Abstract
Anopheles gambiae mosquitoes are the principal vectors of malaria. A major determinant of the capacity of these mosquitoes as disease vectors is their high reproductive rate. Reproduction depends on a single insemination, which profoundly changes the behavior and physiology of females. To identify factors and mechanisms relevant to the fertility of A. gambiae, we performed a comprehensive analysis of the molecular and cellular machinery associated with copulation in females. Initial whole-body microarray experiments comparing virgins with females at 2 h, 6 h, and 24 h after mating detected large transcriptional changes. Analysis of tissue localization identified a subset of genes whose expression was strikingly regulated by mating in the lower reproductive tract and, surprisingly, the gut. In the atrium of virgin females, where the male seminal fluid is received, our studies revealed a “mating machinery” consisting of molecular and structural components that are turned off or collapse after copulation, suggesting that this tissue loses its competence for further insemination. In the sperm storage organ, we detected a number of mating-responsive genes likely to have a role in the maintenance and function of stored sperm. These results identify genes and mechanisms regulating the reproductive biology of A. gambiae females, highlighting considerable differences with Drosophila melanogaster. Our data inform vector control strategies and reveal promising targets for the manipulation of fertility in field populations of these important disease vectors.
Keywords: mosquito, post-mating response, reproduction, microarray, reproductive tract
The mosquito Anopheles gambiae is the principal vector of malaria, a disease that kills over a million people each year. The development of effective tools for controlling vector populations is of paramount importance. Promising genetic control strategies are emerging, such as those based on the sterile insect technique (SIT) (1), or the use of selfish genetic elements to skew the sex ratios of natural populations (2). Many of these measures rely on the ability to manipulate mosquito reproduction, a topic that remains poorly studied.
The explosive reproductive rate of A. gambiae females is an important determinant of their vectorial capacity. The entire reproductive output of a female is contingent on a single mating, which inhibits remating and is sufficient to acquire enough sperm to fertilize a lifetime supply of eggs. The inability to replace aged or depleted sperm means that females must possess a highly reliable mechanism for maintaining the viability of stored sperm. A successful copulation is also essential for ovulation and oviposition; although virgin females can produce mature eggs after blood feeding, they do not lay them until mating has occurred (3). Also, mating may increase the competence of females to respond to blood feeding: when access to a blood meal is limited, females that have mated are more likely to produce eggs than are virgins (4).
Studies on the molecular basis of the female response to mating in insects have so far been mainly limited to Drosophila melanogaster, where postcopulatory changes in gene expression are generally of small scale (<2-fold) (5–8). Across a number of microarray studies, only 1 functional class shows a consistently strong response to mating: immune genes, in particular antimicrobial peptides (AMPs), are highly induced (5–10). In the only other insect analyzed to date, the honey bee Apis mellifera, mating mainly causes transcriptional changes in genes associated with egg production in the ovaries (11).
To identify factors and mechanisms essential for fertility in A. gambiae, we conducted an initial whole-genome microarray analysis of transcript levels comparing virgins with females at different time points after mating, followed by a detailed tissue-specific and temporal analysis of the expression of a large subset of genes by quantitative real-time PCR (qRT-PCR) and by ultrastructural examination of part of the female reproductive tract. Our analyses reveal that unlike Drosophila, A. gambiae females undergo prominent transcriptional changes after mating, and unveil a dedicated “mating machinery” in the reproductive tract, composed of molecular and structural factors that are switched off or profoundly altered after copulation. Combined with the identification of a number of genes potentially important to sperm storage and function, our results have implications for genetic vector control programs and provide targets for the development of new tools for combating malaria.
Results
Transcriptional Responses to Mating in Whole A. gambiae Females.
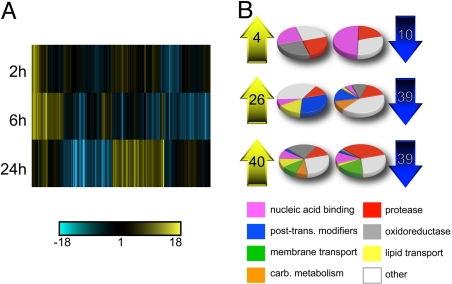
To investigate changes in gene expression induced by mating in female mosquitoes, we compared transcript levels in virgin females with females at 2 h, 6 h, and 24 h after mating. We chose to examine female whole bodies at 3 widely spread time points to capture a comprehensive picture of the transcriptional response to mating. To focus on the genes that exhibited the largest responses to mating, and to reduce the risk of including false-positives, we restricted our analysis to probesets that changed a minimum of 2-fold in mated females at least at 1 time point compared with virgins [Fig. 1A; supporting information (SI) Table S1]. These probesets were mapped to a total of 141 unique VectorBase gene predictions (http://agambiae.vectorbase.org). The majority of the mating-responsive genes could be assigned to 1 of 8 broad functional classes (Fig. 1B). Among the genes analyzed, 60% contained a secretory signal peptide or at least 1 transmembrane domain.
Fig. 1.
Transcriptional response to mating in whole A. gambiae females. (A) Agglomerative cluster using cosine correlation-based similarity of 141 genes exhibiting a >2-fold change in expression at 2 h, 6 h, and 24 h after mating. Each gene is represented by a vertical line. Expression relative to virgins is indicated by a color gradient (see bar). (B) Functional classes of the 141 genes. The number of genes up-regulated (Left) and down-regulated (Right) at each time point are specified in the arrows. Pie charts indicate the proportion of up-regulated (Left) and down-regulated (Right) genes at each time point that belong to each functional category. Genes involved in cell cycle regulation have been included in the “nucleic acid binding” group.
The number of genes differentially expressed in mated females increased with time. At 2 h postmating, only 14 genes exhibited >2-fold changes relative to virgins: 4 were up-regulated and 10 were down-regulated (Fig. 1B; Table S1). Intriguingly, 8 of the 10 down-regulated genes, most of which are involved in nucleic acid binding and cell cycle control, were shown to be up-regulated in the ovaries in response to a blood meal (12). A gene encoding a putative andropin-like AMP (AGAP009429, also found in the A. gambiae male accessory glands; see ref. 13) was “switched on” at this early time point, and remained highly expressed at 6 h (Table S1).
By 6 h postmating, the number of differentially expressed genes increased to 65, with 26 up-regulated and 39 down-regulated genes (Fig. 1B). The gene showing the largest response to mating, the Kunitz-type protease inhibitor AGAP009766, was switched on at this time point (Table S1). Several up-regulated genes were involved in lipid transport, including the yolk protein precursor vitellogenin (AGAP004203) and cathepsin b (AGAP004534, thought to assist vitellogenin function in the ovaries; see ref. 14). This result was unexpected, because vitellogenesis does not occur in nonbloodfed females and vitellogenin has not been posited to have a role outside of egg maturation in mosquitoes. Other up-regulated genes included a number of putative juvenile hormone (JH)-re- sponsive genes (AGAP003757, AGAP007946, AGAP005065, and AGAP000750). JH levels determine the competence of the ovaries and fat body to respond to blood feeding (15). The up-regulation of JH-responsive genes is consistent with the hypothesis that JH produced by the male accessory glands is transferred to the female during mating (16), and could also underlie the increased competence of mated females to produce mature eggs in response to a blood meal (4). The largest class of genes down-regulated at 6 h postmating comprised integral membrane transporters (Fig. 1B; Table S1), including the ATP-binding cassette (ABC) AGAP011518. ABC transporters transfer lipids, among other molecules, from inside cells across the plasma membrane to extracellular lipid transport proteins (17).
By 24 h postmating, 40 genes exhibited >2-fold up-regulation and 39 an equivalent down-regulation (Fig. 1B). Many of the genes up-regulated at this time point were oxidoreductases, including peroxidase 4b (AGAP010810), which was switched on by mating. Two more genes were switched on, the zinc carboxypeptidase AGAP008373 and the fibrinogen AGAP012000 (Table S1). Strikingly, transcripts for 13 proteases were dramatically down-regulated (Table S1). Of these, 8 encoded secreted serine proteases (trypsins and chymotrypsins) and 5 belonged to classes typically associated with the processing of peptide hormones (18), including a zinc metalloprotease (AGAP000885), a glutamyl aminopeptidase (AGAP003077), a dipeptidyl-peptidase (AGAP008176), and 2 neprilysins (AGAP001791 and AGAP009791). Two ABC transporters were also down-regulated at 24 h, AGAP011518, which was also down-regulated at 6 h, and AGAP001858 (Table S1).
From previous work on D. melanogaster, we expected that mating would result in heightened expression of immune genes. Surprisingly, no gene with a demonstrated function in the mosquito immune system (19) was found to change >2-fold in response to mating. To confirm the array results, we tested 5 genes encoding AMPs (Gambicin, Defensin 1, Cecropin 1, Cecropin 2, and Cecropin 3) alongside 3 other immune genes [Thioester-containing protein 1 (TEP1), Leucine-rich immune protein 1 (LRIM1), and C-type lectin 4 (CTL4)] by qRT-PCR on whole females. In line with the array results, we found no consistent >2-fold increase in expression associated with mating in any of the genes tested (Fig. S1).
Tissue-Specific Changes in Gene Expression.
The analysis of transcriptional levels in whole females revealed major changes after mating. To ascertain the tissue localization of these changes, we analyzed by qRT-PCR a total of 20 mating responsive genes in 5 different female tissues: the lower reproductive tract (LRT), the ovaries, the head, the gut, and the carcass (Fig. 2). Because few changes in gene expression were observed on the array at 2 h postmating, we restricted our analysis to comparisons between virgins and females at 6 h or 24 h postmating. Among these genes were 10 of the 13 proteases down-regulated at 24 h, 5 genes switched on by mating, and 3 lipid transport genes including vitellogenin and the ABC transporter AGAP011518. All 20 genes encoded proteins predicted to be secreted or localized to the plasma membrane.
Fig. 2.
Tissues of expression of mating responsive genes. Schematic representation of a whole female mosquito, showing the tissues examined by qRT-PCR and the genes detected in each tissue, listed in the insets of the corresponding color. The tissues tested were the whole head (head capsule and brain), the gut (midgut, hindgut, and rectum), the ovaries, the LRT (atrium, spermatheca, and parovarium), and the carcass (cuticle, muscle, and fat body). A schematic representation of the female reproductive tract is also provided, showing the atrium (yellow) and the spermatheca (brown). The ovaries and the parovarium are greyed as they were not analyzed further. In bold are genes that are primarily expressed in 1 tissue (at least 10-fold higher than in any other tissue, after correcting for control gene levels). For genes expressed in the LRT and gut, mean fold changes from 3 biological replicates are indicated (Fold change) and the time point showing the largest change (Time, h). The levels of expression of each gene in virgins (V) and at 6 h and 24 h, normalized against the control gene RpL19, are indicated by a color gradient (see bar). The last column in the LRT inset indicates whether a gene was expressed primarily in the atrium (A) or spermatheca (S). All changes are significant at the level of P < 0.05 unless otherwise indicated (ns). †, also down-regulated at 24 h (−3.9-fold); ‡, also up-regulated at 24 h (73.3-fold).
The vast majority of genes (17/20) were found to be expressed predominantly or exclusively in a single tissue (Fig. 2). Most were detected in the LRT and a conspicuous number, surprisingly, in the gut. The 3 remaining genes were detected in multiple tissues. No gene was primarily expressed in the head or the carcass, although some were detected at low levels in these tissues. Remarkably, none of the genes tested was expressed at detectable levels in the ovaries of virgin or mated females at any time point (Fig. 2).
A. LRT Genes.
A total of 14 genes were detected in the LRT, 11 expressed almost exclusively in this tissue (Fig. 2). They included 8 secreted proteases, 6 of which showed large, statistically significant decreases in expression at 24 h. AGAP000885, AGAP009791, AGAP005194, and AGAP005195 were expressed at high levels in the virgin LRT, whereas baseline expression levels of AGAP001791 and AGAP005196 were considerably lower. Another protease, AGAP003077, was also expressed in other tissues and did not show any significant response to mating (Fig. 2). Also, 4 genes switched on (the andropin-like AGAP009429, the Kunitz-type protease inhibitor AGAP009766, and the peroxidase AGAP010810) or strongly induced (the gene of unknown function AGAP002620) by mating were found in the LRT (Fig. 2). AGAP009429 was also detected at low levels in the head, where it showed no response to mating. Among the lipid transport genes, the yolk protein vitellogenin (AGAP004203) was predominantly expressed in the LRT, where it was surprisingly up-regulated >200-fold at 6 h (Fig. 2). A low level of vitellogenin expression was also detected in the head and the carcass. The ABC transporter AGAP011518 was also expressed exclusively in this tissue, where it was significantly down-regulated at both 6 h and 24 h (Fig. 2).
To gain further insight into the tissue-localization of these mating-responsive genes, we compared their levels of expression in the 2 principal organs of the LRT: the atrium [where the male seminal fluid is received in the form of a gelatinous “mating plug” (20)] and the spermatheca (where sperm are stored) (Fig. 2). We found that most of the down-regulated proteases were expressed primarily in the atrium. Two exceptions were AGAP005196 (predominant in the spermatheca) and AGAP001791 (similar in both organs). Interestingly, the protease inhibitor AGAP009766, which is switched on at 6 h, was also strongly expressed in the atrium, suggesting a role in the inhibition of the atrial proteases. In contrast to the proteases, both lipid transport genes, vitellogenin and the ABC transporter, were expressed primarily in the spermatheca, and so was peroxidase 4b. The remaining genes switched on in the LRT were expressed either in the atrium (AGAP002620) or detected in both tissues (AGAP009429) (Fig. 2).
B. Gut Genes.
Among the 20 genes analyzed by qRT-PCR, 8 were detected in the gut, 6 of which were expressed primarily in this tissue (Fig. 2). This result was unexpected, because the digestive apparatus has never been associated with mating-induced responses in insects. We observed strong up-regulation of the endopeptidase AGAP006385 at 6 h. This gene also exhibited a large increase in the LRT at the same time point. A second protease, AGAP004809, showed a significant down-regulation at 6 h. The remaining gut-enriched mating responsive genes were expressed at low levels and did not show any significant change after mating.
Mating Induces Permanent Changes in Females.
We next investigated whether mating was inducing long-lasting transcriptional responses in females beyond the time points analyzed in our experiments. To this aim, we compared the expression of 10 genes, 7 that were strongly down-regulated and 3 that were highly up-regulated at 24 h after mating (Table S1; Fig. 2), in virgins and females 4 days postmating. Remarkably, many of the genes tested showed permanent regulation by mating (Fig. S2). Among the genes expressed in the atrium, the proteases AGAP000885 and AGAP009791 remained strongly down-regulated, the protease AGAP005194 and the neprilysin AGAP001791 showed reduced expression, whereas only the protease AGAP005195 returned to virgin levels. Expression of AGAP002620 at 4-days postmating was variable, but in general remained up-regulated, as did the andropin-like AGAP009429. Two spermatheca-specific genes, the peroxidase b AGAP010810 and the ABC transporter AGAP011518, remained respectively up-regulated and repressed, whereas the serine proteases AGAP005196 returned to levels similar to those observed in virgins (Fig. S2).
The Ultrastructure of the Atrium Is Profoundly Altered by Mating.
Our analyses showed that the majority of the transcriptional changes induced by mating occurred in the atrium. To determine whether these extensive transcriptional responses were mirrored by morphological changes, we used transmission electron microscopy (TEM) to compare the ultrastructure of the atrium in virgins and females 8 h after mating (Fig. 3).
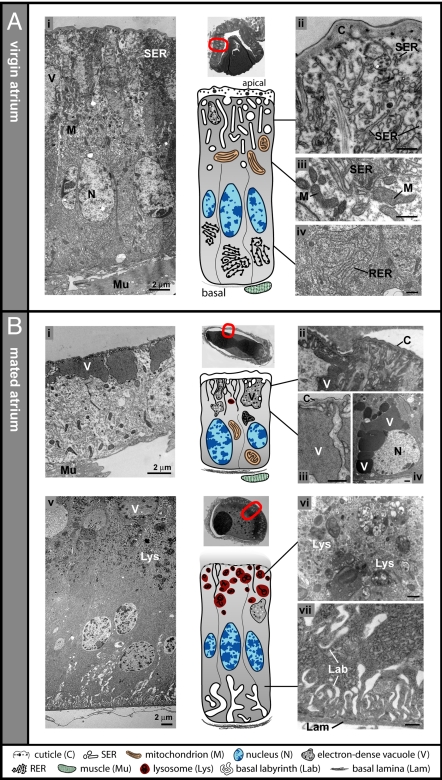
Fig. 3.
Transmission electron micrographs of atrium cells. In virgin mosquitoes (A), and in mated mosquitoes (8 h) (B). (Ai) Representative atrial cell from a virgin, showing SER and storage vacuoles populating the apical cytoplasm (ii, detail), mitochondria alongside the SER (iii, detail), and abundant RER at the basal pole (iv, detail). (Bi): Atrial cell after mating, showing electron-dense vacuoles at the apical surface with collapsed SER membranes (ii and iii, detail from this and neighbouring cells). Other cells contain vacuoles of varying composition that cluster basally (iv, detail). Cell in direct contact with the mating plug in a different mated female (v). Endosomes and lysosomes occur densely at the apical pole (vi, detail), the basal labyrinth is expanded and the basal lamina is distinct (vii, detail). Some vacuoles are also visible (v). Thumbnail images above the diagrams show low-magnification sections of the whole atrium indicating the micrograph locations. Images are orientated with the apical surface uppermost. (Scale bar, 500 nm unless otherwise stated.)
TEM analysis of the atrium of virgin females (with the exception of cells in a region of tight contact with embedding-resistant external structures; see Fig. 3A, thumbnail) showed highly polarized cells (Fig. 3Ai) typical of class 1 insect epithelia (21). Like most insect transporting epithelia (22), the luminal surface of these cells was coated with a cuticle, which lacked any obvious channels or pores (Fig. 3Aii). Strikingly, the apical (luminal) cytoplasm was filled with strongly developed irregular tubular and vesicular smooth endoplasmic reticulum (SER) (Fig. 3Aii), containing heterogeneous material. High numbers of mitochondria surrounded the SER cisternae (Fig. 3Aiii). The apical regions of some cells contained large storage organelles (or vacuoles) that did not appear to be actively secreting their granular contents (Fig. 3Ai). Free polyribosomes were distributed throughout the cytoplasm. The basal poles of the cells lacked a well-developed basal labyrinth and were packed with rough endoplasmic reticulum (RER), indicating a high capacity of these cells for protein synthesis (Fig. 3Aiv).
After mating, profound transformations were observed and most of the features that characterized virgin atrial cells were no longer identifiable. Remarkably, the extensive apical SER detected in virgins was mostly lost (Fig. 3Bi), although in places we observed some membranous material, seemingly originated from SER fused with the cuticle (Fig. 3 Bii–Biii). Mitochondria were no longer confined to the apical half of the cell but more evenly distributed, and the previously dense basal RER was greatly reduced (Fig. 3Bi). Many of the epithelial cells were cuboidal (Fig. 3Bi), possibly from distension caused by the presence of the mating plug or from cytoplasmic recession due to the loss of organelles.
Another hallmark of the mated atrium 8 h postcopulation was the appearance of several new features not detected in virgins. At the apical poles of some cells, we observed large electron-dense vacuoles of uniform granular composition (Fig. 3 Bi–Biii) that were continuous with the cuticle, suggesting a large scale transfer of material between the atrial lumen and the epithelial cells. Occasionally, we found electron-dense vacuoles of variable composition aggregated near the basal surface (Fig. 3Biv). In regions where the mating plug was observed in direct contact with the epithelium, the exchange of material was so extensive that it was impossible to identify the boundary between the epithelium and the plug itself (Fig. 3Bv). These cells often exhibited a strikingly dense apical accumulation of endosomes and lysosomes (Fig. 3 Bv and Bvi), accompanied by a comprehensive expansion of the basal labyrinth and lamina (Fig. 3Bvii), suggestive of an active uptake of plug material by the atrial cells.
Discussion
The data reported here identify previously undescribed factors and mechanisms shaping the mating response of A. gambiae females. The combined results from the array, qRT-PCR, and TEM strongly indicate that the elements required for successful insemination are present in the atria of female A. gambiae before mating. This mating machinery consists of structural and molecular components including a highly developed SER and a number of highly expressed secreted proteases that are successively turned off or profoundly altered after mating. Several of these proteases are relatively nonspecific digestive enzymes, whereas others are normally associated with processing of neuropeptides. An intriguing hypothesis is that, on mating, the first class breaks down the mating plug (formed by the male seminal fluid) into its component parts, which are then processed by the second class. Existing data suggests that in A. gambiae such processing of the mating plug in the female atrium is necessary for triggering postmating responses, that are indeed not elicited when delivery of male seminal fluids bypasses the mating machinery (3).
Extensive SER and large numbers of mitochondria are generally associated with production and secretion of lipids. However, in our TEM analysis, the lipid droplets and/or glycogen deposits that usually accompany these organelles were conspicuously absent from the virgin atrium. These observations point to a role of the SER in the storage and secretion of proteins, similar to the secretory mammalian epididymis (23, 24). Interestingly, the disappearance of the SER after mating combined with the parallel permanent down-regulation of most of the proteases suggests that the former is involved in the synthesis and secretion of the latter. Preliminary TEM analyses carried out at 24 h indicate that the SER is not reformed at later time points after mating (data not shown). The loss of the mating machinery may represent a physiological barrier against remating, making mated females simply not competent for subsequent insemination. This observation would have important implications for genetic vector control strategies such as SIT, because the evolution of polyandry would likely hamper their success (25). The lack of multiple mating in mosquitoes might also explain the absence of a mating-induced immune response in our study, because immune responses associated with protection against sexually transmitted diseases, cryptic female choice, and sperm competition (26) are not likely in monandrous species.
Although the virgin atrium is clearly ready to respond to insemination, the spermatheca seems to rely on the mating-induced regulation of a number of genes for sperm storage and function. One gene that exhibited a massive response to mating in the spermatheca is vitellogenin. Interestingly, yolk proteins have also been found in the spermatheca of D. melanogaster (27). Because this protein transports lipids from the fat body to the ovaries during vitellogenesis, and the spermatheca is surrounded by fat body, it is possible that vitellogenin transports lipids into the spermathecal capsule to provision sperm (28). Also, the down-regulation of 2 ABC transporters (AGAP001858 and AGAP011518, the second being expressed primarily in the spermatheca) could help to regulate the lipid reserves required for sperm storage. Additionally or alternatively, vitellogenin might help “prepare” the spermatheca for long-term sperm storage by acting as a powerful antioxidant removing sperm-damaging free radicals (29). The parallel activation of the peroxidase AGAP010810 (as well as other oxidoreductases; see Table S1) observed mostly by 24 h may be associated with the acquisition of sperm motility, which is strongly regulated by lipid peroxidation of sperm membranes (30). Intriguingly, in A. gambiae sperm are immotile when transferred to the female until at least 17 h after mating (31). Therefore, our analysis unveils possible mechanisms for sperm storage, nutrition, and activation, which deserves further investigation.
We were surprised to find that some mating responsive genes were expressed primarily or exclusively in the gut, because this tissue has traditionally been overlooked in studies of insect mating. However, the gut is an important endocrine tissue, releasing peptides similar to the gut-brain hormones of vertebrates that are crucial modulators of reproductive physiology (32). It will be interesting to determine whether the endocrine cells of the gut respond to copulation. However, we observed no transcriptional response to mating in the ovaries, contrary to data from A. mellifera and, to a minor extent, D. melanogaster (7, 11). The observed difference may rely on the fact that, whereas in both A. mellifera and D. melanogaster egg maturation is mostly mating-dependent, oogenesis in A. gambiae additionally requires blood feeding.
Importantly, our data reveal that failure to control the mating status of females in gene expression experiments could affect the interpretation of the results obtained. For example, some mating-responsive genes down-regulated at 24 h were previously found to be repressed in response to blood feeding (12). However, for most of these genes, we were unable to confirm any significant effect of blood feeding on their expression in virgin females (Fig. S3). Because the mating status of females used in the previous study was unknown, it is possible that the observed changes in these particular genes were caused by copulation and not by blood feeding.
The results reported here reinforce the notion that mating profoundly affects the biology and physiology of female Anopheles mosquitoes, and strongly contribute to an improved understanding of the factors and mechanisms shaping reproductive success in these important malaria vectors, providing powerful new targets for the manipulation of their fertility at the population level.
Materials and Methods
Mosquito Procedures.
A. gambiae mosquitoes from a laboratory colony of the G3 strain were separated by sex as pupae to ensure their status as virgins. Experimental matings were carried out as described in the SI Experimental Procedures. In all experiments, virgin females were raised under identical conditions, and dissected at the same age, as mated females.
Microarray Analysis.
Total RNA was extracted from 15 females for 3 biological replicates at each time point (virgins, 2 h, 6 h, and 24 h), and then processed, labeled, and hybridized to Affymetrix GeneChip Plasmodium/Anopheles genome arrays by using standard protocols. Data analysis was carried out by using Rosetta Resolver 7.1 (Rosetta Biosoftware). Full details of the analysis are provided in the SI Experimental Procedures.
Quantitative RT-PCRs.
In all analyses, genes were considered to be expressed in a particular tissue if the highest level of expression (at any time point) was >2% the level of expression of the ribosomal RpL19 control gene. SYBR green-based detection was used to quantify the expression of all genes, except immune genes, which were analyzed by using TaqMan gene expression assays (primers and probes are listed in Tables S2 and S3). Full details of these methods are provided in the SI Experimental Procedures. Data were analyzed by using one-tailed Student's t tests for changes in the direction indicated by the microarray data (unless otherwise specified).
TEM.
Atria were dissected from virgins and mated females in 0.1 M phosphate buffer pH 7.2 at 8 h after mating, and fixed overnight with 2.5% glutaraldehyde. Samples were postfixed in 1% osmium tetroxide in phosphate buffer, rinsed, dehydrated through a graded ethanol series, and embedded by using 4 changes of araldite resin. After screening toluidine blue stained semithin sections, several ultrathin sections per sample were transferred to copper grids, stained with uranyl acetate, observed at 60 kV on a Hitachi 7500 transmission electron microscope.
Supplementary Material
Acknowledgments.
We thank all members of the Levashina laboratory for their generous assistance, Andrea Crisanti for suggestions and support, and Valérie Demais (l'Institut Fédératif de Recherche Neurosciences, Strasbourg) for the use of the TEM platform. This work was supported by an Medical Research Council Career Development Award G0600062, Agreement ID 78415 (to F.C. and D.W.R.); Centre National de la Recherche Scientifique (E.A.L.), the Royal Society International Research Travel Grant 2007/R2-IJP (to F.C.), the Foundation for the National Institutes of Health through the Grand Challenges in Global Health initiative grant (J.T.), and a European Science Foundation Exchange Grant 1738 (to M.M.A.W.). E.A.L. is an International Research Scholar of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
Data deposition: The microarray data reported in this paper have been deposited with Arrayexpress, http://www.ebi.ac.uk/microarray-as/ae (accession number M-EXP-1868).
This article contains supporting information online at www.pnas.org/cgi/content/full/0809723105/DCSupplemental.
References
- 1.Benedict MQ, Robinson AS. The first releases of transgenic mosquitoes: An argument for the sterile insect technique. Trends Parasitol. 2003;19:349–355. doi: 10.1016/s1471-4922(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 2.Burt A. Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proc R Soc Lond Ser B: Biol Sci. 2003;270:921–928. doi: 10.1098/rspb.2002.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klowden MJ. Sexual receptivity in Anopheles gambiae mosquitoes: Absence of control by male accessory gland substances. J Insect Physiol. 2001;47:661–666. doi: 10.1016/s0022-1910(00)00127-x. [DOI] [PubMed] [Google Scholar]
- 4.Klowden MJ, Russell RC. Mating affects egg maturation in Anopheles gambiae Giles (Diptera : Culicidae) J Vector Ecol. 2004;29:135–139. [PubMed] [Google Scholar]
- 5.Lawniczak MK, Begun DJ. A genome-wide analysis of courting and mating responses in Drosophila melanogaster females. Genome. 2004;47:900–910. doi: 10.1139/g04-050. [DOI] [PubMed] [Google Scholar]
- 6.Mack PD, Kapelnikov A, Heifetz Y, Bender M. Mating-responsive genes in reproductive tissues of female Drosophila melanogaster. Proc Natl Acad Sci USA. 2006;103:10358–10363. doi: 10.1073/pnas.0604046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGraw LA, Clark AG, Wolfner MF. Post-mating gene expression profiles of female Drosophila melanogaster in response to time and to four male accessory gland proteins. Genetics. 2008;179:1395–1408. doi: 10.1534/genetics.108.086934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGraw LA, Gibson G, Clark AG, Wolfner MF. Genes regulated by mating, sperm, or seminal proteins in mated female Drosophila melanogaster. Curr Biol. 2004;14:1509–1514. doi: 10.1016/j.cub.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Domanitskaya EV, Liu H, Chen S, Kubli E. The hydroxyproline motif of male sex peptide elicits the innate immune response in Drosophila females. FEBS J. 2007;274:5659–5668. doi: 10.1111/j.1742-4658.2007.06088.x. [DOI] [PubMed] [Google Scholar]
- 10.Peng J, Zipperlen P, Kubli E. Drosophila sex-peptide stimulates female innate immune system after mating via the Toll and Imd pathways. Curr Biol. 2005;15:1690–1694. doi: 10.1016/j.cub.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 11.Kocher SD, Richard FJ, Tarpy DR, Grozinger CM. Genomic analysis of post-mating changes in the honey bee queen (Apis mellifera) BMC Genomics. 2008;9:232. doi: 10.1186/1471-2164-9-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marinotti O, et al. Genome-wide analysis of gene expression in adult Anopheles gambiae. Insect Mol Biol. 2006;15:1–12. doi: 10.1111/j.1365-2583.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- 13.Dottorini T, et al. A genome-wide analysis in Anopheles gambiae mosquitoes reveals 46 male accessory gland genes, possible modulators of female behavior. Proc Natl Acad Sci USA. 2007;104:16215–16220. doi: 10.1073/pnas.0703904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho WL, et al. Mosquito cathepsin B-like protease involved in embryonic degradation of vitellin is produced as a latent extraovarian precursor. J Biol Chem. 1999;274:13311–13321. doi: 10.1074/jbc.274.19.13311. [DOI] [PubMed] [Google Scholar]
- 15.Noriega FG. Nutritional regulation of JH synthesis: A mechanism to control reproductive maturation in mosquitoes? Insect Biochem Mol Biol. 2004;34:687–693. doi: 10.1016/j.ibmb.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 16.Borovsky D, Carlson DA, Hancock RG, Rembold H, van Handel E. De novo biosynthesis of juvenile hormone III and I by the accessory glands of the male mosquito. Insect Biochem Mol Biol. 1994;24:437–444. doi: 10.1016/0965-1748(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 17.Oram JF, Heinecke JW. ATP-binding cassette transporter A1: A cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev. 2005;85:1343–1372. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- 18.Riehle MA, Garczynski SF, Crim JW, Hill CA, Brown MR. Neuropeptides and peptide hormones in Anopheles gambiae. Science. 2002;298:172–175. doi: 10.1126/science.1076827. [DOI] [PubMed] [Google Scholar]
- 19.Christophides GK, Vlachou D, Kafatos FC. Comparative and functional genomics of the innate immune system in the malaria vector Anopheles gambiae. Immunol Rev. 2004;198:127–148. doi: 10.1111/j.0105-2896.2004.0127.x. [DOI] [PubMed] [Google Scholar]
- 20.Giglioli MEC. The female reproductive system of Anopheles gambiae melas. The structure and function of the genital ducts and associated organs. Riv Malariol. 1963;42:149–176. [PubMed] [Google Scholar]
- 21.Noirot C, Quennedey A. Fine structure of insect epidermal glands. Annu Rev Entomol. 1974;19:61–80. [Google Scholar]
- 22.Cioffi M. Comparative ultrastructure of arthropod transporting epithelia. Am Zool. 1984;24:139–156. [Google Scholar]
- 23.Dacheux F, Dacheux JL. Androgenic control of antagglutinin secretion in the boar epididymal epithelium. An immunocytochemical study. Cell Tissue Res. 1989;255:371–378. doi: 10.1007/BF00224120. [DOI] [PubMed] [Google Scholar]
- 24.Flickinger CJ, Wilson KM, Gray HD. The secretory pathway in the mouse epididymis as shown by electron microscope radioautography of principal cells exposed to monensin. Anat Rec A Discov Mol Cell Evol Biol. 1984;210:435–448. doi: 10.1002/ar.1092100304. [DOI] [PubMed] [Google Scholar]
- 25.Whitten M, Mahon R. In: Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management. Dyck VA, Hendrichs J, Robinson AS, editors. Dordrecht, The Netherlands: Springer; 2005. pp. 601–626. [Google Scholar]
- 26.Lawniczak MK, et al. Mating and immunity in invertebrates. Trends Ecol Evol. 2007;22:48–55. doi: 10.1016/j.tree.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Allen AK, Spradling AC. The Sf1-related nuclear hormone receptor Hr39 regulates Drosophila female reproductive tract development and function. Development. 2008;135:311–321. doi: 10.1242/dev.015156. [DOI] [PubMed] [Google Scholar]
- 28.Werner M, Simmons LW. Insect sperm motility. Biol Rev Camb Philos Soc. 2008;83:191–208. doi: 10.1111/j.1469-185X.2008.00039.x. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura A, et al. Vitellogenin-6 is a major carbonylated protein in aged nematode, Caenorhabditis elegans. Biochim Biophys Acta. 1999;264:580–583. doi: 10.1006/bbrc.1999.1549. [DOI] [PubMed] [Google Scholar]
- 30.Aitken RJ, Baker MA. Oxidative stress, sperm survival and fertility control. Mol Cell Endocrinol. 2006;250:66–69. doi: 10.1016/j.mce.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 31.Verhoek BA, Takken W. Age effects on the insemination rate of Anopheles gambiae s. l. in the laboratory. Entomol Exp Appl. 1994;72:167–172. [Google Scholar]
- 32.Zitnan D, Sauman I, Sehnal F. Peptidergic innervation and endocrine cells of insect midgut. Arch Insect Biochem Physiol. 1993;22:113–132. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.