the circuitry of the hippocampal system might contribute significantly to age-associated memory impairment. Unlike Alzheimer's disease, which encompasses profound neuronal death and extensive loss of cholinergic input in the medial temporal region [74], normal aging is associated with a far more subtle pattern of deterioration in hippocampal circuitry leading to a shift in the balance of the components of the neural network. Nevertheless, these subtle changes might account for a subset of the cognitive deficits that accompany normal aging in some individuals.
Information processing by hippocampal place cells of aged animals
To examine how subtle age-related changes in hippocampal circuits affect information coding, we and others have recorded the activity of hippocampal neurons in behaving animals. As a rat explores a spatial environment, CA1 and CA3 neurons fire selectively when the animal occupies particular locations within the environment. These ‘place cells’ are believed to reflect elements of a cognitive map of the environment [75] or representations of places where significant events occur within episodic memories [76,77]. Characterization of age-associated changes in the firing patterns of place cells might shed light on disturbances in memory processing in the aged hippocampus.
Spatial coding by place cells is altered in aged rats in several experimental settings [64] (I. Wilson, PhD thesis, University of Kuopio, Finland, 2005). Of particular importance here, numerous studies have shown that CA3 and CA1 place cells of aged rats fail to encode new spatial or task information rapidly [78-82]. After young rats have explored one environment until it becomes highly familiar, introduction into a novel environment typically results in the creation of a new spatial representation, reflected in spatial firing patterns of hippocampal neurons that are drastically different from those associated with the familiar environment [83,84]. By contrast, in aged rats, place cells often retain the spatial firing patterns observed in the familiar environment when the animal is moved to the novel environment (Figure 2). Furthermore, the extent of the ‘rigidity’ of place cells in old rats predicts the magnitude of spatial memory impairment [80]. Remarkably, rigid hippocampal representations occur almost exclusively in the CA3 subregion and not among CA1 cells [82]. These observations parallel the impairments that arise with proactive interference in aged humans, suggesting that the aged hippocampus fails to encode appropriate new contextual information owing to proactive interference from prior related experiences.
Figure 2.
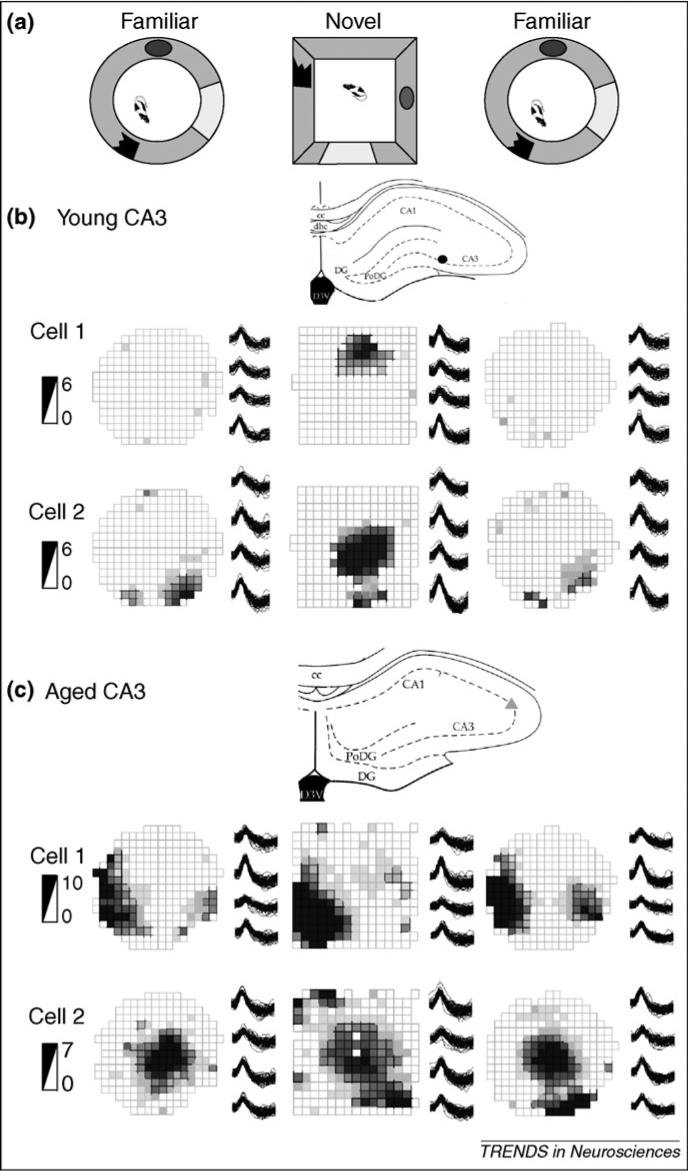
Examples of young and aged CA3 place fields. (a) Experimental setup, in which rats explore a familiar cylindrical open-field environment and a novel square open-field environment, and then are re-exposed to the familiar environment. (b,c) The recording sites, the place fields, the tetrode waveforms and the firing-rate scale in spikes per second are shown for two cells recorded simultaneously in CA3 of a young rat (b) and in CA3 of an aged rat (c). Each row represents the activity of one cell over the entire experiment shown in the firing-rate maps. Note that young CA3 cells created new spatial representations and often some were active in only one environment (e.g. (b) cell 1). By contrast, the aged CA3 cells used similar place-field representations for both environments and scarcely changed their abnormally high firing rates. Adapted, with permission, from Ref. [82].
Studies of place cells have also provided a window into failures by the aged hippocampus in retrieval of previous experiences. Barnes et al. [85] characterized the spatial representations of CA1 cells in young and aged rats exploring an initial environment. Then, following a tour of several new environments (without recording cellular activity), the rats were placed back into the original arena. In young rats, the same spatial representations were consistently re-generated, whereas in aged rats, some cells exhibited a distinct and new pattern of spatial activity. In this situation, where substantial proactive interference was applied, the hippocampus of aged rats failed to recall an earlier learned spatial context [86], and instead lapsed into fluctuations between multiple stable representations.
The failure to retrieve a correct representation reliably might arise from a failure to encode the representation sufficiently strongly. CA1 place fields of aged rats demonstrate less backward expansion of their fields than do those of young rats as they run repeatedly around a circular track [87]. This well-studied plasticity-dependent phenomenon shows that repeated neuronal sequences in young rats become strong predictors of future sequences, therefore eliciting earlier activity from subsequent neurons [88,89]. Importantly with respect to aged memory-impaired rats, it illustrates that deficient synaptic plasticity can have significant effects on processing [87] and possibly even retrieval [85] of spatial information.
Thus, studies of place cells are helping to explain failures by the aged hippocampus to encode and retrieve memories. They suggest that encoding of new spatial contexts is weakened by proactive interference from representations of already-stored contexts, and the associations between external context and internal representation are apparently not strong enough to reliably support recall following interfering experiences.
A model of information processing in the aged hippocampus
Based on the neurobiological and neurophysiological findings, we suggest a model of information processing in the aged hippocampus (Figure 1) that might account for failures to encode new contextual details due to interference from previously stored memories. Each hippocampal sub-region might make its own unique contribution to the impairment, as indicated in the model.
Models of information processing describe two major functions that take place in the hippocampal network, pattern separation in the DG and pattern completion in CA3 [26,27]. For young rats, the hippocampal system is simulated as an attractor network, capable of maintaining a representation despite minor changes in external input from the EC. However, when the change in external context is sufficient, the circuit switches to a dramatically different representation [28,90-93]. Upon entry into an environment, the balance of incoming sensory and stored information causes the hippocampal network to settle upon either the previously stored representation or a newly created representation. As revealed in place-cell recordings, in young rats a relatively modest change in environment or in task demands is sufficient to induce the switch from recalling a stored representation of the familiar environment to creating a new representation for the changed environment [83,84]. By contrast in aged rats, CA3 place-cell recordings indicate a propensity to recall stored representations rather than create new ones [82]. Several factors affecting the DG and CA3 in the aged brain are consistent with a condition in which the balance between the processes of pattern separation and pattern completion is altered, and these alterations, in addition to other intrinsic changes, also alter CA1 function. These features are incorporated into our model of neurocognitive aging.
Dentate gyrus
Processing in the DG of aged individuals suffers from reduced synaptic input and reduced electrophysiological excitation from EC layer II [35,94]. Reduced inputs might not transmit sufficient details to resolve changes in external contextual information that would normally be used to induce a switch from pattern completion to pattern separation. Based on this, the rigidity of encoding observed downstream in CA3 place cells [78-82] might be due, at least in part, to failure in the DG to reduce the similarity of input patterns sufficiently (e.g. pattern separation). Notably, reduced input from EC layer II in rats is paralleled in humans [37], and indications of DG hypofunction have been reported in aged rats, non-human primates and humans [95,96]. Indeed, Small et al. have emphasized the DG as a signature region of dysfunction in normal aging [95,96]. Thus, our model suggests that failures to encode new memories in aged individuals arise from insufficient pattern separation in a hypoactive DG.
CA3
In area CA3 of memory-impaired individuals, reduced inputs from the EC and reduced modulation by ACh might impair processing, forcing CA3 to rely on its own internal storage of the external world. In the young brain, ACh-mediated modulation is thought to contribute to switching between modes of recall and storage within the hippocampus. Increased activity of the cholinergic system seems to set the stage for new learning, whereas decreased activity encourages maintenance of an existing representation [29]. In CA3, the auto-associative network of powerful recurrent collaterals promotes completion of the familiar pattern, and reduced cholinergic input releases the CA3 auto-associative fibers from inhibition [29]. Thus, in aged animals, decreased cholinergic input might reduce the relative influence of new information through the perforant path, and subsequently favor the reactivation of stored information in the CA3 auto-associative network [50,51]. Reduced ACh-mediated modulation, in combination with fewer external details arriving at the reduced number of entorhinal synapses, might therefore bias the aged CA3 subregion towards maintenance of the original representation, and this is exemplified by rigidity of CA3 place cells [82]. Owing to the combined effects of aging in this sector of the hippocampus (i.e. effects on EC–DG–CA3 connections), our neurobiological model asserts that the CA3 of aged individuals strongly favors pattern completion to a greater extent than the CA3 of young animals (Figure 3).
Figure 3.
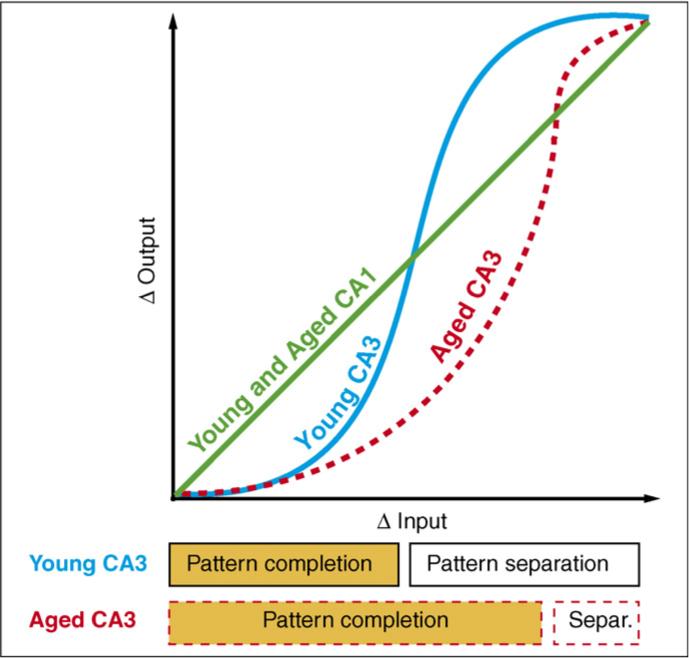
Model of CA1 and CA3 transformations of input. Age-related changes are denoted by red dashed lines. The CA1 subregion responds in a linear manner to changes in input. In response to two inputs, CA1 processing of both young and aged individuals generates two outputs whose difference is equal to the difference between the two inputs. The CA3 region responds in a non-linear manner to changes in input. In response to two inputs, CA3 processing of aged individuals generates two outputs whose difference is relatively smaller than that generated by CA3 processing of young individuals. Thus, the CA3 of aged individuals performs excessive pattern completion. In this model, the linear responses of aged CA1, with its greater independence from CA3 (see main text), are not affected by the CA3 deficiencies. Adapted, with permission, from Ref. [28].
An additional consequence of the alterations in functional circuitry described so far is hyperactivity of CA3 cells in aged rats. Using slice recordings, Hasselmo et al. [29] showed that reduced cholinergic input, similar to that seen in aging, releases the CA3 auto-association fibers from inhibition. Consistent with this observation in the slice preparation, aged CA3, but not CA1, place cells have abnormally high firing rates [82]. In addition to factors such as reduced function of cholinergic neurons, alterations intrinsic to CA3 might contribute to altered excitability. Notably, in situ hybridization has shown that expression of GABA receptor α5 subunit (Gabra5) mRNA is robustly reduced in CA3 (but not in CA1) of cognitively impaired aged rats compared with young rats (M. Gallagher and R. Haberman, personal communication); this form of the GABAA receptor mediates tonic inhibition of pyramidal neurons in the hippocampus [97]. Thus, age-related hypofunction in the DG, together with hyperfunction in CA3, might fundamentally change the ability of the hippocampus to switch from previously stored to newly created representations.
CA1
The effects of aging on the information processing functions of CA1 reflect not only the downstream consequences of alterations in the DG and CA3 but also changes intrinsic to the CA1 subregion itself. A common feature of CA1 place cells as studied in many different settings is a loss of reliable encoding in aged rats. The inconsistencies in CA1 encoding are perhaps best exemplified by the fluctuations between multiple stable representations observed when aged rats are returned repeatedly to the same environment after intervening experience [85]. With similar inconsistency, spatial representations in aged rats rotate with rotated landmarks on some occasions but not others [81]. Furthermore, rigidity can be evident in the encoding properties of CA1 cells [78-81], but this is not as invariable in CA1 as it seems to be in CA3 [82]. As we now describe further, in our model these inconsistencies in CA1 might represent shifts between dependence on CA3 inputs and direct EC inputs to the CA1 subregion.
In young rats, encoding in CA1 can arise independently of changes in CA3 encoding [98,99], and CA1 place cells are able to encode environment-specific information despite a lesion of CA3 [100] or suppression of CA3 output by septal inactivation [101]. Information processing in CA1 that lacks CA3 inputs relies on the direct input provided by EC layer III [100]. In aged rats, several factors might combine to increase the impact on CA1 of direct EC input and reduce the impact of input from CA3. First, in contrast to the loss of EC layer II input to the DG and CA3 regions, the synaptic input from EC layer III to CA1 is preserved in aged rats [35]. Second, reduced dopamine-mediated modulation in aging might bias CA1 activity towards dependence on its EC inputs. In young individuals, modulation by dopamine has a critical role in facilitating plasticity in the CA1 hippocampus when novelty has been detected, by reducing the impact of direct cortical inputs to CA1, while having little effect on the input from CA3 [61,102,103]. The age-related weakening of dopamine-mediated modulation might shift this balance by strengthening the impact of the EC inputs on the CA1 subregion while not affecting the impact of its CA3 inputs. Third, CA1 cells of aged rats are less excited by CA3 stimulation than are CA1 cells of young rats [72], perhaps arising from the smaller, less effective synapses found at CA3–CA1 connections [71]. These factors would be expected to alter the comparator function of CA1 in processing inputs from CA3 and those coming directly from the EC.
Regardless of the input source, the properties of aged CA1 cells themselves further compromise their ability to encode new information with sufficient strength to support reliable representations. In considering this deficient encoding in CA1, we note the weakened synaptic plasticity of aged CA1, where LTP is not able to reach the same level of potentiation as in young rats [31]. In the CA1 of aged memory-impaired rats, the afterhyperpolarization is prolonged [63], decreased ACh input reduces neuronal excitability [73], the synapses might not contain as many AMPA receptors [71], and the regulation of intracellular Ca2+ levels is dysfunctional [62]. These factors might combine such that representations supported by CA1 plasticity have reduced strength. One specific example of weakened plasticity in aged CA1 representations is a failure of place-field expansion, which is normally observed as a function of experience in young rats [87].
Summary of the model
According to the model presented here, alterations in each subregion of the hippocampus combine to the detriment of overall hippocampal function. The DG might not manage to resolve and reduce the similarity of new input patterns, the auto-associative fibers of CA3 might be overly entrenched in pattern completion, and the CA1 might be unable to encode with sufficient strength. As a result, age-related memory impairments emerge from failures by the aged hippocampal system to encode new contexts with sufficient distinction from already-stored memories.
Concluding remarks
Memory serves to guide our actions based on past experience. We learn from new situations so that future challenges can be addressed appropriately and quickly. Our brain is designed to take away the surprises and dangers of novelty, seeking to find familiarity in the regeneration of previous patterns of representation when confronted with new situations. Aging therefore might be considered a lifelong adaptation in which the acquisition of wisdom is associated with the gradual elimination of novelty. An untoward consequence of this adaptive strategy is that many aged individuals are beset by interference from older, stored memories when confronted with new contexts and episodes that should evoke new learning rather than recovered memories. Here, we have argued that the aged hippocampal network might account for such impairment in memory associated with aging. Information processing in the DG and CA3 subregions is biased towards maintenance of representations of familiar contexts. Normal aging is associated with highly specific alterations in the balance of information processing, resulting in weakened processing of new information and consequent strengthening of the processing of previously stored patterns.
Acknowledgements
This work was supported by the National Institute on Aging, by the Academy of Finland, by the Northern Savonia Cultural Foundation, and by the Research and Science Foundation of Farmos.
References
- 1.Zacks RT, et al. Human Memory. In: Salthouse TA, Craik FIM, editors. Handbook of Aging and Cognition. Erlbaum; 2000. pp. 293–357. [Google Scholar]
- 2.Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat. Rev. Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu. Rev. Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- 4.Burke DM, Mackay DG. Memory, language, and ageing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1997;352:1845–1856. doi: 10.1098/rstb.1997.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalfonte BL, Johnson MK. Feature memory and binding in young and older adults. Mem. Cognit. 1996;24:403–416. doi: 10.3758/bf03200930. [DOI] [PubMed] [Google Scholar]
- 6.Uttl B, Graf P. Episodic spatial memory in adulthood. Psychol. Aging. 1993;8:257–273. doi: 10.1037//0882-7974.8.2.257. [DOI] [PubMed] [Google Scholar]
- 7.Wilkniss SM, et al. Age-related differences in an ecologically based study of route learning. Psychol. Aging. 1997;12:372–375. doi: 10.1037//0882-7974.12.2.372. [DOI] [PubMed] [Google Scholar]
- 8.Newman MC, Kaszniak AW. Spatial memory and aging: performance on a human analog of the Morris water maze. Aging Neuropsychol. Cogn. 2000:86–93. [Google Scholar]
- 9.Henkel LA, et al. Aging and source monitoring: cognitive processes and neuropsychological correlates. J. Exp. Psychol. Gen. 1998;127:251–268. doi: 10.1037//0096-3445.127.3.251. [DOI] [PubMed] [Google Scholar]
- 10.Parkin AJ, et al. Data-driven recognition memory: a new technique and some data on age differences. Psychon. Bull. Rev. 2001;8:812–819. doi: 10.3758/bf03196222. [DOI] [PubMed] [Google Scholar]
- 11.Lustig C, Hasher L. Interference. In: Maddox GL, editor. The Encyclopedia of Aging. 3rd edn Springer; 2001. pp. 553–555. [Google Scholar]
- 12.Kane MJ, Hasher L. Interference. In: Maddox GL, editor. The Encyclopedia of Aging. Springer; 1995. pp. 514–516. [Google Scholar]
- 13.Ranganath C, Rainer G. Neural mechanisms for detecting and remembering novel events. Nat. Rev. Neurosci. 2003;4:193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- 14.Friedman D. Event-related brain potential investigations of memory and aging. Biol. Psychol. 2000;54:175–206. doi: 10.1016/s0301-0511(00)00056-9. [DOI] [PubMed] [Google Scholar]
- 15.Fjell AM, Walhovd KB. Age-sensitivity of P3 in high-functioning adults. Neurobiol. Aging. 2005;26:1297–1299. doi: 10.1016/j.neurobiolaging.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Daffner KR, et al. Age-related differences in novelty and target processing among cognitively high performing adults. Neurobiol. Aging. 2005;26:1283–1295. doi: 10.1016/j.neurobiolaging.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Ford JM, et al. Elderly men and women are less responsive to startling noises: N1, P3 and blink evidence. Biol. Psychol. 1995;39:57–80. doi: 10.1016/0301-0511(94)00959-2. [DOI] [PubMed] [Google Scholar]
- 18.Ellwanger J, et al. The relationship of age to prepulse inhibition and habituation of the acoustic startle response. Biol. Psychol. 2003;62:175–195. doi: 10.1016/s0301-0511(02)00126-6. [DOI] [PubMed] [Google Scholar]
- 19.Kirasic KC. Spatial cognition and behavior in young and elderly adults: implications for learning new environments. Psychol. Aging. 1991;6:10–18. doi: 10.1037//0882-7974.6.1.10. [DOI] [PubMed] [Google Scholar]
- 20.Cohen NJ, Eichenbaum H. Memory, Amnesia, and the Hippocampal System. MIT Press; 1993. [Google Scholar]
- 21.Wood ER, et al. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 22.Frank LM, et al. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27:169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- 23.Ferbinteanu J, Shapiro ML. Prospective and retrospective memory coding in the hippocampus. Neuron. 2003;40:1227–1239. doi: 10.1016/s0896-6273(03)00752-9. [DOI] [PubMed] [Google Scholar]
- 24.Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The Rat Nervous System. 2nd edn Academic Press; 1995. pp. 443–493. [Google Scholar]
- 25.Boss BD, et al. On the number of neurons in the dentate gyrus of the rat. Brain Res. 1985;338:144–150. doi: 10.1016/0006-8993(85)90257-4. [DOI] [PubMed] [Google Scholar]
- 26.McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 1987;10:408–415. [Google Scholar]
- 27.McClelland JL, Goddard NH. Considerations arising from a complementary learning systems perspective on hippocampus and neocortex. Hippocampus. 1996;6:654–665. doi: 10.1002/(SICI)1098-1063(1996)6:6<654::AID-HIPO8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 28.Guzowski JF, et al. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Hasselmo ME, et al. Dynamics of learning and recall at excitatory recurrent synapses and cholinergic modulation in rat hippocampal region CA3. J. Neurosci. 1995;15:5249–5262. doi: 10.1523/JNEUROSCI.15-07-05249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raz N, et al. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol. Aging. 2004;25:377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- 31.Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 32.Terry RD. Cell death or synaptic loss in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2000;59:1118–1119. doi: 10.1093/jnen/59.12.1118. [DOI] [PubMed] [Google Scholar]
- 33.Nicolle MM, et al. No loss of synaptic proteins in the hippocampus of aged, behaviorally impaired rats. Neurobiol. Aging. 1999;20:343–348. doi: 10.1016/s0197-4580(99)00054-8. [DOI] [PubMed] [Google Scholar]
- 34.Geinisman Y, et al. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- 35.Smith TD, et al. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J. Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geinisman Y, et al. Aging, spatial learning, and total synapse number in the rat CA1 stratum radiatum. Neurobiol. Aging. 2004;25:407–416. doi: 10.1016/j.neurobiolaging.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Scheff SW, et al. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol. Aging. 2005 doi: 10.1016/j.neurobiolaging.2005.09.012. DOI: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Kalus P, et al. Examining the gateway to the limbic system with diffusion tensor imaging: the perforant pathway in dementia. Neuroimage. 2005;30:713–720. doi: 10.1016/j.neuroimage.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 39.Chouinard ML, et al. Hippocampal muscarinic receptor function in spatial learning-impaired aged rats. Neurobiol. Aging. 1995;16:955–963. doi: 10.1016/0197-4580(95)02015-2. [DOI] [PubMed] [Google Scholar]
- 40.Nicolle MM, et al. Metabotropic glutamate receptor-mediated hippocampal phosphoinositide turnover is blunted in spatial learning-impaired aged rats. J. Neurosci. 1999;19:9604–9610. doi: 10.1523/JNEUROSCI.19-21-09604.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugaya K, et al. Septo–hippocampal cholinergic and neurotrophin markers in age-induced cognitive decline. Neurobiol. Aging. 1998;19:351–361. doi: 10.1016/s0197-4580(98)00072-4. [DOI] [PubMed] [Google Scholar]
- 42.Fischer W, et al. Degenerative changes in forebrain cholinergic nuclei correlate with cognitive impairments in aged rats. Eur. J. Neurosci. 1989;1:34–45. doi: 10.1111/j.1460-9568.1989.tb00772.x. [DOI] [PubMed] [Google Scholar]
- 43.Fischer W, et al. NGF improves spatial memory in aged rodents as a function of age. J. Neurosci. 1991;11:1889–1906. doi: 10.1523/JNEUROSCI.11-07-01889.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gallagher M, et al. Markers for biogenic amines in the aged rat brain: relationship to decline in spatial learning ability. Neurobiol. Aging. 1990;11:507–514. doi: 10.1016/0197-4580(90)90111-c. [DOI] [PubMed] [Google Scholar]
- 45.Luine V, Hearns M. Spatial memory deficits in aged rats: contributions of the cholinergic system assessed by ChAT. Brain Res. 1990;523:321–324. doi: 10.1016/0006-8993(90)91507-d. [DOI] [PubMed] [Google Scholar]
- 46.Stroessner-Johnson HM, et al. Cholinergic cell loss and hypertrophy in the medial septal nucleus of the behaviorally characterized aged rhesus monkey. J. Neurosci. 1992;12:1936–1944. doi: 10.1523/JNEUROSCI.12-05-01936.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen J, Barnes CA. Age-related decrease in cholinergic synaptic transmission in three hippocampal subfields. Neurobiol. Aging. 1996;17:439–451. doi: 10.1016/0197-4580(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 48.Nicolle MM, et al. Visualization of muscarinic receptor-mediated phosphoinositide turnover in the hippocampus of young and aged, learning-impaired Long Evans rats. Hippocampus. 2001;11:741–746. doi: 10.1002/hipo.1089. [DOI] [PubMed] [Google Scholar]
- 49.Perry EK, et al. Convergent cholinergic activities in aging and Alzheimer's disease. Neurobiol. Aging. 1992;13:393–400. doi: 10.1016/0197-4580(92)90113-c. [DOI] [PubMed] [Google Scholar]
- 50.Hasselmo ME, Schnell E. Laminar selectivity of the cholinergic suppression of synaptic transmission in rat hippocampal region CA1: computational modeling and brain slice physiology. J. Neurosci. 1994;14:3898–3914. doi: 10.1523/JNEUROSCI.14-06-03898.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hasselmo ME, Wyble BP. Free recall and recognition in a network model of the hippocampus: simulating effects of scopolamine on human memory function. Behav. Brain Res. 1997;89:1–34. doi: 10.1016/s0166-4328(97)00048-x. [DOI] [PubMed] [Google Scholar]
- 52.Vela J, et al. Rat hippocampal GABAergic molecular markers are differentially affected by ageing. J. Neurochem. 2003;85:368–377. doi: 10.1046/j.1471-4159.2003.01681.x. [DOI] [PubMed] [Google Scholar]
- 53.Stanley DP, Shetty AK. Aging in the rat hippocampus is associated with widespread reductions in the number of glutamate decarboxylase-67 positive interneurons but not interneuron degeneration. J. Neurochem. 2004;89:204–216. doi: 10.1111/j.1471-4159.2004.02318.x. [DOI] [PubMed] [Google Scholar]
- 54.Cadacio CL, et al. Hilar neuropeptide Y interneuron loss in the aged rat hippocampal formation. Exp. Neurol. 2003;183:147–158. doi: 10.1016/s0014-4886(03)00126-2. [DOI] [PubMed] [Google Scholar]
- 55.Shi L, et al. Stereological quantification of GAD-67-immunoreactive neurons and boutons in the hippocampus of middle-aged and old Fischer 344 × Brown Norway rats. J. Comp. Neurol. 2004;478:282–291. doi: 10.1002/cne.20303. [DOI] [PubMed] [Google Scholar]
- 56.Siddiqi Z, et al. Age-related neuronal loss from the substantia nigra-pars compacta and ventral tegmental area of the rhesus monkey. J. Neuropathol. Exp. Neurol. 1999;58:959–971. doi: 10.1097/00005072-199909000-00006. [DOI] [PubMed] [Google Scholar]
- 57.Salvatore MF, et al. Decreased plasma membrane expression of striatal dopamine transporter in aging. Neurobiol. Aging. 2003;24:1147–1154. doi: 10.1016/s0197-4580(03)00129-5. [DOI] [PubMed] [Google Scholar]
- 58.Stemmelin J, et al. Immunohistochemical and neurochemical correlates of learning deficits in aged rats. Neuroscience. 2000;96:275–289. doi: 10.1016/s0306-4522(99)00561-8. [DOI] [PubMed] [Google Scholar]
- 59.Hemby SE, et al. Neuron-specific age-related decreases in dopamine receptor subtype mRNAs. J. Comp. Neurol. 2003;456:176–183. doi: 10.1002/cne.10525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaasinen V, et al. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol. Aging. 2000;21:683–688. doi: 10.1016/s0197-4580(00)00149-4. [DOI] [PubMed] [Google Scholar]
- 61.Lisman JE, Grace AA. The hippocampal–VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Foster TC. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res. Rev. 1999;30:236–249. doi: 10.1016/s0165-0173(99)00017-x. [DOI] [PubMed] [Google Scholar]
- 63.Wu WW, et al. Age-related biophysical alterations of hippocampal pyramidal neurons: implications for learning and memory. Ageing Res. Rev. 2002;1:181–207. doi: 10.1016/s1568-1637(01)00009-5. [DOI] [PubMed] [Google Scholar]
- 64.Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog. Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- 65.Barnes CA, et al. Electrophysiolocial markers of cognitive aging: region specificity and computational consequences. Semin. Neurosci. 1994;6:359–367. [Google Scholar]
- 66.Barnes CA, et al. LTP induction threshold change in old rats at the perforant path–granule cell synapse. Neurobiol. Aging. 2000;21:613–620. doi: 10.1016/s0197-4580(00)00163-9. [DOI] [PubMed] [Google Scholar]
- 67.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 68.Dieguez D, Jr, Barea-Rodriguez EJ. Aging impairs the late phase of long-term potentiation at the medial perforant path-CA3 synapse in awake rats. Synapse. 2004;52:53–61. doi: 10.1002/syn.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee HK, et al. NMDA receptor-independent long-term depression correlates with successful aging in rats. Nat. Neurosci. 2005;8:1657–1659. doi: 10.1038/nn1586. [DOI] [PubMed] [Google Scholar]
- 70.Norris CM, et al. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J. Neurosci. 1996;16:5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nicholson DA, et al. Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments. J. Neurosci. 2004;24:7648–7653. doi: 10.1523/JNEUROSCI.1725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosenzweig ES, et al. Role of temporal summation in age-related long-term potentiation-induction deficits. Hippocampus. 1997;7:549–558. doi: 10.1002/(SICI)1098-1063(1997)7:5<549::AID-HIPO10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 73.Disterhoft JF, Matthew Oh M. Modulation of cholinergic transmission enhances excitability of hippocampal pyramidal neurons and ameliorates learning impairments in aging animals. Neurobiol. Learn. Mem. 2003;80:223–233. doi: 10.1016/j.nlm.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 74.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 75.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. 1978 Clarendon. [Google Scholar]
- 76.Eichenbaum H, et al. The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 77.Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 78.Tanila H, et al. Brain aging: impaired coding of novel environmental cues. J. Neurosci. 1997;17:5167–5174. doi: 10.1523/JNEUROSCI.17-13-05167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oler JA, Markus EJ. Age-related deficits in the ability to encode contextual change: a place cell analysis. Hippocampus. 2000;10:338–350. doi: 10.1002/1098-1063(2000)10:3<338::AID-HIPO14>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 80.Wilson IA, et al. Place cell rigidity correlates with impaired spatial learning in aged rats. Neurobiol. Aging. 2003;24:297–305. doi: 10.1016/s0197-4580(02)00080-5. [DOI] [PubMed] [Google Scholar]
- 81.Wilson IA, et al. Cognitive aging and the hippocampus: how old rats represent new environments. J. Neurosci. 2004;24:3870–3878. doi: 10.1523/JNEUROSCI.5205-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilson IA, et al. Age-associated alterations of hippocampal place cells are subregion specific. J. Neurosci. 2005;25:6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- 84.Leutgeb S, et al. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- 85.Barnes CA, et al. Multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388:272–275. doi: 10.1038/40859. [DOI] [PubMed] [Google Scholar]
- 86.Redish AD, et al. Hippocampus. 1998;8:438–443. doi: 10.1002/(SICI)1098-1063(1998)8:5<438::AID-HIPO4>3.0.CO;2-Z. Reconciling Barnes et al. (1997) and Tanila et al. (1997a,b) [DOI] [PubMed] [Google Scholar]
- 87.Shen J, et al. The effect of aging on experience-dependent plasticity of hippocampal place cells. J. Neurosci. 1997;17:6769–6782. doi: 10.1523/JNEUROSCI.17-17-06769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mehta MR, et al. Experience-dependent, asymmetric expansion of hippocampal place fields. Proc. Natl. Acad. Sci. U. S. A. 1997;94:8918–8921. doi: 10.1073/pnas.94.16.8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ekstrom AD, et al. NMDA receptor antagonism blocks experience-dependent expansion of hippocampal ‘place fields’. Neuron. 2001;31:631–638. doi: 10.1016/s0896-6273(01)00401-9. [DOI] [PubMed] [Google Scholar]
- 90.McNaughton BL, et al. Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J. Exp. Biol. 1996;199:173–185. doi: 10.1242/jeb.199.1.173. [DOI] [PubMed] [Google Scholar]
- 91.Redish AD, Touretzky DS. Cognitive maps beyond the hippocampus. Hippocampus. 1997;7:15–35. doi: 10.1002/(SICI)1098-1063(1997)7:1<15::AID-HIPO3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 92.Samsonovich A, McNaughton BL. Path integration and cognitive mapping in a continuous attractor neural network model. J. Neurosci. 1997;17:5900–5920. doi: 10.1523/JNEUROSCI.17-15-05900.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Redish AD. The hippocampal debate: are we asking the right questions? Behav. Brain Res. 2001;127:81–98. doi: 10.1016/s0166-4328(01)00356-4. [DOI] [PubMed] [Google Scholar]
- 94.Barnes CA. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 1994;17:13–18. doi: 10.1016/0166-2236(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 95.Small SA, et al. Imaging hippocampal function across the human life span: is memory decline normal or not? Ann. Neurol. 2002;51:290–295. doi: 10.1002/ana.10105. [DOI] [PubMed] [Google Scholar]
- 96.Small SA, et al. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc. Natl. Acad. Sci. U. S. A. 2004;101:7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Glykys J, Mody I. Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABAA receptor α5 subunit-deficient mice. J. Neurophysiol. 2006;95:2796–2807. doi: 10.1152/jn.01122.2005. [DOI] [PubMed] [Google Scholar]
- 98.Lee I, et al. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature. 2004;430:456–459. doi: 10.1038/nature02739. [DOI] [PubMed] [Google Scholar]
- 99.Leutgeb S, et al. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- 100.Brun VH, et al. Place cells and place recognition maintained by direct entorhinal–hippocampal circuitry. Science. 2002;296:2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- 101.Mizumori SJ, et al. Preserved spatial coding in hippocampal CA1 pyramidal cells during reversible suppression of CA3c output: evidence for pattern completion in hippocampus. J. Neurosci. 1989;9:3915–3928. doi: 10.1523/JNEUROSCI.09-11-03915.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Otmakhova NA, Lisman JE. Dopamine selectively inhibits the direct cortical pathway to the CA1 hippocampal region. J. Neurosci. 1999;19:1437–1445. doi: 10.1523/JNEUROSCI.19-04-01437.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11:551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]