Abstract
The overlapping histological and biochemical features underlying the beneficial effect of deacetylase inhibitors and NO donors in dystrophic muscles suggest an unanticipated molecular link among dystrophin, NO signaling, and the histone deacetylases (HDACs). Higher global deacetylase activity and selective increased expression of the class I histone deacetylase HDAC2 were detected in muscles of dystrophin-deficient MDX mice. In vitro and in vivo siRNA-mediated down-regulation of HDAC2 in dystrophic muscles was sufficient to replicate the morphological and functional benefits observed with deacetylase inhibitors and NO donors. We found that restoration of NO signaling in vivo, by adenoviral-mediated expression of a constitutively active endothelial NOS mutant in MDX muscles, and in vitro, by exposing MDX-derived satellite cells to NO donors, resulted in HDAC2 blockade by cysteine S-nitrosylation. These data reveal a special contribution of HDAC2 in the pathogenesis of Duchenne muscular dystrophy and indicate that HDAC2 inhibition by NO-dependent S-nitrosylation is important for the therapeutic response to NO donors in MDX mice. They also define a common target for independent pharmacological interventions in the treatment of Duchenne muscular dystrophy.
Keywords: epigenetic, skeletal muscle
Recent studies performed in mouse models of muscular dystrophy have reported on the therapeutic potential of pharmacological interventions that target events downstream to the genetic defect responsible for the disease. For instance, histone deacetylase inhibitors (DIs) and NO donors countered the progression of muscular dystrophy in MDX mice (1, 2). Intriguingly, the beneficial effect of both treatments relies, at least in part, on the formation of myofibers larger than normal and on the transcriptional activation of the myostatin antagonist follistatin (1, 3, 4). This evidence suggests a common mechanism linking the effect of NO donors and that of DIs in dystrophic muscles.
Targeting the follistatin–myostatin pathway by either direct myostatin blockade or follistatin overexpression has revealed valuable therapeutic potential in the treatment of muscular dystrophies (5–8). Thus, the understanding of the mechanism by which compounds of pharmacological interest, such as DIs and NO donors, target the follistatin–myostatin pathway is instrumental to develop effective strategies in the treatment of muscular dystrophies. Follistatin expression in skeletal muscles is regulated by class I histone deacetylases (HDACs) (1, 4), which inhibit the activity of MyoD (9, 10) and additional transcription factors, such as CREB and NFAT, possibly recruited to the follistatin promoter (4).
In the present study we have investigated the individual role of class I HDACs in the progression of muscular dystrophy in MDX mice and evaluated the possibility that DIs and NO signaling could converge on HDAC blockade. We show that class I HDAC2 expression and activity are increased in skeletal muscle from MDX mice. HDAC2 down-regulation by siRNA enhances the ability of MDX derived-satellite cells to form multinucleated myotubes in vitro and is sufficient to counter the disease progression in vivo, when delivered to MDX muscles. Finally, we show that HDAC2 S-nitrosylation by NO donors impairs its enzymatic function. These results indicate that HDAC2 is an important common pharmacological target of distinct pharmacological interventions aimed at Duchenne muscular dystrophy (DMD) treatment and suggest a novel mechanism of HDAC2 inhibition by NO-dependent cysteine S-nitrosylation.
Results and Discussion
Previous studies have reported on the impaired NO production in dystrophic muscles due to the disruption of the association between sarcolemmal neuronal NOS and the dystrophin–sarcoglycan complex (11–14). Consistently, the reconstitution of NO levels ameliorates the dystrophic phenotype in MDX mice (14). Additional evidence shows that agents that promote NO release stimulate myoblast fusion into multinucleated myotubes with an increased size via follistatin up-regulation (4)—an effect reminiscent of that observed upon treatment with DIs (2).
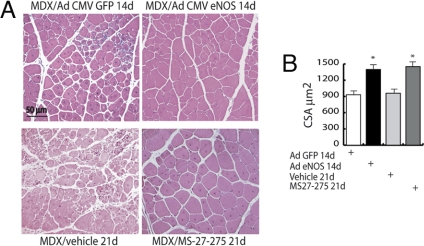
We found that the injection of an adenoviral vector encoding for a constitutively active endothelial NOS (eNOS) mutant in MDX adductor muscle [supporting information (SI) Fig. S1a] led to the recovery of muscle morphology (Fig. 1A) and to the increase in muscle fiber cross-sectional area that are equivalent to those observed upon treatment with the class I HDAC inhibitor MS27-275 (Fig. 1B and Fig. S1a). These results suggest that class I HDACs could be a common target for DIs and NO donors.
Fig. 1.
Adenovirus-mediated eNOS expression restores muscle differentiation in MDX mice. (A) H&E-stained sections of MDX adductor muscles injected with either GFP or eNOS adenovirus (1 × 108 pfu per muscle) and evaluated 14 days after infection, or adductor muscles from MDX treated for 21 days with the class I HDAC inhibitor MS27-275 or the vehicle alone. (B) The bar graph shows the quantification of muscle fiber cross-sectional area (CSA) of the adductor from MDX mice injected with GFP or eNOS adenovirus and evaluated 14 days after infection, or adductor muscles from MDX treated for 21 days with the class I HDAC inhibitor MS27-275 or the vehicle alone. Cross-sectional area is expressed as mean ± SE (P < 0.05).
The regulation of individual HDACs in dystrophic muscles and their potential role in disease pathogenesis and progression in MDX mice have never been addressed. Thus, we have monitored the global HDAC activity in muscles from normal vs. MDX mice. Whole muscles isolated from MDX mice showed a higher HDAC activity as compared with the normal counterpart (Fig. 2A). This activity was eliminated by the class I DI MS27-275 (Fig. 2A), indicating a deregulated expression and/or activity of class I HDACs in dystrophic muscles. Among the class I HDAC members analyzed by us, only the expression levels of HDAC2 were increased in lysates from whole muscles (Fig. 2B). Likewise, satellite cells derived from single myofibers isolated by MDX mice showed an increased global deacetylase activity (Fig. 2C) and higher expression levels of HDAC2 (Fig. 2D). These data indicate a special contribution from HDAC2 to the pathogenesis and/or disease progression in MDX mice. They also significantly narrow down the spectrum of potential targets of selective pharmacological strategies that could replicate the beneficial effect of DIs in the treatment of DMD. Importantly, an independent screening of siRNA-mediated down-regulation of each individual HDAC (from HDAC1 to HDAC11; data not shown) in primary human skeletal muscle cells showed that HDAC2 siRNA replicates the morphological effect of DIs (fusion into multinucleated myotubes that are larger than controls) more than any other individual HDAC siRNA (G.M., P.G., and P.L.P., unpublished data). Note that this effect was accompanied by the up-regulation of follistatin (Fig. S1b), which is consistent with our previous finding that HDAC2 occupies follistatin promoter in myoblasts (2).
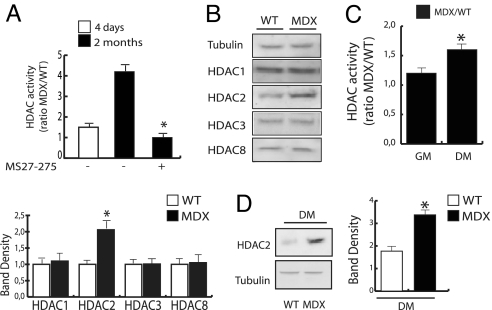
Fig. 2.
Class I HDAC expression and function is altered in MDX skeletal muscle and satellite cells. (A) Assessment of global HDAC activity was performed in whole lysates from WT and MDX mice adductor muscle by deacetylation assay. MS27-275 was added to fresh lysates at the concentration of 1 μM. Enzymatic activity is represented as the MDX/WT ratio. Experiments were performed 4 days and 2 months after birth (n = 6; P < 0.05). (B Upper) Western blotting analysis of class I HDACs in WT vs. MDX adductor skeletal muscle lysates. (B Lower) Data normalization by densitometric analysis. (C) Assessment of global HDAC activity was performed in lysates from primary satellite cells derived from single myofibers isolated from WT and MDX mice and cultured in growth medium (GM) or differentiation medium (DM). Enzymatic activity is represented as the MDX/WT ratio (n = 4; P < 0.05). (D) HDAC2 protein expression levels were measured by Western blot in primary satellite cells derived from single myofibers isolated from WT and MDX mice and cultured in DM. Data were normalized by densitometric analysis.
Thus, deregulation of HDAC2 levels and activity appears to be a hallmark of MDX muscles and suggests that selective neutralization of HDAC2 could have a beneficial effect in these mice.
siRNA was therefore used to selectively down-regulate HDAC2 in MDX muscles in vitro and in vivo and to assess the effect on regeneration and morphological and functional parameters. HDAC2 knockdown in satellite cells derived from MDX mice was achieved by delivery of siRNA oligonucleotides (Fig. 3A). Satellite cells receiving the HDAC2 siRNA showed enhanced fusion into myotubes when compared with controls (70–80% increase in the fusion index of HDAC2 siRNA-treated MDX satellite cells vs. controls) (Fig. 3B Upper). Down-regulation of HDAC2 was estimated at ≈50% by Western blotting analysis (Fig. 3A), which corresponds to a decrease in HDAC2-specific activity paralleled by a global deacetylase activity reduction (Fig. 3B Lower). Thus, in MDX satellite cells, it is sufficient to reduce the levels and activity (Fig. 3B) of the aberrantly expressed HDAC2 to obtain morphological features that are reminiscent of those achieved with NO donors and DIs treatment (3, 4, 13). Consistently, myosin heavy chain (MHC) expression was increased in siRNA-treated MDX satellite cells when compared with control cells (Fig. 3B Lower Left).
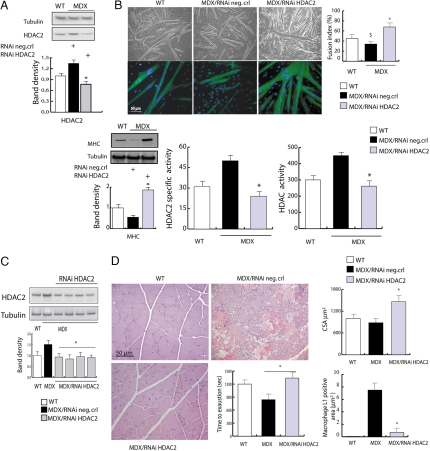
Fig. 3.
siRNA-mediated down-regulation of HDAC2 reproduces the effects of DIs and NO on MDX skeletal muscle. (A) Representative Western blotting analysis of HDAC2 levels in satellite cells from normal (WT) mice and MDX mice after delivery of scramble siRNA (control) or siRNA HDAC2. The densitometric analysis (Lower) shows that HDAC2 knockdown reduced the levels of endogenous HDAC2 to ≈50% of the endogenous one in MDX satellite cells. (B) The ability to differentiate into multinucleated myofibers was monitored in satellite cells from normal (WT) mice and MDX mice after delivery of scramble siRNA (control) or siRNA HDAC2. Shown are representative microphotographs of WT, MDX, and siRNA-treated MDX satellite cells at 72 h after differentiation. (B Upper) Phase contrast. (B Lower) MHC immunofluorescence and DAPI staining. Similar results were obtained with all of the oligonucleotides tested. Satellite cell differentiation has been evaluated by Western blotting analysis of the expression of MHC (Lower Left) and by the fusion index determined as the number of nuclei in myosin-expressing cells with >2 nuclei versus the total number of nuclei. Results are shown in the bar graph in Upper Right. HDAC global activity and HDAC2-specific activity have been evaluated in satellite cells treated with HDAC2-specific RNAi or control oligonucleotides and induced to differentiation. These results are shown in the lower, middle, and right panels of B. (C) Representative Western blotting analysis of HDAC2 levels in whole adductor muscles from normal (WT) mice vs. MDX mice treated with HDAC2 siRNAi or the scramble siRNA (control). Relative densitometric normalization was performed as described above (P < 0.05). (D) The in vivo HDAC2 knockdown shows morphological and functional recovery in MDX mice. The picture shows H&E staining of muscle sections and the treadmill test performed on WT and MDX mice injected with specific HDAC2 RNAi or scrambled oligonucleotides (neg.crl). The bar graph in Upper Right shows the average cross-section area in normal mice (white) and in MDX mice treated with HDAC2 RNAi (gray) or control oligonucleotides (black). The graph in Lower Right shows the presence of inflammatory infiltrate measured as macrophage L1-positive areas.
Based on this evidence we sought to evaluate whether in vivo down-regulation of HDAC2 in muscles of MDX mice could replicate the beneficial effect of NO donors and DIs. Systemic delivery of HDAC2 siRNAi in MDX mice was achieved by daily i.p. injections for 30 days and led to a down-regulation of HDAC2 in the skeletal muscles that was comparable to that obtained in satellite cells (Fig. 3C). Notably, systemic siRNA-mediated HDAC2 down-regulation resulted in a morphological (Fig. 3D) and functional (Fig. 3D) recovery of dystrophic muscles. Although the strategy adopted in this experiment (systemic delivery of HDAC2 siRNA) does not rule out that HDAC2 down-regulation in other tissues and organs could indirectly contribute to the effect on muscles, this possibility appears remote because HDAC2 is increased only in muscles of MDX mice (data not shown).
The data presented so far suggest that in dystrophic muscles an impaired dystrophin–NO signaling to HDAC2 can provide a selective target for pharmacological interventions. This information assumes a particular relevance within the current effort toward the identification of individual HDACs that should be targeted to achieve the same beneficial effect observed with general DIs.
HDAC2 regulation by NO has recently been reported in neurons (15, 16). Nott et al. (16) identified 2 cysteines at positions 262 and 274 targeted by NO-dependent S-nitrosylation. The S-nitrosylation of these residues determines an impairment of HDAC2 binding to target DNA sequences (16). A comparison analysis of the amino acidic sequence of all mouse and human class I HDACs revealed that these cysteines are well-conserved among the different class I members, suggesting the possibility that other HDACs, including HDAC1 and HDAC3, could be regulated by S-nitrosylation (see Fig. S2). We therefore investigated whether NO-mediated S-nitrosylation of HDAC1, HDAC2, and HDAC3 could be a relevant intracellular signaling altered in dystrophic muscles. Cysteine-S-nitrosylation of endogenous HDAC2, but not that of HDAC1 and HDAC3, was detected in C2C12 myoblasts exposed to NO donors (Fig. 4A). This cysteine-S-nitrosylation correlated with a significant reduction in the enzymatic activity of HDAC2 (Fig. 4B). Similarly, HDAC2 S-nitrosylation was increased in MDX adductor muscles injected with the eNOS adenovirus (Fig. 4C), leading to the reduction of HDAC2-specific activity, which is typically elevated in MDX muscles (Fig. 4D). This evidence links the effect of restoring the NO signaling in dystrophin-deficient muscle with a beneficial effect due to HDAC2 inhibition via S-nitrosylation. Surprisingly, we have detected a reduced activity of HDAC1 in lysates from C2C12 myoblasts and from MDX adductor muscle despite the fact that HDAC2 is not S-nitrosylated by DETA-NO (Fig. 4 B–D). In contrast, the enzymatic activity of HDAC3 was unaffected by NO. We further investigate the relationship between NO-mediated S-nitrosylation and the enzymatic activity of HDAC1, HDAC2, and HDAC3 by measuring in vitro the enzymatic activity of the recombinant enzymes purified from Escherichia coli in the presence of NO donors or the DI SAHA, as control. NO donors markedly reduced the enzymatic activity of HDAC2, minimally influenced that of HDAC1, and were ineffective on HDAC3 (Fig. 4E). In agreement with these results Western blotting analysis of nitrosylated recombinant HDAC1 and HDAC2 protein showed a strong S-nitrosylation signal in HDAC2 that was not detectable in HDAC1 (Fig. 4F). These results indicate that direct regulation of deacetylase activity by NO-mediated S-nitrosylation is restricted to HDAC2 and that NO-mediated inhibition of HDAC1 can occur by an S-nitrosylation-independent mechanism. Collectively, our findings indicate that HDAC2 inhibition, either via direct blockade or by S-nitrosylation, mediate the beneficial effects of DIs and NO, respectively. This information provides the mechanism that accounts for the overlapping features of 2 distinct pharmacological interventions in DMD.
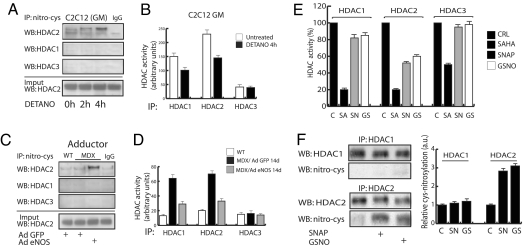
Fig. 4.
NO regulates the enzymatic activity of HDAC2 by cysteine-S-nitrosylation. (A) Western blotting analysis of HDAC1, HDAC2, and HDAC3 in C2C12 myoblasts after immunoprecipitation with anti S-nitroso-cysteine-specific antibody. An increased cysteine-S-nitrosylation of HDAC2 was detectable after 4 h of treatment with DETA-NO. (B) The bar graph shows the HDAC1-, HDAC2-, and HDAC3-specific activity in C2C12 evaluated in the presence or absence of DETA-NO treatment for 4 h. (C) Anti-S-nitroso-cysteine antibody immunoprecipitation and Western blotting analysis of HDAC1, HDAC2, and HDAC3 in WT and MDX mice infected with either eNOSS1177A or GFP adenovirus adductor muscles. An increased cysteine-S-nitrosylation of HDAC2 was detectable after 14 days from AdeNOS infection. (D) The bar graph shows in vivo the HDAC1-, HDAC2-, and HDAC3-associated enzymatic activity evaluated in adenovirus-infected WT and MDX mice expressing GFP or eNOS, respectively, in the adductor muscles. Assays were performed on total muscle lysates after immunoprecipitation with anti-HDAC1-, anti-HDAC2-, and anti-HDAC3-specific antibodies. (E) Evaluation of NO effect on recombinant E. coli GST-purified HDAC1-, HDAC2-, and HDAC3-specific activities in the presence or absence of HDAC inhibitor SAHA (SA, 5 μM) or NO donors SNAP (SN, 205 μM) or GSNO (GS, 250 μM). (F) Western blotting analysis of S-nitroso-cysteine on recombinant HDAC1 and HDAC2 proteins after NO donors SNAP (SN, 10 mM) or GSNO (GS, 10 mM). Data were normalized by densitometric analysis (Right).
The key role of class I HDACs in DMD pathogenesis and progression was previously suggested by the beneficial effect exerted by MS27-275 in MDX mice (2). Our current data further extend this notion and indicate that selective blockade of HDAC2 is a primary target for therapeutic strategies.
HDAC2 S-nitrosylation is emerging as a nodal regulatory mechanism for signal-dependent control of gene expression (15, 16). Our finding that HDAC2 is specifically up-regulated in dystrophic muscles offers an interesting model for the exploitation of pharmacological manipulation of HDAC2 via S-nitrosylation as a selective intervention in a genetic disease. (i.e., muscular dystrophies). The precise role of HDAC2 cysteine S-nitrosylation in the regulation of gene expression by cGMP-activated NO signaling (4, 17, 18) during skeletal myogenesis remains to be determined. However, our data indicate that derepression of HDAC2-regulated genes (19–23), such as follistatin, might produce compensatory responses (e.g., increased regeneration) that counterbalance muscle degeneration in muscle wasting disorders. Future work should determine the identity of the genes affected by HDAC2 inhibition via NO-mediated S-nitrosylation and establish whether inhibition of HDAC2 by distinct treatments in dystrophic muscles has synergistic effects. Moreover, the key role of HDAC2 in mediating corticosteroid-regulated gene expression (24, 25) suggests that pharmacological modulation of HDAC2 could also be exploited to modulate the effect of glucocorticoids in the current treatment of DMD (26).
Given the physical and functional relationship between dystrophin (and possibly other components of the dystrophin-associated complex) and NO, and because of the impaired NO signaling in dystrophic muscles, future studies should be devoted to investigating whether reduced HDAC2 S-nitrosylation is responsible for the deregulated gene expression in dystrophic muscles (27). This analysis should determine the contribution of HDAC2 up-regulation in the pathogenesis of muscular dystrophies. Overall, these studies will establish a link between the genetic defect responsible for DMD and epigenetic modifications underlying the disease progression that could be targeted by epigenetic drugs without correcting the primary genetic defect.
Methods
Animals and Treatments.
Eight-week-old normal WT C57BL/10 and MDX mice were used in the experiments. MDX mice received i.p. daily injections of MS27-275 at a dose of 0.5 mg/kg or saline for 21 days. All experimental procedures were approved by the internal Animal Research Ethical Committee (protocol HH39) according to the Italian Ministry of Health and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Cell Cultures and Treatments.
C2C12 myoblasts were maintained at subconfluence in DMEM supplemented with 20% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin. DETANO (500 μM) was administered for 2 and 4 h to C2C12 cells in growing conditions. Satellite cells were isolated from single fibers as described in ref. 9 and maintained in growing conditions or induced to differentiate in low-serum medium.
Infections.
C2C12 myoblasts and satellite cells were infected with either adenoviruses CMV-GFP encoding for the constitutively active isoform of eNOS (eNOS S1177D) or CMV-GFP-null. In in vivo experiments, adductor muscles were injected with 1 × 108 pfu per animal of virus according to previously published procedures (28, 29).
RNA Interference.
Satellite cells were transfected with 3 different oligonucleotides for HDAC2 siRNA (30 nM each; Ambion) by using siPORT NeoFX according to instructions from the manufacturer.
Protein Analysis.
Western blot was performed on tissue protein extracts after lysis in 50 mM Tris·HCl (pH 8.0), 125 mM NaCl, 1 mM DTT, 5 mM MgCl2, 1 mM EDTA, 10% glycerol, and 0.1% Nonidet P-40 supplemented with 1 mM PMSF and protease inhibitor mix. The extracts were resolved in SDS/PAGE and transferred on nitrocellulose membrane (Hybond ECL; Amersham) at 50 V for 24 h at 4 °C before immunoblotting assays.
Fluorimetric Human Recombinant HDAC Assays.
The HDAC fluorescent activity assay for HDAC1, HDAC2, and HDAC3 has been carried out according to the supplier's instructions (BioMol) with an incubation time of 30 min. For HDAC1, HDAC2, and HDAC3, 250 ng of recombinant proteins have been used per assay, respectively. Full-length HDAC1, HDAC2, and HDAC3 were purified by using GST fusion proteins expressed in the E. coli strain BL21 as described previously (30). For HDAC3 enzyme activity full-length recombinant human NCOR2 was coincubated.
Immunohistochemistry and Immunofluorescence.
Cells were fixed in 4% paraformaldehyde, permeabilized with 0.2% Triton, blocked in 10% BSA/PBS, and subjected to immunofluorescence. Sections from adductor muscles were deparaffinized and processed for H&E staining or subjected to microwave for antigen retrieval in a solution of 0.01 M sodium citrate buffer for immunofluorescence analysis. Macrophage L1 antibody was used to detect inflammatory cells in tissue sections. Cross-sectional area and treadmill tests were performed as described in ref. 2.
For additional information, see SI Text.
Supplementary Material
Acknowledgments.
This work was partially supported by FIRB Grant RBLA035A4X-1-FIRB (to M.C.C.); European Union FP6 Grant UE-LHSB-CT-04-502988 (to M.C.C.); Parent Project Onlus Grant and AFM Grants MNM2-06 (to C.G.) and DdT2-06 (to M.C.C.); Muscular Dystrophy Association Grant 88202 (to C.G.); Grant EU-LSHC-CT2005-518417 to L.A.; and Telethon Special Grant, Muscular Dystrophy Association, Parent Project Onlus, and Compagnia San Paolo di Torino grants (to P.L.P.). E.C. is a recipient of the Telethon Grant GGP07006 and a Research Grant from AFM; A.M. is a recipient of an AIRC grant 2007 and the PRIN Grant 2006038137_005; G.P. is supported by AIRC, ISS-ACC, and Ministero della Sanita Grant ICS-120.4/RA00-90;R.F.02/184; C.M. is a recipient of an AFM fellowship and G.M. is a recipient of a Parent Project Onlus fellowship. P.L.P. is an Associate Telethon scientist at the Dulbecco Telethon Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805514105/DCSupplemental.
References
- 1.Brunelli S, et al. Nitric oxide release combined with nonsteroidal antiinflammatory activity prevents muscular dystrophy pathology and enhances stem cell therapy. Proc Natl Acad Sci USA. 2007;104:264–269. doi: 10.1073/pnas.0608277104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minetti GC, et al. Functional and morphological recovery of dystrophic muscles in mice treated with deacetylase inhibitors. Nat Med. 2006;12:1147–1150. doi: 10.1038/nm1479. [DOI] [PubMed] [Google Scholar]
- 3.Iezzi S, et al. Deacetylase inhibitors increase muscle cell size by promoting myoblast recruitment and fusion through induction of follistatin. Dev Cell. 2004;6:673–684. doi: 10.1016/s1534-5807(04)00107-8. [DOI] [PubMed] [Google Scholar]
- 4.Pisconti A, et al. Follistatin induction by nitric oxide through cyclic GMP: A tightly regulated signaling pathway that controls myoblast fusion. J Cell Biol. 2006;172:233–244. doi: 10.1083/jcb.200507083. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Bogdanovich S, et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418–421. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- 6.Haidet AM, et al. Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc Natl Acad Sci USA. 2008;105:4318–4322. doi: 10.1073/pnas.0709144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakatani M, et al. Transgenic expression of a myostatin inhibitor derived from follistatin increases skeletal muscle mass and ameliorates dystrophic pathology in mdx mice. FASEB J. 2008;22:477–487. doi: 10.1096/fj.07-8673com. [DOI] [PubMed] [Google Scholar]
- 8.Wagner KR, McPherron AC, Winik N, Lee SJ. Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann Neurol. 2002;52:832–836. doi: 10.1002/ana.10385. [DOI] [PubMed] [Google Scholar]
- 9.Mal A, et al. A role for histone deacetylase HDAC1 in modulating the transcriptional activity of MyoD: Inhibition of the myogenic program. EMBO J. 2001;20:1739–1753. doi: 10.1093/emboj/20.7.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puri PL, et al. Class I histone deacetylases sequentially interact with MyoD and pRb during skeletal myogenesis. Mol Cell. 2001;8:885–897. doi: 10.1016/s1097-2765(01)00373-2. [DOI] [PubMed] [Google Scholar]
- 11.Brenman JE, et al. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- 12.Chao DS, et al. Selective loss of sarcolemmal nitric oxide synthase in Becker muscular dystrophy. J Exp Med. 1996;184:609–618. doi: 10.1084/jem.184.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iezzi S, et al. Stage-specific modulation of skeletal myogenesis by inhibitors of nuclear deacetylases. Proc Natl Acad Sci USA. 2002;99:7757–7762. doi: 10.1073/pnas.112218599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riccio A, et al. A nitric oxide signaling pathway controls CREB-mediated gene expression in neurons. Mol Cell. 2006;21:283–294. doi: 10.1016/j.molcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Nott A, et al. S-nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 2008;455:411–415. doi: 10.1038/nature07238. [DOI] [PubMed] [Google Scholar]
- 17.Sciorati C, et al. Ex vivo treatment with nitric oxide increases mesoangioblast therapeutic efficacy in muscular dystrophy. J Cell Sci. 2006;119:5114–5123. doi: 10.1242/jcs.03300. [DOI] [PubMed] [Google Scholar]
- 18.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- 19.Laherty CD, et al. Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell. 1997;89:349–356. doi: 10.1016/s0092-8674(00)80215-9. [DOI] [PubMed] [Google Scholar]
- 20.Rountree MR, Bachman KE, Baylin SB. DNMT1 binds HDAC2 and a new co-repressor, DMAP1, to form a complex at replication foci. Nat Genet. 2000;25:269–277. doi: 10.1038/77023. [DOI] [PubMed] [Google Scholar]
- 21.Zhang HS, et al. Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell. 2000;101:79–89. doi: 10.1016/S0092-8674(00)80625-X. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, et al. Histone deacetylases and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell. 1997;89:357–364. doi: 10.1016/s0092-8674(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, et al. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 1999;13:1924–1935. doi: 10.1101/gad.13.15.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnes PJ. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br J Pharmacol. 2006;148:245–254. doi: 10.1038/sj.bjp.0706736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito K, Barnes PJ, Adcock IM. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1beta-induced histone H4 acetylation on lysines 8 and 12. Mol Cell Biol. 2000;20:6891–6903. doi: 10.1128/mcb.20.18.6891-6903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muntoni F, Fisher I, Morgan JE, Abraham D. Steroids in Duchenne muscular dystrophy: From clinical trials to genomic research. Neuromuscul Disord. 2002;12(Suppl 1):S162–S165. doi: 10.1016/s0960-8966(02)00101-3. [DOI] [PubMed] [Google Scholar]
- 27.Pescatori M, et al. Gene expression profiling in the early phases of DMD: A constant molecular signature characterizes DMD muscle from early postnatal life throughout disease progression. FASEB J. 2007;21:1210–1226. doi: 10.1096/fj.06-7285com. [DOI] [PubMed] [Google Scholar]
- 28.Zaccagnini G, et al. Telomerase mediates vascular endothelial growth factor-dependent responsiveness in a rat model of hind limb ischemia. J Biol Chem. 2005;280:14790–14798. doi: 10.1074/jbc.M414644200. [DOI] [PubMed] [Google Scholar]
- 29.Teupe C, et al. Vascular gene transfer of phosphomimetic endothelial nitric oxide synthase (S1177D) using ultrasound-enhanced destruction of plasmid-loaded microbubbles improves vasoreactivity. Circulation. 2002;105:1104–1109. doi: 10.1161/hc0902.104720. [DOI] [PubMed] [Google Scholar]
- 30.Guan KL, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: An improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.