Abstract
Traits associated with seed dispersal vary tremendously among sympatric wind-dispersed plants. We used two contrasting tropical tree species, seed traps, micrometeorology, and a mechanistic model to evaluate how variation in four key traits affects seed dispersal by wind. The conceptual framework of movement ecology, wherein external factors (wind) interact with internal factors (plant traits) that enable movement and determine when and where movement occurs, fully captures the variable inputs and outputs of wind dispersal models and informs their interpretation. We used model calculations to evaluate the spatial pattern of dispersed seeds for the 16 factorial combinations of four traits. The study species differed dramatically in traits related to the timing of seed release, and a strong species by season interaction affected most aspects of the spatial pattern of dispersed seeds. A rich interplay among plant traits and seasonal differences in atmospheric conditions caused this interaction. Several of the same plant traits are crucial for both seed dispersal and other aspects of life history variation. Observed traits that limit dispersal are likely to be constrained by their life history consequences.
Keywords: atmospheric turbulence, conditional seed release, Coupled Eulerian-Lagrangian closure (CELC) model, long distance dispersal, tropical forest
Seed dispersal allows plants to colonize new habitats, reach sites where resources favor regeneration, and escape pests and competition with siblings and mother and sets the spatial template for all post dispersal processes (1, 2). A mechanistic understanding of seed dispersal could lead to progress on many fronts but requires models that recreate the complex interactions between plants and seed dispersal vectors. Mechanistic models are perhaps most advanced for seeds dispersed by wind (3). We use a wind dispersal model developed and validated for forests and grasslands (4–6) to compare spatial patterns of seed dispersal for factorial combinations of four key plant traits observed for two contrasting tropical tree species. These comparisons, made within the conceptual framework provided by movement ecology (7), provide insight into the complex interplay between atmospheric conditions and plant traits that influence seed dispersal by wind.
Seed fate motivates seed dispersal through natural selection (2). Wind dispersal models have traditionally focused on a single aspect of seed fate, the distance moved from the mother (3). Long dispersal distances sample more potential regeneration sites and minimize negative interactions with siblings and mother. The implications of coincident arrival in close proximity have been overlooked for wind-dispersed seeds (but see ref. 8 for animal-dispersed seeds). Coincident arrival of siblings increases the potential for sibling competition and pest facilitation, reduces the number of potential regeneration sites reached, and leads to future inbreeding among adults. Thus, coincident arrival impacts seed fate negatively. Dispersal distance and directionality influence coincident arrival. Wind directionality is highly variable in time and space and determines dispersal directionality in wind-dispersed seeds (9). For these reasons, we evaluate seed deposition in two dimensions to explore how atmospheric conditions and plant traits influence coincident arrival as well as dispersal distance.
Mechanistic wind dispersal models are readily interpreted within the conceptual framework of movement ecology, which begins with external and internal factors that determine movement (7). The variable inputs to wind dispersal models are atmospheric conditions (external factors) and plant traits (internal factors) (3). For a given set of atmospheric conditions, internal factors determine how an individual moves (motion capacity), when and where it moves (navigation capacity), and why it moves (the ultimate and proximate motivation to move determined by natural selection). Key plant traits include seed terminal velocity and the height and timing of seed release. Terminal velocity affects the time seeds remain aloft and their trajectories and is the primary determinant of motion capacity. Release height also affects the time seeds remain aloft and, because winds change predictably with height, where (how far) and possibly when seeds move. Thus, release height contributes to motion and navigation capacity. Non-random seed release with respect to atmospheric conditions is the only other control plants exercise over when and where seeds move and is the primary determinant of navigation capacity. Finally, the output of wind dispersal models consists of metrics that describe seed movement in space. These metrics are chosen to summarize aspects of the spatial pattern of seed deposition that influence seed fate and the motivation for movement through natural selection. Thus, the conceptual framework of movement ecology fully captures the inputs and outputs of wind dispersal models and informs their interpretation.
We consider two tree species with contrasting seed release from tropical moist forest on Barro Colorado Island (BCI), Panama (9°9′N, 79°51′W). Tabebuia rosea (Bertol.) DC matures fruit in the dry season, as do most wind-dispersed species on BCI (10). Jacaranda copaia (Aubl.) D. Don is the only abundant wind-dispersed species to mature fruit in the wet season. During the dry season (December–April), the Inter Tropical Convergence Zone (ITCZ) lies to the south of BCI, the Trade Winds rush toward the ITCZ, mean windspeeds are maximal, and the wind is consistently from the north. During the wet season (May-December), the ITCZ hovers over BCI, mean windspeeds are slower, and winds are, on average, adirectional (11). Turbulent vertical velocity fluctuations also vary seasonally and are important because they provide lift, prolong time aloft, and expose seeds to greater windspeeds with height. Convection and shear produce turbulent kinetic energy (TKE) within the daytime atmospheric surface layer (ASL). Convective turbulence occurs when incident solar radiation causes large sensible heat fluxes to the atmosphere, which generates convective eddies by introducing air density gradients with warmer, lighter air near the surface. Shear-induced or mechanical turbulence occurs when surface drag imposed by the canopy generates vertical gradients in the mean horizontal velocity. High windspeeds above the canopy cause high rates of mechanical TKE production. Thus, windspeed, directionality, and mechanical turbulence are greater in the dry season than in the wet season.
Botanists and ecologists have long assumed that the Trade Winds favor dry-season dispersal of wind-dispersed seeds (12). We confirm this expectation. Our focus, however, is on the contrast between dry and wet season atmospheric conditions and their implications for the spatial pattern of seed dispersal. Seasonal differences in atmospheric conditions create at least two potential tradeoffs in this context. The first involves convective and mechanical turbulence. The high windspeeds that cause mechanical TKE production are often associated with low surface heating and low convective TKE production. Thus, mechanical and convective TKE tend to be associated with different atmospheric conditions and different seed dispersal patterns. The second tradeoff involves windspeed and directionality. If windspeed and directionality are independent, faster winds and larger dispersal distances will reduce coincident arrival. If windspeed and directionality are positively correlated, this might not be true. These tradeoffs raise the possibility that a plant trait or combination of traits that enhances dispersal in one season might reduce dispersal in another season.
We use model calculations to explore how plant traits and atmospheric conditions influence dispersal. We measure key traits for Tabebuia and Jacaranda including terminal velocity, fertile tree height, and the timing of seed release. We compute wind statistics for entire fruiting seasons calibrating short-term, high-frequency observations taken with sonic anemometers with long-term, low-frequency observations taken at a nearby (1.2 km) meteorological station. We simulate 283 million seed trajectories using factorial combinations of plant traits and seasonal atmospheric conditions as input to a coupled Eulerian–Lagrangian closure (CELC) model (4–6). We compare simulated seed dispersal for observed combinations of plant traits and atmospheric conditions and artificial combinations that contrast traits and seasons and ask how internal and external factors interact to determine seed dispersal patterns. The simulated seed trajectories provide a comprehensive overview of dispersal outcomes for contrasting plant traits and divergent atmospheric conditions. We find a rich interplay among plant traits, atmospheric conditions, and dispersal patterns.
Results
Plant Traits.
Terminal velocity averaged 0.526 (±0.116 = 1 SD) and 1.142 (±0.302) m s−1 for Jacaranda and Tabebuia, respectively. We used diameter at breast height (DBH) and asymptotic height-DBH relationships [r2 ≥ 0.95, Eq. 4, supporting information (SI) Fig. S1A] to estimate the heights of 184 and 51 fertile Jacaranda and Tabebuia, respectively. Fertile trees averaged 28.0 (±2.4) and 31.6 (±5.0) m tall (Fig. S1B), and we used 28- and 32-m heights to represent Jacaranda and Tabebuia, respectively.
We consider the timing of seed release for seasonal and 20-min time scales and use “seed release” to refer to the latter. Dispersal season, defined as the 31 consecutive days that included ≥95% of annual seed capture, included Julian dates 100–130, 93–123, and 100–130 for Tabebuia and 228–258, 195–225, and 230–260 for Jacaranda in 2005, 2006, and 2007, respectively.
We evaluated only daytime seed release because daytime seed arrival (S, seeds m−2 hr−1) averaged 11 and 12 times greater than nighttime arrival for Tabebuia (0.023 vs. 0.0021) and Jacaranda (0.010 vs. 0.00084), respectively. In bivariate analyses, Tabebuia seed arrival increased with mean air temperature (T̄, r2 = 0.18, P < 0.001), horizontal windspeed (ū, r2 = 0.13, P < 0.01), and the standard deviation of the vertical wind component (σW, r2 = 0.08, P < 0.05). T̄, ū, and σW entered a forward stepwise multiple regression analysis in that order, and σW was negatively related to the log(S) − T̄ − ū residuals. The final regression model (r2 = 0.42, F3,66 = 15.7, P < 0.001) was
Relative humidity is inversely related to diurnal changes in T̄ in tropical forest canopies, abscission is inversely related to humidity (9) and these two relationships are likely to explain the positive relationship between log(S) and T̄2.
Jacaranda seed arrival was unrelated to ū or ū2 (r2 = 0.060, 0.0035, 0.062, and 0.0011 for ū and ū2 for 29 and 20 trap censuses, respectively) but responded to sensible heat flux at the canopy top (w′T′). The final multiple regression model for the 29-trap census (r2 = 0.12, F3,134 = 7.15, P < 0.001) was
The final model for the 20-trap census (r2 = 0.12, F1,86 = 12.5, P < 0.001) was
We used the linear model (Eq. 3) because most Jacaranda seeds arrived over the increasing portion of the relationship for both censuses.
Wind Conditions.
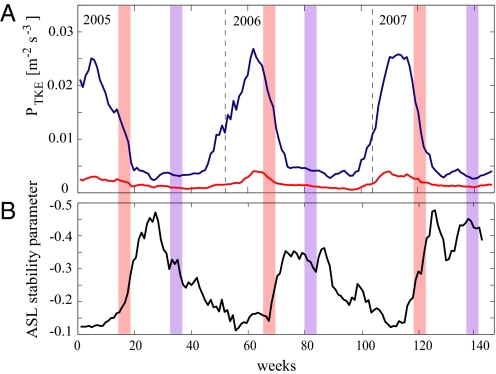
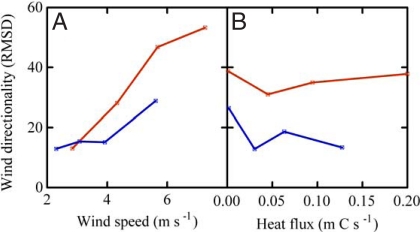
Windspeed, wind directionality, σW, and mechanical, convective and total TKE production were greater during the seed dispersal season of Tabebuia than Jacaranda (Fig. 1, Fig. S2). The ASL stability parameter was more negative (more convective) during the dispersal season of Jacaranda (Fig. 1). Wind directionality increased with horizontal windspeed and was independent of sensible heat flux in both seasons (Fig. 2).
Fig. 1.
Average weekly (A) production rates of mechanical (blue line) and convective (red line) total kinetic energy (TKE) and (B) the atmospheric surface layer (ASL) stability parameter (black line). The ASL stability parameter measures the ratio of convective and mechanical TKE production and excludes stable periods. Red and blue shading represent Tabebuia and Jacaranda dispersal seasons, respectively.
Fig. 2.
Wind directionality increases with wind speed (A) and is independent of sensible heat flux (B). Red and blue lines represent daytime values observed during Tabebuia and Jacaranda dispersal seasons, respectively. Directionality is the unitless root mean square deviation (RMSD) from a uniform angular distribution calculated for 20 18° bins.
Simulated Dispersal.
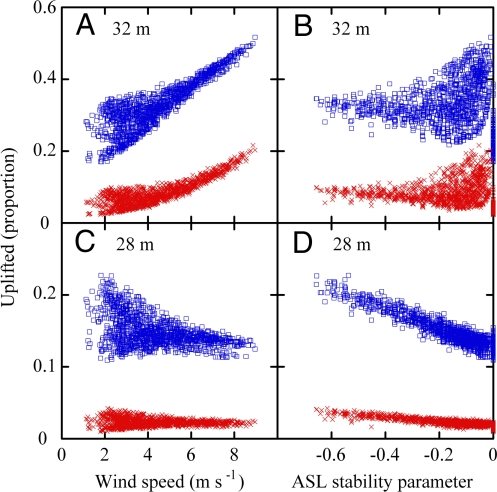
The percentage of seeds uplifted (henceforth uplifting) increased with tree height and decreased with terminal velocity as expected (Fig. 3). The principal drivers of uplifting changed with tree height. Uplifting increased with ū for 32-m trees (Fig. 3A) and decreased with the ASL stability parameter (ζ) for 28-m trees (Fig. 3D). ζ quantifies the relative importance of mechanical and convective TKE production, and smaller values indicate greater convective TKE production. Uplifting was largely independent of ū for 28-m trees (Fig. 3C).
Fig. 3.
Relationships between proportions of seeds lifted above the canopy, windspeed (A, C) and the atmospheric surface layer stability parameter (B and D). Each symbol represents 10,000 seed trajectories simulated for 32 (A and B) or 28 m (C and D) trees, seed terminal velocities characteristic of Jacaranda (blue) or Tabebuia (red), and random seed release with respect to atmospheric conditions.
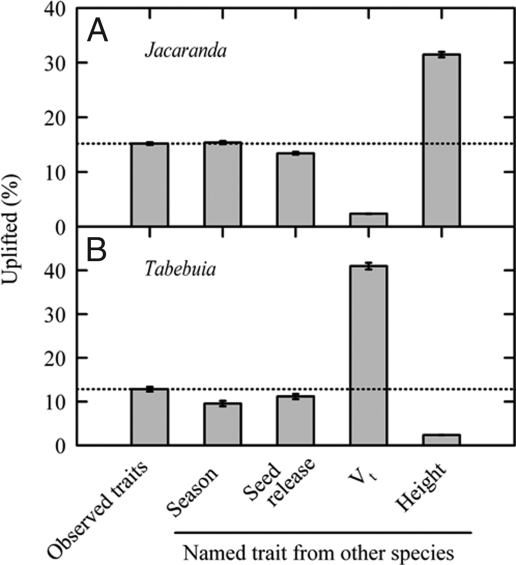
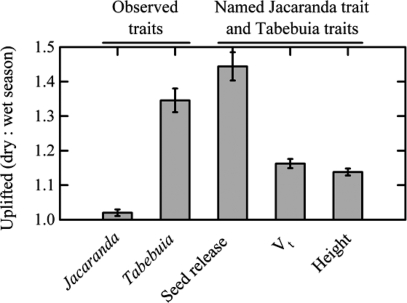
We compared uplifting simulated for trait combinations observed for each species and artificial trait combinations composed of three traits from one species and one trait from the other species (Fig. 4). Uplifting was always greatest for the slower Jacaranda terminal velocity and taller Tabebuia trees, as expected. Uplifting was also greatest for each species under its own seed release function (Eqs. 1 or 3) (compare the first and center bars within panels of Fig. 4). Uplifting was greater for dry season dispersal for Tabebuia as expected, but was virtually unaffected by season for Jacaranda (compare the second bars between panels in Fig. 4).
Fig. 4.
The percentage of seeds lifted above the canopy. The leftmost bars and the dashed horizontal line represent simulations for plant traits observed for Jacaranda (A) and Tabebuia (B). Subsequent bars represent simulations for artificial combinations of three traits from Jacaranda (A) or Tabebuia (B) and one trait named along the abscissa from the other species. Values are means (±1 SE) calculated over three years.
To evaluate the factors underlying this species-season interaction, we calculated dry:wet season ratios for uplifting for observed and artificial trait combinations composed of one Jacaranda and two Tabebuia traits (Fig. 5). The observed trait combinations confirm the species-season interaction. The artificial trait combinations identify Jacaranda traits that minimize seasonal differences in uplifting, including slow terminal velocity and relatively short tree heights.
Fig. 5.
The dry:wet season ratio of percentages of seeds lifted above the canopy. The two leftmost bars represent simulations for plant traits observed for Jacaranda and Tabebuia. Uplift is virtually unaffected by season for Jacaranda but is greatly reduced in the wet season for Tabebuia. The final three bars represent simulations that combine the named trait from Jacaranda with Tabebuia traits. Values are means (±1 SE) calculated over three years.
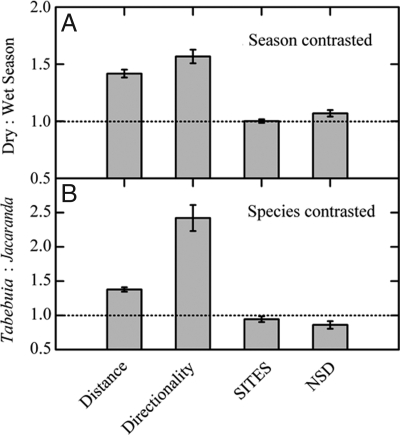
Median dispersal distances were 6–12 times larger for uplifted seeds than non-uplifted seeds (Fig. S3). Short dispersal increases the chance for coincident arrival, making the number of 1-m2 sites reached (SITES) and nearest sibling distances (NSD) important indicators of seed fate of non-uplifted seeds. We calculated dry:wet season ratios of four dispersal metrics for all comparisons of models that changed seasonal atmospheric conditions and held plant traits constant. Dispersal distance and directionality were greater for dry season atmospheric conditions while SITES and NSD were nearly indistinguishable between seasons (Fig. 6A). We also calculated Tabebuia:Jacaranda ratios for models that used observed trait combinations. Species-specific seed release enhanced dispersal directionality above the level caused by seasonal differences in wind directionality (compare directionality in Figs. 6 A and B noting the different vertical scales). As a consequence, SITES and NSD were larger for wet-season dispersed Jacaranda than for dry-season dispersed Tabebuia (Fig. 6B).
Fig. 6.
The impact of seasonal atmospheric conditions (A) and species traits (B) for dispersal of non-uplifted seeds. Paired simulations contrast season and hold other traits constant (A) or contrast Tabebuia and Jacaranda for observed traits (B). The horizontal dashed lines represent equal values. Dispersal distance and directionality are greater in the dry season and for Tabebuia traits and have nearly compensatory effects on numbers of 1-m2 sites reached (SITES) and nearest sibling distance (NSD). Values are mean ratios (±1 SE) calculated over three years. The caption to Fig. 2 defines directionality.
Discussion
Our simulations illuminate a rich interplay among atmospheric conditions and plant traits that influence seed dispersal by wind. Seeds lifted above the forest are particularly important because they disperse long distances (13). Dry season atmospheric conditions favor uplift (Fig. 1), and simulated uplifting was 35% and just 1.3% greater for dry than for wet season conditions for Tabebuia and Jacaranda, respectively (Fig. 5). This species-season interaction had three causes. The seasonal difference is smaller in Jacaranda because (1) its slower terminal velocity allows uplifting by weaker wet season eddies, (2) its shorter seed release height reduces uplifting associated with windspeed (Fig. 3C), which is greater in the dry season, and (3) its shorter release height increases uplifting associated with convective eddies (Fig. 3D), which are more frequent in the wet season (Fig. 1). Most seeds are not uplifted (complement of values in Fig. 4), and dry season conditions favor longer dispersal in these seeds too. Our simulations confirmed this expectation; however, wind directionality also increased with windspeed (Fig. 2). Seasonal differences in directionality and distance were compensatory so that non-uplifted seeds reached similar numbers of sites and were similarly likely to land near siblings in both seasons (Fig. 6A). Jacaranda seed release was also more sensitive to sensible heat flux than to windspeed (Eq. 2 and 3). This contributed to uplift under convective conditions and also reduced dispersal directionality and increased sites reached and nearest sibling distances in non-uplifted seeds (Figs. 2 and 6B). To summarize, slow terminal velocity, short seed release height, seed release in response to sensible heat flux and a direct relationship between windspeed and directionality all contributed to minimize seasonal differences in Jacaranda dispersal. Several of the same plant traits have life history implications that provide additional insight.
Life History and Seed Dispersal.
The life history of Jacaranda is extreme. Jacaranda ranked first and Tabebuia ranked 47th among the 73 most abundant BCI tree species in an analysis of dependence of regeneration on forest gaps (14). Gaps permit rapid regeneration when low light limits seedlings because bright light penetrates to the forest floor for a short time after a canopy tree death opens a gap and before rapid herb, seedling, shrub, and sapling growth closes the gap at ground level. Compared with Tabebuia, Jacaranda produces more (89 seeds cm−2 basal area yr−1 vs. 18) and smaller seeds (dry mass of endosperm plus embryo averages 2.16 vs. 18.1 mg) whose survival to the seedling stage is lower (0.051% vs. 1.3%) due to its dependence on gaps (14, 15). Jacaranda seeds are also recalcitrant (germinate immediately), which is unusual among small-seeded, gap-dependent species. Jacaranda saplings grow extremely rapidly (14), and we observed seed production in an individual known to be less than 18 years old (S.J.W., unpublished data). We speculate that successful wet season dispersal is linked to these life history traits.
The link is established by seed arrival in gaps and incidental reductions in the negative impact of wet season dispersal associated with key life history traits. The probability of arrival in a newly created gap is greatest in the wet season for two reasons. First, the rate of gap creation on BCI is lowest in the dry season and greatest in August and September (16) when recalcitrant Jacaranda seeds disperse. Second, gaps are equally likely in all directions, and adirectional wet season winds increase the number of gaps within reach of non-uplifted seeds. Small seed mass permits an extremely small wing loading and slow terminal velocity—the smallest and second slowest among 34 wind dispersed BCI species, respectively (17). The early onset of reproduction curtails height growth as resources are directed to reproduction. Thus, we speculate that (1) the probability of arriving in a recently created gap selects for wet season dispersal of tiny, recalcitrant Jacaranda seeds and (2) correlated life history traits contribute to slow terminal velocity and relatively short adult stature that reduce the negative impact of wet season dispersal. Thus, life history traits and successful wet season dispersal by wind might be linked in Jacaranda.
Limitations and Future Directions.
Canopy scale models like ours cannot accommodate processes at very small spatial scales. We used terminal velocity to represent seeds and neglected aerodynamic shape, inertia, and collisions with vegetation inside the canopy. Aerodynamic shape may introduce Bernoulli sailing (18), uplift on wing surfaces (19), and rotation (20). The effects of rotation can be parameterized by terminal velocity (21). Resolving other aerodynamic effects would require <1 mm resolution, which is currently impossible in canopy scale models. We assumed seeds lack inertia and are coupled with the wind flow excepting a vertical component due to terminal velocity. This assumption does not bias model calculations (22). Collisions with vegetation affect the full flight trajectory of non-uplifted seeds and the final descent of all seeds (23); these collisions would reduce dispersal distances, bringing our results more closely in line with independent empirical estimates for our focal species (24, 25). The spatial structure of stems, branches, and leaves would be required to model these collisions, and this again is currently impossible in canopy scale models.
The different drivers of uplift for 28- and 32-m trees were unexpected (Fig. 3). Uplift is influenced by the coherence of vertical velocity fluctuations (henceforth eddy coherence). Eddy coherence is influenced by the ratio of total production (PTKE) of TKE to its dissipation rate. Increased mean horizontal wind velocity (ū) increases mechanical and total PTKE (Fig. 1) and the potential for uplift. Convective TKE generation by buoyancy increases both TKE and its dissipation and has relatively little impact on their ratio and eddy coherence. Hence, the frequency of uplifting should depend on ū (26), as we found for 32-m trees. The dependence of uplift on the ASL stability parameter for 28-m trees is not easily explained. In our simulations, we released seeds from the local canopy top, canopy height averaged 30 m, and seed release heights averaged (±1 SD) 25.2 (1.4) and 28.8 (1.6) m for 28- and 32-m trees, respectively. Mechanical, buoyant, and wake production and non-local flux transport all dissipate TKE at low points at the top of an undulating canopy. This makes the prediction of eddy coherence more difficult and the relationship with ū potentially more complex (27). Although a complete understanding of the drivers of uplift awaits further research, the simulated dependence on the ASL stability parameter for 28-m trees (Fig. 3D) is consistent with the measured dependence of seed arrival on sensible heat flux in Jacaranda (Eq. 2 and 3).
We used 20-min censuses of seed arrival to quantify non-random seed release because the statistics of bursts sufficiently organized to maintain high velocity over 10–100 s and dislodge seeds are tightly linked to the organization of wind flow at the integral time scale (28). The integral time scale is determined by forest surface properties, the mean TKE (measured at a 20–30 min scale), its dissipation rate and the stability regime measured by the ASL stability parameter. The averaging period of 20–30 min used to measure mean TKE, friction velocity, dissipation rate, mean wind speed, and heat flux is long enough to capture a representative number of organized bursts and short enough to consider changes in the mean velocity (and the influence of the daily cycle) negligible. Hence, a statistical description of the ASL and turbulence properties at the time scale of 20–30 min provides an excellent description of the distribution of organized bursts that dislodge seeds.
Alternative methods to quantify non-random seed release include wind tunnels and video photography. These alternatives optimize spatial and temporal resolution but sample few individuals and dates (video photography) or introduce treatment effects as branches are cut and transported to wind tunnels. Results from our wind tunnel experiments were severely compromised by the latter problem (JK Best and HC M-L, personal observation), and prolonged dispersal seasons stress the importance of the former limitation. For now, fruit morphology and canopy observations are consistent for Jararanda. Fruit point upwards from distal branch tips (10). Two valves dry and fold back to cradle the seeds from below. The seeds are fully exposed to the sun and rain and disperse in large numbers when dried sufficiently by strong incident radiation. These conditions correspond with large sensible heat flux.
Movement Ecology of Plants.
The conceptual framework of movement ecology contributed to our study through a delineation of the complete set of internal and external factors that influence seed dispersal and their interactions. The framework describes the state and movement related traits of individuals—their internal motivation to move (why move?) and the traits that enable motion (how to move?) and navigation (when and where to move?) (7). The framework is readily applied to plants if these properties are evaluated in an evolutionary context with respect to selection for movement (see Introduction, ref. 29). Substituting proxies for these traits is unlikely to capture fully the interactions among traits and external factors that determine movement (29). In our study, a detailed mechanistic wind dispersal model enabled identification of a rich interplay among external factors and plant traits that influence the spatial pattern of seed deposition. Slow seed terminal velocity, short seed release heights, seed release in response to sensible heat flux and seasonal differences in wind directionality combined to offset seasonal differences in windspeed and turbulence. The conceptual framework of movement ecology allowed these complex interactions to be described in a way that will facilitate comparison with other studies of seed dispersal and enable generalization.
Materials and Methods
Wind Measurements.
We used sonic anemometers (RM-Young 81000) to record temperature (Ts) and wind velocity (u, v and w aligned along the longitudinal, lateral and vertical directions, respectively) at 10 Hz and a radiometer (Kipp&Zonen CNR1) to record net incoming solar radiation at 1 Hz. We mounted the radiometer 39 m and the anemometers 58, 40, and 25 m above the ground on towers near the center of BCI. The nearby canopy was ≈30 m tall. Moisture caused frequent instrument failures, and we used wind statistics and air temperature recorded at 15-min intervals at a tower 1.2 km distant to reconstruct long-term 30-min time series as described in SI Appendix and Figs. S4 and S5.
Plant Traits.
We measured terminal velocity by recording seed free fall with a digital video camera (30 frames per second) in a closed 2 × 2 × 2.5 (height) m chamber. Customized image analysis software calculated aerodynamic properties from 54 seed trajectories for each species.
We estimated heights (H) of reproductive trees from censuses of mapped trees whose diameters at breast height (DBH) were known and H-DBH allometry. We measured H and DBH for 36 Jacaranda and 20 Tabebuia as described in (30) and used nonlinear regression to fit these data to the following relationship:
where Hmax (asymptotic maximum height), a and b are fitted parameters (31). We evaluated reproductive status in ≥2 yrs between 2004 and 2007 for 252 Jacaranda and 115 Tabebuia, including all individuals larger than 20 cm DBH in 70 ha.
We used weekly censuses of 250 seed traps to quantify the seasonal timing of seed dispersal (14, 15) and 20-min censuses of seed traps and above-canopy sonic anemometry to quantify non-random seed release in response to wind conditions (henceforth, seed release). We counted seeds at 20-min intervals from dawn to dusk for 22 traps for 3 d for Tabebuia and 29 traps for 7 d and another 20 traps for 5 d for Jacaranda. The 22 and 29 traps were located on a square 15-m grid in one hectare of forest with a tower at its center. The 20 traps were about 200 m from the 29 traps. Rain delayed many Jacaranda censuses, and we excluded census intervals longer than 30 min. We measured nighttime seed arrival (S, seeds m−2 min−1) when a census ending after 17:30 h was followed the next morning by a census beginning before 06:30 h. Daytime sample sizes were 70 (106), 138 (126) and 88 (60) 20-min intervals (seeds) for Tabebuia and the 29 and 20 trap Jacaranda censuses, respectively. We used multiple regression analyses to evaluate relationships between S and atmospheric conditions for the preceding 20 min. Logarithms of S improved normality. Independent variables included: windspeed (ū, m·s−1), temperature (T̄, degrees Celsius), maximum vertical wind velocity (Wmax, m·s−1), standard deviation of vertical wind velocity (σW, m2·s−2), TKE (m2·s−2), sensible heat flux (w′T′, °C m·s−1), and Reynolds stress (w′u′, m2·s−2), where overbars and primes mark 20-min means and perturbations around means, respectively. We evaluated linear and quadratic fits between log (S) and independent variables and included independent variables with marginally significant fits (P < 0.1) in an initial regression model (32). Insignificant variables (P > 0.05) were removed one at a time. The final models included significant terms only (adjusted P < 0.05).
Simulations.
We used a Coupled Eulerian-Lagrangian closure (CELC) model to simulate seed dispersal (4–6). SI Appendix describes the CELC approach (Fig. S6 and Table S1). CELC input parameters include leaf area density profile, foliage drag coefficient, terminal velocity, seed release height and 30-min friction velocity and sensible heat flux time series measured above the canopy. Dry and wet season leaf area density profiles from (33) summed to leaf area indices of 5.12 and 5.43, respectively. The foliage drag coefficient was assumed to be 0.1 (34). Terminal velocity, release heights and seasonal wind climatology are described in Results and SI Appendix. The five input parameters are consistent with the “minimum necessary” to describe seed dispersal (3).
We simulated dispersal from 28, 30, 32, and 34 m trees to capture most of the height variation observed among fertile trees. Both species fruit at apical meristems exposed to full sunlight (S. J. W, personal observation). Canopy contour maps indicate that the upper 20% of most tree crowns receive full sunlight on BCI (S. Bohlman, personal communication). We therefore simulated mean seed release heights (±1 SD) equal to 90% (±5%) of tree height. We set an average canopy top height of 30 m. The mean seed release height of 32 m trees (28.8 m) was lower than the canopy top height.
We simulated 32 scenarios including all combinations of four tree heights, two terminal velocities, two seasons, and two seed release functions. For each scenario, we simulated 10,000 seeds for all 30-min wind statistics observed between 0600 and 1830 h with non negative surface sensible heat flux (rain can cause negative daytime heat flux) and a neutral or dynamic convective ASL. We incorporated non-random seed release by drawing subsamples of the simulated seed trajectories weighted according to the 30-min wind statistics used as input and the measured dependence of seed release on wind statistics (Eq. 1 and 3). We repeated simulations for wind statistics observed when Jacaranda and Tabebuia dispersed seeds in 2005, 2006, and 2007 and calculated standard errors over years.
Dispersal metrics included the percentage of seeds uplifted, dispersal distance, nearest sibling distance (NSD) and the number of 1-m2 sites reached (SITES). We recorded the maximum height reached and the x-y coordinates where the ground was contacted for each seed trajectory. Seeds were uplifted if maximum height exceeded 110% of canopy height for trees shorter than the 30 m canopy or 110% of tree height for taller trees and not uplifted otherwise. We used the x-y coordinate of ground contact to calculate dispersal distance, NSD and SITES. We do not consider NSD and SITES for uplifted seeds because large dispersal distances (Fig. S3) ensure that each seed reaches a new 1-m2 site and NSD is large. NSD and SITES depend on seed number, which we standardized to 30,000 randomly drawn non-uplifted seeds. We calculated rmsd relative to uniform angular distributions to quantify directionality.
Supplementary Material
Acknowledgments.
We thank O Altstein, D Brassfield, O Calderon, S Paton, P Ramos, and R Rios for assistance. This work was supported by National Science Foundation Grant DEB-0453665, 0453445 and 0453296 (to R.N., S.J.W., H.C.M. and R. Avissar), a Harvard University Center for the Environment John and Elaine French fellowship (to G.B.), a Packard fellowship (to H.C.M. and F.A.J.), the HSBC Climate Partnership (H.C.M.), a Smithsonian Tupper postdoctoral fellowship (F.A.J.), and Israel Science Foundation Grant ISF-FIRST 1316/15, US–Israel Binational Science Foundation Grant BSF 124/2004, and the Friedrich Wilhelm Bessel Award, Humboldt Foundation (to R.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802697105/DCSupplemental.
References
- 1.Howe HF, Smallwood J. Ecology of seed dispersal. Annu Rev Ecol Syst. 1982;13:201–228. [Google Scholar]
- 2.Levin SA, Muller-Landau HC, Nathan R, Chave J. The ecology and evolution of seed dispersal: A theoretical perspective. Annu Rev Ecol Evol Syst. 2003;34:575–604. [Google Scholar]
- 3.Kuparinen A. Mechanistic models for wind dispersal. Trends Plants Sci. 2006;11:296–301. doi: 10.1016/j.tplants.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Nathan R, et al. Mechanisms of long-distance dispersal of seeds by wind. Nature. 2002;418:409–413. doi: 10.1038/nature00844. [DOI] [PubMed] [Google Scholar]
- 5.Soons MB, Heil GW, Nathan R, Katul GG. Determinants of long-distance seed dispersal by wind in grasslands. Ecology. 2004;85:3056–3068. [Google Scholar]
- 6.Nathan R, Katul GG. Foliage shedding in deciduous forests lifts up long-distance seed dispersal by wind. Proc Natl Acad Sci USA. 2005;102:8251–8256. doi: 10.1073/pnas.0503048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathan R, et al. A movement ecology paradigm for unifying organismal movement research. Proc Natl Acad Sci USA. 2008;105:19052–19059. doi: 10.1073/pnas.0800375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schupp EW, Milleron T, Russo SE. In: Seed Dispersal and Frugivory: Ecology, Evolution and Conservation. Levey DJ, Silva WR, Galetti M, editors. Oxfordshire, UK: CABI International; 2002. pp. 19–33. [Google Scholar]
- 9.Greene DF, Quesada M, Calogeropoulos C. Dispersal of seeds by the tropical sea breeze. Ecology. 2008;89:118–125. doi: 10.1890/06-0781.1. [DOI] [PubMed] [Google Scholar]
- 10.Croat TB. Flora of Barro Colorado Island. Stanford, CA: Stanford Univ Press; 1978. [Google Scholar]
- 11.Windsor DM. Climate and moisture variability in a tropical forest: Long-term records from Barro Colorado Island, Panama. Smithsonian Contributions to the Earth Sciences. 1990;29:1–146. [Google Scholar]
- 12.Janzen DH. Synchronization of sexual reproduction of trees within dry season in Central America. Evolution. 1967;21:620–637. doi: 10.1111/j.1558-5646.1967.tb03416.x. [DOI] [PubMed] [Google Scholar]
- 13.Nathan R. Long-distance dispersal of plants. Science. 2006;313:786–788. doi: 10.1126/science.1124975. [DOI] [PubMed] [Google Scholar]
- 14.Wright SJ, Muller-Landau HC, Condit R, Hubbell SP. Shade tolerance, realized vital rates, and size distributions of tropical trees. Ecology. 2003;84:3174–3185. [Google Scholar]
- 15.Wright SJ, Muller-Landau HC, Calderón O, Hernández A. Annual and spatial variation in seedfall and seedling recruitment in a Neotropical forest. Ecology. 2005;86:848–860. [Google Scholar]
- 16.Brokaw NVL. In: The Ecology of a Tropical Forest: Seasonal Rhythms and Long-Term Changes. Leigh EG Jr, Rand AS, Windsor DM, editors. Washington, DC: Smithsonian Institution; 1996. [Google Scholar]
- 17.Augspurger CK. Morphology and dispersal potential of wind-dispersed diaspores of neotropical trees. Am J Bot. 1986;73:353–363. [Google Scholar]
- 18.Horn HS, Nathan R, Kaplan SR. Long-distance dispersal of tree seeds by wind. Ecol Res. 2001;16:877–885. [Google Scholar]
- 19.Greene DF, Johnson EA. A model of wind dispersal of winged or plumed seeds. Ecology. 1989;70:339–347. [Google Scholar]
- 20.Azuma A, Okuno Y. Flight of a samara, Alsomitra macrocarpa. J Theor Biol. 1987;129:263–274. [Google Scholar]
- 21.Greene DF, Johnson EA. The dispersal of winged fruits and seeds differing in autorotative behavior. Can J Bot. 1990;68:2693–2697. [Google Scholar]
- 22.Bohrer G, Katul GG, Nathan R, Walko RL, Avissar R. Effects of canopy heterogeneity, seed abscission, and inertia on wind-driven dispersal kernels of tree seeds. J Ecol. 2008;96:569–580. [Google Scholar]
- 23.Pounden E, Greene DF, Quesada M, Contreras Sánchez JM. The effect of collisions with vegetation elements on the dispersal of winged and plumed seeds. J Ecol. 2008;96:591–598. [Google Scholar]
- 24.Muller-Landau HC, Wright SJ, Calderon O, Condit R, Hubbell SP. Interspecific variation in seed dispersal in a primary tropical forest. J Ecol. 2008;96:653–667. [Google Scholar]
- 25.Jones FA, Muller-Landau HC. Measuring long distance seed dispersal in complex natural environments: Evaluation and integration of the contributions of classical and genetic methods. J Ecol. 2008;96:642–652. [Google Scholar]
- 26.Wesson K, Katul GG, Siqueira M. Quantifying organization of atmospheric turbulent eddy motion using nonlinear time series analysis. Boundary-Layer Meteorol. 2003;106:507–525. [Google Scholar]
- 27.Poggi D, Katul GG, Albertson JD. Momentum transfer and turbulent kinetic energy budgets within a dense model canopy. Boundary-Layer Meteorol. 2004;111:589–614. [Google Scholar]
- 28.Katul G, Albertson J, Parlang M, Chu C, Sticker H. Conditional Sampling, bursting, and the intermittent structures of sensitble heat flux. J Geophys Res Atmos. 1994;99:22869–22876. [Google Scholar]
- 29.Damschen EI, Brudvig LA, Haddad NM, Levey DJ, Orrock JL, Tewksbury JJ. The movement ecology and dynamics of plant communities in fragmented landscapes. Proc Natl Acad Sci USA. 2008;105:19078–19083. doi: 10.1073/pnas.0802037105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bohlman SA, O'Brien ST. Allometry, adult stature and regeneration requirements of 65 tree species on Barro Colorado Island, Panama. J Trop Ecol. 2006;22:123–136. [Google Scholar]
- 31.Thomas SC. Asymptotic height as a predictor of growth and allometric characteristics of Malaysian rain forest trees. Am J Bot. 1996;83:556–566. [Google Scholar]
- 32.Greene DF, Johnson EA. Fruit abscission in Acer saccharinum with reference to seed dispersal. Can J Bot. 1992;73:1036–1045. [Google Scholar]
- 33.Wirth R, Weber B, Ryel RJ. Spatial and temporal variability of canopy structure in a tropical moist forest. Acta Oecologica. 2001;22:235–244. [Google Scholar]
- 34.Cescatti A, Marcolla B. Drag coefficient and turbulence intensity in conifer canopies. Agric For Meteor. 2004;121:197–206. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.