Abstract
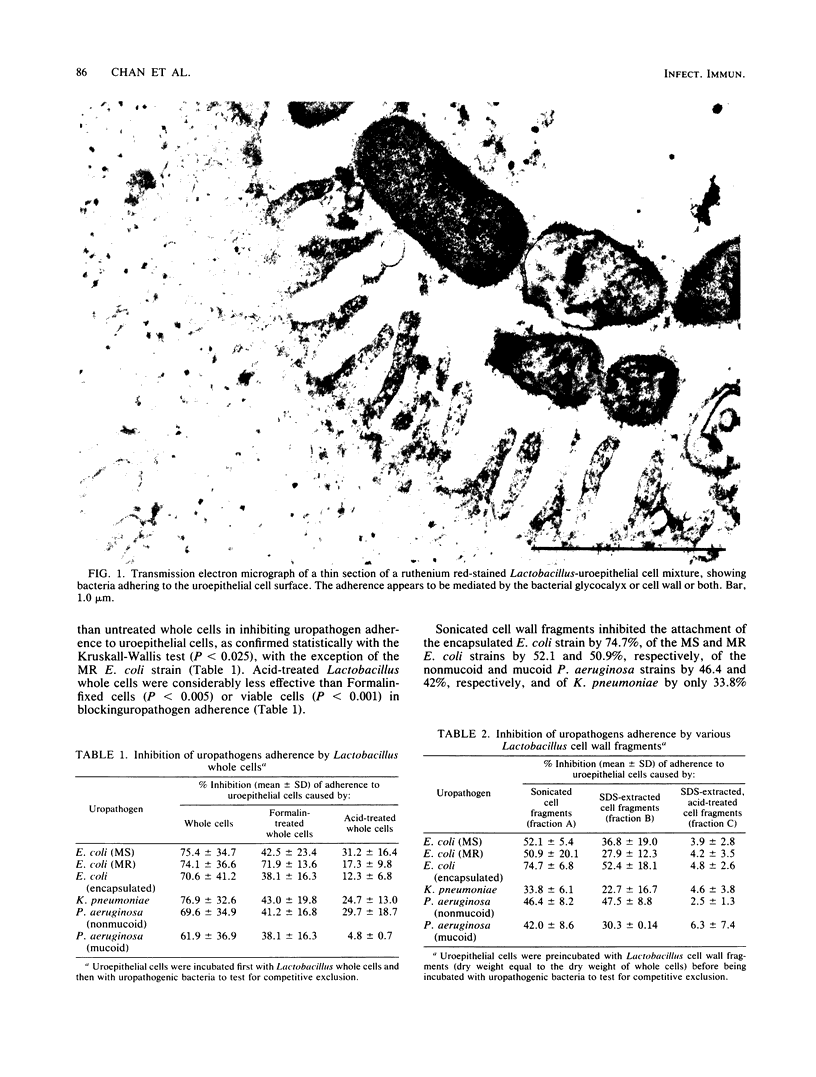
Previous studies have shown that indigenous bacteria isolated from cervical, vaginal, and urethral surfaces of healthy women are able to adhere to human uroepithelial cells in vitro. Furthermore, these organisms were found to block the adherence of uropathogenic bacteria to uroepithelial cells from women with and without a history of urinary tract infections. In the present study, complete or partial inhibition of the adherence of gram-negative uropathogens was achieved by preincubating the uroepithelial cells with bacterial cell wall fragments isolated from a Lactobacillus strain. Competitive exclusion was most effective with whole viable cells and less effective with cell wall fragments obtained by sonication, extraction with sodium dodecyl sulfate, and treatment with sodium dodecyl sulfate and acid. Analysis of the Lactobacillus cell wall preparations suggested that lipoteichoic acid was responsible for the adherence of the Lactobacillus cells to uroepithelial cells but that steric hindrance was the major factor in preventing the adherence of uropathogens. This conclusion was also supported by blockage studies with reconstituted lipoteichoic acid-peptidoglycan, which was more effective at blocking adherence than lipoteichoic acid or peptidoglycan alone. The results suggest that the normal flora of the urinary tract may be used to protect against the attachment of uropathogens to the surfaces of uroepithelial cells. The long-term implications of these findings may lead to alternative methods for the management and prevention of recurrent urinary tract infections in females.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Beachey E. H., Simpson W. A. Adherence of streptococcus pyogenes, Escherichia coli, and Pseudomonas aeruginosa to fibronectin-coated and uncoated epithelial cells. Infect Immun. 1983 Sep;41(3):1261–1268. doi: 10.1128/iai.41.3.1261-1268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibel D. J., Aly R., Bayles C., Strauss W. G., Shinefield H. R., Maibach H. I. Competitive adherence as a mechanism of bacterial interference. Can J Microbiol. 1983 Jun;29(6):700–703. doi: 10.1139/m83-114. [DOI] [PubMed] [Google Scholar]
- Bruce A. W., Chan R. C., Pinkerton D., Morales A., Chadwick P. Adherence of gram-negative uropathogens to human uroepithelial cells. J Urol. 1983 Aug;130(2):293–298. doi: 10.1016/s0022-5347(17)51115-5. [DOI] [PubMed] [Google Scholar]
- Chan R. C., Bruce A. W., Reid G. Adherence of cervical, vaginal and distal urethral normal microbial flora to human uroepithelial cells and the inhibition of adherence of gram-negative uropathogens by competitive exclusion. J Urol. 1984 Mar;131(3):596–601. doi: 10.1016/s0022-5347(17)50512-1. [DOI] [PubMed] [Google Scholar]
- Chan R. C., Bruce A. W. The influence of growth media on the morphology and in vitro adherence characteristics of gram-negative urinary pathogens. J Urol. 1983 Feb;129(2):411–417. doi: 10.1016/s0022-5347(17)52128-x. [DOI] [PubMed] [Google Scholar]
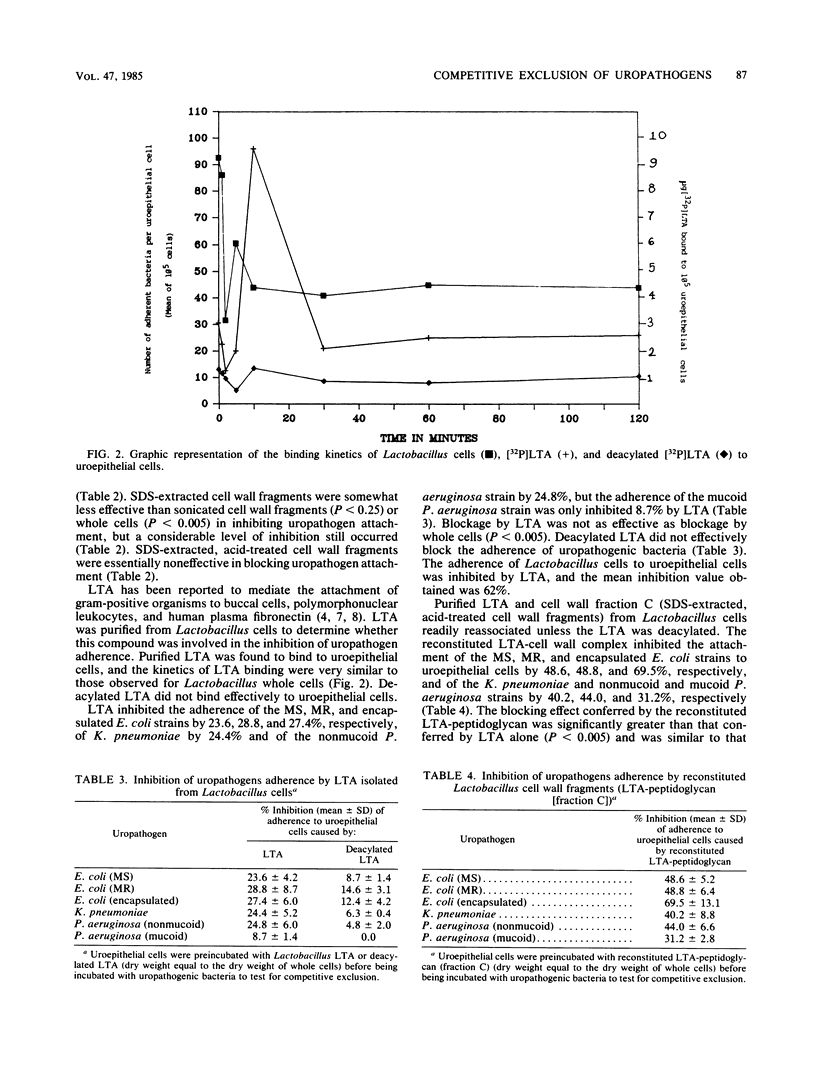
- Courtney H. S., Simpson W. A., Beachey E. H. Binding of streptococcal lipoteichoic acid to fatty acid-binding sites on human plasma fibronectin. J Bacteriol. 1983 Feb;153(2):763–770. doi: 10.1128/jb.153.2.763-770.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney H., Ofek I., Simpson W. A., Beachey E. H. Characterization of lipoteichoic acid binding to polymorphonuclear leukocytes of human blood. Infect Immun. 1981 May;32(2):625–631. doi: 10.1128/iai.32.2.625-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén C. S., Freter R., Hagberg L., Hull R., Hull S., Leffler H., Schoolnik G. Inhibition of experimental ascending urinary tract infection by an epithelial cell-surface receptor analogue. Nature. 1982 Aug 5;298(5874):560–562. doi: 10.1038/298560a0. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Hanson L. A., Jodal U., Lindberg U., Akerlund A. S. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet. 1976 Sep 4;1(7984):490–492. [PubMed] [Google Scholar]
- Fowler J. E., Jr, Latta R., Stamey T. A. Studies of introital colonization in women with recurrent urinary infections. VIII. The role of bacterial interference. J Urol. 1977 Aug;118(2):296–298. doi: 10.1016/s0022-5347(17)57978-1. [DOI] [PubMed] [Google Scholar]
- Källenius G., Svenson S. B., Möllby R., Korhonen T., Winberg J., Cedergren B., Helin I., Hultberg H. Carbohydrate receptor structures recognized by uropathogenic E. coli. Scand J Infect Dis Suppl. 1982;33:52–60. [PubMed] [Google Scholar]
- Lomberg H., Hanson L. A., Jacobsson B., Jodal U., Leffler H., Edén C. S. Correlation of P blood group, vesicoureteral reflux, and bacterial attachment in patients with recurrent pyelonephritis. N Engl J Med. 1983 May 19;308(20):1189–1192. doi: 10.1056/NEJM198305193082003. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Swantee C. A., Hartlen M. Aerobic and anaerobic urethral flora of healthy females in various physiological age groups and of females with urinary tract infections. J Clin Microbiol. 1980 Jun;11(6):654–659. doi: 10.1128/jcm.11.6.654-659.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G., Brooks H. J., Bacon D. F. In vitro attachment of Escherichia coli to human uroepithelial cells: variation in receptivity during the menstrual cycle and pregnancy. J Infect Dis. 1983 Sep;148(3):412–421. doi: 10.1093/infdis/148.3.412. [DOI] [PubMed] [Google Scholar]
- Roberts J. A. Pathogenesis of pyelonephritis. J Urol. 1983 Jun;129(6):1102–1106. doi: 10.1016/s0022-5347(17)52592-6. [DOI] [PubMed] [Google Scholar]
- SHINEFIELD H. R., RIBBLE J. C., EICHENWALD H. F., BORIS M., SUTHERLAND J. M. Bacterial interference: its effect on nursery-acquired infection with Staphylococcus aureus. V. An analysis and interpretation. Am J Dis Child. 1963 Jun;105:683–688. [PubMed] [Google Scholar]
- Sanders E. Bacterial interference. I. Its occurrence among the respiratory tract flora and characterization of inhibition of group A streptococci by viridans streptococci. J Infect Dis. 1969 Dec;120(6):698–707. doi: 10.1093/infdis/120.6.698. [DOI] [PubMed] [Google Scholar]
- Sprunt K., Leidy G. A., Redman W. Prevention of bacterial overgrowth. J Infect Dis. 1971 Jan;123(1):1–10. doi: 10.1093/infdis/123.1.1. [DOI] [PubMed] [Google Scholar]