Abstract
The ultimate goal for the treatment of autoimmunity is to restore immunological tolerance. Regulatory T cells (Treg) play a central role in immune tolerance, and Treg functional abnormalities have been identified in different autoimmune diseases, including rheumatoid arthritis (RA). We have previously shown that natural Treg from RA patients are competent at suppressing responder T cell proliferation but not cytokine production. Here, we explore the hypothesis that this Treg defect in RA is linked with abnormalities in the expression and function of CTLA-4. We demonstrate that CTLA-4 expression on Treg from RA patients was significantly reduced compared with healthy Treg and is associated with an increased rate of CTLA-4 internalization. Regulation of T cell receptor signaling by CTLA-4 was impaired in RA Treg and associated with delayed recruitment of CTLA-4 to the immunological synapse. Artificial induction of CTLA-4 expression on RA Treg restored their suppressive capacity. Furthermore, CTLA-4 blockade impaired healthy Treg suppression of T cell IFN-γ production, but not proliferation, thereby recapitulating the unique Treg defect in RA. Our results suggest that defects in CTLA-4 could contribute to abnormal Treg function in RA and may represent a target for therapy for inducing long-lasting remission.
Keywords: autoimmunity, Foxp3, IL-17
Regulatory T cells (Treg) are responsible for controlling immune responses to self-antigens and promoting tolerance. Deficiencies in Treg function have been identified in a wide variety of human autoimmune disorders, including rheumatoid arthritis (RA) (1–3). We have previously shown that Treg isolated from patients with active RA are competent at suppressing conventional T cell proliferation but not cytokine production (2). Both in health and disease, numerous mechanisms of Treg-mediated suppression have been proposed, but it is unclear whether abnormalities in these processes account for the Treg defects found in autoimmunity. The identification of the molecular basis for the Treg defect in RA would be critical in manipulating Treg for therapy in autoimmune disorders.
Recently, we have demonstrated that anti-TNF therapy can induce a potent population of Treg in patients with RA, but the natural Treg defect persists in responding patients after anti-TNF treatment (4). Although TNF blockade in RA patients has been a major advance in therapy, these drugs do not provide a cure because disease returns if treatment is discontinued. The natural Treg defect, which remains after anti-TNF therapy, could provide an explanation for disease flare after cessation of therapy. Here, we explore the hypothesis that this persisting natural Treg defect in RA is linked with abnormalities in CTLA-4, a marker for Treg (5) and a candidate susceptibility gene for RA (6). CTLA-4 has been extensively investigated in conventional T cells as a pivotal negative regulator of T cell signaling but much less is known about how CTLA-4 modulates T cell signaling in Treg. Indeed, the focus of investigation in the context of Treg has been whether CTLA-4 has a functional role in suppression of responder T cells (Tresp), rather than as an intrinsic regulator of T cell receptor (TCR) signaling. However, these 2 roles could be complementary with CTLA-4-driven changes in Treg signaling influencing suppression of target cells.
The analytical tools available to define Treg have been called into question in a number of human studies. In particular, Foxp3 expression increases upon TCR stimulation of human Tresp in vitro (7–9). This up-regulation in Foxp3 expression is accompanied by substantial production of IL-2 by the CD4+Foxp3+ cells themselves. In an inflammatory environment, these in vitro phenomena may be reflected in vivo by increased numbers of Foxp3+“Treg” expressing IL-2 and IFN-γ. For the current study we have assayed Il-2 and IFN-γ production by Foxp3+ T cells as an aid in the identification of bona fide Treg.
In this study we have identified reduced expression and functional abnormalities in Treg-associated CTLA-4, which could account for the Treg defect in patients with RA. Ligation of CTLA-4 reduced the downstream signaling events after activation of Treg from healthy individuals, but not Treg from patients with RA. The observation that induced accumulation of CTLA-4 at the membrane of RA Treg by phorbol 12-myristate 13-acetate (PMA) restored their suppressive activity raises the possibility that targeted manipulation of CTLA-4 expression could represent a therapeutic strategy for patients with RA.
Results
Foxp3 Efficiently Suppresses Cytokine Production in Healthy and RA Treg.
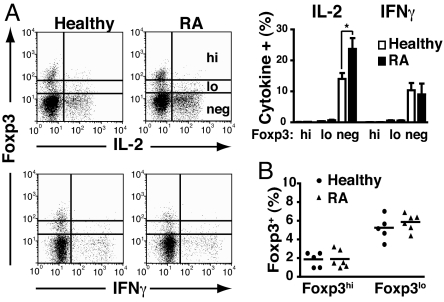
Given that expression of Foxp3 has been associated with activated, rather than regulatory, T cells in humans (7–9), we assayed IL-2 and IFN-γ production by Foxp3+ Treg from RA patients and healthy controls. The vast majority of IL-2 and IFN-γ was produced by conventional CD4+Foxp3− T cells, isolated from both healthy individuals and patients with active RA (Fig. 1A). Indeed, there was a significant increase in IL-2 detected in the CD4+Foxp3− T cell population from RA patients compared with those isolated form healthy individuals. However, very little IL-2 or IFN-γ was detected in the CD4+Foxp3+ population from Treg from healthy controls or RA patients. A small, but distinct, subset (<0.5%) of CD4+Foxp3lo from both healthy individuals and RA patients expressed IL-2 or IFN-γ, but production of both of these cytokines was negligible in the Foxp3hi population (Fig. 1A). A number of groups, including our own, have found similar numbers of Treg in patients with established active RA compared with healthy individuals (2, 3, 10). We confirmed this finding when enumerating Treg defined as CD4+Foxp3hi (Fig. 1B).
Fig. 1.
Foxp3 suppresses cytokine production in RA Treg. (A) Ex vivo PBMC from healthy controls and patients with active RA were stained for CD4, Foxp3, and IL-2 or IFN-γ. Representative FACS plots for IL-2 and IFN-γ production are shown. The bar graph shows the cumulative data of cytokine levels in healthy and RA Foxp3high (hi), low (lo), and negative (neg) cells (gated on CD4+ T cells). (B) Percentage of Foxp3hi and Foxp3lo CD4 T cells in healthy controls and patients with active RA is shown. *, P < 0.05
CTLA-4 Expression Is Diminished on Treg from Patients with RA.
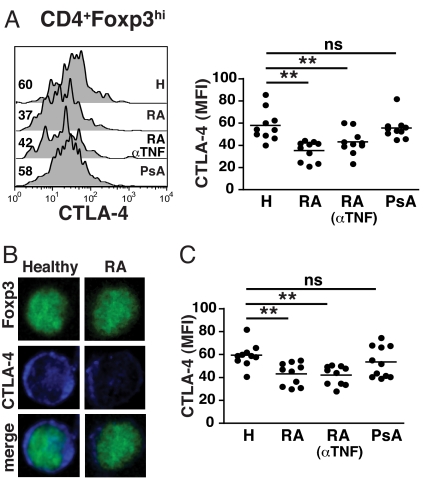
We next measured CTLA-4 expression in the CD4+Foxp3hi subset from patients with RA, psoriatic arthritis (PsA), and healthy controls. There was a significant reduction in CTLA-4 expression in Treg from patients with RA compared with Treg from healthy individuals (Fig. 2A). In contrast, CTLA-4 expression in Treg from PsA patients was normal. Confocal microscopy confirmed a marked reduction in CTLA-4 expression in the Foxp3+ Treg compartment from patients with RA compared with healthy controls (Fig. 2B). Reduced CTLA-4 expression was also confirmed when Treg were defined as CD4+CD25+CD127− (Fig. 2C) (11), thereby facilitating their purification and further analysis. Interestingly, anti-TNF (infliximab) therapy did not normalize CTLA-4 expression on Treg (Fig. 2 A and C). CTLA-4 and CD28 have opposing actions on T cell activation and compete for the same ligands (5, 12). Therefore, we assessed CD28 expression on Treg from RA patients. Flow cytometry analysis of Foxp3hi Treg from healthy controls and RA patients showed similar levels of CD28 expression (Fig. S1A). This finding would be in accord with evidence that CD28, unlike CTLA-4, although important for the development of Treg, is not directly involved in Treg function (5). Comparable expression of CD28 (Fig. S1A) and CTLA-4 (Fig. S1B) was noted in Tresp from healthy controls and patients with RA. Moreover, the kinetics of CTLA-4 expression in activated Tresp from both healthy controls and patients with RA were very similar (Fig. S1 C and D), indicating that abnormalities in CTLA-4 expression are confined to Treg in RA.
Fig. 2.
Reduced expression of CTLA-4 in Treg from RA patients. (A) PBMC were surface-stained for CD4 followed by intracellular staining for Foxp3 and CTLA-4. Representative histograms [mean fluorescence intensity (MFI) indicated] and cumulative data for CTLA-4 MFI in (ex vivo) CD4+Foxp3hi cells from healthy individuals, RA, anti-TNF-treated RA patients and PsA patients; 10 healthy controls and 10 patients in each disease group were studied. (B) Ex vivo CD4+ cells were fixed/permeabilized, stained for Foxp3 (green) and CTLA-4 (blue) and analyzed by confocal microscopy. Merged pictures show combined staining for Foxp3 and CTLA-4. Images are representative of at least 25 cells from 5 healthy controls and 5 patients with RA. (Magnification: 63×.) (C) Representative histograms and cumulative data for CTLA-4 MFI in ex vivo healthy, RA, and PsA Treg defined as CD4+CD25+CD127−; 10 healthy controls and 10 patients in each disease group were studied. *, P < 0.05; **, P < 0.01.
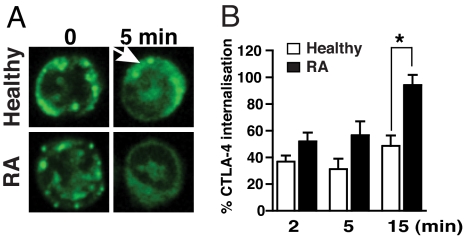
CTLA-4 expression at the cell surface of Tresp is regulated by exocytosis–endocytosis and once internalized is targeted to lysosomes for degradation (13, 14). Given the reduction in CTLA-4 expression in RA Treg, we investigated whether this change is accompanied by alterations in CTLA-4 recycling. PMA has been shown to markedly increase the migration of CTLA-4 to the plasma membrane via a phospholipase D-dependent mechanism and can be used to study the dynamics of CTLA-4 internalization (13). Confocal microscopy analysis indicated intense staining for CTLA-4 localized at or close to the plasma membrane in both healthy and RA Treg after exposure to PMA (Fig. 3A). These areas of CTLA-4 aggregation persisted in PMA-treated Treg from healthy controls, but not from RA patients, suggesting an increased rate of internalization in the latter. FACS analysis confirmed an increased rate of CTLA-4 internalization in RA Treg compared with Treg from healthy controls (Fig. 3B), which could account for its decreased expression on RA Treg.
Fig. 3.
Increased internalization rates of CTLA-4 in RA Treg. (A) FACS-sorted Treg (CD4+CD25+CD127−) were treated with 10 ng/ml PMA to induce CTLA-4 accumulation at the cell membrane, stained for CTLA-4, and then incubated for 5 min to allow internalization of CTLA-4. Confocal microscopy analysis showed areas of CTLA-4 aggregation in healthy Treg (arrow) compared with a diffuse staining pattern in RA Treg. Pictures are representative of Treg-sorted samples derived from 4 healthy controls and 4 RA patients. (Magnification: 63×.) (B) CTLA-4 internalization after treatment with 10 ng/ml PMA for 1 h at 37 °C was determined by FACS and expressed as percentage of CTLA-4 internalization. Results represent the mean ± SE of 4 healthy controls and 6 patients with active RA. *, P < 0.05.
Defective CTLA-4 Mediated Inhibition of TCR-Associated Signaling in RA Treg.
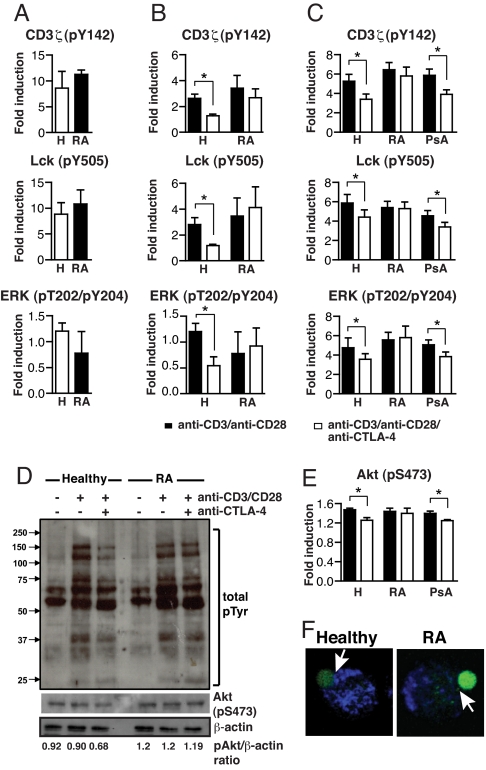
Next, we investigated whether we could detect an altered signaling response to CTLA-4/TCR coligation between Treg isolated from RA patients and healthy individuals. Initially, using phospho-site-specific mAbs, we noted similar levels of phosphorylation of CD3ζ, Lck, and ERK after CD3/CD28 ligation in Tresp purified from either healthy individuals or RA patients (Fig. 4A). The response of Treg to CD3/CD28 triggering was diminished 2- to 3-fold compared with Tresp with respect to CD3ζ and Lck phosphorylation. However, there were no significant differences in the signaling pattern in response to CD3/CD28-mediated activation between Treg from RA patients and healthy controls (Fig. 4B). Having shown a normal response of RA Treg to CD3/CD28 stimulation, we tested the effects of CTLA-4 cross-linking. Whereas CTLA-4 engagement significantly reduced the CD3/CD28-induced phosphorylation of CD3ζ, Lck, and ERK in Treg from healthy controls, no such effect was apparent in Treg from RA patients (Fig. 4B). We confirmed these observations by studying the induction of phosphorylation of the CD4+Foxp3hi population in fresh peripheral blood mononuclear cells (PBMC), thereby avoiding any variation introduced by cell purification. Fig. 4C shows that CTLA-4 engagement did not alter the induction of phosphorylation in RA Treg after TCR stimulation, whereas there was a significant reduction in the induction of phosphorylation in healthy Treg. Moreover, Treg from PsA patients exhibited a similar response to CTLA-4 ligation compared with healthy Treg, consistent with their normal CTLA-4 expression (Fig. 2A).
Fig. 4.
CTLA-4-mediated regulation of TCR-associated signaling is impaired in RA Treg. (A) FACS-sorted Tresp (CD4+CD25−CD127+) from healthy controls and RA patients were sorted and stimulated with anti-CD3 (1 μg/106 cells)/CD28 (1 μg/106 cells) antibodies for 10 min at 37 °C. Results represent the mean ± SE from 6 healthy individuals and 6 patients with RA. (B) FACS-sorted Treg (CD4+CD25+CD127−) were stimulated with anti-CD3 (1 μg/106 cells)/CD28 (1 μg/106 cells) ± anti-CTLA-4 (5 μg/106 cells) antibodies for 10 min. Phosphorylation of signaling molecules was evaluated by flow cytometry after intracellular staining with phosphospecific antibodies. Shown are percentage increases in protein tyrosine-phosphorylation (p) levels of TCR-associated signaling proteins in stimulated Treg. Results represent the mean ± SE from 6 healthy individuals and 6 patients with RA. (C) PBMC were stimulated as in B and the induction of phosphorylation was evaluated in the CD4+Foxp3hi subset. Results represent the mean ± SE from 6 healthy individuals, 6 patients with RA, and 6 patients with PsA. (D) Analysis of total protein-tyrosine phosphorylation in Treg from patients with RA and healthy controls. FACS-sorted Treg were stimulated for 10 min at 37 °C with anti-CD3 (1 μg/106 cells)/CD28 (1 μg/106 cells) ± anti-CTLA-4 (5 μg/106 cells). Samples from 3 different healthy individuals or 3 different RA patients (matched for disease activity score) were pooled before loading onto gels for protein phosphorylation analysis by Western blot. The lower gels show incubation of membranes with anti-S 473-Akt and anti-β-actin antibodies. Also indicated are the phospho Akt/β-actin ratios obtained from densitometry analysis. (E) Induction of phosphorylation of Akt in CD4+Foxp3hi T cells, PBMC stimulated as in B. Results represent the mean ± SE from 6 healthy individuals, 6 patients with RA, and 6 patients with PsA. (F) Purified Treg were mixed with beads coated with anti-CD3/CD28 antibodies. Activation was stopped at 10 min with 2% paraformaldehyde, and cells were stained for Foxp3 and CTLA-4. Recruitment of CTLA-4 (blue) to the immunological synapse (beads:Foxp3+ cell depicted) is indicated (arrow). Confocal microscopy images are representative of at least 25 conjugates from 4 healthy controls and 4 patients with RA. (Magnification: 63×.) *, P < 0.05.
Western blot analysis of global protein-tyrosine phosphorylation confirmed impaired function of CTLA-4 in RA Treg (Fig. 4D). Upon TCR-mediated stimulation, both healthy and RA Treg increased overall tyrosine phosphorylation. Cross-linking of CTLA-4 down-regulated the TCR-induced tyrosine phosphorylation of proteins with molecular masses of 60–75 and 120–150 kDa in Treg from healthy individuals, but not in Treg from patients with RA. It has been recently shown that reduced phosphorylation of Akt is required for the functional activity of Treg (15). Importantly, CTLA-4 signaling inhibits Akt phosphorylation in Tresp (16). We found a CTLA-4-mediated inhibition of Akt phosphorylation in Treg from healthy controls, but not in RA Treg (Fig. 4D). FACS analysis confirmed a reduction in Akt phosphorylation after CTLA-4 ligation in Treg from healthy controls and PsA patients, but not in RA Treg (Fig. 4E).
We also determined the pattern of CTLA-4 recruitment to the immunological synapse, which could impact the suppressive capacity of Treg. Confocal microscopy of conjugates of Treg and TCR-stimulating beads showed an accumulation of CTLA-4 at the immunological synapse in healthy Treg but not in RA Treg at 10 min (Fig. 4F). The lack of CTLA-4 at the synapse provides an anatomical basis for the failure of RA Treg to efficiently suppress Tresp. Collectively, these results indicate the presence of a CTLA-4-mediated attenuation of CD3/CD28 signaling in healthy Treg, which is lost in Treg derived from RA patients with active disease.
Restoration of RA Treg Function Through Manipulation of CTLA-4 Expression.
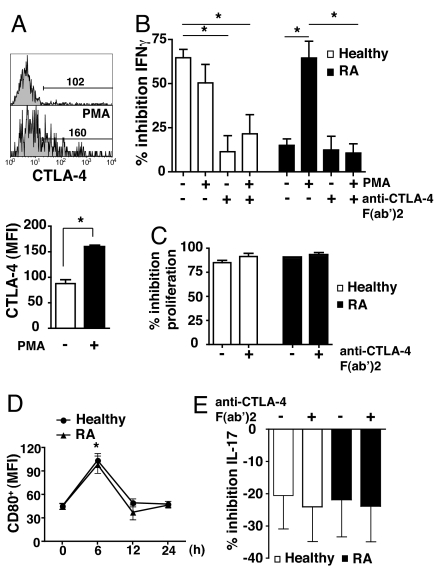
We next tested whether an increase in CTLA-4 expression could restore the function of RA Treg. We used the distinct properties of PMA in driving CTLA-4 to the plasma membrane (13). Thus, purified Treg were treated with PMA, which significantly increased surface CTLA-4 expression (Fig. 5A), and subsequently fixed with paraformaldehyde. Paraformaldehyde-fixed Treg show similar regulatory capacity to their viable counterparts (17). We confirmed our previous finding that Treg from healthy donors, but not from patients with RA, suppressed the production of IFN-γ by Tresp (Fig. 5B) (2). PMA treatment of Treg from RA patients restored their suppressive capacity, whereas the suppressive ability of similarly treated Treg from healthy individuals was unaffected. The use of a blocking F(ab′)2 anti-CTLA-4 antibody demonstrated the specific role of CTLA-4 in Treg suppression of IFN-γ production in both PMA-treated RA Treg and healthy controls. Interestingly, blockade of CTLA-4 did not abrogate Treg-mediated inhibition of Tresp proliferation in either healthy controls or RA patients (Fig. 5C). The anti-CTLA-4 F(ab′)2 antibody did not affect proliferation of Tresp cultured alone (data not shown). Furthermore, we found that CD80, but not CD86 (data not shown), was expressed by Tresp at early time points after activation (Fig. 5D), supporting the possibility of a CTLA-4/CD80 interaction between Treg and Tresp (18).
Fig. 5.
Restoration of suppressive capacity of RA Treg after induced delivery of CTLA-4 to the cell surface. (A) Histogram of surface CTLA-4 expression in isolated RA Treg before and after incubation with 10 ng/ml PMA for 1 h, and the cumulative data are indicated. MFI was calculated by using the indicated gate based on isotype control. Results represent the mean ± SE of 6 RA patients. (B) Cocultures of Treg (CD4+CD25+CD127−) with or without exposure to 10 ng/ml PMA for 1 h, and Tresp (CD4+CD25−CD127+) (1:2) from RA patients and healthy individuals were incubated in the presence of anti-CD3 (1 μg/ml)/CD28 (2 μg/ml) ± anti-CTLA-4 (10 μg/ml). After 3 days, cocultures were stained for IFN-γ, before analysis by FACS. Results represent the mean ± SE of 6 patients and 6 healthy controls and are expressed as the percentage inhibition of IFN-γ production compared with Tresp alone. (C) Cocultures of Treg and Tresp (1:2) were incubated in the presence of anti-CD3 (1 μg/ml)/CD28 (2 μg/ml) ± anti-CTLA-4 (10 μg/ml). Proliferation after 4 days was determined by 3[H]Tdr incorporation, and results are expressed as percentage inhibition of proliferation. Results represent the mean ± SE of 5 healthy controls and 5 patients with RA. (D) PBMC were stimulated with CD3 (1 μg/ml)/CD28 (2 μg/ml) antibodies for up to 24 h. The graphs show the time course for CD80 expression in Tresp. Results represent the mean ± SE of 5 healthy controls and 5 patients with RA. (E) Cocultures of Treg and Tresp (1:2) and Tresp alone were incubated as in B. Results represent the mean ± SE of 4 RA patients and 4 healthy controls and are expressed as the percentage inhibition of IL-17 production compared with Tresp alone. *, P < 0.05.
Given that Th17 is an important effector population in inflammation (19), we examined the capacity of Treg to suppress IL-17 production as a prelude to defining the role of CTLA-4 in this context. The data in Fig. 5E reveal that Treg from healthy individuals or RA patients were unable to suppress CD4+ IL-17 production. Indeed there was a modest increase in IL-17 production when Treg were cultured with Tresp. Incubation of these cocultures with anti-CTLA-4 antibody did not influence this lack of suppression.
Discussion
The role of CTLA-4 in immunoregulation has been extensively investigated. Despite its constitutive expression on Treg, its precise contribution to their suppressive function has not been fully defined. In this study, we have identified abnormalities in the expression and function of CTLA-4 in Treg isolated from patients with active RA.
In addition to a reduction in CTLA-4 expression and function, 2 other strands of evidence support the notion that CTLA-4 could be responsible for Treg abnormalities in RA patients. First, when CTLA-4 is artificially driven to the membrane surface of RA Treg by PMA, their suppressive function is restored. This restoration in function can be reversed by CTLA-4 blockade, further supporting its role in both Treg function and the defect identified in RA Treg. Second, targeting CTLA-4 impairs the ability of Treg from healthy individuals to suppress IFN-γ production while sparing suppression of T cell proliferation. This result parallels our previous findings showing that Treg isolated from patients with active RA, although competent at suppressing T cell proliferation, lack the capacity to suppress cytokine production by Tresp (2).
The balance of evidence in the literature implicates CTLA-4 as a mediator of the suppressive effects of Treg. For instance, using murine models of disease, blockade of CTLA-4 abrogates the suppressor function of WT Treg (20, 21). It is likely that CTLA-4 participates in multiple mechanisms to empower Treg with suppressive properties. One mechanism that has been proposed involves a direct interaction with CD80/CD86 expressed on activated Tresp (18, 22). However, a later publication (23) that refuted this model demonstrated that WT Treg were efficient suppressors of conventional T cell proliferation from CD80/CD86-deficient mice, but were impaired in their ability to inhibit IFN-γ production. Our results parallel this dissociation between inhibition of proliferation and cytokine production in CD80/CD86-deficient mice. Additional work is needed to explore the potential differences in the pathways used by Treg to suppress proliferation as compared with cytokine production in healthy Treg. Nevertheless, our data implicating CTLA-4 may afford some mechanistic insight into the Treg defect found in RA patients.
Although genetically targeted mice have revealed that Treg possess suppressor activity in the absence of CTLA-4, this observation could be explained by the potential for Treg to use alternate compensatory mechanisms, such as TGF-β production (21, 24). After anti-TNF therapy, the expression of CTLA-4 on Treg was not returned to normal, which is in accord with our previous data indicating that TNF blockade does not restore natural Treg function in patients with RA (4). Rather, anti-TNF therapy induced a distinct Treg population, which suppresses proinflammatory cytokine production via TGFβ, as opposed to a predominantly contact-dependent mechanism involving CTLA-4. It is tempting to draw a parallel with CTLA-4-deficient mice in which Treg use a TGF-β-dependent compensatory suppressive mechanism (24). Indeed, similarly to the increased latency-associated peptide (LAP) expression on Treg from CTLA-4-deficient mice, Treg isolated from RA patients after infliximab therapy also have an increase in LAP expression. The notion that a reduction in CTLA-4 expression on RA Treg is a consequence of the inflammatory milieu, although possible, is unlikely given that anti-TNF therapy did not restore its expression. Moreover, there was no reduction in CTLA-4 expression on Treg from patients with PsA.
Pertinent to the functional attributes of Treg are our observations that Treg do not suppress IL-17 production in either healthy people or patients with RA. There has been considerable interest in Th17 as an effector population with strong evidence from murine studies implicating this cytokine in inflammation (19). However, its role in patients with RA is less clear. Although some reports (25, 26) demonstrated increased IL-17 production in RA synovium, others (27) have indicated that Th1 cells secreting IFN-γ rather than Th17 cells are the predominant T cell subset. In a further study (28) an increase in Th17 cells has been observed in patients with spondylarthritides, but not in patients with RA. Notwithstanding the issue of a Th1 or Th17 predominance in RA, our data suggest that human natural Treg are unable to suppress Th17 in vitro, which is consistent with recent reports suggesting that Treg can either secrete IL-17 or promote its production (29, 30). Moreover, in a murine model of inflammation, natural Treg were found to be impaired in their ability to suppress Th17 compared with Th1 (31). The fact that healthy Treg are unable to inhibit IL-17 production, despite normal CTLA-4 expression and function, raises the possibility that this molecule may not be relevant in the suppression of IL-17.
Treg have a markedly different response to TCR engagement compared with conventional T cells, which is likely to be reflected in their intracellular signaling responses (15, 32, 33). Our findings suggest that the phosphorylation of TCR signaling responses in Treg, whether isolated from healthy individuals or patients with RA, are muted compared with Tresp. In view of data indicating a role for CTLA-4 in dampening proximal signaling molecules in conventional T cells (12, 34), it is tempting to speculate that CTLA-4 could play a similar role in altering Treg signaling responses. Indeed, the effects of CTLA-4 cross-linking reduced the TCR-driven response in Treg from healthy individuals. However, Treg from RA patients were resistant to the effects of CTLA-4 cross-linking with respect to phosphorylation of a number of TCR-associated signaling molecules and global tyrosine phosphorylation. We propose that the decreased level of CTLA-4 and its delayed recruitment to the immune synapse could contribute to the defective regulation of protein phosphorylation by CTLA-4 in RA Treg.
Recent evidence has linked alterations in the phosphorylation of specific proteins with Treg function. For instance, a reduced phosphorylation of AKT is associated with the functional activity of Treg (15). Importantly, CTLA-4 signaling inhibits Akt phosphorylation in Tresp via recruitment of specific phosphatases (16). Similarly, our data reveal that CTLA-4 mediated a reduction in Akt phosphorylation in Treg from healthy controls but not from patients with RA. In addition to a role in dephosphorylation of Akt, it is possible that phosphatases recruited by CTLA-4 engagement might also affect its stability at the membrane surface (34). If expression of CTLA-4 on the surface of Treg is important for its function, it is tempting to speculate that failure of CTLA-4 to recruit phosphatases could reduce CTLA-4 membrane stability and expression, thereby impairing Treg function. Indeed, we noted an increase in CTLA-4 internalization, which could be a direct consequence of reduced membrane stability of CTLA-4.
The expression of Foxp3 has been associated with production of IL-2 and IFN-γ after in vitro activation, suggesting that mere expression of Foxp3 is insufficient to confer a regulatory phenotype (7–9). In contrast to these in vitro studies, we found that Foxp3, particularly at high levels of expression, effectively abolished cytokine synthesis in healthy individuals but also in patients with RA where T cell activation is likely to be present. The IL-2 and IFN-γ secretion by Foxp3-negative Tresp was far higher than in the Foxp3lo subset, suggesting that even low expression of Foxp3 has some inhibitory action upon IL-2 and IFN-γ production. In contrast to the potent intrinsic inhibition of cytokine production by Foxp3 in RA Treg, their extrinsic suppressive actions on conventional T cells is impaired. These observations, taken together with our data showing abnormalities in CTLA-4 function, are compatible with the notion that CTLA-4 mediates extrinsic, as opposed to intrinsic, suppression of cytokine production in Treg.
In summary, the data presented here suggest that abnormal CTLA-4 biology may underlie the defect found in RA Treg. We have provided insight into the relatively unexplored area of CTLA-4 modulation of Treg signaling and have identified abnormalities in the action of CLTA-4 on the signaling pathways in RA Treg compared with healthy Treg. Furthermore, the demonstration that an increase in CTLA-4 expression on RA Treg can lead to restoration of suppressor function highlights Treg-associated CTLA-4 as a potential therapeutic target. Of note, CTLA-4 expression on Treg from RA patients responding to anti-TNF therapy was not restored to normal. Rather, the improvement in disease is associated with the appearance of a functionally and phenotypically distinct adaptive Treg population that functions through a TGF-β-dependent mechanism (4). Therefore, we propose that the rapid flare of patients with RA after cessation of anti-TNF therapy could be explained by persistent defects in the natural Treg pool, which is not corrected by anti-TNF therapy. Our data provide a rationale for a dual approach in the treatment of RA: blockade of inflammation combined with manipulation of the natural Treg population. This strategy could be extended to other autoimmune diseases where Treg defects have been noted in a setting where inflammation is a major component. This approach could result in an enduring remission associated with restoration of tolerance.
Methods
Patient Population.
Peripheral blood was obtained from patients with active RA and active PsA attending the rheumatology clinic at University College Hospital, London. All RA patients had a disease activity score (DAS28) of >5.1. PsA patients had at least 3 swollen and tender joints. See Table S1 for drug treatment. Some patients with RA were analyzed after 4 months of anti-TNF therapy (infliximab) and had responded to treatment with a reduction of DAS28 >1.2. Sex- and aged-matched healthy individuals were used as controls. The study was approved by the University College London Hospital ethics committee.
Cell Isolation.
PBMC were separated on Ficoll-Hypaque (Amersham Pharmacia Biotech), and CD4+ lymphocytes were purified by negative selection with magnetic beads (Miltenyi Biotech). Treg (CD4+CD25+CD127−) and Tresp (CD4+CD25+CD127−) were isolated by FACSAria (BD).
Antibodies.
See SI Text.
Flow Cytometry.
Membrane staining for phenotyping was performed with the relevant antibodies by using 1% FCS-PBS for 30 min at room temperature. Intracellular staining was carried out after fixation/permeabilization. Intracellular staining for IL-2 and IFN-γ was carried out after incubation with 50 ng/mL PMA, 250 ng/mL ionomycin, and 2 μM monensin for 4 h at 37 °C for ex vivo and in vitro analysis. Cells were read in a BD-LSR flow cytometer and analyzed with FlowJo.
Confocal Microscopy.
Ex vivo- and in vitro-activated cells were stained, fixed/permeabilized, and mounted on glass slides. Pictures were taken with a 63× objective in a Leica DMIRE2 microscope.
CTLA-4 Recycling.
FACS-sorted Treg were rested for 1 h at 37 °C, and then stimulated for 1 h at 37 °C with PMA (10 ng/mL). Cells were membrane-stained with FITC-CTLA-4 for 1 h in ice, incubated at 37 °C for 5 min, and fixed and mounted for confocal microscopy analysis. Alternatively, CTLA-4 recycling was analyzed by using FACS as described (13).
Cell Signaling.
Sorted Treg and Tresp were rested for 1 h in medium. Cells were stimulated by incubating with anti-CD3/CD28 ± anti-CTLA-4 for 20 min at 4 °C. Cells were washed with ice-cold PBS followed by the addition of goat anti-mouse IgG F(ab′)2 and incubated at 4 °C for 20 min. Cells were resuspended in warm PBS and incubated for 10 min at 37 °C. Cells were fixed/permeabilized and stained with the appropriate antibodies.
Western Blot Analysis.
FACS-sorted Treg were activated as described for cell signaling above and lysed in Triton X-100 lysis buffer. Proteins samples (from 0.5 × 106 cells) were resolved by PAGE, transferred to PVDF membranes (Millipore), and blotted with phospho-specific antibodies. Bound antibodies were revealed with HRP-conjugated secondary antibodies by using enhanced chemiluminescence (Amersham Pharmacia Biotech).
Treg Functional Assays.
Cocultures of Treg and Tresp (1:2), or Tresp alone, were stimulated with anti-CD3/anti-CD28 ± anti-CTLA-4 F(ab′)2 antibodies. After 3 days, cells were permeabilized, stained for intracellular IFN-γ, and analyzed by flow cytometry. For proliferation assays, cocultures of Treg and Tresp, or Tresp alone, were incubated in the presence of anti-CD3/CD28 ± anti-CTLA-4 F(ab′)2 for 4 days and then pulsed with [3H]thymidine. Cultures were incubated for 16 h before harvesting and analyzed in a scintillation counter.
Statistics.
All values are expressed as means ± SEM. We performed analysis of significance in Prism (GraphPad) by the Mann–Whitney test.
Supplementary Material
Acknowledgments.
We thank Jamie Evans for providing technical assistance with FACS sorting; Dr. Panos Kabouridis and Dr. Torben Lund for helpful comments; and the Arthritis Research Campaign for providing Equipment Grant 17746 for purchasing a FACS sorter (FACSAria). F.F.-B. is supported by Arthritis Research Campaign Grants 16309 and 17707. E.C.J., an Arthritis Research Campaign Career Development Fellow, is supported by Arthritis Research Campaign Grants 17319 and 18106.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806855105/DCSupplemental.
References
- 1.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehrenstein MR, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNF-α therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valencia X, et al. TNF down-modulates the function of human CD4+CD25hi T regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadkarni S, Mauri C, Ehrenstein MR. Anti-TNF-α therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-β. J Exp Med. 2007;204:33–39. doi: 10.1084/jem.20061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sansom DM, Walker LSK. The role of CD28 and cytotoxic T lymphocyte antigen-4 (CTLA-4) in regulatory T cell biology. Immunol Rev. 2006;212:131–148. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 6.Han S, Li Y, Mao Y, Xie Y. Meta-analysis of the association of CTLA-4 exon-1 +49A/G polymorphism with rheumatoid arthritis. Hum Genet. 2005;118:123–132. doi: 10.1007/s00439-005-0033-9. [DOI] [PubMed] [Google Scholar]
- 7.Gavin MA, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci USA. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allan SE, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 9.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T cell receptor stimulation is transforming growth factor-β-dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Amelsfort JM, et al. CD4+CD25+ regulatory T cells in rheumatoid arthritis: Differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–2785. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelhardt JJ, Sullivan TJ, Allison JP. CTLA-4 overexpression inhibits T cell responses through a CD28–B7-dependent mechanism. J Immunol. 2006;177:1052–1061. doi: 10.4049/jimmunol.177.2.1052. [DOI] [PubMed] [Google Scholar]
- 13.Mead KI, et al. Exocytosis of CTLA-4 is dependent on phospholipase D and ADP ribosylation factor-1 and stimulated during activation of regulatory T cells. J Immunol. 2005;174:4803–4811. doi: 10.4049/jimmunol.174.8.4803. [DOI] [PubMed] [Google Scholar]
- 14.Iida T, et al. Regulation of cell surface expression of CTLA-4 by secretion of CTLA-4-containing lysosomes upon activation of CD4+ T cells. J Immunol. 2000;165:5062–5068. doi: 10.4049/jimmunol.165.9.5062. [DOI] [PubMed] [Google Scholar]
- 15.Crellin NK, Garcia RV, Levings MK. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood. 2007;109:2014–2022. doi: 10.1182/blood-2006-07-035279. [DOI] [PubMed] [Google Scholar]
- 16.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dieckmann D, et al. Human CD4+CD25+ regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells. J Exp Med. 2002;196:247–253. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manzotti CN, et al. Inhibition of human T cell proliferation by CTLA-4 utilizes CD80 and requires CD25+ regulatory T cells. Eur J Immunol. 2002;32:2888–2896. doi: 10.1002/1521-4141(2002010)32:10<2888::AID-IMMU2888>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 19.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, et al. B7 interactions with CD28 and CTLA-4 control tolerance or induction of mucosal inflammation in chronic experimental colitis. J Immunol. 2001;167:1830–1838. doi: 10.4049/jimmunol.167.3.1830. [DOI] [PubMed] [Google Scholar]
- 21.Read S, et al. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci USA. 2004;101:10398–10403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.May KF, Jr, et al. B7-deficient autoreactive T cells are highly susceptible to suppression by CD4+CD25+ regulatory T cells. J Immunol. 2007;178:1542–1552. doi: 10.4049/jimmunol.178.3.1542. [DOI] [PubMed] [Google Scholar]
- 24.Tang Q, et al. Distinct roles of CTLA-4 and TGF-β in CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 25.Chabaud M, et al. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–970. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 26.Raza K, et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther. 2005;7:R784–R795. doi: 10.1186/ar1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada H, et al. Th1 but not Th17 cells predominate in the joints of patients with rheumatoid arthritis. Ann Rheum Dis. 2007;67:1299–1304. doi: 10.1136/ard.2007.080341. [DOI] [PubMed] [Google Scholar]
- 28.Jandus C, et al. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58:2307–2317. doi: 10.1002/art.23655. [DOI] [PubMed] [Google Scholar]
- 29.Evans HG, et al. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc Natl Acad Sci USA. 2007;104:17034–17039. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koenen HJ, et al. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17 producing cells. Blood. 2008;112:2340–2352. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 31.Stummvoll GH, et al. Th1, Th2, and Th17 effector T cell-induced autoimmune gastritis differs in pathological pattern and in susceptibility to suppression by regulatory T cells. J Immunol. 2008;181:1908–1916. doi: 10.4049/jimmunol.181.3.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, et al. CD4+CD25+ regulatory T cell lines from human cord blood have functional and molecular properties of T cell anergy. Blood. 2005;106:3068–3073. doi: 10.1182/blood-2005-04-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsang JY, et al. Altered proximal T cell receptor (TCR) signaling in human CD4+CD25+ regulatory T cells. J Leukocyte Biol. 2006;80:145–151. doi: 10.1189/jlb.0605344. [DOI] [PubMed] [Google Scholar]
- 34.Valk E, Rudd CE, Schneider H. CTLA-4 trafficking and surface expression. Trends Immunol. 2008;29:272–279. doi: 10.1016/j.it.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.