Abstract
Mistranslation is toxic to bacterial and mammalian cells and can lead to neurodegeneration in the mouse. Mistranslation is caused by the attachment of the wrong amino acid to a specific tRNA. Many aminoacyl-tRNA synthetases have an editing activity that deacylates the mischarged amino acid before capture by the elongation factor and transport to the ribosome. For class I tRNA synthetases, the editing activity is encoded by the CP1 domain, which is distinct from the active site for aminoacylation. What is not clear is whether the enzymes also have an editing activity that is separable from CP1. A point mutation in CP1 of class I leucyl-tRNA synthetase inactivates deacylase activity and produces misacylated tRNA. In contrast, although deletion of the entire CP1 domain also disabled the deacylase activity, the deletion-bearing enzyme produced no mischarged tRNA. Further investigation showed that a second tRNA-dependent activity prevented misacylation and is intrinsic to the active site for aminoacylation.
Keywords: amino acid editing, fidelity, protein synthesis
The aminoacyl-tRNA synthetases (aaRSs) are responsible for the first step of protein synthesis, which links a specific amino acid to its correct tRNA isoacceptor. About half of the aaRSs misactivate noncognate amino acids that are similar to their cognate substrates (1). These enzymes have acquired an amino acid editing mechanism to clear mistakes before they can be misincorporated into proteins during translation. In some cases, editing can also occur in trans by a non-aaRS protein (2–4). Amino acid editing is essential to the viability of microbes and mammalian cells (5–7). Mammalian aaRSs that have defective editing mechanisms can also cause neurological diseases (8).
Aminoacylation occurs in a two-step reaction pathway that relies on an aminoacyl adenylate intermediate:
Amino acid editing has been proposed to occur at either of the two steps (9). Posttransfer editing cleaves mischarged tRNA in a domain that is distinct from the synthetic aminoacylation domain (10–16).
 |
Pretransfer editing, which clears the aminoacyl adenylate intermediate, has been much more controversial.
 |
Multiple sites of hydrolysis for pretransfer editing have been suggested (17–22). In addition, pretransfer editing has been reported to be RNA-dependent (9, 23) and independent (20, 21).
X-ray structures for a number of class I and II editing aaRSs have shown that aminoacyl adenylate analogs bind in the editing active site, which is responsible for posttransfer editing similar to the mischarged adenosine end of the tRNA (18, 22, 24). Mutational analysis also suggests that at least portions of the pretransfer and posttransfer editing active sites coincide (18, 25). However, translocation of the labile adenylate over 30 Å between the aminoacylation and editing active site remains dubious. It is clear that some aaRSs can eject misactivated adenylates from the synthetic active site in a selective release mechanism that preserves the correctly activated adenylate for aminoacylation (17, 20, 21, 26), but in this case the target could be a remote site on the aaRS or its aqueous environment. In a third scenario, hydrolysis of adenylate intermediates that are associated with the canonical aminoacylation core of the aaRS have also been reported (19, 21).
Leucyl-tRNA synthetase (LeuRS) and the homologous isoleucyl-tRNA synthetase (IleRS) and valyl-tRNA synthetase (ValRS) use an amino acid editing domain called CP1 to maintain fidelity (10–12). It is inserted into the Rossmann fold, which comprises the aminoacylation active site, but folds into a discrete domain that is linked to the canonical core via two flexible β-strand linkers (Fig. 1) (27–29).
Fig. 1.
LeuRS CP1 deletion design. (A) The aminoacylation core and CP1 domain of the E. coli LeuRS (ecLeuRS) homology model (31) are orange and blue, respectively. (B) Sequence of ecLeuRS and yeast mitochondrial LeuRS (ymLeuRS) with marked site of CP1 deletion (blue) that was replaced by insertion of three- and two-alanine peptides (black), respectively.
Escherichia coli LeuRS has been suggested to edit exclusively by a posttransfer editing mechanism (18, 30). However, key mutations on the surface of the CP1 domain or canonical core in a posttransfer-editing-defective E. coli LeuRS activated a dormant pretransfer-editing-like mechanism to enhance fidelity (26). To distinguish the origins of this pretransfer-editing activity, we deleted the entire CP1 domain of E. coli and yeast mitochondrial LeuRSs. Surprisingly, the ancient canonical core of LeuRS maintained fidelity, even in the absence of its editing domain. We hypothesized that a pretransfer editing activity was responsible for fidelity and showed that amino acid fidelity depended on the tRNA.
Results
The CP1 domain of LeuRS is tethered to the canonical aminoacylation core via two flexible β-strands (18, 27) (Fig. 1A). We showed that an active CP1 domain with its extended β-strands could be expressed independent of the full-length LeuRS (10). Herein, we deleted the CP1 domain and replaced it and a portion of each N- and C-terminal β-strand with short linkers consisting of alanine residues to generate a mutant ΔCP1 LeuRS from E. coli (ecΔCP1) and yeast mitochondria (ymΔCP1). Based on homology models (31, 32), three alanine residues were inserted into E. coli LeuRS between Arg-226 and Tyr- 415 and two alanines linked Ser-242 and Arg-439 in ymΔCP1 (Fig. 1B).
The wild type and each of the ΔCP1 deletion mutants were stably expressed and isolated by affinity chromatography using an N-terminal six-histidine tag. Subsequent ion-exchange and gel-exclusion purification steps were carried out to ensure complete separation of the deletion mutant from endogenous wild-type LeuRS. The ΔCP1 deletion mutants retained significant aminoacylation activity, albeit reduced compared with the respective wild-type enzymes from E. coli and yeast mitochondria (Fig. 2). In both cases, the Km for tRNA aminoacylation was increased at least 20-fold (Table 1), supporting the conclusion that the CP1 domain stabilizes tRNA interactions with the enzyme. This is consistent with recent suggestions that the tRNA 3′ end binds initially near the CP1 domain and sweeps through the hydrolytic editing active site en route to the synthetic aminoacylation site (33). Thus, it is likely that deletion of the CP1 domain destabilized efficient tRNALeu binding.
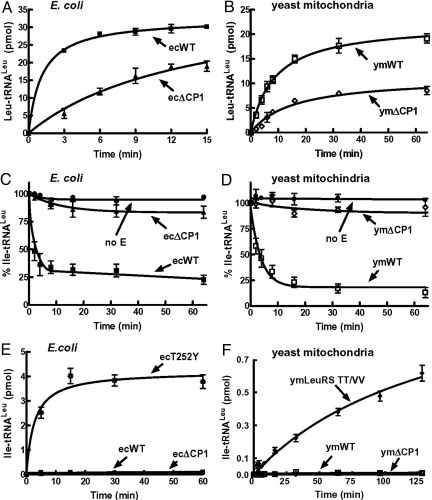
Fig. 2.
Aminoacylation and deacylation activity. Leucylations by E. coli LeuRS and ecΔCP1 (A) or ymLeuRS and ymΔCP1 (B) were carried out by using 4 μM tRNALeu and 50 nM enzyme. Deacylation reactions for E. coli LeuRS (C) and ymLeuRS (D) wild type and ΔCP1 deletion mutants contained ≈6.5 μM [3H]Ile-tRNALeu and 100 nM enzyme. Isoleucine mischarging by E. coli LeuRS (E) and ymLeuRS (F) wild type and ΔCP1 deletion mutants incorporated 21 μM [3H]isoleucine (166 Ci/mmol) and 1 μM enzyme. Editing-defective E. coli T252Y LeuRS (34) and TT/VV ymLeuRS (5) were used as positive controls. ■, E. coli LeuRS wild type (ecWT); (▲, E. coli LeuRS ΔCP1 (ecΔCP1); □, ymLeuRS wild type (ymWT); ◇, ymLeuRS ΔCP1 (ymΔCP1);  , E. coli T252Y LeuRS (ecT252Y); ♦, ymLeuRS TT/VV; ●, no enzyme control (no E). Error bars represent triplicated reactions.
, E. coli T252Y LeuRS (ecT252Y); ♦, ymLeuRS TT/VV; ●, no enzyme control (no E). Error bars represent triplicated reactions.
Table 1.
Kinetic parameters for wild type and ΔCP1 LeuRS mutants
| Enzyme | Aminoacylation |
Amino acid activation |
||||
|---|---|---|---|---|---|---|
|
Km, μM |
kcat, s−1 |
Km, mM |
kcat, s−1 |
|||
| Leu | Leu | Leu | Ile | Leu | Ile | |
| ecLeuRS WT | 0.7 ± 0.1 | 9.6 ± 2.7 | 0.018 ± 0.008 | 1.0 ± 0.3 | 71 ± 25 | 4.7 ± 1.3 |
| ecLeuRSΔCP1 | 23.1 ± 3.6* | 5.2 ± 1.3* | 0.021 ± 0.006 | 3.4 ± 0.9 | 0.23 ± 0.07 | 2.2 ± 1.3 |
| ymLeuRS WT | 0.7 ± 0.12 | 5.06 ± 0.25 | 0.17 ± 0.08† | 10.52 ± 0.92† | 3.3 ± 0.6† | 0.95 ± 0.2† |
| ymLeuRSΔCP1 | 19.9 ± 1.2* | 1.05 ± 0.75* | 38 ± 1.25* | ‡ | 0.32 ± 0.02* | ‡ |
We also tested deacylation activity of both ΔCP1 mutants (Fig. 2 C and D). As would be expected in the absence of the CP1 editing domain, the ΔCP1 LeuRS deletion mutants from E. coli and yeast mitochondria did not hydrolyze mischarged Ile-tRNALeu. Surprisingly, however, both the ecΔCP1 and ymΔCP1 LeuRS deletion mutants failed to produce tRNALeu that was misaminoacylated with isoleucine, even at high enzyme concentrations of 1 μM (Fig. 2 E and F). Controls using editing-defective E. coli (34) and yeast mitochondrial (5) LeuRS mutants yielded significant levels of mischarged Ile-tRNALeu. The mischarging activity for each of the ΔCP1 deletion mutants was comparable to the wild-type enzyme, which maintains its editing function.
It is possible that isoleucine is not misactivated by either of the ΔCP1 mutant LeuRSs. We used pyrophosphate exchange assays to determine whether the ΔCP1 LeuRS deletion mutants activate isoleucine and other non-leucine amino acids in the first step (Eq. 1) of the aminoacylation reaction. The ecΔCP1 LeuRS mutant misactivated isoleucine, methionine, and valine at levels similar to those of the wild-type enzyme (Fig. 3). However, pyrophosphate exchange activity for leucine and the noncognate norvaline were significantly lower for ecΔCP1 compared with the wild-type LeuRS. The decrease in leucine activation for the E. coli LeuRS deletion mutant was largely due to a kcat that differed by 300-fold (Table 1). Only small changes of 2- to 3-fold for the Km and kcat of isoleucine activation were measured for the wild-type E. coli LeuRS and ecΔCP1.
Fig. 3.
E. coli LeuRS pyrophosphate exchange. (A) Reaction mixtures contained 1 mM amino acid and 200 nM enzyme. (B) Magnified noncognate amino acid-dependent activity from A. E. coli LeuRS wild type (ecWT) with leucine (■), isoleucine (△), valine (▽), methionine (×), or norvaline (●); E. coli LeuRS ΔCP1 (ecΔCP1) with leucine (▲), isoleucine (+), valine (▽), methionine (◇), or norvaline (○). Error bars represent triplicated reactions.
Amino acid activation by the ymΔCP1 LeuRS deletion mutant was greatly decreased in the presence of leucine or isoleucine to levels that were below isoleucine-dependent pyrophosphate exchange for the wild-type yeast mitochondrial LeuRS (Table 1). Because isoleucine was misactivated by ecΔCP1, but not stably linked to tRNALeu, we hypothesized that, at least in the case of E. coli LeuRS, a latent pretransfer editing mechanism is activated upon deletion of the CP1 editing domain. It is also possible that ymLeuRS editing occurs by a pretransfer mechanism that is tRNA-independent. In this scenario, pyrophosphate exchange activity would appear negligible because isoleucyl adenylate could be preferentially cleared, rather than consumed in the back reaction.
In these particular cases where the CP1 domain has been deleted, pretransfer editing would originate from the ancient canonical core of the LeuRS. It has been proposed that hydrolysis of the adenylate intermediate can occur within the aminoacylation active site (19, 21). Alternatively, isoleucyl adenylate may be selectively ejected by the aminoacylation active site to clear its mistakes (20). For this latter example, it would be difficult to distinguish for the ecΔCP1 mutant if ejection targets translocation of the labile adenylate to the missing CP1 domain for editing (18, 22) or if it is simply, but specifically, released into the cellular milieu, where it would be hydrolytically degraded (20).
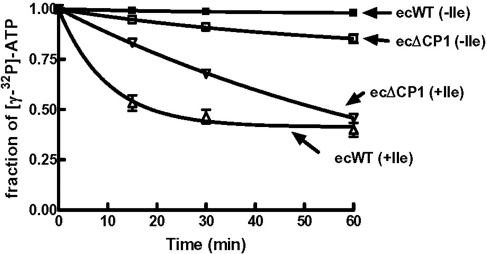
We measured amino acid-dependent ATPase activity of E. coli LeuRS and its CP1 deletion mutant to further investigate whether isoleucine was misactivated and then cleared. The E. coli LeuRS ΔCP1 mutant exhibited significant isoleucine-dependent ATPase activity (Fig. 4). Although the activity was slower compared with the wild-type enzyme, it was robust and consistent with the isoleucine-dependent pyrophosphate exchange analysis. Because the ecΔCP1 mutant does not charge tRNA with isoleucine and its posttransfer editing active site in the CP1 domain has been deleted, we hypothesized that a pretransfer editing mechanism in the E. coli LeuRS enzyme had been activated. Thus, its fidelity mechanism has shifted from a pathway that relies on posttransfer editing to one that depends on a pretransfer editing activity.
Fig. 4.
Isoleucine-dependent ATPase activity of E. coli LeuRS wild type and ΔCP1 deletion mutant. ■, E. coli LeuRS wild type (ecWT) without isoleucine; □, E. coli LeuRS ΔCP1 (ecΔCP1) without isoleucine; △, ecWT with isoleucine; ▽, ecΔCP1 with isoleucine. Error bars represent triplicated reactions.
Pretransfer editing has been proposed to be tRNA-dependent for IleRS and LeuRS (30, 35–38). We used unchargeable tRNA analogs in which the 2′-hydroxyl group of the terminal adenosine was replaced by a hydrogen (2′dA tRNALeu). As would be expected for this class I enzyme, the 2′dA tRNALeu was not aminoacylated by LeuRS or the ecΔCP1 mutant (data not shown). However, 2′dA tRNALeu stimulated isoleucine-dependent ATPase activity to a level that was comparable to the wild-type tRNA that lacked modifications at the 2′ end (Fig. 5). Because the 2′dA tRNALeu could not be charged, this supports the conclusion that isoleucine is misactivated to form an isoleucyl adenylate intermediate and then hydrolyzed. We hypothesize that in the absence of a viable mechanism for posttransfer editing, to ensure fidelity E. coli LeuRS activates an inherent pretransfer editing mechanism that is tRNA-dependent.
Fig. 5.
Amino acid and tRNA-dependent ATPase activity of E. coli wild-type LeuRS and CP1 deletion mutant. Reaction mixtures contained 10 μM tRNALeu or a tRNA analog with 2′-deoxyadenosine substituted for the A76 base (2′dA) and 1 μM enzyme. Shown is ATP consumption during ATPase reaction by E. coli wild type (A) and CP1 deletion mutant (B). □, plus isoleucine and tRNA; △, plus isoleucine and 2′dA tRNA; ◇, plus 2′dA tRNA only; ○, plus isoleucine only. Error bars represent triplicated reactions for all time points.
Discussion
The CP1 domain has been proposed to be an ancient addition to the aminoacylation core of LeuRS, ValRS, and IleRS (39–42). As protein synthesis gained sophistication and the thresholds for fidelity became more stringent, aaRSs such as LeuRS, ValRS, and IleRS, which were more prone to errors in amino acid selection, adapted so that they could clear their mistakes. These enzymes differ in discrimination requirements for noncognate amino acids that challenge them. For example, IleRS must distinguish isoleucine from valine, which differs only by a missing methyl group and results in a discrimination factor of ≈4 (28, 43). By contrast, LeuRS has a larger discrimination factor (Table 1) (44) for other standard amino acids, because it relies in part on differences in substrate shape because of diversity in the location of the branched methyl group of these aliphatic amino acids. However, LeuRS must also accommodate challenges from a range of amino acids, including nonstandard amino acids such as norvaline (6). Significantly, at least 10 of the aaRSs, representing both of the enzyme family's distinct classes, acquired editing domains to clear mistakes that are generated by a range of insufficient discrimination factors that compromise their fidelity in the synthetic site (45).
The CP1 domain can function autonomously (10–12), albeit its tRNA deacylation activity is less efficient in the absence of the rest of the aaRS. This is likely due to additional RNA binding regions from the canonical core that stabilize tRNA binding in the editing complex (10, 18, 27, 29, 46, 47). Binding of the tRNA that spans the CP1 domain and other parts of the LeuRS enzyme also promotes greater specificity in the editing active site (10). Here, we demonstrate not only that LeuRS has become dependent on the CP1 domain for fidelity but also that the CP1 domain influences activities that originate in the canonical aminoacylation core (see below).
Pretransfer editing has been proposed to be dependent on the tRNA for some aaRSs (9, 11, 23, 30, 35–38, 48–53). In the case of E. coli LeuRS, the presence of a tRNALeu analog stimulated pretransfer editing to facilitate clearance of the adenylate intermediate. The molecular determinants of tRNALeu that triggered pretransfer editing remain undefined because the tRNALeu analog is missing only the ribose hydroxyl that is covalently linked to the amino acid during aminoacylation.
An inherent tRNA-dependent pretransfer editing activity in the canonical core supports the idea that a primitive LeuRS, before addition of the CP1 domain, likely contained a rudimentary fidelity mechanism that coevolved to be dependent on its RNA cofactor. This early pretransfer editing mechanism was not as efficient or accurate as the modern LeuRS, but it was sufficient to provide a level of fidelity as well as enough charged tRNALeu for protein synthesis such that an ancient organism could survive. Its imperfections may have also been an important factor in the evolution of the cell and the aaRS (54, 55).
The addition of the CP1 domain to LeuRS not only added a second, more efficient, mechanism for fidelity but also appears to play a significant role in regulating the aminoacylation reaction and pretransfer editing of the aminoacyl adenylate intermediate. We hypothesize this effector role is due in part to interactions between the CP1 domain and tRNA that control access of the tRNA to the aminoacylation active site. It is likely that a similar paradigm exists for the homologous IleRS and ValRS as well as LeuRSs from other organisms. However, we also propose that the CP1-dependent balance between pretransfer and posttransfer editing could shift between these redundant fidelity mechanisms.
A long-term shift would likely be due to evolutionary pressures that induce more permanent changes in aaRS structure–function relationships. This might include historical changes that occurred during the expansion of the genetic code or more modern adaptations of an aaRS for alternative functions. As one example, the CP1 editing domain of ymLeuRS is also an RNA splicing domain (32). Although it maintains a robust posttransfer editing activity, intracellular fidelity mechanisms of the ymLeuRS may be independent of the CP1 domain-based hydrolytic site (5).
Short-term shifts between editing mechanisms may occur readily in some aaRSs, such as IleRS, where both pretransfer and posttransfer editing activities have been reported to occur within the same enzyme (23, 56–58). These rapid and reversible shifts might be dependent on intracellular substrate levels of tRNA and amino acid. It is also possible that pretransfer editing preferentially targets one noncognate amino acid, whereas posttransfer editing is more efficient with another structurally related noncognate amino acid. This would be akin to the “triple” sieve models that have been discovered for ProRS (2, 3, 59) and AlaRS (4, 59).
The predominance of a pretransfer or posttransfer editing mechanism within a single aaRS could be dependent on a number of factors, including the site of the CP1 domain insertion, which differs among the LeuRS, IleRS, and ValRS subclass (60). Significantly, this ability to shift would provide an evolutionary advantage to accommodate other molecular and functional adaptations of the aaRS to its cellular environment.
Materials and Methods
E. coli and yeast mitochondrial tRNALeu were prepared by in vitro transcription (61) and (mis)charged as described (5, 62). A 2′-deoxyadenosine analog was introduced at the 3′ end of E. coli crude tRNA according to a previously reported method using CCA-adding enzyme (63). Aminoacylation assays were carried out to confirm that negligible “unmodified” tRNA was produced during the enzymatic synthesis of the 3′-CCdA end. A Chroma spin 30 column (Clontech) that had been washed with water was used for purification of tRNA derivatives.
Plasmids encoding the ecΔCP1 (p15MTVecΔCP1) and ymΔCP1 (p32MBymΔCP1) deletion mutants were generated by PCR mutagenesis (5). Protein was expressed and isolated by using Affinity Gel His-Select Nickel resin (Sigma). Deletion mutants were then purified by Mono Q 5/50 (GE Healthcare) ion exchange and eluted with a NaCl gradient. In some cases, a third purification step used a HiLoad 16/60 size-exclusion Superdex 200 purification column (GE Healthcare).
Charging and deacylation reactions were carried out as previously described (5). For ymLeuRS, 150 mM KCl was added to the reaction (46). Kinetic parameters were measured by using 0.2–20 μM tRNALeu and 10 nM freshly purified enzyme.
ATPase assays containing 50 mM Tris·HCl (pH 7.5), 10 mM MgCl2, 5 mM DTT, 10 μM tRNALeu, 18 μM radiolabeled ATP, and 1 μM protein were initiated with 2.5 mM amino acid. Inorganic pyrophosphate (PPi) exchange assays contained 50 mM Hepes (pH 8.0), 10 mM MgCl2, 1 mM ATP, 1 mM [32P]PPi (2.5 μCi/ml), 1 mM DTT, and 1 mM amino acid. Kinetic parameters were measured by using 1–400 μM leucine or 0.5–35 mM isoleucine. Reaction aliquots of 2 μL were quenched by spotting on PEI-cellulose TLC plates (prerun in water). The plates were developed in 750 mM KH2PO4 (pH 3.5) containing 4 M urea. Radiolabeled bands were quantitated by phosphorimaging.
Acknowledgments.
We are grateful to Prof. M. Sprinzl for providing modified tRNA samples and Prof. M. Mörl for valuable advice in the preparation of tRNA derivatives. This work was supported by National Institutes of Health Grants GM63789 and GM63107.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 19031.
References
- 1.Hendrickson TL, de Crécy-Lagard V, Schimmel P. Incorporation of nonnatural amino acids into proteins. Annu Rev Biochem. 2004;73:147–176. doi: 10.1146/annurev.biochem.73.012803.092429. [DOI] [PubMed] [Google Scholar]
- 2.An S, Musier-Forsyth K. Trans-editing of Cys-tRNAPro by Haemophilus influenzae YbaK protein. J Biol Chem. 2004;279:42359–42362. doi: 10.1074/jbc.C400304200. [DOI] [PubMed] [Google Scholar]
- 3.Ruan B, Söll D. The bacterial YbaK protein is a Cys-tRNAPro and Cys-tRNACys deacylase. J Biol Chem. 2005;280:25887–25891. doi: 10.1074/jbc.M502174200. [DOI] [PubMed] [Google Scholar]
- 4.Chong YE, Yang XL, Schimmel P. Natural homolog of tRNA synthetase editing domain rescues conditional lethality caused by mistranslation. J Biol Chem. 2008;283:30073–30078. doi: 10.1074/jbc.M805943200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karkhanis VA, Boniecki MT, Poruri K, Martinis SA. A viable amino acid editing activity in the leucyl-tRNA synthetase CP1-splicing domain is not required in the yeast mitochondria. J Biol Chem. 2006;281:33217–33225. doi: 10.1074/jbc.M607406200. [DOI] [PubMed] [Google Scholar]
- 6.Karkhanis VA, Mascarenhas AP, Martinis SA. Amino acid toxicities of Escherichia coli that are prevented by leucyl-tRNA synthetase amino acid editing. J Bacteriol. 2007;189:8765–8768. doi: 10.1128/JB.01215-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nangle LA, Motta CM, Schimmel P. Global effects of mistranslation from an editing defect in mammalian cells. Chem Biol. 2006;13:1091–1100. doi: 10.1016/j.chembiol.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Lee JW, et al. Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature. 2006;443:50–55. doi: 10.1038/nature05096. [DOI] [PubMed] [Google Scholar]
- 9.Schimmel P, Schmidt E. Making connections: RNA-dependent amino acid recognition. Trends Biochem Sci. 1995;20:1–2. doi: 10.1016/s0968-0004(00)88937-9. [DOI] [PubMed] [Google Scholar]
- 10.Betha AK, Williams AM, Martinis SA. Isolated CP1 domain of Escherichia coli leucyl-tRNA synthetase is dependent on flanking hinge motifs for amino acid editing activity. Biochemistry. 2007;46:6258–6267. doi: 10.1021/bi061965j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin L, Hale SP, Schimmel P. Aminoacylation error correction. Nature. 1996;384:33–34. doi: 10.1038/384033b0. [DOI] [PubMed] [Google Scholar]
- 12.Zhao MW, et al. Leucyl-tRNA synthetase from the ancestral bacterium Aquifex aeolicus contains relics of synthetase evolution. EMBO J. 2005;24:1430–1439. doi: 10.1038/sj.emboj.7600618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong FC, Beuning PJ, Nagan M, Shiba K, Musier-Forsyth K. Functional role of the prokaryotic proline-tRNA synthetase insertion domain in amino acid editing. Biochemistry. 2002;41:7108–7115. doi: 10.1021/bi012178j. [DOI] [PubMed] [Google Scholar]
- 14.Roy H, Ibba M. Phenylalanyl-tRNA synthetase contains a dispensable RNA-binding domain that contributes to the editing of noncognate aminoacyl-tRNA. Biochemistry. 2006;45:9156–9162. doi: 10.1021/bi060549w. [DOI] [PubMed] [Google Scholar]
- 15.Dock-Bregeon A, et al. Transfer RNA-mediated editing in threonyl-tRNA synthetase. The class II solution to the double discrimination problem. Cell. 2000;103:877–884. doi: 10.1016/s0092-8674(00)00191-4. [DOI] [PubMed] [Google Scholar]
- 16.Dock-Bregeon AC, et al. Achieving error-free translation; the mechanism of proofreading of threonyl-tRNA synthetase at atomic resolution. Mol Cell. 2004;16:375–386. doi: 10.1016/j.molcel.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Bishop AC, Nomanbhoy TK, Schimmel P. Blocking site-to-site translocation of a misactivated amino acid by mutation of a class I tRNA synthetase. Proc Natl Acad Sci USA. 2002;99:585–590. doi: 10.1073/pnas.012611299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lincecum TL, Jr, et al. Structural and mechanistic basis of pre- and posttransfer editing by leucyl-tRNA synthetase. Mol Cell. 2003;11:951–963. doi: 10.1016/s1097-2765(03)00098-4. [DOI] [PubMed] [Google Scholar]
- 19.Gruic-Sovulj I, Uter N, Bullock T, Perona JJ. tRNA-dependent aminoacyl-adenylate hydrolysis by a nonediting class I aminoacyl-tRNA synthetase. J Biol Chem. 2005;280:23978–23986. doi: 10.1074/jbc.M414260200. [DOI] [PubMed] [Google Scholar]
- 20.Hati S, et al. Pre-transfer editing by class II prolyl-tRNA synthetase: Role of aminoacylation active site in “selective release” of noncognate amino acids. J Biol Chem. 2006;281:27862–27872. doi: 10.1074/jbc.M605856200. [DOI] [PubMed] [Google Scholar]
- 21.Splan KE, Ignatov ME, Musier-Forsyth K. Transfer RNA modulates the editing mechanism used by class II prolyl-tRNA synthetase. J Biol Chem. 2008;283:7128–7134. doi: 10.1074/jbc.M709902200. [DOI] [PubMed] [Google Scholar]
- 22.Fukunaga R, Fukai S, Ishitani R, Nureki O, Yokoyama S. Crystal structures of the CP1 domain from Thermus thermophilus isoleucyl-tRNA synthetase and its complex with l-valine. J Biol Chem. 2004;279:8396–8402. doi: 10.1074/jbc.M312830200. [DOI] [PubMed] [Google Scholar]
- 23.Bishop AC, Beebe K, Schimmel PR. Interstice mutations that block site-to-site translocation of a misactivated amino acid bound to a class I tRNA synthetase. Proc Natl Acad Sci USA. 2003;100:490–494. doi: 10.1073/pnas.0237335100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukunaga R, Yokoyama S. Structural basis for non-cognate amino acid discrimination by the valyl-tRNA synthetase editing domain. J Biol Chem. 2005;280:29937–29945. doi: 10.1074/jbc.M502668200. [DOI] [PubMed] [Google Scholar]
- 25.Hendrickson TL, Nomanbhoy TK, Schimmel P. Errors from selective disruption of the editing center in a tRNA synthetase. Biochemistry. 2000;39:8180–8186. doi: 10.1021/bi0004798. [DOI] [PubMed] [Google Scholar]
- 26.Williams AM, Martinis SA. Mutational unmasking of a tRNA-dependent pathway for preventing genetic code ambiguity. Proc Natl Acad Sci USA. 2006;103:3586–3591. doi: 10.1073/pnas.0507362103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tukalo M, Yaremchuk A, Fukunaga R, Yokoyama S, Cusack S. The crystal structure of leucyl-tRNA synthetase complexed with tRNALeu in the post-transfer-editing conformation. Nat Struct Mol Biol. 2005;12:923–930. doi: 10.1038/nsmb986. [DOI] [PubMed] [Google Scholar]
- 28.Nureki O, et al. Enzyme structure with two catalytic sites for double-sieve selection of substrate. Science. 1998;280:578–582. doi: 10.1126/science.280.5363.578. [DOI] [PubMed] [Google Scholar]
- 29.Fukai S, et al. Structural basis for double-sieve discrimination of l-valine from l-isoleucine and l-threonine by the complex of tRNA(Val) and valyl-tRNA synthetase. Cell. 2000;103:793–803. doi: 10.1016/s0092-8674(00)00182-3. [DOI] [PubMed] [Google Scholar]
- 30.Englisch S, Englisch U, von der Haar F, Cramer F. The proofreading of hydroxy analogues of leucine and isoleucine by leucyl-tRNA synthetases from E. coli and yeast. Nucleic Acids Res. 1986;14:7529–7539. doi: 10.1093/nar/14.19.7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee KW, Briggs JM. Molecular modeling study of the editing active site of Escherichia coli leucyl-tRNA synthetase: Two amino acid binding sites in the editing domain. Proteins. 2004;54:693–704. doi: 10.1002/prot.10300. [DOI] [PubMed] [Google Scholar]
- 32.Rho SB, Lincecum TL, Jr, Martinis SA. An inserted region of leucyl-tRNA synthetase plays a critical role in group I intron splicing. EMBO J. 2002;21:6874–6881. doi: 10.1093/emboj/cdf671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rock FL, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759–1761. doi: 10.1126/science.1142189. [DOI] [PubMed] [Google Scholar]
- 34.Mursinna RS, Martinis SA. Rational design to block amino acid editing of a tRNA synthetase. J Am Chem Soc. 2002;124:7286–7287. doi: 10.1021/ja025879s. [DOI] [PubMed] [Google Scholar]
- 35.Ibba M, Hong KW, Sherman JM, Sever S, Söll D. Interactions between tRNA identity nucleotides and their recognition sites in glutaminyl-tRNA synthetase determine the cognate amino acid affinity of the enzyme. Proc Natl Acad Sci USA. 1996;93:6953–6958. doi: 10.1073/pnas.93.14.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hale SP, Auld DS, Schmidt E, Schimmel P. Discrete determinants in transfer RNA for editing and aminoacylation. Science. 1997;276:1250–1252. doi: 10.1126/science.276.5316.1250. [DOI] [PubMed] [Google Scholar]
- 37.Hale SP, Schimmel P. Protein synthesis editing by a DNA aptamer. Proc Natl Acad Sci USA. 1996;93:2755–2758. doi: 10.1073/pnas.93.7.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baldwin AN, Berg P. Transfer ribonucleic acid-induced hydrolysis of valyladenylate bound to isoleucyl ribonucleic acid synthetase. J Biol Chem. 1966;241:839–845. [PubMed] [Google Scholar]
- 39.O'Donoghue P, Luthey-Schulten Z. On the evolution of structure in aminoacyl-tRNA synthetases. Microbiol Mol Biol Rev. 2003;67:550–573. doi: 10.1128/MMBR.67.4.550-573.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schimmel P, Ribas De Pouplana L. Footprints of aminoacyl-tRNA synthetases are everywhere. Trends Biochem Sci. 2000;25:207–209. doi: 10.1016/s0968-0004(00)01553-x. [DOI] [PubMed] [Google Scholar]
- 41.Ribas de Pouplana L, Schimmel P. Aminoacyl-tRNA synthetases: Potential markers of genetic code development. Trends Biochem Sci. 2001;26:591–596. doi: 10.1016/s0968-0004(01)01932-6. [DOI] [PubMed] [Google Scholar]
- 42.Ribas de Pouplana L, Schimmel P. A view into the origin of life: Aminoacyl-tRNA synthetases. Cell Mol Life Sci. 2000;57:865–870. doi: 10.1007/PL00000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pauling L. Festschrift für Prof. Dr. Arthur Stöll. Basel, Switzerland: Birkhauser; 1958. The probability of errors in the process of synthesis of protein molecules; pp. 597–602. [Google Scholar]
- 44.Lue SW, Kelley SO. An aminoacyl-tRNA synthetase with a defunct editing site. Biochemistry. 2005;44:3010–3016. doi: 10.1021/bi047901v. [DOI] [PubMed] [Google Scholar]
- 45.Mascarenhas AP, Martinis SA, An S, Rosen AE, Musier-Forsyth K. Fidelity mechanisms of the aminoacyl-tRNA synthetases. In: Rajbhandary UL, Koehrer C, editors. Protein Engineering. New York: Springer; 2009. pp. 153–200. [Google Scholar]
- 46.Hsu JL, Rho SB, Vannella KM, Martinis SA. Functional divergence of a unique C-terminal domain of leucyl-tRNA synthetase to accommodate its splicing and aminoacylation roles. J Biol Chem. 2006;281:23075–23082. doi: 10.1074/jbc.M601606200. [DOI] [PubMed] [Google Scholar]
- 47.Silvian LF, Wang J, Steitz TA. Insights into editing from an Ile-tRNA synthetase structure with tRNAIle and mupirocin. Science. 1999;285:1074–1077. [PubMed] [Google Scholar]
- 48.Schmidt E, Schimmel P. Residues in a class I tRNA synthetase which determine selectivity of amino acid recognition in the context of tRNA. Biochemistry. 1995;34:11204–11210. doi: 10.1021/bi00035a028. [DOI] [PubMed] [Google Scholar]
- 49.Tardif KD, Horowitz J. Transfer RNA determinants for translational editing by Escherichia coli valyl-tRNA synthetase. Nucleic Acids Res. 2002;30:2538–2545. doi: 10.1093/nar/30.11.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tardif KD, Liu M, Vitseva O, Hou YM, Horowitz J. Misacylation and editing by Escherichia coli valyl-tRNA synthetase: Evidence for two tRNA binding sites. Biochemistry. 2001;40:8118–8125. doi: 10.1021/bi0103213. [DOI] [PubMed] [Google Scholar]
- 51.Larkin DC, Williams AM, Martinis SA, Fox GE. Identification of essential domains for Escherichia coli tRNALeu aminoacylation and amino acid editing using minimalist RNA molecules. Nucleic Acids Res. 2002;30:2103–2113. doi: 10.1093/nar/30.10.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nomanbhoy TK, Hendrickson TL, Schimmel P. Transfer RNA-dependent translocation of misactivated amino acids to prevent errors in protein synthesis. Mol Cell. 1999;4:519–528. doi: 10.1016/s1097-2765(00)80203-8. [DOI] [PubMed] [Google Scholar]
- 53.Nordin BE, Schimmel P. Transiently misacylated tRNA is a primer for editing of misactivated adenylates by class I aminoacyl-tRNA synthetases. Biochemistry. 2003;42:12989–12997. doi: 10.1021/bi035052q. [DOI] [PubMed] [Google Scholar]
- 54.Bacher JM, Waas WF, Metzgar D, de Crécy-Lagard V, Schimmel P. Genetic code ambiguity confers a selective advantage on Acinetobacter baylyi. J Bacteriol. 2007;189:6494–6496. doi: 10.1128/JB.00622-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bacher JM, Schimmel P. An editing-defective aminoacyl-tRNA synthetase is mutagenic in aging bacteria via the SOS response. Proc Natl Acad Sci USA. 2007;104:1907–1912. doi: 10.1073/pnas.0610835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eldred EW, Schimmel PR. Rapid deacylation by isoleucyl transfer ribonucleic acid synthetase of isoleucine-specific transfer ribonucleic acid aminoacylated with valine. J Biol Chem. 1972;247:2961–2964. [PubMed] [Google Scholar]
- 57.Fersht AR. Editing mechanisms in protein synthesis. Rejection of valine by the isoleucyl-tRNA synthetase. Biochemistry. 1977;16:1025–1030. doi: 10.1021/bi00624a034. [DOI] [PubMed] [Google Scholar]
- 58.Norris AT, Berg P. Mechanism of aminoacyl RNA synthesis: Studies with isolated aminoacyl adenylate complexes of isoleucyl RNA synthetase. Proc Natl Acad Sci USA. 1964;52:330–337. doi: 10.1073/pnas.52.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahel I, Korencic D, Ibba M, Söll D. Trans-editing of mischarged tRNAs. Proc Natl Acad Sci USA. 2003;100:15422–15427. doi: 10.1073/pnas.2136934100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cusack S, Yaremchuk A, Tukalo M. The 2 Å crystal structure of leucyl-tRNA synthetase and its complex with a leucyl-adenylate analogue. EMBO J. 2000;19:2351–2361. doi: 10.1093/emboj/19.10.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sampson JR, Uhlenbeck OC. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc Natl Acad Sci USA. 1988;85:1033–1037. doi: 10.1073/pnas.85.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhai Y, Martinis SA. Two conserved threonines collaborate in the Escherichia coli leucyl-tRNA synthetase amino acid editing mechanism. Biochemistry. 2005;44:15437–15443. doi: 10.1021/bi0514461. [DOI] [PubMed] [Google Scholar]
- 63.Sprinzl M, Cramer F. Site of aminoacylation of tRNAs from Escherichia coli with respect to the 2′- or 3′-hydroxyl group of the terminal adenosine. Proc Natl Acad Sci USA. 1975;72:3049–3053. doi: 10.1073/pnas.72.8.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]