Abstract
Allen's Rule documents a century-old biological observation that strong positive correlations exist among latitude, ambient temperature, and limb length in mammals. Although genetic selection for thermoregulatory adaptation is frequently presumed to be the primary basis of this phenomenon, important but frequently overlooked research has shown that appendage outgrowth is also markedly influenced by environmental temperature. Alteration of limb blood flow via vasoconstriction/vasodilation is the current default hypothesis for this growth plasticity, but here we show that tissue perfusion does not fully account for differences in extremity elongation in mice. We show that peripheral tissue temperature closely reflects housing temperature in vivo, and we demonstrate that chondrocyte proliferation and extracellular matrix volume strongly correlate with tissue temperature in metatarsals cultured without vasculature in vitro. Taken together, these data suggest that vasomotor changes likely modulate extremity growth indirectly, via their effects on appendage temperature, rather than vascular nutrient delivery. When combined with classic evolutionary theory, especially genetic assimilation, these results provide a potentially comprehensive explanation of Allen's Rule, and may substantially impact our understanding of phenotypic variation in living and extinct mammals, including humans.
Keywords: Allen's Rule, bone growth, bone tissue culture, cartilage biology, thermoregulation
Ecogeographical rules relating climate to extremity length and body mass have long been central tenets of biology, and are among the best supported observations in natural-dwelling species (1). Allen's Rule codifies the observation that appendages (ears, limbs, and tails) of animals living in cold geographical regions are consistently shorter than those of closely related counterparts occupying warmer climes (2). Shortened extremities minimize heat loss by reducing surface area relative to volume and have long been viewed as genetically determined thermoregulatory adaptations (1). However, the heritability of extremity length is largely unknown, because similar phenotypes can be reproduced in laboratory mammals by modifying their ambient rearing temperature (3–6) (Fig. 1). The mechanism by which environmental temperature modulates extremity growth has remained elusive (7, 8). Understanding growth plasticity is critical to basic evolutionary analyses, because many characters that have long been hypothesized to be adaptations may instead be partial or even entirely effects of ambient temperature (9, 10). Moreover, knowledge of this phenotypic plasticity will be a key factor in ecological conservation strategies for anticipated changes in global climate that may have direct impacts on human economics and sustainability (11).
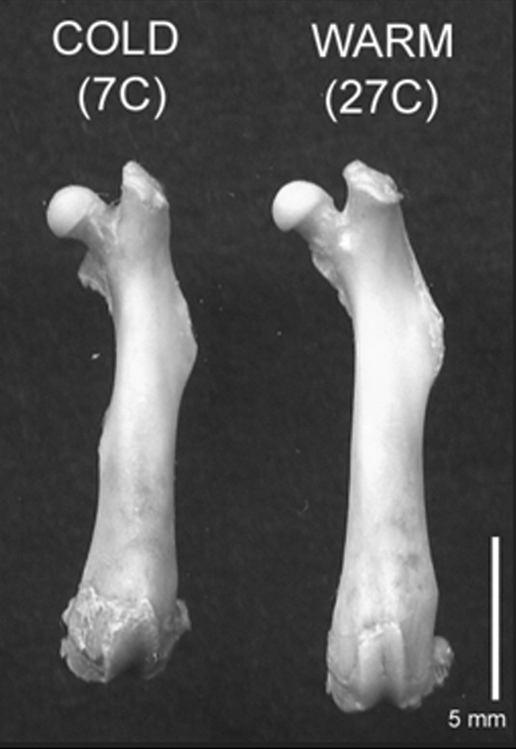
Fig. 1.
Temperature effects on femur length. Representative femora from mice housed at cold (7 °C) and warm (27 °C) temperatures from weaning age to adulthood showing the effect of ambient temperature on extremity size. The underlying cause of such effects is not immediately obvious because homeotherms maintain tightly regulated internal body temperatures independent of their external environment. For discussion see text.
The traditional explanation for temperature-growth effects in skeletal extremities is an altered supply of essential nutrients and growth factors via increased or decreased blood flow that results from changes in vasomotor tone (i.e., temperature-induced vasoconstriction/vasodilation) (5, 12). However, no study to date has tested this central hypothesis directly. Impairment of bone growth by disruption of blood flow is well-known (13), as are correlations of enhanced blood flow with bone elongation (14) and ear enlargement (15). It is equally well established that cold-induced vasoconstriction shunts blood away from theperiphery as a heat-conserving mechanism (1, 9), just as warm temperature enhances heat dissipation via vasodilation (1). Prior work has demonstrated that abrupt (acute) heat or cold exposure indeed affects bone perfusion (16), but despite frequent citation in explanatory models of climate-phenotype interactions (5, 12), no study has yet addressed the effect of long-term temperature exposure on skeletal blood supply and its potential relationship to bone elongation. Here we address the vascularity hypothesis that bone perfusion and elongation are directly related to ambient temperature.
Temperature and Extremity Growth.
We housed outbred mice continuously at cold (7 °C), control-intermediate (21 °C), or warm (27 °C) ambient temperatures from weaning through maturity (3.5–12 weeks age). Two intermediate endpoints were evaluated (4.5 and 6.5 weeks) in separate trials to assess temperature effects during ontogeny (see supporting information (SI) Table S1). Consistent with prior research (3, 4, 6–8), we confirmed that the ears, limbs, and tails of warm-reared mice were significantly longer than those of siblings raised in the cold (Figs. 1 and 2A, C, and D and Table S2), but with no change in total body mass (Fig. 2B). Differences in core organ size appeared to account for the latter—hearts and kidneys were enlarged in cold-reared mice (Table S2)—but differences in limb length were not explicable by diet and/or activity level, because cold-reared mice consumed substantially more food and were more active than their warm and control counterparts (Figs. S1 and S2), two factors reasonably presumed to be associable with increased rather than decreased limb length (17). There were, nevertheless, significant positive correlations among ambient and appendage temperatures, and associated growth of the limbs, tails (Fig. 2C) and ears (Fig. 2D). Although not subject to formal regression analysis, these data suggest that in vivo extremity temperature is a good predictor of extremity growth.
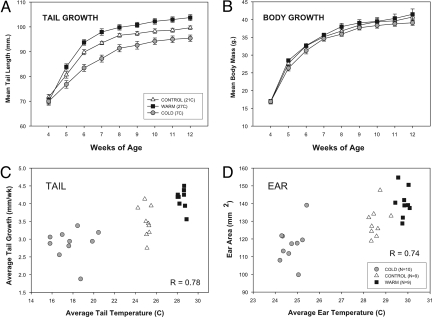
Fig. 2.
Extremity growth under different ambient rearing temperatures. Mouse tail length (A) and body mass (B) measured at weekly intervals beginning at weaning. Means ± 1 SEM shown (n = 7/group). Tail elongation, significantly reduced in the cold after only 1 week (P < 0.05, one-way ANOVA), follows a predictable temperature gradient, but body mass remains similar among all 3 groups (P > 0.10). Average peripheral temperature, (C) and (D), measured weekly by using a noncontact thermometer at the tail base and ear show strong positive correlations with growth (Pearson's r in figure). These results suggest direct temperature effects on developing cartilaginous tissues.
Blood Flow Analysis.
To determine whether a cold-induced reduction in skeletal blood supply contributed to extremity foreshortening, we measured relative bone blood flow (BBF) to the hindlimb bones and tail base of juvenile mice by using fluorescent microspheres, a reliable standard for quantifying regional organ and bone perfusion (18). We predicted that BBF would positively covary with rearing temperature and bone elongation as previously hypothesized. When compared with the two warmer-temperature groups, mice raised at the coldest temperature (7 °C) indeed had reduced femur, tibia, and hindpaw BBF at 4.5 weeks of age (after 1 week in the cold) (Fig. 3 A–C), and reduced tibia, paw, and tail BBF at 6.5 weeks (Fig. 3 B–D) (all confirmed by ANOVA and Tukey posthoc test at P < 0.05 as detailed in Fig. 3). Because BBF precipitously declines with age (19, 20), we neither expected nor found statistically significant temperature effects on bone perfusion in 12-week animals. We did observe an interesting decrease in paw blood flow in cold-reared adults consistent with the younger age points (Fig. 3C), but this was not statistically significant and so should be interpreted cautiously.
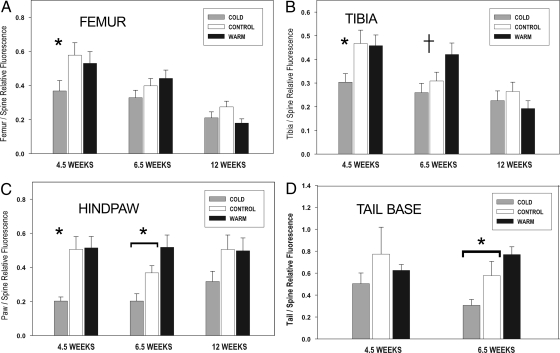
Fig. 3.
Relative hindlimb and tail blood flow measured by fluorescent microsphere deposition. Effects of chronic rearing temperature on blood flow to the femur (A), tibia (B), hindpaw (C), and tail base (D) by using the thoracic spine as an internal reference. Rearing temperature is shown by shaded bars within each age cluster. Means + 1 SEM plotted (n = 9 minimum per age and group; Table S1). For each age, a single asterisk indicates significant reduction in blood flow in the cold (P < 0.05, Tukey test) compared with either control or warm groups (e.g., 4.5-week femur, tibia, and paw). An asterisk overlying a horizontal bracket indicates that pairwise comparisons among all 3 groups were significant (e.g., 6.5-week paw and tail), and a crossbar (†) denotes significantly increased blood flow in the warm group compared with both cold and control (6.5-week tibia), all confirmed by Tukey test at α = 0.05. Tail samples were not collected at 12 weeks.
Our results do not fully support the vascularity hypothesis because the highest growth rates were observed in the warmest reared mice (i.e., Figs. 1 and 2A), but these animals did not also exhibit the correspondingly highest predicted BBF (Fig. 3 A–D, detailed in legend). The coldest-reared mice, however, did show the lowest growth rate and markedly reduced BBF. This, together with the fact that the two warmer-reared groups were more similar to one another in terms of housing temperature, growth rate, and BBF, raises the possibility of a threshold response to cold.
Blood not only provides developing limbs with a reservoir of essential growth factors, nutrients, and oxygen (13), it is also an important source of heat (1, 9). If this heat source is altered, temperature-sensitive processes will be affected. Cartilage growth, which largely determines bone growth rate via endochondral ossification, is one process that may be sensitive to ambient temperature. Therefore, the amount of blood arriving at an extremity might modulate its temperature and thereby its rate of bone elongation via this route (7). Indeed, many arctic mammals rely on countercurrent vascular heat exchange mechanisms to prevent excessive heat loss in their extremities, and by doing so reduce limb temperatures to near ambient conditions (1, 9).
Metatarsal Organ Cultures.
We examined whether temperature, as a controlled independent variable, could itself enhance or reduce bone elongation. We compared growth of neonatal mouse metatarsals (MTs) in culture maintained at cold (32 °C), control (37 °C) or warm (39 °C) temperatures, holding all other factors constant. This rodent MT model should be largely free of mechanical, dietary, vascular and systemic hormonal influences, save those impacted directly or indirectly by tissue temperature. Incubation temperature is already known to affect osteoblast activity (21), glycosaminoglycan synthesis (22), and collagen calcification (23) in vitro; however, these studies were conducted at the cellular level, whereas our experiments, that rely on a heterogeneous tissue complex (entire MTs), provide a model more similar to an intact organism.
In vitro temperature had an extraordinary impact on MT elongation (Fig. 4A). MTs from all three groups showed increases in length from baseline at two- and four-day time intervals, but total accrued growth was strikingly temperature dependent (Fig. 4B). Interestingly, although the control and warm groups differed only slightly in temperature (2 °C), this small increase was still sufficient to produce significantly greater growth in warm MTs compared with both other groups (Fig. 4B, pairwise comparisons significant at P ≤ 0.01 by using a Tukey test at each time point). All increases in MT size (length and width) occurred at the cartilaginous ends of the bone; as expected, the mineralized diaphyses did not differ among groups (Fig. 4A), nor did they elongate under these avascular culture conditions (data not shown). Histological analyses (Fig. 4 C–E) demonstrated higher rates of mitosis measured by BrdU incorporation (Fig. 4D) and increased extracellular matrix volume (Fig. 4E) at warmer temperatures, suggesting that chondrocyte proliferation and matrix (synthesis, secretion, and/or stability) respond directly to temperature and likely underlie the observed growth differences. In all cases, there were more BrdU-positive cells in the epiphyseal region of the MTs when compared with the columnar region of the growth plate (Fig. 4 C and D), as is expected because much early proliferation in vivo occurs in the rounded epiphyseal chondrocytes before the establishment of a mature growth plate (24, 25). Our experiments did not extend beyond four days in culture, and so it is possible that a differential temperature effect on the cell populations might occur later in growth.
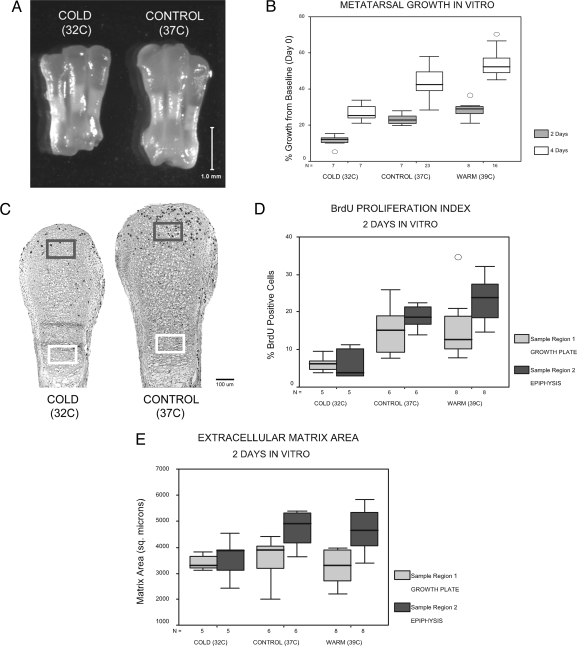
Fig. 4.
Effects of incubation temperature on metatarsal growth in vitro. Representative comparison of metatarsals from the same individual (left–right antimeres) grown in culture at cold and control temperatures for 4 days (A). Percentage growth from baseline plotted at 2- and 4-day time points (B) shows that accrued growth is directly proportionate to temperature (P ≤ 0.01 as noted in text). In histological analyses of left–right antimeres from 2-day cultures kept at cold or control temperatures (C), 150-μm × 100-μm sample regions in the growth plate columnar zone (light gray) and distal epiphysis (dark gray), showed increased chondrocyte proliferation (C and D) (BrdU positive cells indicated by dark stained nuclei in C) and extracellular matrix area (E) at warmer temperatures, closely matching the growth patterns revealed in (B). Within regions, BrdU staining was significantly increased in the warm group relative to cold in the growth plate (region 1) and in both control and warm groups in the distal epiphysis (region 2) (D). Matrix area (E) was also significantly increased in control and warm groups relative to cold in the distal epiphysis. Significance verified at α = 0.05 by using ANOVA and Tukey test. Boxes show interquartile range (middle 50%), whiskers denote upper 10th and 90th percentiles, circles indicate outliers, and horizontal line denotes the median. Sample size (n) listed in the horizontal axis of each graph.
A compelling comparison with our whole animal experiments reveals that these in vitro results closely match data from living mice in that peripheral ear and tail temperatures strongly correlated with extremity growth (Fig. 2 C and D). This provides evidence of similar direct and local tissue responses to ambient temperature in both live mice and MT cultures. However, care must be taken in interpreting our results with regard to the effects of temperature on cartilage (as a precursor to bone) versus bone itself. Our data do not negate possible temperature effects on vascular perfusion of the periosteum or other regions of actively growing bone, and such effects might well be synergistic to the ones that we have shown. Our experiments did not specifically address this issue, but previous work does suggest that it clearly merits further investigation (21, 23). Nevertheless, the marked growth response in non-osseous tissue (i.e., ears) confirms the important role of temperature on the chondrocyte.
Discussion
These data suggest that environmental temperature may modulate extremity growth by inducing physiological responses in peripheral tissue temperature, rather than by affecting vascular nutrient delivery, and that such temperature lability may then impact extremity size via its direct effect on cell proliferation and matrix production in cartilage (i.e., epiphyseal plates in long bones and tails, and cartilage mass in ears). Thus, the effects of vascular modification on bone growth are likely indirect. That is, vasoconstriction and vasodilation may modulate limb growth not by affecting arrival rate of nutrients and hormones, but by modulating temperature within developing cartilage. From an evolutionary perspective, Allen's “extremity size rule” may not actually reflect a functional genotypic adaptation in some or even many homeotherms (9, 10), but may instead be partially or wholly dependent on environmental temperature; that is, a secondary growth response to “facultative extremity heterothermy” in mammals that maintain constant core body temperatures.
Our results are particularly intriguing because cold-dwelling humans, especially Neandertals, possess disproportionately shortened long bones (12). Moreover the crural index (tibia/femur length ratio) exhibits a strikingly positive correlation with mean annual temperature (tibias shorten relative to the femur with decreasing temperature) (12). This relationship is especially relevant because the tibia is directly exposed to ambient temperature by virtue of its immediately subcutaneous anterior surface and relatively poor blood supply (19). This results in the shank having markedly cooler temperatures than more proximal bones of the limb.
Our experiments, however, may not fully resolve the complex genetic basis of observed clines in mammalian limb length. Clearly the degree of genotype-environment interaction remains an unaddressed issue, and future experiments will be required to assess the effects of heritability. Studies that incorporate genomic screening in models such as those presented here may be particularly informative. In fact, environmentally-induced physiological changes are not limited to bone or cartilage. Temperature has been shown to have direct effects on skin pigmentation in rabbits (26), cell proliferation in turtle brains (27), tumor metastases (28), slime mold differentiation (29), plant leaf growth (30), mammalian hair growth (7), and invertebrate cell size (31). Indeed, temperature effects have been suggested to impact mutation rates (32), and thus molecular clocks based on them.
In addition to reported temperature effects on the expression of heat-shock proteins (21) and cell-cycle control genes in cultured bone cells (33), there are several plausible mechanisms by which temperature may impact limb elongation via effects on growth plate chondrocytes. Temperature variation may induce physiochemical changes in extracellular matrix proteins and thereby lead to altered matrix-integrin interactions and/or altered diffusion rates of paracrine growth regulators. Recent work has shown that specific molecular signaling pathways regulate the chondrocyte response to loss of homeostasis, including endoplasmic reticulum stress (34), and these pathways modulate responses such as proliferation, apoptosis, and extracellular matrix synthesis (35). Altered temperature could directly or indirectly trigger these stress pathways, resulting in downstream effects on limb elongation. Concordantly, induction of endoplasmic reticulum stress in the growth plate has recently been linked to disrupted chondrocyte function and limb growth (36). Additional pathways regulating growth plate cell-cycle and metabolism, such as the Ihh–PTHrP feedback loop (37) or expression of growth hormone, IGF1, and/or its receptor (38) may also have temperature-sensitive elements. Elucidation of these mechanisms may be key to unveiling environment-genotype interactions.
Some years ago, Waddington imposed artificial selection in Drosophila by exposing multiple generations to near-fatal hot temperatures (39). This resulted in an eventual fixation of novel traits first elicited in response to heat but subsequently expressed in its absence, a phenomenon he defined as “genetic assimilation” (39). Clinal distributions of limb length may therefore represent a complex amalgam of genetic assimilation after generations of selection in combination with direct temperature responses in growing cartilage. Future investigations of similar temperature response pathways are likely to provide key insights into the evolutionary history of mammals, including humans.
Materials and Methods
In Vivo Temperature Experiments.
All procedures were carried out in accordance with IACUC guidelines at the Northeastern Ohio Universities College of Medicine. Male out-bred CF-1 mice (n = 95 partitioned into age and group, Table S1) were shipped from Charles River Laboratories at weaning. Based on described methods (6), 3.5-week-old mice were randomly assorted into 3 sized-matched groups and individually housed at 7 °C, 21 °C, or 27 °C in controlled environmental chambers (Serrat Heating and Cooling) under otherwise identical conditions: Plastic caging with pine bedding, 12-hour light/dark cycle, and ad lib access to food and water. Body mass and tail length were measured once weekly. At that time skin temperatures (ear and tail base) were also recorded by using a noncontact infrared thermometer (Kent Scientific).
Blood Flow Measurements by Using Fluorescent Microspheres.
At 4.5-, 6.5-, and 12- week age points, anesthetized mice received an intracardiac injection of fluorescent-labeled microspheres (15 μm, Molecular Probes) to measure regional blood flow as previously described (40). Femur, tibia, paw, and tail vertebrae (caudal 1–4) were immediately harvested and cleaned of soft tissue. Microspheres were recovered after published digestion/filtration methods (18, 41–43). In brief, individual bones were weighed, demineralized in Cal-Ex (Fisher Scientific), and digested in ethanolic KOH. The homogenate was filtered through polyamide mesh (Sefar Nitex 03–7/2), which was soaked in Cellosolve acetate (Sigma Aldrich) to dissolve the spheres and release the fluorescent dyes. Aliquots of the solvent containing the dissolved spheres were transferred in triplicate to a 96-microwell plate (Nunc) and fluorescence was measured by using a Typhoon 8610 Variable Mode Imager (Amersham Biosciences) and quantified by using ImageQuant software (Molecular Dynamics). For conventional size-correction (43), the fluorescence of each sample was standardized by its mass to create a relative density. The thoracic spine (T10–13), similarly sized among groups (P = 0.49, one-way ANOVA) and located within the body core, served as a standard reference for evaluating microsphere density in the hindlimb and tail.
Whole-Bone Metatarsal Organ Cultures.
The middle 3 metatarsal bones (MT2–4) were harvested from left and right hindpaws of neonatal C57BL/6J mice (housed at room temperature) on the day of birth after decapitation under deep halothane-induced anesthesia as detailed elsewhere (24). Each side was cultured separately at 32 °C, 37 °C, or 39 °C in DMEM growth medium (10% FBS, L-glutamine, fungizone, and gentamycin; Sigma) in humidified incubators with 5% CO2. At 2- or 4-day endpoints, culture medium was replaced with new containing 1:100 BrdU (Zymed) to identify proliferating cells. After a 12-hour labeling period, bones were removed from culture to assess their longitudinal growth (expressed as a percentage change from baseline averaged among MT2–4), fixed and decalcified in Cal-Ex II (Fisher), and processed for routine histology. Serial sections were stained with either Safranin-O for morphological evaluation (25) or BrdU by using a commercially available kit (BD Biosciences). Cells stained positively for BrdU were manually counted in 150-μm × 100-μm sample regions from the growth plate columnar zone and distal epiphysis of MT3 by using an Olympus BH-2 microscope interfaced to Bioquant Osteo-II image analysis software (BQIAC) (Fig. 4C). Matrix area was quantified in the same regions based on Safranin-O-defined thresholds in Bioquant.
Statistical Analysis.
Among-group differences were assessed by using one-way analysis of variance (ANOVA). No significant temperature-age interactions were present in the sample (confirmed by two-way factorial ANOVA), so age groups were analyzed separately to best discriminate temperature effects. Pairwise differences were revealed by using Tukey posthoc test. Product-moment correlation (Pearson) was applied to test for significant correlations between variables. Analyses were performed by using SPSS 11.0 software and α = 0.05 for all procedures.
Supplementary Material
Acknowledgments.
We thank the National Science Foundation, Kent State University Graduate Student Senate, and NEOUCOM Skeletal Biology Research Focus Area for generous financial support to M.A.S. Environmental chambers were designed and built by Marcos Serrat (Serrat Heating and Cooling, N. Royalton, OH). W. Horne, J. Jacobi, D. Rurak, R. Glenny, and the Fluorescent Microsphere Resource Center (Seattle, WA) provided valuable input on microsphere injection and recovery protocols, and A. Carey (Sefar America, Depew, NY) helped obtain essential filtration material. W. Horton and P. Reno suggested and facilitated the in vitro experiments. We thank J. Kriz, C. Vinyard, J. Stalvey, R. Meindl, C. Farnum, H. Thewissen, J. Hardwick, L. Yang, D. Dutton, M. Moser, J. Thomas, D. McBurney, B. Lowder, A. Nugent, B. Armfield, H. McEwen, and many others for discussions and technical assistance. We thank P. Reno, M. McCollum, W. Horton, M. Cohn, the Editor, and two anonymous reviewers for critical reading of the manuscript and helpful commentary. We are grateful to Evan Bailey for executing graphic file conversions.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. N.J. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803319105/DCSupplemental.
References
- 1.Feldhamer GA, Drickamer LC, Vessey SH, Merritt JF. Mammalogy: Adaptation, Diversity, Ecology. New York: McGraw Hill; 2004. [Google Scholar]
- 2.Allen JA. The influence of physical conditions in the genesis of species. Radical Review. 1877;1:108–140. [Google Scholar]
- 3.Sumner FB. Some effects of external conditions upon the white mouse. J Exp Zool. 1909;7:97–155. [Google Scholar]
- 4.Ashoub MA. Effect of two extreme temperatures on growth and tail-length of mice. Nature. 1958;181:284. doi: 10.1038/181284a0. [DOI] [PubMed] [Google Scholar]
- 5.Weaver ME, Ingram DL. Morphological changes in swine associated with environmental temperature. Ecology. 1969;50:710–713. [Google Scholar]
- 6.Al-Hilli F, Wright EA. The effects of changes in the environmental temperature on the growth of the tail bones in the mouse. Br J Exp Pathol. 1983;64:34–42. [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison GA. Environmental modification of mammalian morphology. Man. 1960;60:3–6. [Google Scholar]
- 8.Harland SC. Effect of temperature on growth in weight and tail-length of inbred and hybrid mice. Nature. 1960;186:446. [Google Scholar]
- 9.Scholander PF. Evolution of climatic adaptation in homeotherms. Evolution. 1955;9:15–26. [Google Scholar]
- 10.Steegmann AT. Human cold adaptation: An unfinished agenda. Am J Hum Biol. 2007;19:218–227. doi: 10.1002/ajhb.20614. [DOI] [PubMed] [Google Scholar]
- 11.Weladji RB, Holand O. Global climate change and reindeer: Effects of winter weather on the autumn weight and growth of calves. Oecologia. 2003;136:317–323. doi: 10.1007/s00442-003-1257-9. [DOI] [PubMed] [Google Scholar]
- 12.Trinkaus E. Neanderthal limb proportions and cold adaptation. In: Stringer CB, editor. Aspects of Human Evolution. London: Taylor and Francis; 1981. pp. 187–224. [Google Scholar]
- 13.Brookes M, Revell WJ. Blood Supply of Bone: Scientific Aspects. NY: Springer; 1998. [Google Scholar]
- 14.Trueta J. The influence of the blood supply in controlling bone growth. Bull Hosp Joint Disease. 1953;14:147–157. [PubMed] [Google Scholar]
- 15.Ingram DL, Weaver ME. A quantitative study of the blood vessels of the pig's skin and the influence of environmental temperature. Anat Rec. 1969;164:1–8. doi: 10.1002/ar.1091630404. [DOI] [PubMed] [Google Scholar]
- 16.Schoutens A, Bergmann P, Verhas M. Bone flood flow measured by 85Sr microspheres and bone seeker clearances in the rat. Am J Physiol. 1979;236:H1–H6. doi: 10.1152/ajpheart.1979.236.1.H1. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg ME, Trueta J. Effects of activity on bone growth and development in the rat. Clin Orthop Relat Res. 1981;156:52–60. [PubMed] [Google Scholar]
- 18.Anetzberger H, Thein E, Becker M, Zwissler B, Messmer K. Microspheres accurately predict regional bone blood flow. Clin Orthop Relat Res. 2004;424:253–265. doi: 10.1097/01.blo.0000128281.67589.b4. [DOI] [PubMed] [Google Scholar]
- 19.Tothill P, MacPherson JN. The distribution of blood flow to the whole skeleton in dogs, rabbits and rats measured with microspheres. Clin Phys Physiol Meas. 1986;7:117–123. doi: 10.1088/0143-0815/7/2/002. [DOI] [PubMed] [Google Scholar]
- 20.Prisby RD, et al. Aging reduces skeletal blood flow, endothelium-dependent vasodilation and nitric oxide bioavailability in rats. J Bone Miner Res. 2007;22:1280–1288. doi: 10.1359/jbmr.070415. [DOI] [PubMed] [Google Scholar]
- 21.Shui C, Scutt A. Mild heat shock induces proliferation, alkaline phosphatase activity, and mineralization in human bone marrow stromal cells and Mg-63 cells in vitro. J Bone Miner Res. 2001;16:731–741. doi: 10.1359/jbmr.2001.16.4.731. [DOI] [PubMed] [Google Scholar]
- 22.Wei G, Bai X, Esko JD. Temperature-sensitive glycosaminoglycan biosynthesis in a Chinese hamster ovary cell mutant containing a point mutation in glucuronyltransferase I. J Biol Chem. 2004;279:5693–5698. doi: 10.1074/jbc.M311621200. [DOI] [PubMed] [Google Scholar]
- 23.Wadkins CL. Experimental factors that influence collagen calcification in vitro. Calcif Tissue Res. 1968;2:214–228. doi: 10.1007/BF02279209. [DOI] [PubMed] [Google Scholar]
- 24.Reno PL. Kent, OH: Kent State University; 2006. Ossification of the mammalian metatarsal: Proliferation and differentiation in the presence/absence of a defined growth plate. PhD thesis. [Google Scholar]
- 25.Reno PL, McBurney DL, Lovejoy CO, Horton WE. Ossification of the mouse metatarsal: Differentiation and proliferation in the presence/absence of a defined growth plate. Anat Rec. 2006;288A:104–118. doi: 10.1002/ar.a.20268. [DOI] [PubMed] [Google Scholar]
- 26.Stern C. Genetic Mosaics and Other Essays. Cambridge, MA: Harvard Univ Press; 1968. [Google Scholar]
- 27.Radmilovich M, Fernández A, Trujillo-Cenóz O. Environmental temperature affects cell proliferation in the spinal cord and brain of juvenile turtles. J Exp Biol. 2003;206:3085–3093. doi: 10.1242/jeb.00515. [DOI] [PubMed] [Google Scholar]
- 28.De Neve W, et al. The influence of ambient temperature on tumor growth, metastasis and survival in mice. Clin Exp Metastasis. 1988;6:213–219. doi: 10.1007/BF01782481. [DOI] [PubMed] [Google Scholar]
- 29.Maeda M. Control of cellular differentiation by temperature in the cellular slime mould Dictyostelium discoideum. J Cell Sci. 1984;69:159–165. doi: 10.1242/jcs.69.1.159. [DOI] [PubMed] [Google Scholar]
- 30.Rymen B, et al. Cold nights impair leaf growth and cell cycle progression in maize through transcriptional changes of cell cycle genes. Plant Physiol. 2007;143:1429–1438. doi: 10.1104/pp.106.093948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arendt J. Ecological correlates of body size in relation to cell size and cell number: Patterns in flies, fish, fruits and foliage. Biol Rev Camb Philos Soc. 2007;82:241–256. doi: 10.1111/j.1469-185X.2007.00013.x. [DOI] [PubMed] [Google Scholar]
- 32.Gillooly JF, Allen AP, West GB, Brown JH. The rate of DNA evolution: Effects of body size and temperature on the molecular clock. Proc Natl Acad Sci USA. 2005;102:140–145. doi: 10.1073/pnas.0407735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takaoki M, Murakami N, Gyotoku J. C-fos expression of osteoblast-like MC3T3–E1 cells induced either by cooling or by fluid flow. Biol Sci Space. 2004;18:181–182. [PubMed] [Google Scholar]
- 34.Yang L, McBurney D, Tang SC, Carlson SG, Horton WE., Jr A novel role for Bcl-2 associated-athanogene-1 (Bag-1) in regulation of the endoplasmic reticulum stress response in mammalian chondrocytes. J Cell Biochem. 2007;102:786–800. doi: 10.1002/jcb.21328. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, Carlson SG, McBurney D, Horton WE., Jr Multiple signals induce endoplasmic reticulum stress in both primary and immortalized chondrocytes resulting in loss of differentiation, impaired cell growth, and apoptosis. J Biol Chem. 2005;280:31156–31165. doi: 10.1074/jbc.M501069200. [DOI] [PubMed] [Google Scholar]
- 36.Tsang KY, et al. Surviving endoplasmic reticulum stress is coupled to altered chondrocyte differentiation and function. PLoS Biol Mar. 2007;5:e44. doi: 10.1371/journal.pbio.0050044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Eerden BC, Karperien M, Gevers EF, Löwik CW, Wit JM. Expression of Indian hedgehog, parathyroid hormone-related protein, and their receptors in the postnatal growth plate of the rat: Evidence for a locally acting growth restraining feedback loop after birth. J Bone Miner Res. 2000;15:1045–1055. doi: 10.1359/jbmr.2000.15.6.1045. [DOI] [PubMed] [Google Scholar]
- 38.Serrat MA, Lovejoy CO, King D. Age- and site-specific decline in insulin-like growth factor-I receptor expression is correlated with differential growth plate activity in the mouse hindlimb. Anat Rec. 2007;290:375–381. doi: 10.1002/ar.20480. [DOI] [PubMed] [Google Scholar]
- 39.Waddington CH. The Strategy of the Genes. New York: Macmillan; 1957. [Google Scholar]
- 40.Jacobi J, et al. Adenoviral gene transfer with soluble vascular endothelial growth factor receptors impairs angiogenesis and perfusion in a murine model of hindlimb ischemia. Circulation. 2004;110:2424–2429. doi: 10.1161/01.CIR.0000145142.85645.EA. [DOI] [PubMed] [Google Scholar]
- 41.Tan W, Riggs KW, Theis RL, Rurak DW. Use of an automated fluorescent microsphere method to measure regional blood flow in the fetal lamb. Can J Physiol Pharmacol. 1997;75:959–968. [PubMed] [Google Scholar]
- 42.Raab S, Thein E, Harris AG, Messmer K. A new sample-processing unit for the fluorescent microsphere method. Am J Physiol. 1999;276:H1801–H1806. doi: 10.1152/ajpheart.1999.276.5.H1801. [DOI] [PubMed] [Google Scholar]
- 43.Fluorescent Microsphere Resource Center. Manual for Using Fluorescent Microspheres to Measure Regional Organ Perfusion. Seattle, WA: University of Washington; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.