Abstract
A conceptual model of movement ecology has recently been advanced to explain all movement by considering the interaction of four elements: internal state, motion capacity, navigation capacities, and external factors. We modified this framework to generate predictions for species richness dynamics of fragmented plant communities and tested them in experimental landscapes across a 7-year time series. We found that two external factors, dispersal vectors and habitat features, affected species colonization and recolonization in habitat fragments and their effects varied and depended on motion capacity. Bird-dispersed species richness showed connectivity effects that reached an asymptote over time, but no edge effects, whereas wind-dispersed species richness showed steadily accumulating edge and connectivity effects, with no indication of an asymptote. Unassisted species also showed increasing differences caused by connectivity over time, whereas edges had no effect. Our limited use of proxies for movement ecology (e.g., dispersal mode as a proxy for motion capacity) resulted in moderate predictive power for communities and, in some cases, highlighted the importance of a more complete understanding of movement ecology for predicting how landscape conservation actions affect plant community dynamics.
Keywords: corridors, dispersal, diversity, life-history traits, species richness
The pervasive negative effects of habitat fragmentation on biodiversity and reduced opportunities to preserve large landscapes have led to widespread use of corridors, thin strips of habitat that connect otherwise isolated habitat patches in fragmented landscapes. Successful corridor function is based on the assumption that movement of organisms increases among connected patches, increasing colonization and gene flow, with a net benefit for biodiversity.
Yet not all species respond to corridors in the same way, and there is a growing need for frameworks that allow corridor effectiveness to be predicted across species (1, 2). Life-history theory (3) may present such a framework by providing insight into tradeoffs that constrain movement abilities, but the broad scope and generality of life-history theory may fail to capture all dynamics of movement. An alternative theoretical foundation focused explicitly on movement has recently been proposed (4). This framework aims to provide a unified theory of movement that links basic aspects of life history and behavior (internal state, motion capacity, navigation capacity) with environmental variables (external factors). The movement ecology framework was conceived to explain movement of individuals. Here, we attempt to apply aspects of this framework to predict the dynamics of communities. In particular, we track the outcomes of colonization and persistence in rapidly changing plant communities in experimentally fragmented landscapes, linking proxies of plant motion capacity (i.e., dispersal modes) with external, experimentally controlled landscape features. Admittedly, our approach overlaps incompletely with the movement ecology framework; the dynamics of community assembly over time are influenced by factors that the movement ecology framework does not consider (e.g., the effect of competition on plant establishment), and the lack of species-specific, community-wide data on many aspects of the movement ecology framework limits our options as we scale up from individuals to communities. Yet our question is central to movement ecology, as it evaluates the general importance of motion capacities interacting with external factors in shaping entire plant communities. It is also critical for conservation, as it focuses on the interactions between landscape management and community response.
The Movement Ecology of Plants
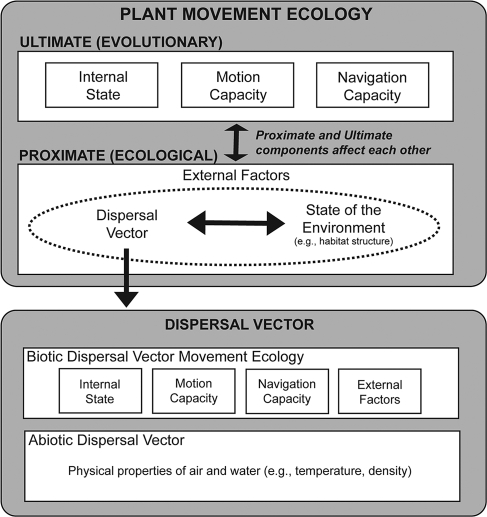
The movement ecology of most plants differs considerably from the movement ecology of most animals. Unlike animals, plant movement is restricted to reproductive structures: pollen, seeds, and some vegetative tissues. As a result, the movement ecology framework applied to animals includes a rich tapestry of elements determined over both ecological (proximate) and evolutionary (ultimate) time spans, whereas the same framework applied to plants creates a more obvious dichotomy between these evolutionary and ecological elements (Fig. 1). For example, the internal state of plant reproductive structures could include seed dormancy and seed release characteristics, which are determined over evolutionary rather than ecological time scales. Similarly, a plant's motion capacity is largely determined by evolved structures (e.g., wing of a samara) and its navigation capacity may be lacking or intimately linked to the movement ecology of an external dispersal vector (e.g., seed-dispersing birds or wind). Although there are rare examples of plant species that have internal states and navigation capacities that affect plant movement over ecological time scales (e.g., walking and crevice-following plants; ref. 5), in general, the short-term physiological, reflexive, neurological, and cognitive elements that play such a large role in describing animal movement (4, 5) do not apply to plants.
Fig. 1.
A movement ecology framework for plants separating ecological (proximate) and evolutionary (ultimate) processes. Interactions between proximate and ultimate factors and between external factors and seed dispersal vectors are critical for understanding plant movement.
Yet plants do not live in their own world; external factors such as the abundance and behavior of dispersal vectors, the quality and quantity of plant habitat, and the interactions between the framework's elements influence plant movement over both ecological and evolutionary time. External factors may interact strongly with other elements, such as motion capacity and internal states, to influence seed movement, plant recruitment, population spread, and community composition. This interaction among elements leads to two useful observations about the movement ecology framework as applied to plants, one simplifying and one complicating. The framework becomes simpler because for most plants, there is generally one primary type of movement, dispersal of propagules (but see ref. 5 for exceptions). We focus on seed movement because it is the sole opportunity for most plant species to physically move [i.e., it is the sole movement phase (4)]. Functionally, this sole opportunity to move makes the movement path of a seed equivalent to the lifetime track of the sedentary plant it will become (4).
In another way, applying the movement ecology framework is more complicated for plants than for many animals. Because external dispersal vectors (e.g., animals, wind) are linked to the movement of plants, one must integrate two simultaneous phenomena: movement of the vector and movement of the seed (Fig. 1). We see dispersal vectors as one of two large classes of external factors influencing the movement ecology of most plants (the second being environmental states), and we believe it is the interaction between these two external factors and their combined interaction with motion capacities that are most important for determining plant movement over ecological time scales (Fig. 1).
Although there are many possible environmental states, our study relates movement ecology to landscape-level dispersal. We focus on habitat structure, including the amount of suitable habitat, boundaries between suitable and unsuitable (matrix) habitat, and connectivity between suitable habitat patches. We focus on these elements because changes in basic landscape properties (presence or absence of a corridor, proximity to an edge) can have profound consequences for the movement of dispersal vectors (6), creating the potential for broad-reaching interactions between the motion capacity of plants and a set of easily defined and often manipulated external factors.
Predicting Plant Community Dynamics in Fragmented Landscapes
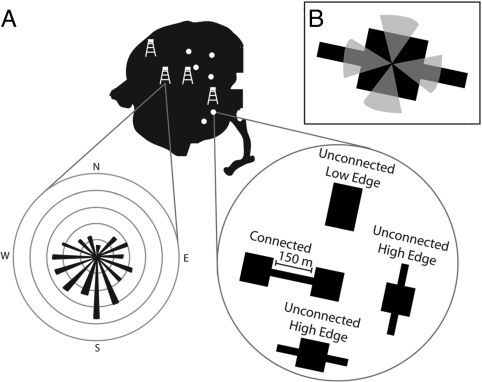
We conducted our study within experimental landscapes created to test for the effects of corridors as movement conduits, while controlling for differences in habitat area and amount of edge that also change when corridors are implemented (Fig. 2). We used six sets of five ≈1-ha open habitat patches surrounded by dense pine plantation forest (“experimental landscapes”), which have been censused over time to determine total species richness for the entire plant community [see supporting information (SI) Text for detailed methods]. Each experimental landscape contained a center patch connected by a 150-m-long corridor to a patch of the same size and shape, i.e., “connected” patches. Each landscape also contained two types of unconnected patches of equal area but different shapes, separated from the center patch by 150 m of unsuitable matrix habitat. “High-edge” patches had dead-end corridors extending from each side, whereas “low-edge” patches were rectangular in shape. Connected, unconnected low-edge, and unconnected high-edge patches were equal in area, but connected and high-edge patches had similarly greater edge-to-area ratio than low-edge patches. Comparisons of plant species richness between connected and unconnected high-edge patches tested whether corridors function as movement conduits, channeling seed dispersal between patches (“connectivity effect;” Figs. 2 and 3). Comparisons of species richness between unconnected high-edge and unconnected low-edge patches tested whether habitat patches with large amounts of edge increase seed colonization relative to similar patches of equal area but less edge (“edge effect;” Figs. 2 and 3).
Fig. 2.
Locations of study sites and wind measurements. (A) Location of six experimental landscapes (dots) and four wind towers (tower icons) at the Savannah River Site near Aiken, SC. The wind rose demonstrates the type of data used in analyses and represents the frequency of wind observations within directional bins from one tower in 2006–2007. (B) Shown are 45° bins perpendicular to the landscape orientation of each patch where wind observations were summed (i.e., wind incidence; see SI Text).
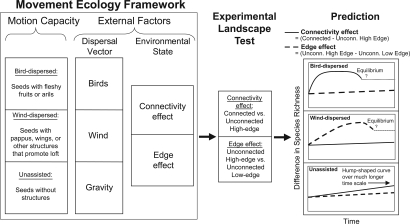
Fig. 3.
Predictions generated by extending the movement ecology framework to plant species richness dynamics within experimental landscapes. Species were separated into three groups based on motion capacity (bird, wind, unassisted), and predictions were generated for how each group would respond to two external landscape features over time: patch connectivity (solid lines) and patch edge effects (dashed lines). Predicted connectivity and edge effects may increase to an asymptote over time or may peak and then relax to an unknown equilibrium point (indicated by question marks and dotted lines).
We have demonstrated that corridors increase species richness of the plant community (7). A critical next step for understanding how corridors function is to understand the degree to which this connectivity effect can be predicted by plant traits. Our aim is to determine whether readily available plant trait information (e.g., dispersal modes) can be used as a proxy for a more detailed understanding of movement ecology. This is an important practical question: connectivity has the potential to benefit many species, but even in the most well studied plant communities, the detailed, species-specific data needed to predict community response is often lacking.
Using dispersal modes as a proxy for motion capacities, we focus on the interaction between motion capacities and external factors for two reasons. First, this interaction has already been useful for predicting the movement of bird-dispersed species in our system (8, 9) and other recent studies of plant communities in fragmented landscapes (10). Second, in a review of movement papers, Holyoak et al. (5) found that 62% of plant movement studies focus on this interaction. We see this as overwhelming support for the importance of the interaction between these two components of the movement ecology framework.
We divide the community of plants in our landscapes into three groups that have different motion capacities: bird-dispersed species, wind-dispersed species, and species without dispersal structures (unassisted species) (Fig. 3). We then use a 7-year time series to examine whether corridors increase plant species richness for each group.
We predict that the difference in diversity of bird-dispersed plants between connected and unconnected patches will increase most quickly (Fig. 3; ref. 10). At least one species of seed-dispersing bird common at our study site moves more frequently between connected patches than to either high-edge or low-edge unconnected patches (6). In addition, we know that seed dispersal from the central patch into unconnected high- and low-edge patches does not differ (8). However, because birds are not restricted to moving just between connected patches in our landscapes (9), we expect that connectivity effects may weaken over time as species accumulate in all patch types.
Our predictions for wind-dispersed species are based on our rudimentary understanding of wind dynamics in fragmented landscapes. We predict that differences in the species richness of wind-dispersed plants will increase more rapidly in response to increased amount of patch edge and more slowly in response to connectivity (Fig. 3; ref. 10). Wind often moves across landscapes in one predominant direction, making the likelihood of a wind-dispersed seed encountering an open habitat patch greater in elongated patches (e.g., with corridors). Over longer time periods, however, connectivity effects on wind-dispersed species may emerge because seeds blown into a suitable corridor, rather than matrix habitat, may result in successive generations of adult plants eventually traveling down the corridor into a connected patch.
For unassisted plant species, our predictions should be treated as a null model; these plants have no obvious dispersal structures, and thus we cannot use properties of their dispersal vectors to make predictions about their movement. We recognize that vector-assisted, long-distance dispersal does likely play a role in the movement of these species (11). But without detailed information on which vectors are most important, predictions regarding the relative importance of corridors or edges are impossible. We provisionally predict that dispersal in this group is primarily local (within patch) and that neither the connectivity nor the edginess provided by corridors will affect species richness over the time scale of our study (Fig. 3). Over longer time scales, we would expect unassisted species to eventually travel down corridors over successive generations into connected patches.
Three types of analyses were used to test our predictions (see SI Text for a full description of analyses). To test for overall effects of connectivity and edges on species richness, we used a mixed-model repeated-measures analysis of covariance (ANCOVA). To assess whether connectivity or edge effects could account for the changes in species richness over time, a separate ANCOVA was performed on the differences in species richness among patch types (connectivity effects = connected − unconnected high edge; edge effects = unconnected high edge − unconnected low edge). Finally, we directly tested for the differences among slopes for connectivity and edge effects for each dispersal mode.
Results
Our findings generally conform to our predictions, but mirror our levels of confidence for how corridors affect species richness of plants with different motion capacities. Species dispersed by birds and wind showed largely predictable responses; however, unassisted species did not follow predictions of a null response to the two environmental states, connectivity and edginess.
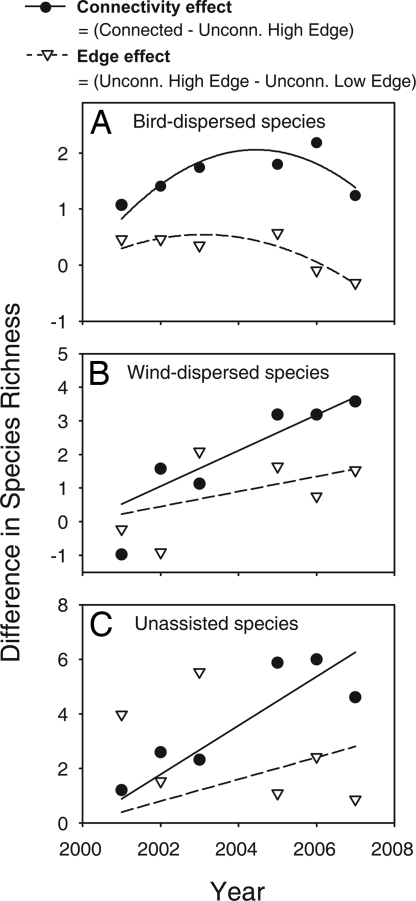
Bird-dispersed species exhibited trends consistent with predicted connectivity effects: rapid increases in species richness in connected patches relative to unconnected patches, followed by a gradual “catching up” of unconnected patches. The difference in bird-dispersed species richness between connected and unconnected high-edge patches increased over time and peaked in year 6 when connected patches showed a trend for more species than unconnected high-edge patches (linear contrasts within repeated-measures model, P = 0.06). This pattern matches our conceptual predictions (Fig. 3) as it is best described by a quadratic relationship (repeated-measures analysis using differences as a dependent variable, time covariate F1,6 = 7.74, P = 0.03, time2 covariate F1,6 = 8.43, P = 0.03) that was strongest for connectivity effects (Fig. 4A; quadratic model in simple linear regression, r2 = 0.98, F2,4 = 112.82, P < 0.001). Because of the transience of this pattern, the main effect of connectivity on species richness was weakened: connected patches showed only a trend for more bird-dispersed species averaged across all years of the study (Fig. 4A; linear contrast comparing connected and unconnected high-edge patches in a repeated-measures model, F1,15.1 = 3.11, P = 0.10). The amount of edge had no effect on bird-dispersed species richness: there was no difference between unconnected high-edge and unconnected low-edge patches (Fig. 4A; linear contrast comparing high- and low-edge patches in a repeated-measures model, F1,15.7 = 0.09, P = 0.77). Although there were no significant differences in species richness between high- and low-edge patches, the difference in richness between these two patch types also exhibited a quadratic trend with time (Fig. 4A; quadratic model in simple regression, r2 = 0.86, F2,4 = 12.58, P < 0.02). Comparison of slopes suggests that the difference increased more quickly between connected and unconnected high-edge patches (95% confidence limits for time: 0.46 to 0.92) compared with the rate of change between high- and low-edge unconnected high-edge patches (0.13 to 0.45). However, the rate at which the difference decreased did not differ between connected vs. unconnected high-edge patches (95% confidence limits for time2: −0.10 to −0.03) and high- vs. low-edge unconnected patches (−0.06 to −0.02). These findings are supported by a formal test of differences between the two curves (ANCOVA comparing the interaction between the type of richness difference (i.e., connected minus unconnected high-edge or unconnected high-edge minus unconnected low-edge) and either time F1,6 = 8.05, P < 0.03, and time2, F1,6 = 0.66, P = 0.45).
Fig. 4.
The difference in species richness between patch types showing the relative importance of connectivity and edginess over time for bird-dispersed (A), wind-dispersed (B), and unassisted (C) species. Significant relationships are depicted by lines indicating the best fit via least-squares regression. Solid lines and circles indicate the difference in connected and unconnected high-edge patches and represent the importance of connectivity for species richness dynamics. Dashed lines and triangles indicate the difference between unconnected high- and low-edge patches and represent the importance of patch edge effects for species richness dynamics.
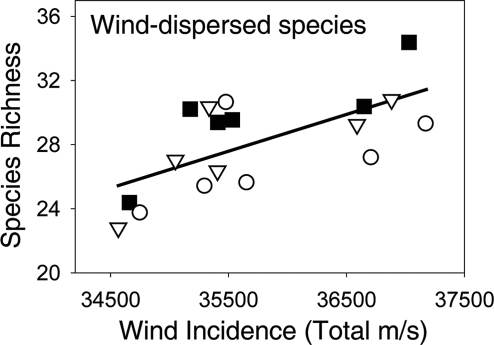
Wind-dispersed species exhibited a linear increase in the difference in species richness between connected and unconnected high-edge patches (repeated-measures analysis using differences as a dependent variable, year covariate F1,8 = 15.46, P < 0.01). This strong time effect was largely caused by the difference in richness between connected and unconnected high-edge patches (Fig. 4B; F1,5 = 39.76, r2 = 0.88, P < 0.01). Controlling for year, the effect of connectivity was significant for wind-dispersed species (Fig. 4B; linear contrast comparing connected and unconnected high-edge patches in repeated-measures model, F1,30.6 = 5.99, P = 0.02). As was found for bird-dispersed species, amount of edge had no effect on differences in species richness of wind-dispersed seeds (Fig. 4B; linear contrast comparing high- and low-edge patches in repeated-measures model, F1,30.7 = 1.42, P = 0.24). However, unlike bird-dispersed seeds, the difference in species richness between unconnected high- and low-edge patches showed an increasing trend over time (Fig. 4B; F1,5 = 6.10, r2 = 0.55, P = 0.056). The rate at which the difference in richness increased was similar when comparing connected and unconnected high-edge patches (95% confidence limits for slope: 0.26 to 0.81) or when comparing high- and low-edge unconnected patches (95% confidence limits for slope: −0.007 to 0.374; ANCOVA comparing the interaction between time and the type of richness difference, F1,8 = 2.44, P = 0.16). The prevailing direction of wind relative to the orientation of the patches was also a significant predictor of the richness of wind-dispersed plants (testing wind as a covariate in repeated-measures model, F1,31 = 6.32, P < 0.02): as the frequency of wind events (number of observations) passing perpendicular to a patch edge increased (which we refer to as “wind incidence”), so did the richness of wind-dispersed plant species (Fig. 5 and SI Text).
Fig. 5.
Evidence of the strong role of wind incidence in affecting the richness of wind-dispersed plant species. Each point represents the average richness of wind-dispersed species as a function of average wind incidence (see SI Text) for each patch type in each year of the study (three patch types in each of 6 years). Connected patches are represented by squares, unconnected low-edge patches are represented by circles, and unconnected high-edge patches are represented by triangles. Line indicates fitted by least-squares regression (r2 = 0.43). Repeated measures analysis supports the significance of the depicted relationship (F1,17 = 12.07, P = 0.003).
There was a strong positive relationship between time and the difference in richness of unassisted species between connected and unconnected high-edge patches (Fig. 4C; F1,5 = 98.76, r2 = 0.96, P < 0.01), resulting in significantly more species in connected patches relative to unconnected patches averaged over the last 3 years of the study (linear contrast in repeated-measures model, F1,16.8 = 5.18, P = 0.04). However, because of high levels of year-to-year variability, there was no difference in richness of unassisted species caused by connectivity across all years (Fig. 4C; linear contrast comparing connected and unconnected high-edge patches in repeated-measures model, F1,15.2 = 2.51, P = 0.13). There was no difference in richness among high- and low-edge unconnected patches (Fig. 4C; F1,5 = 3.64, r2 = 0.42, P = 0.11), but the rate at which the difference in unassisted species richness changed was significantly greater when comparing connected and unconnected high-edge patches (95% confidence limits for slope: 0.56 to 0.94) than when comparing high- and low-edge unconnected patches (95% confidence limits for slope: −0.13 to 0.86; ANCOVA comparing the interaction between time and the type of richness difference, F1,8 = 8.94, P < 0.02). As with bird- and wind-dispersed species, edge effects did not affect the richness of unassisted species (Fig. 4C; linear contrast comparing unconnected high- and low-edge patches in repeated-measures model, F1,15.4 = 1.42, P = 0.25).
Discussion
Predicting Community Response in Fragmented Landscapes.
Our results demonstrate that seed dispersal modalities, a part of the motion capacity of plants, influence plant community responses to habitat structure. As we suspected, we were most successful in our predictions for bird-dispersed plant species, for which we know the most about mechanisms, and least successful for unassisted plant species, about which we know the least. Some of our predictions for wind-dispersed species were supported, whereas others were rejected, reflecting our rudimentary understanding of the relationship between seed morphologies, wind dynamics, and landscape features.
Our previous work has shown that connected patches receive greater bird-dispersed seed input than unconnected patches (8), so it may not be surprising that bird-dispersed species richness was rapidly elevated in connected relative to unconnected patches. Yet this effect appears transient, likely because birds visit all patches in our experiment with some regularity (9) and thus even isolated patches occasionally receive new colonists. Likewise, the lack of difference in species richness between high-edge and low-edge patches fits with our knowledge of bird behavior, because birds disperse equal numbers of seeds from the center patch into both unconnected patch types (8).
Contrary to our predictions, the richness of wind-dispersed species exhibited stronger connectivity effects than edge effects (Fig. 4B). This finding demonstrates the need for a more mechanistic understanding of the relationship between the morphological and physiological traits that make up the motion capacities of wind-dispersed plants, the dynamics of the dispersal vector (wind) and the environmental states that interact with these variables (in our landscapes – edginess and connectivity). We are hopeful that a union between these factors is possible, and the relationship between wind dynamics and species richness support this view (Fig. 5). An obvious step forward includes more fine-grain measurements of wind dynamics as it relates to patch geometry, as recent studies have found that habitat edges between forested and open habitats alter wind circulation patterns (12–14).
Our results challenge the notion that unassisted species are truly unassisted in their dispersal, corroborating evidence from several other studies (11, 15). Unassisted species showed an increasing connectivity effect, which occurred much more quickly than we had expected, and no edge effect for the duration of our study. These results demonstrate the importance of understanding long-distance dispersal based on seed morphology, as the primary dispersal mode for all species in this group should be gravity. However, gravity dispersal from low-growing shrubs, forbs, and grasses, which typically moves a seed no more than a few meters per year, cannot account for the rapid colonization of connected patches 150 m distant. Many “unassisted” species are clearly getting assistance, and that assistance appears to preferentially move them down corridors to connected patches. A more mechanistic understanding of the dispersal of this group of species is necessary, starting with the identification of the vectors responsible for long-distance dispersal.
The movement ecology framework was not designed to interpret the dynamics of entire communities, so our approach risks overlooking important tradeoffs that may direct evolutionary trajectories (3, 16–19) and constrain community assembly (e.g., ref. 20). Colonization is only a first step to plant establishment at a site, as plant establishment can be limited by suitable microsites or seed mortality caused by predators or pathogens (e.g., ref. 21). Yet despite the many other drivers of plant community dynamics, our categorization of plants by dispersal mode did provide insights into the dynamics of entire communities. However, this basic classification only worked to create reliable predictions when it was combined with a detailed, mechanistic understanding of the behavior of the dispersal vector, and the interactions of that vector with environmental states that are likely to influence its behavior. We believe that the movement ecology framework can be extended to entire communities, as long as the primary dispersal agents and mechanisms that govern the interactions between motion capacity and environmental states are well understood.
Our results also demonstrate an important temporal component to plant movement and resulting community dynamics. Bird-dispersed species responded faster than wind-dispersed or unassisted species, suggesting that the impact of colonization events happens more quickly for bird-dispersed plants than other groups, corroborating other recent evidence of plant response to habitat fragmentation (10). This result begs an additional question: are the biodiversity benefits of corridors ephemeral whereby all plant species eventually colonize unconnected patches, or will corridors always provide a boost to colonization in the face of plant extinction dynamics? We suspect the latter, but answers to these questions can only be tested over longer time periods.
Finally, it is important to note that the rates of change we observed are dynamic and related to the spatial and temporal scales of fragmentation. This is important in a conservation context, where patch isolation is often much greater than in our experiment. When isolation is greater and corridors are longer, it may take longer time periods to observe responses by plant communities. Furthermore, plants may never disperse between patches separated by very long distances in one generation, requiring establishment, growth, and reproduction within the corridor. This recognition of spatial and temporal scaling underscores the importance of considering increasing corridor quality and width in larger landscapes in which longer corridors are used to connect isolated patches.
The Movement Ecology of Plants.
One of the most important advances of our work is the linkage of the movement ecology framework to corridors. Given the diversity of species in need of conservation in fragmented landscapes, there is a pressing need for broad generalizations that will allow managers to predict the full consequences of management actions. Our work suggests that these generalizations will not come easy. Although life-history traits related to dispersal allowed us to detect differences in community assembly in response to connectivity and edginess, two factors that are often altered by management actions, our predictive power was inconsistent. Movement ecology offers a framework that may take us closer to predictive generalizations.
In our application of movement ecology to plant communities, we found some aspects of the movement ecology framework that were helpful (motion capacities, external factors), and others that were more difficult to apply (internal state, navigation capacities). Although considering internal states and navigation capacities may refine our understanding of landscape impacts on plant communities by including factors such as the timing of seed release (e.g., refs. 22 and 23) or the details of seed dormancy and germination (e.g., refs. 24 and 25), we think the most rapid progress will be made through a redoubled effort to understand the mechanistic relationships between various aspects of motion capacity and two key external factors, the behavior of dispersal vectors and their interaction with environmental states. In particular, key aspects of motion capacities (e.g., seed terminal velocities and seed release heights; see ref. 23) and environmental states (e.g., wind dynamics within seed dispersal seasons, see ref. 23) should be characterized so these traits and states can be identified and prioritized.
Movement ecology seeks to change our thinking about how we study movement (4). Still, it is important to ask what is gained by adopting this new framework and what risks there might be. On the positive side, this new framework allows us to use movement as an organizing principle and more specific and consistent language to understand why and how organisms move, and how the environment changes that movement. However, the advantages of this framework will be most effectively implemented when they are complemented by an explicit recognition of the constraints and tradeoffs that are the hallmark of life history.
Materials and Methods
We conducted this study in six 50-ha experimental landscapes at the Savannah River Site near Aiken, SC, which consisted of five open habitats (patches) within a matrix of pine forest (Fig. 2). Patches were of three types: connected, unconnected high-edge, and unconnected low-edge. Since 2001, all plant species have been censused within each patch. Each species was classified by dispersal mode and ANCOVA was used to compare plant community responses for each group over a 7-year time series. A complete description of the landscapes, plant surveys, and data analysis methods can be found in SI Text.
Supplementary Material
Acknowledgments.
We thank J. Segar, C. Hobson, J. Demas, J. Blake, E. Olson, and the U.S. Department of Agriculture Forest Service-Savannah River for site creation and maintenance; the Atmospheric Technologies Group at the Savannah River National Laboratory for wind data; A. Krings, J. Stucky, T. Wentworth, and the North Carolina State Herbarium for plant identification; K. Pollock and K. Gross for statistical advice; and many field technicians for data collection and management. This research was supported by National Science Foundation Grants DEB-9907365, DEB-0733746, DEB-0613701, and DEB-061375) and funds provided to the Department of Agriculture Forest Service, Savannah River, under Interagency Agreement DE-AI09-00SR22188 with the Department of Energy, Aiken, SC.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.N. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802037105/DCSupplemental.
References
- 1.Haddad NM, et al. Corridor use by diverse taxa. Ecology. 2003;84:609–615. [Google Scholar]
- 2.Hudgens BR, Haddad NM. Predicting which species will benefit from corridors in fragmented landscapes from population growth models. Am Nat. 2003;161:808–820. doi: 10.1086/374343. [DOI] [PubMed] [Google Scholar]
- 3.Stearns SC. Life-history tactics: A review of the ideas. Q Rev Biol. 1976;51:3–47. doi: 10.1086/409052. [DOI] [PubMed] [Google Scholar]
- 4.Nathan R, et al. A movement ecology paradigm for unifying organismal movement research. Proc Natl Acad Sci USA. 2008;105:19052–19059. doi: 10.1073/pnas.0800375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holyoak M, Casagrandi R, Nathan R, Revilla E, Spiegel O. Trends and missing parts in the study of movement ecology. Proc Natl Acad Sci USA. 2008;105:19060–19065. doi: 10.1073/pnas.0800483105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levey DJ, Bolker BM, Tewksbury JJ, Sargent S, Haddad NM. Bird foraging behaviors scale up to predict corridor effects on seed dispersal. Science. 2005;309:146–148. doi: 10.1126/science.1111479. [DOI] [PubMed] [Google Scholar]
- 7.Damschen EI, Haddad NM, Orrock JL, Tewksbury JJ, Levey DJ. Corridors increase plant species richness at large scales. Science. 2006;313:1284–1286. doi: 10.1126/science.1130098. [DOI] [PubMed] [Google Scholar]
- 8.Tewksbury JJ, et al. Corridors affect plants, animals, and their interactions in fragmented landscapes. Proc Natl Acad Sci USA. 2002;99:12923–12926. doi: 10.1073/pnas.202242699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey DJ, Tewksbury JJ, Bolker BM. Modeling long-distance seed dispersal in heterogeneous landscapes. J Ecol. 2008;96:599–608. [Google Scholar]
- 10.Montoya D, Zavala MA, Rodriguez MA, Purves DW. Animal versus wind dispersal and the robustness of tree species to deforestation. Science. 2008;320:1502–1504. doi: 10.1126/science.1158404. [DOI] [PubMed] [Google Scholar]
- 11.Higgins SI, Nathan R, Cain ML. Are long-distance dispersal events in plants usually caused by nonstandard means of dispersal? Ecology. 2003;84:1945–1956. [Google Scholar]
- 12.Nathan R, et al. Long-distance biological transport processes through the air: Can nature's complexity be unfolded in silico? Diversity Distributions. 2005;11:131–137. [Google Scholar]
- 13.Bohrer G, Katul GG, Nathan R, Walko RL, Avissar R. Effects of canopy heterogeneity, seed abscission, and inertia on wind-driven dispersal kernels of tree seeds. J Ecol. 2008;96:568–580. [Google Scholar]
- 14.Detto M, Katul G, Siqueira M, Yuang JY, Stoy P. The structure of turbulence near a tall forest edge: The backward facing step flow analogy revisited. Ecol Appl. 2008;18:1420–1435. doi: 10.1890/06-0920.1. [DOI] [PubMed] [Google Scholar]
- 15.Vellend M, Myers JA, Gardescu S, Marks PL. Dispersal of Trillium seeds by deer: Implications for long-distance migration of forest herbs. Ecology. 2003;84:1067–1072. [Google Scholar]
- 16.Roff DA, Mostowy S, Fairbairn DJ. The evolution of trade-offs: Testing predictions on response to selection and environmental variation. Evolution. 2002;56:84–95. doi: 10.1111/j.0014-3820.2002.tb00851.x. [DOI] [PubMed] [Google Scholar]
- 17.Roff DA. Life History Evolution. Sunderland, MA: Sinauer; 2002. [Google Scholar]
- 18.Stearns SC. Evolution of Life Histories. New York: Oxford Univ Press; 1992. [Google Scholar]
- 19.Grime JP. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat. 1977;111:1169–1194. [Google Scholar]
- 20.Ackerly DD, Cornwell WK. A trait-based approach to community assembly: Partitioning of species trait values into within- and among-community components. Ecol Lett. 2007;10:135–145. doi: 10.1111/j.1461-0248.2006.01006.x. [DOI] [PubMed] [Google Scholar]
- 21.Orrock JL, Danielson BJ, Burns MJ, Levey DJ. Spatial ecology of predator–prey interactions: Corridors and patch shape influence seed predation. Ecology. 2003;84:2589–2599. [Google Scholar]
- 22.Greene DF. Dispersal of seeds by the tropical sea breeze. Ecology. 2008;89:118–125. doi: 10.1890/06-0781.1. [DOI] [PubMed] [Google Scholar]
- 23.Wright SJ, et al. Understanding strategies for seed dispersal by wind under contrasting atmospheric conditions. Proc Natl Acad Sci USA. 2008;105:19084–19089. doi: 10.1073/pnas.0802697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donohue K. Germination timing influences natural selection on life-history characters in Arabidopsis thaliana. Ecology. 2002;83:1006–1016. [Google Scholar]
- 25.Bazzaz FA. Habitat selection in plants. Am Nat. 1991;137:S116–S130. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.