Abstract
Aquatic ecosystems around the world face serious threats from anthropogenic contaminants. Results from 8 years of field and laboratory investigations indicate that sublethal contaminant exposure is occurring in the early life stages of striped bass in the San Francisco Estuary, a population in continual decline since its initial collapse during the 1970s. Biologically significant levels of polychlorinated biphenyls, polybrominated diphenyl ethers, and current-use/legacy pesticides were found in all egg samples from river-collected fish. Developmental changes previously unseen with standard methods were detected with a technique using the principles of unbiased stereology. Abnormal yolk utilization, brain and liver development, and overall growth were observed in larvae from river-collected fish. Histopathological analyses confirmed and identified developmental alterations. Using this methodology enabled us to present a conclusive line of evidence for the maternal transfer of xenobiotics and their adverse effects on larval striped bass in this estuary.
Keywords: Morone saxatilis, contaminants, biomarkers, histopathology, unbiased stereology
Over the past few decades the world's aquatic environments have been severely impacted by anthropogenic activities. Contaminants from industry, agriculture, urban runoff, and other sources have found their way into these environments, affecting all levels of biological organization, from the individual to the entire ecosystem (1–3). Consequently, fish species assemblages have shifted (4), and major fish population declines have occurred (5, 6). Understanding the role contaminants play in contributing to these declines is essential and will ultimately help elucidate their effects on human populations.
Many classes of contaminants can bioaccumulate in muscle and fat tissue. These compounds can affect physiological processes and the overall health and fitness of the individual, possibly leading to population-level effects (7). Lipophilic compounds bioaccumulate in fish and wildlife, as well as in human tissues, and can be transferred maternally to offspring, generating deleterious effects (8–10).
The striped bass (Morone saxatilis) population, along with such other pelagic fish as delta smelt (Hypomesus transpacificus), longfin smelt (Spirinchus thaleichthys), and threadfin shad (Dorosoma petenense), in the San Francisco Estuary has suffered significant declines in the past decades (6, 11). Striped bass were part of a thriving commercial and sport fishery. In 1935 the commercial fishery was closed and striped bass were designated a game fish. The population continued to thrive through the 1960s. The first population crash occurred in the 1970s (6) followed by significant declines in the mid-1980s and mid-1990s (5, 12). The most recent step change raises significant concerns because it has occurred during a period of moderate weather conditions and river flows that typically result in at least minor population recovery (12). The past 3 decades of monitoring data and various studies suggest that some factors causing the striped bass population decline occur in the early life stages and before the 38-mm young of the year index (3, 13, 14), an index that has been used to accurately measure population abundance of striped bass for more than 40 years (15).
During this extended period of population decline, several factors have been identified as possible causes, indicating that multiple stressors are affecting the aquatic fauna in this dynamic and complex ecosystem: entrainment and death of fish due to water diversions for agriculture and human usage, food limitation and larval starvation, introduced species, climate change, and contaminants (5, 6, 11). However, there is evidence that starvation is not occurring (14) and that the combined effects of water diversions and introduced species do not account entirely for the recent declines in the population (12).
On the other hand, contaminant inputs are diverse, and sources such as agriculture, industry, and urban runoff into the estuary have been well documented for several decades (16, 17). Effects of contaminants on the early life stages of striped bass have been documented since the late 1980s (3, 14), but comprehensive studies investigating contaminant concentrations, exposure, and effect have not been conducted to this date.
The study presented here is the first of several ongoing investigations we initiated to determine the contributing effects of timing and route of contaminant exposure, and their physiological and pathological significance on the early life stages of striped bass. The potential routes and timing of exposures considered were: (i) maternal transfer of xenobiotics to the egg may affect embryonic and larval development before the onset of exogenous feeding, (ii) exposure to xenobiotics located within the spawning grounds in ovo immediately after fertilization and before water hardening, (iii) larval exposure after hatching to xenobiotics found in the area of hatching and larval development, and (iv) larval exposure to xenobiotics via contaminated food sources in the larval nursery grounds. This study focuses on the first potential route of exposure, the maternal transfer of xenobiotics and effects on early development.
Results
Chemical analysis of the eggs from field-collected striped bass revealed significantly higher levels of polychlorinated biphenyls [PCBs; 400–1,000 parts per billion (ppb)], polybrominated diphenyl ethers (PBDEs; 100–150 ppb), and current-use and legacy pesticides (pesticides that are no longer in use but persist in the environment; 5–750 ppb), than found in the eggs of control hatchery-reared females (Table 1). Egg quality and viability were examined and found to be comparable between eggs from the wild and the hatchery. Eggs from field-collected striped bass were slightly larger and in 1 year (1999) had slightly higher total lipid than the hatchery controls. Other studies have reported similar findings, indicating that river-collected striped bass eggs were of higher quality than hatchery eggs (18, 19).
Table 1.
PBEs, PBDEs, and current and legacy pesticide levels (ppb) in unfertilized striped bass eggs
| Compound | Hatchery (1999) | River (1999) | River (2001) |
|---|---|---|---|
| PCBs | |||
| Congeners 8–209 | 93.9 ± 7.5 | 601.8 ± 107.2* | 426.1 ± 54.4* |
| Aroclors 1248, 1254, and 1260 | 178.7 ± 15.2 | 945.2 ± 158.0* | 678.3 ± 85.5* |
| PBDEs | 12.9 ± 0.3 | 138.0 ± 22.0* | 134.5 ± 29.3* |
| Pesticides | |||
| Chlordane, cis- | 4.9 ± 0.4 | 15.8 ± 2.4* | 15.8 ± 2.5* |
| Chlordane, trans- | 3.0 ± 0.1 | 6.1 ± 1.0 | 5.0 ± 0.6 |
| Chlorpyrifos | ND | 4.5 ± 1.7 | 0.4 ± NA |
| DDD, o,p′- | ND | 6.3 ± 1.5 | 12.2 ± 4.1* |
| DDD, p,p′- | 13.4 ± 1.3 | 68.9 ± 12.1* | 87.3 ± 21.5* |
| DDE, o,p′- | ND | 0.4 ± NA | 5.4 ± 3.1 |
| DDE, p,p′- | 41.2 ± 4.8 | 389.0 ± 60.6* | 566.8 ± 195.8* |
| DDMU, p,p′- | ND | 16.5 ± 4.3 | 16.2 ± 6.9 |
| DDT, o,p′- | ND | ND | 4.3 ± 3.2 |
| DDT, p,p′- | 4.6 ± 0.3 | 21.6 ± 2.8* | 35.8 ± 18.6* |
| Dieldrin | 10.3 ± 0.5 | 19.9 ± 2.9 | 24.7 ± 6.8* |
| Heptachlor epoxide | 1.9 ± 0.1 | 2.5 ± 0.3 | 2.3 ± 0.2 |
| Nonachlor, cis- | 1.9 ± 0.2 | 11.7 ± 1.9* | 10.4 ± 1.7* |
| Nonachlor, trans- | 4.0 ± 0.5 | 24.9 ± 3.6* | 23.5 ± 4.4* |
| Oxychlordane | ND | 2.5 ± 0.4* | 1.8 ± 0.6 |
| Toxaphene | ND | 36.4 ± 27.2 | 91.8 ± 55.4 |
A total of 51 PCBs (congeners 8–209, aroclors 1248, 1254, and 1260) and 12 PBDEs (BDEs 17–190) were analyzed. Duplicate samples of eggs (5–10 g per sample) were collected from hatchery control fish (Hatchery 1999, n = 3) and river-collected females (River 1999, n = 11), and from river-collected females in 2001 (River 2001, n = 10) for chemical analysis. Shown are mean contaminant concentrations in parts per billion (ppb) ± standard error. Concentrations below detection limits are indicated with ND. Standard error is not applicable (NA) when only a single sample showed contaminant concentrations above the detection limit. Significant changes (P ≤ 0.05) in comparison with hatchery control are shown with an asterisk.
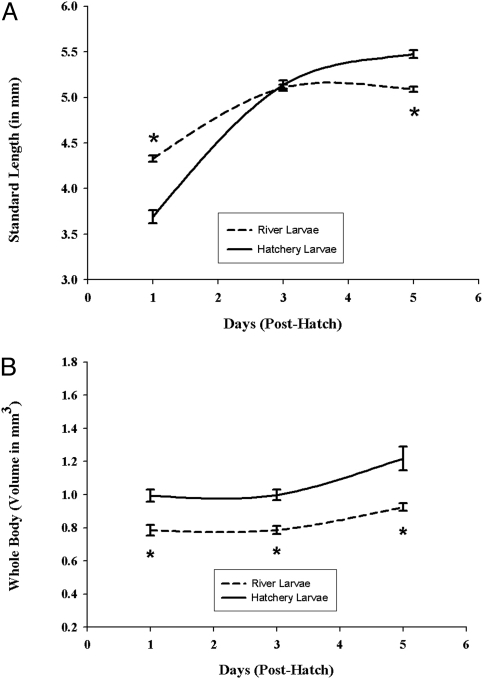
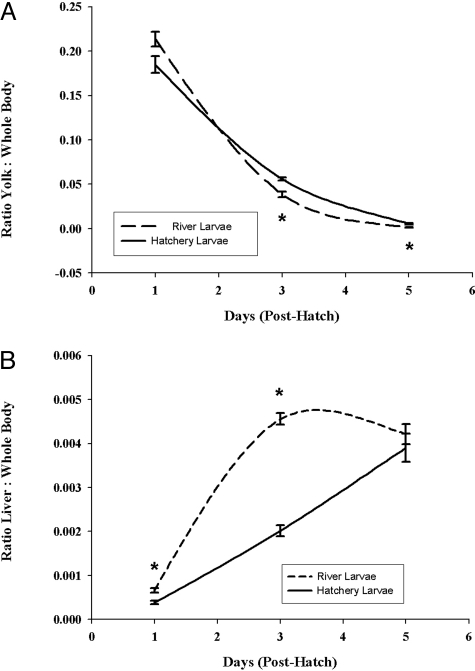
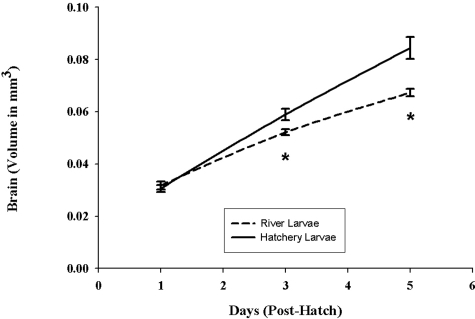
Results from the developmental studies showed that control larvae exhibited normal morphology, growth, and development, whereas larvae from field-collected striped bass (subsequently referred to as river larvae) developed abnormally, grew more slowly, and were significantly smaller. River larvae were longer and thinner than hatchery controls at 24 h posthatch, resembling larvae at a later stage (e.g., body morphology, tail fin, and notochord development resembled 3- to 5-day posthatch larvae). Overall growth of these larvae was significantly slower throughout the examined developmental period of 5 days posthatch (Fig. 1). Yolk sac utilization in river larvae was more rapid, with poorer results than in the hatchery control larvae (Fig. 2A). At 5 days posthatch, river larvae had significantly less yolk or no yolk sac remaining compared with the hatchery controls. Brain growth in the river larvae was slower throughout the developmental period (days 1 through 5 posthatch; Fig. 3). Liver growth and development in river larvae were accelerated significantly during the first 3 days posthatching; however, growth and development became retarded and regressed between days 3 and 5 posthatch (Fig. 2B). In contrast, liver growth in the control group was constant throughout the developmental period, resulting in a significantly larger and better-developed liver at day 5 posthatching.
Fig. 1.
Comparison of the development of larvae from hatchery and river-collected striped bass. (A) Using standard length as a measure of size, this graph represents growth of river and hatchery larvae between day 1 and day 5 posthatching. Results represent the means ± SE of 12–15 hatchery larvae and 36–75 river-collected larvae. *, Significantly different from hatchery larvae; significance level P ≤ 0.001. (B) Using a technique (Cavalieri method) enabled us to compare whole-body volumes of river and hatchery larvae between days 1 and 5. Results represent the means ± SE of 12–15 hatchery larvae and 36–75 river-collected larvae. *, Significantly different from hatchery larvae; significance level P ≤ 0.001.
Fig. 2.
Comparison of organ development relative to whole-body volume of larvae from hatchery and river-collected striped bass. (A) Yolk utilization of river and hatchery larvae between day 1 and day 5. Values are reported as yolk sac volumes relative to whole-body volumes. Results represent the means ± SE of 12–15 hatchery larvae and 36–75 river-collected larvae. *, Significantly different from hatchery larvae; significance level P ≤ 0.007. (B) Comparison of liver growth of river and hatchery larvae between day 1 and day 5. Values are reported as liver volumes relative to whole-body volumes. Results represent the means ± SE of 12–15 hatchery larvae and 36–75 river-collected larvae. *, Significantly different from hatchery larvae; significance level P ≤ 0.016.
Fig. 3.
Comparison of brain development of larvae from hatchery and river-collected striped bass. This graph compares brain volumes of river and hatchery larvae between day 1 and day 5. Results represent the means ± SE of 12–15 hatchery larvae and 36–75 river-collected larvae. *, Significantly different from hatchery larvae; significance level P ≤ 0.021.
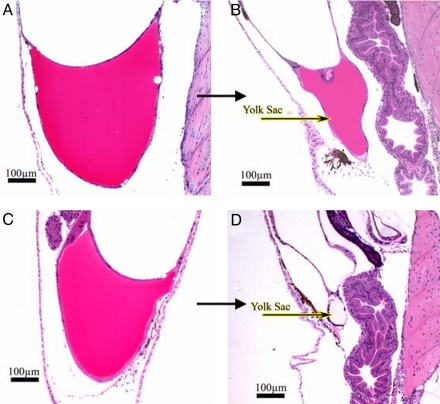
Histopathological evaluations were performed subsequent to morphometric analysis (Figs. 4 and 5). At day 3 posthatch, yolk sacs from the two groups were similar in appearance (Fig. 4 A and C) and highly eosinophilic, indicating abundant protein, but there was significantly less yolk remaining in the river larvae. At day 5 posthatch, the disparity between the 2 groups became more pronounced (Fig. 4 B and D). The hatchery-reared control larvae had a portion of highly eosinophilic yolk sac remaining, whereas the river larvae had very little or no yolk sac remaining. Any residual yolk in river larvae did not stain with eosin, indicating an absence/low concentration of protein remained. In addition, the color and consistency of yolk during the late stages of absorption (days 3 to 5 posthatch) were abnormal. The yolk from river larvae had an inconsistent (bubbly/frothy) appearance, and yolk sac edemas were observed in the majority of these larvae.
Fig. 4.
Histopathological evaluations of yolk sacs of river and hatchery larvae. Striped bass larvae from hatchery females (n = 15) and 3–5 river-collected females (n = 75) were embedded in glycol methacrylate and serial sectioned at 4-μm thickness by using a Sorval JB-4A Microtome. Sections were placed onto coded and numbered glass slides, stained with H&E, and coverslipped by using Shandon-Mount. (A and C) Yolk sac appearance on day 3 of larvae from hatchery striped bass females (A) and river-collected females' larvae (C). (B and D) Yolk sac appearance on day 5 of larvae from hatchery striped bass females (B), and depleted yolk sac from river-collected females' larvae (D).
Fig. 5.
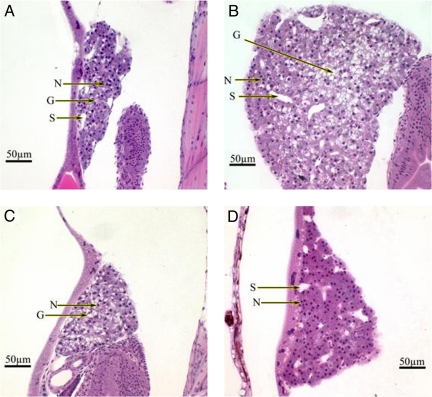
Histopathological evaluations of livers of river and hatchery larvae. Striped bass larvae from 3 hatchery females (n = 15) and 3–5 river-collected females (n = 75) were embedded in glycol methacrylate and serial sectioned at 4-μm thickness by using a Sorval JB-4A Microtome. Sections were placed onto coded and numbered glass slides, stained with H&E, and coverslipped using Shandon-Mount. (A) Liver appearance on day 3 (midorganogenesis) of larvae from hatchery striped bass females. Organ appears normally developed, beginning glycogen storage. (B) Normal glycogen-laden liver with distinct cellular architecture of 5-day hatchery larvae. (C) Liver appearance on day 3 of larvae from river-collected striped bass females. Liver growth and development are accelerated, as demonstrated by distinct cellular architecture and glycogen storage not normally present during this stage of development. Liver appears developmentally similar to a normal larval liver at day 5. (D) Liver appearance on day 5 of larvae from river-collected striped bass females. Growth of the liver is retarded/regressing, cell size is decreased, the cellular architecture is indistinct, glycogen is not detectable, and nuclear bunching can be observed. N, nuclei; G, glycogen storage; S, sinusoids.
Histopathological evaluation of the liver was also performed (Fig. 5). Livers from the control group were beginning to show distinct cellular architecture and glycogen storage 3 days after hatching (Fig. 5A). The livers from river larvae at day 3 posthatch were more highly developed, with very distinct cellular architecture and abundant stores of glycogen, and they were significantly larger than those of the controls (Fig. 5C). At day 5 posthatch, these changes reversed dramatically. Livers from the control group continued to develop, grow, and exhibit advanced cellular architecture, morphology, and abundant stores of glycogen (Fig. 5B). On the contrary, livers from the river larvae had shrunken considerably by day 5 posthatching, cellular architecture became indistinct/regressed, hepatocytes were devoid of glycogen, and nuclear bunching was observed (Fig. 5D).
Discussion
This study provides clear evidence of maternal transfer of xenobiotics and their adverse effects on larval striped bass in the San Francisco Estuary. Chemical analysis of unfertilized eggs from Sacramento River-collected striped bass indicated that maternal transfer of biologically significant lipophilic compounds occurred in all 21 females in this study. Contaminants found in these eggs included PCBs, PBDEs, current-use pesticides, legacy pesticides, and their degradation products. Our results indicate that pesticides not in use for decades, such as DDT and its degradation products, are still persistent in the estuary and are being made bioavailable by recycling through the food chain to apex predators. Furthermore, our results show that these contaminants are being transferred to their progeny in biologically relevant levels. Concentrations of individual contaminants and mixtures determined in this study have been shown to have adverse effects in a wide range of animals, including mammals, reptiles, amphibians, and fish (20–23). Some of the effects described are as follows: alterations of growth and development (24), poor hatching success (22), alterations of the reproductive and nervous system (25, 26), learning and behavioral deficits retained throughout life (27, 28), abnormalities of the liver and other organ systems (29, 30), and endocrine disruption (29, 31). Studies have also shown that these contaminants in combination can increase adverse effects by several orders of magnitude (32, 33). Chemical analysis of hatchery control eggs indicated that fewer compounds are present in comparison with eggs from field-collected females, and that levels of these contaminants are most likely biologically insignificant. Contaminants found in the control eggs are believed to originate from the commercial diet fed to hatchery fish, which is produced from wild fish and other contaminated byproducts (34), and not the hatchery water which is supplied by a deep uncontaminanted well. In addition to current-use pesticides, legacy pesticides, and PCBs, significant levels of PBDEs were also detected in eggs from field-collected female striped bass. This is a reported finding of maternal transfer of PBDEs in fish in the San Francisco Estuary system, a novel group of environmental contaminants that has recently become the focus of many toxicity studies. PBDEs are mainly used as flame retardants and are found in a variety of materials, such as paint, upholstery, carpeting, plastics, textiles, electronic circuits, and insulation (35). PBDEs are now as ubiquitous as PCBs in the aquatic environment, and their levels are rising due to a lack of regulation (35, 36). It is now believed that PBDEs have spread throughout the world's oceans (35, 37), and as a consequence have been found in many aquatic organisms, including fish, where they have bioaccumulated to biologically relevant levels (33). The aquatic environment of the fish we investigated is one of the most contaminated regions worldwide, and studies have shown PBDE levels in breast milk from women living in the San Francisco Bay area up to 100 times greater than those in other regions of the world (8). These compounds have been shown to act as thyroid hormone mimics, induce P450-1A1 and P450-2B2 enzyme systems, have neurotoxic effects, and cause learning and behavioral deficits that remain throughout life in rodents (35). Results presented here support studies indicating PBDE contamination of the San Francisco Estuary (38) and bioaccumulation of these compounds in fish (35). The consequences of PBDE contamination for wildlife and humans are largely unknown (8, 10) and need to be addressed and mitigated.
The study presented here used a method based on the principles of unbiased stereology and the Cavalieri method to measure body and organ volumes of larvae throughout a certain developmental period. This method enabled us to detect developmental changes previously unseen using standard methods. Typically, morphological studies of the early life stages of fish use linear measurements, such as standard length, body depth at the pectoral fins, and body depth at the anus, to determine size and growth relationships (14, 39, 40). However, in our opinion these types of metrics do not provide an accurate representation of growth and development. According to the methods in which linear measurements are used, the progeny from river-collected striped bass were significantly larger at day 1 posthatching than the hatchery larvae, similar in size at day 3, and significantly smaller at day 5 (Fig. 1A). In contrast, our method revealed that river larvae were significantly smaller and grew slower throughout the developmental sampling period of day 1 to day 5 posthatching (Fig. 1B). River larvae at day 1 posthatching, although significantly smaller by volume, were longer and thinner than hatchery larvae, resembling a later and more advanced larval stage. We concluded that development of river larvae is abnormal, with some processes accelerated (tailfin, notochord, and liver) and others retarded (brain and liver from days 3 to 5 posthatching). In addition, standard methods provide data only on exterior body dimensions and do not address the morphometry and condition of developing organ systems. Morphometric and histopathological results of yolk, liver, and brain corroborated findings of larval body development (Figs. 2 and 3). Brain growth was retarded in larvae from river-collected striped bass throughout the developmental period, resulting in a significantly smaller brain than hatchery controls at day 5 posthatching (Fig. 3). The hatchery-reared control larvae at day 5 posthatch just before exogenous feeding had significant yolk remaining as an energy store to aid in finding their first food, as well as a well-developed, glycogen-laden liver (additional energy store) capable of metabolizing food and other exogenous compounds (Figs. 4B and 5B). In contrast, river larvae had little or no yolk remaining at 5 days posthatching, and any yolk remaining was devoid of protein (Figs. 2A and 4D). Therefore, just before first feeding, these larvae had virtually no source of energy available to search for food or avoid predators. Abnormal liver growth and development in river larvae were extreme. Liver development and growth were accelerated during days 1 and 3 posthatching in the river larvae (Figs. 2B and 5C). The liver deteriorated and regressed between days 3 and 5 posthatching, leading to an extremely small liver devoid of glycogen just before first feeding (Fig. 5D). Thus, abnormal liver growth and development in river larvae can be categorized as severe, and the liver is likely much less than optimally functional at day 5 posthatching. Chemical analysis of the eggs coupled with the morphometric and histopathological results from the brain and liver indicate that contaminants are maternally transferred, and that compounds may be causing endocrine disruption during early development of the river larvae. Contaminants found in the eggs of river-collected females are known endocrine disrupters, and effects observed correspond to endocrine disruption during early development (41, 42). We suggest that the combination of the abnormal development, yolk deficiency, and an altered shrunken liver devoid of glycogen at day 5 posthatching adversely affect subsequent growth and survival of larvae from river-caught females. These findings were possible through application of a newly developed morphometric method focusing on whole-body and organ volumes. Coupled with histopathological analysis, this approach overcomes the shortcomings of other, established methods. It provides much more accurate morphometry, representation of body growth, organ growth, organ development, and organ condition in early-life stage striped bass larvae.
The vast majority of maternal transfer studies in fish have been limited to either analysis of eggs from field-collected animals or laboratory studies injecting compounds directly into the developing embryo or female (43, 44). These studies are important sources of toxicological and developmental information, but they are limited to parent compounds, their metabolites, and their effects. These studies fail to reflect the true nature of maternal transfer of complex combinations of xenobiotics affecting fish and wildlife populations. Especially in the aquatic environment today, complex mixtures of xenobiotics, metabolites, and degradation products, not individual compounds, are present and are being bioaccumulated. Accordingly, interactions of complex mixtures detected in the environment may alter effects seen in laboratory studies, and they can cause additional problems not observed when compounds are tested individually or in simple mixtures. To understand the effects of maternally transferred xenobiotics in the environment, it is vital to determine to what extent these real-world complex mixtures are affecting progeny.
The study presented here demonstrates that complex mixtures of contaminants are being maternally transferred to developing progeny, describes developmental alterations detected by using a new technique, and corroborates these findings with histopathological analyses. Laboratory studies can be designed subsequently to investigate the mechanisms involved and to determine which xenobiotics singly or in combination are causing the observed effects. Decisions can then be made to regulate the use of these compounds and their release into the environment to mitigate problems identified by these studies. The results from this study clearly demonstrate that xenobiotics are adversely affecting early-life-stage striped bass in the San Francisco Estuary and need to be considered as one of multiple stressors affecting the continuing population decline.
Methods
Adult male and female striped bass were collected between Knights Landing and Colusa, CA, on the Sacramento River weekly during the spawning seasons of 1999 and 2001 (April to June) by using standard electrofishing methods. Hatchery-reared F2 generation striped bass were used as controls. This domestic striped bass broodstock was created by using striped bass captured in the same location on the Sacramento River during previous years. Just before fertilization, subsets of egg samples were collected for chemical analysis from both hatchery-reared controls and river-collected fish. River-collected and hatchery-reared females were spawned, and larvae were reared under identical conditions. Larvae were sampled and preserved in 10% neutral-buffered formalin at 3 critical periods during development representing early organogenesis (day 1 posthatch), late organogenesis (day 3 posthatch), and completed organogenesis just before the onset of exogenous feeding (day 5 posthatch). Larvae were embedded in glycol methacrylate and serial sectioned at thicknesses of 4 μm. A technique using the Cavalieri method (45, 46) was used to determine whole-body, brain, liver, and yolk sac volumes during development. Organ and yolk sac volumes were normalized to whole-body volumes such that development and yolk sac utilization between the 2 groups could be assessed. Histopathological analysis of the yolk, liver, and brain was used to describe and corroborate morphometric findings.
Twenty-four duplicate samples of eggs were collected for chemical analysis, with one set processed for organic analysis and the other set for trace element analysis. Eleven samples from river-collected females in 1999, 10 from river-collected females in 2001, and 3 from the hatchery controls were analyzed.
Additional details concerning striped bass capture, spawning, sample collection at spawning and during development of larvae, egg analysis, developmental morphometry, and statistical analysis are provided in supporting information (SI) Materials and Methods.
Supplementary Material
Acknowledgments.
D.J.O. thanks J. Zinkl for his advice, critical scientific input, and support; D. Hyde for his critical scientific input and encouragement to develop the new technique, for providing access to the CAST grid system and his technical staff; W. Bennett for his advice and for providing me the initial opportunity to become involved in striped bass research; J. Low-Marchelli for managing the project during illness; K. Eder and J. Cech for critical discussions, reading, and editing the manuscript; I. Werner for her review of the work; T. Vaught and Professional Aquaculture Services for providing support and the facilities to perform all developmental experiments; D. Crane and the California Department of Game's Fish and Wildlife Water Pollution Control Laboratory for chemical analysis and support; B. Osburn and the University of California, Davis, School of Veterinary Medicine for providing the majority of funding for the project; and T. Sommer, F. Feyrer, and the Interagency Ecological Program Pelagic Organism Decline management team for their support and partial funding under Department of Water Resources Contract 4600004664; and most of all to my father, S. Ostrach, without whose advice and comments on language and content during the writing of this paper, ethical training, and continued support and encouragement, this project and my career in science would not have been possible.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.L. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802616105/DCSupplemental.
References
- 1.Schwarzenbach RP, et al. The challenge of micropollutants in aquatic systems. Science. 2006;313:1072–1077. doi: 10.1126/science.1127291. [DOI] [PubMed] [Google Scholar]
- 2.Fent K. Ecotoxicological effects at contaminated sites. Toxicology. 2004;205:223–240. doi: 10.1016/j.tox.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 3.Bailey HC, DiGiorgio C, Doroshov S, Hinton DE. The effect of agricultural discharge on striped bass (Morone saxatilis) in California's Sacramento-San Joaquin drainage. Ecotoxicology. 1994;3:123–142. doi: 10.1007/BF00143410. [DOI] [PubMed] [Google Scholar]
- 4.Matern SA, Moyle PB, Pierce LC. Native and alien fishes in a California estuarine marsh: Twenty-one years of changing assemblages. Trans Am Fish Soc. 2002;131:797–816. [Google Scholar]
- 5.Moyle PB, Williams JE. Biodiversity loss in the temperature zone: Decline of the native fish fauna of California. Conserv Biol. 1990;4:275–284. [Google Scholar]
- 6.Stevens DE, Kohlhorst DW, Miller LW, Kelley DW. The decline of striped bass in the Sacramento-San Joaquin Estuary, California. Trans Am Fish Soc. 1985;114:12–30. [Google Scholar]
- 7.Arkoosh MR, Collier TK. Ecological risk assessment paradigm for salmon: Analyzing immune function to evaluate risk. Hum Ecol Risk Assess. 2002;8:265–276. [Google Scholar]
- 8.She J, et al. PBDEs in the San Francisco Bay Area: Measurements in harbor seal blubber and human breast adipose tissue. Chemosphere. 2002;46:697–707. doi: 10.1016/s0045-6535(01)00234-x. [DOI] [PubMed] [Google Scholar]
- 9.Borga K, Fisk AT, Hoekstra PF, Muir DCG. Biological and chemical factors of importance in the bioaccumulation and trophic transfer of persistent organochlorine contaminants in arctic marine food webs. Environ Toxicol Chem. 2004;23:2367–2385. doi: 10.1897/03-518. [DOI] [PubMed] [Google Scholar]
- 10.McDonald TA. A perspective on the potential health risks of PBDEs. Chemosphere. 2002;46:745–755. doi: 10.1016/s0045-6535(01)00239-9. [DOI] [PubMed] [Google Scholar]
- 11.Bennett WA, Moyle PB. Where have all the fishes gone? Interactive factors producing fish declines in the Sacramento-San Joaquin Estuary. In: Hollibaugh JT, editor. San Francisco Bay: The Ecosystem. San Francisco: American Association for the Advancement of Science; 1996. pp. 519–542. [Google Scholar]
- 12.Sommer T, et al. The collapse of pelagic fishes in the upper San Francisco Estuary. Fisheries. 2007;32:270–277. [Google Scholar]
- 13.Chadwick HK, Stevens DE, Miller LW. Proceedings of the Conference on Assessing the Effects of Power-Plant-Induced Mortality on Fish Populations; Gatlinburg, TN: Pergamon; 1977. pp. 18–35. [Google Scholar]
- 14.Bennett WA, Ostrach DJ, Hinton DE. Larval striped bass condition in a drought-stricken estuary: Evaluating pelagic food-web limitation. Ecol Appl. 1995;5:680–692. [Google Scholar]
- 15.Kimmerer WJ, Cowan JH, Miller LW, Rose KA. Analysis of an estuarine striped bass (Morone saxatilis) population: Influence of density-dependent mortality between metamorphosis and recruitment. Can J Fish Aquat Sci. 2000;57:478–486. [Google Scholar]
- 16.Moore M, Mulville A, Weinberg M. Water allocation in the American West: Endangered fish versus irrigated agriculture. Nat Resour J. 1996;36:319–357. [Google Scholar]
- 17.Thompson B, Hoenicke R, Davis JA, Gunther A. An overview of contaminant-related issues identified by monitoring in San Francisco Bay. Environ Monit Assess. 2000;64:409–419. [Google Scholar]
- 18.Harrell RM, Woods LC. Comparative fatty acid composition of eggs from domesticated and wild striped bass (Morone saxatilis) Aquaculture. 1995;133:225–233. [Google Scholar]
- 19.Gallagher ML, Paramore L, Alves D, Rulifson RA. Comparison of phospholipid and fatty acid composition of wild and cultured striped bass eggs. J Fish Biol. 1998;52:1218–1228. [Google Scholar]
- 20.Jepson PD, et al. Relationships between polychlorinated biphenyls and health status in harbor porpoises (Phocoena phocoena) stranded in the United Kingdom. Environ Toxicol Chem. 2005;24:238–248. doi: 10.1897/03-663.1. [DOI] [PubMed] [Google Scholar]
- 21.Bergeron JM, Crews D, McLachlan JA. PCBs as environmental estrogens: Turtle sex determination as a biomarker of environmental contamination. Environ Health Perspect. 1994;102:780–781. doi: 10.1289/ehp.94102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spies RB, Rice DWJ. Effects of organic contaminants on reproduction of the starry flounder Platichthys stellatus in San Francisco Bay. Mar Biol. 1988;98:191–200. [Google Scholar]
- 23.Gutleb AC, et al. Delayed effects of pre- and early-life time exposure to polychlorinated biphenyls on tadpoles of two amphibian species (Xenopus laevis and Rana temporaria) Environ Toxicol Chem. 1999;8:1–14. doi: 10.1016/s1382-6689(99)00023-x. [DOI] [PubMed] [Google Scholar]
- 24.Njiwa JRK, Müller P, Klein R. Life cycle stages and length of zebrafish (Danio rerio) exposed to DDT. J Health Sci. 2004;50:220–225. [Google Scholar]
- 25.Waring CP, Moore A. Sublethal effects of a carbamate pesticide on pheromonal mediated endocrine function in mature male Atlantic salmon (Salmo salar L. ) Parr. Fish Physiol Biochem. 1997;17:203–211. [Google Scholar]
- 26.Billard R, Roubaud P. The effect of metals and cyanide on fertilization in rainbow trout (Salmo gairdneri) Water Res. 1985;19:209–214. [Google Scholar]
- 27.Scholz NL, et al. Diazinon disrupts antipredator and homing behaviors in chinook salmon (Oncorhynchus tshawytscha) Can J Fish Aquat Sci. 2000;57:1911–1918. [Google Scholar]
- 28.Sandahl JF, Baldwin DH, Jenkins JJ, Scholz NL. Comparative thresholds for acetylcholinesterase inhibition and behavioral impairment in coho salmon exposed to chlorpyrifos. Environ Toxicol Chem. 2005;24:136–145. doi: 10.1897/04-195r.1. [DOI] [PubMed] [Google Scholar]
- 29.Hinton DE, Klaunig JE, Lipsky MM. PCB-induced alterations in teleost liver: A model for environmental disease in fish. Mar Fish Rev. 1978;40:47–50. [Google Scholar]
- 30.Huggett RJ, Kimerle RAJ, Mehrle PMJ, Bergman HL. Biomarkers: Biochemical, Physiological, and Histological Markers of Anthropogenic Stress. Boca Raton, FL: Lewis; 1992. [Google Scholar]
- 31.Donohoe RM, Curtis LR. Estrogenic activity of chlordecone, o,p′-DDT and o,p′-DDE in juvenile rainbow trout: Induction of vitellogenesis and interaction with hepatic estrogen binding sites. Aquat Toxicol. 1996;36:31–52. [Google Scholar]
- 32.James MO, et al. Increased toxicity of benzo[a}pyrene-7,8-dihydrodiol in the presence of polychlorobiphenylols. Mar Environ Res. 2004;58:343–346. doi: 10.1016/j.marenvres.2004.03.079. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt K, Steinberg CEW, Staaks GBO, Pflugmacher S. Influence of a xenobiotic mixture (PCB and TBT) compared to single substances on swimming behavior or reproduction of Daphnia magna. Acta Hydrochim Hydrobiol. 2005;33:287–300. [Google Scholar]
- 34.Easton MDL, Luszniak D, Von der Geest E. Preliminary examination of contaminant loadings in farmed salmon, wild salmon and commercial salmon feed. Chemosphere. 2002;46:1053–1074. doi: 10.1016/s0045-6535(01)00136-9. [DOI] [PubMed] [Google Scholar]
- 35.de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- 36.Rayne S, Ikonomou MG, Antcliffe B. Rapidly increasing polybrominated diphenyl ether concentrations in the Columbia River system from 1992 to 2000. Environ Sci Technol. 2003;37:2847–2854. doi: 10.1021/es0340073. [DOI] [PubMed] [Google Scholar]
- 37.Ueno D, et al. Global pollution monitoring of polybrominated diphenyl ethers using skipjack tuna as a bioindicator. Environ Sci Technol. 2004;38:2312–2316. doi: 10.1021/es035323k. [DOI] [PubMed] [Google Scholar]
- 38.Oros DR, et al. Levels and distribution of polybrominated diphenyl ethers in water, surface sediments, and bivalves from the San Francisco Estuary. Environ Sci Technol. 2005;39:33–41. doi: 10.1021/es048905q. [DOI] [PubMed] [Google Scholar]
- 39.Theilacker GH. Effect of starvation on the histological and morphological characteristics of jack mackerel, Trachurus symmetricus, larvae. US Fish Bull. 1978;76:403–414. [Google Scholar]
- 40.Setzler-Hamilton EM, Wright DA, Martin FD, Millsaps CV, Whitlow SI. Analysis of nutritional condition and its use in predicting striped bass recruitment: Field studies. Am Fish Soc Symp. 1987;2:115–128. [Google Scholar]
- 41.Crews D, Willingham E, Skipper JK. Endocrine disruptors: Present issues, future directions. Q Rev Biol. 2000;75:243–260. doi: 10.1086/393498. [DOI] [PubMed] [Google Scholar]
- 42.Zhou T, Taylor MM, DeVito MJ, Crofton KM. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol Sci. 2002;66:105–116. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]
- 43.Miller MA. Maternal transfer of organochlorine compounds in salmonines to their eggs. Can J Fish Aquat Sci. 1993;50:1405–1413. [Google Scholar]
- 44.Westerlund L, et al. Early life-stage mortality in zebrafish (Danio rerio) following maternal exposure to polychlorinated biphenyls and estrogen. Environ Toxicol Chem. 2000;19:1582–1588. [Google Scholar]
- 45.Howard CV, Reed M. Volume and surface-area estimation from microscopic images. J Microsc. 1998;190:291–291. doi: 10.1046/j.1365-2818.1998.00331.x. [DOI] [PubMed] [Google Scholar]
- 46.Gundersen HJG, Jensen EBV, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology—Reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.