Abstract
Relative hypoxia is essential in wound healing since it normally plays a pivotal role in regulation of all the critical processes involved in tissue repair. Hypoxia-inducible factor (HIF) 1α is the critical transcription factor that regulates adaptive responses to hypoxia. HIF-1α stability and function is regulated by oxygen-dependent soluble hydroxylases targeting critical proline and asparaginyl residues. Here we show that hyperglycemia complexly affects both HIF-1α stability and activation, resulting in suppression of expression of HIF-1 target genes essential for wound healing both in vitro and in vivo. However, by blocking HIF-1α hydroxylation through chemical inhibition, it is possible to reverse this negative effect of hyperglycemia and to improve the wound healing process (i.e., granulation, vascularization, epidermal regeneration, and recruitment of endothelial precursors). Local adenovirus-mediated transfer of two stable HIF constructs demonstrated that stabilization of HIF-1α is necessary and sufficient for promoting wound healing in a diabetic environment. Our findings outline the necessity to develop specific hydroxylase inhibitors as therapeutic agents for chronic diabetes wounds. In conclusion, we demonstrate that impaired regulation of HIF-1α is essential for the development of diabetic wounds, and we provide evidence that stabilization of HIF-1α is critical to reverse the pathological process.
Keywords: angiogenesis, chronic complications, hypoxia, hyperglycemia, chronic ulcers
Diabetic chronic foot ulceration represents a major medical, social, and economic problem. Worldwide, it is believed that a lower limb is lost as a result of diabetes every 30 seconds (1). As no efficient therapy is available, it is a high priority to develop new strategies for treatment of this devastating complication (2, 3).
The pathogenesis of chronic ulcers in diabetes is still unclear. A critical stimulus for normal wound healing is relative hypoxia (4), and an impaired reaction to hypoxia could contribute to impaired wound healing. Local relative hypoxia in wounds was proved by direct measurement of the local oxygen pressure together with the necessity of maintaining hypoxic gradients for good angiogenesis in the wound healing process (5). Hypoxia has been shown to induce major cytokines (VEGF, TGF-β, PDGF), to stimulate proliferation and migration of fibroblasts and keratinocytes (recently reviewed in ref. 4). Cellular adaptive responses to hypoxia are mediated by the hypoxia-inducible factor (HIF) 1, which is a heterodimeric transcription factor complex. Regulation of HIF-1 activity is dependent of the degradation of the HIF-1α subunit in normoxia. The molecular basis of its degradation is O2-dependent hydroxylation of at least one of the two proline residues (6, 7) in the oxygen-dependent degradation domain of HIF-1α (8) by specific Fe2+- and oxoglutarate-dependent prolyl 4-hydroxylases (PHDs) (9, 10). In its hydroxylated form, HIF-1α binds to the von Hippel-Lindau (VHL) tumor suppressor protein that is part of an E3 ubiquitin ligase complex targeting HIF-1α for proteasomal degradation (8). HIF transactivation is also subjected to regulation by oxygen. Two transactivation domains have been identified, termed the amino-terminal transactivation domain (NTAD) and the carboxy-terminal transactivation domain (CTAD) (11). The NTAD overlaps with the oxygen-dependent degradation domain (ODD), and its transcriptional activity is largely coupled with protein stability. However, the transcriptional activity of the CTAD is independent of protein stability. Regulation of CTAD activity involves hydroxylation of a critical asparagine residue through a reaction catalyzed by factor inhibiting HIF (FIH), which is another iron- and oxoglutarate-dependent oxygenase (12). Under hypoxic conditions, HIF-1α is stabilized against degradation and transactivates and up-regulates a series of genes that enable cells to adapt to reduced oxygen availability (11).
HIF-1α plays a pivotal role in wound healing, and its expression in the multistage process of normal wound healing has been well characterized (13). In essence, HIF-1α is necessary for expression of multiple angiogenic growth factors (14), cell motility (15), and recruitment of endothelial progenitor cells (16). We and others have shown that hyperglycemia impairs HIF-1α stability and function (17–19), and we were able to demonstrate low levels of HIF-1α expression in foot ulcer biopsies in patients with diabetes (17). We therefore hypothesized that the defect in wound healing present in diabetes is a result of an inhibition of HIF-1 activity. To demonstrate this, we investigated the relevance of hyperglycemia-induced destabilization of HIF-1α for wound healing in the diabetic db/db mouse, an established model for studying diabetic wounds (20).
Results
Hyperglycemia Destabilizes HIF-1α and Impairs Its Function.
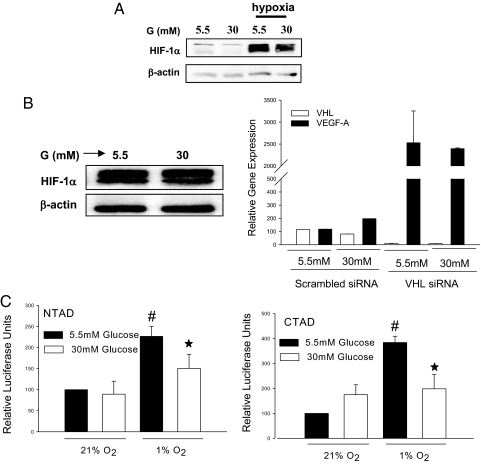
Hyperglycemia affects different levels of HIF-1 regulation. Hyperglycemia destabilized HIF-1α in db/db mouse primary fibroblasts (Fig. 1A). The effect of glucose was dependent on the VHL-mediated degradation mechanism, since hyperglycemia did not modulate HIF-1α in renal carcinoma cells that lack functional pVHL (Fig. 1B Left). Furthermore, VEGF, which is a classical target gene for HIF, was no longer modulated by hyperglycemia when VHL expression was down-regulated following treatment of human fibroblasts with siRNA (Fig. 1B Right). To further evaluate the effect of hyperglycemia on HIF-1 function, we investigated the effect of glucose on each of the two transactivation domains using GAL4-fusion constructs coupled with a GAL4-driven reporter gene. The negative regulatory effect of glucose was not only restricted to stability of HIF-1α but also targeted both the NTAD and the CTAD of HIF-1α, which are critically important for HIF-1α function (Fig. 1C). As a consequence, hyperglycemia caused down-regulation of several HIF-1α target genes essential for wound healing such as heat shock protein 90 (HSP-90), VEGF-A, VEGF-R1, stromal cell-derived factor (SDF)-1α, and stromal cell factor (SCF) (Fig. S1).
Fig. 1.
Hyperglycemia-dependent destabilization and inhibition of HIF-1α. (A) Immunoblot detecting HIF-1α in db/db mouse primary fibroblasts cultured for 48 h in different glucose concentrations (5.5 mM and 30 mM) and then exposed for 6 h in either normoxia (21% O2) or hypoxia (1% O2). (B Left) immunoblot detecting HIF-1α in renal carcinoma cells (SKRC7) expressing functionally inactive VHL cells (SKRC7). B (Right) Relative expression of VEGF in human dermal fibroblast cells exposed to different glucose concentrations after transfection with VHL-specific siRNA or scrambled siRNA. (C) Relative luciferase activity in the extract of 3T3 cells exposed to different oxygen and glucose concentrations after co-transfection with CTAD (GAL4/mHIF-1α 772–822) or NTAD (GAL4/mHIF1α 531–584) and GAL4-responsive reporter gene plasmid (*, P < 0.05, 5.5 mM glucose vs. 30 mM glucose).
Inhibition of HIF Hydroxylases Is Able to Counteract the Repressive Effect of Hyperglycemia on HIF Function.
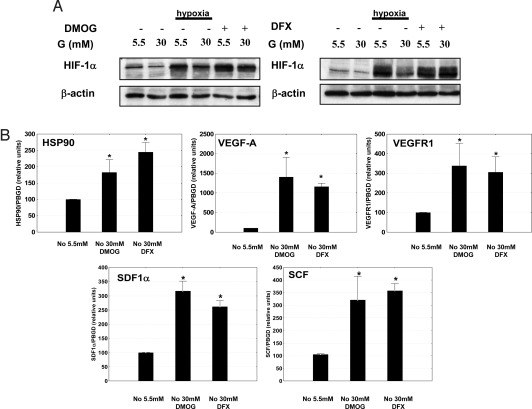
Taking into account the critical role of prolyl and asparaginyl hydroxylation in the regulation of HIF-1α stability and repression, respectively (6, 7, 12), we examined if inhibition of hydroxylase activity could counteract the negative regulatory effect of hyperglycemia. For this purpose we used two different hydroxylase inhibitors, i.e., the 2-oxoglutarate analogue dimethyloxalylglycine (DMOG) and the iron chelator deferoxamine (DFX), which are structurally different and act through different mechanisms, but which both stabilize and activate HIF-1 (21). In primary db/db mouse skin fibroblasts, both DMOG and DFX treatment resulted in stabilization of HIF-1α in the presence of high glucose under normoxic conditions. These levels of HIF-1α were very similar to those produced by hypoxia in normal glucose concentrations (Fig. 2A). Moreover, the compounds induced expression of HIF target genes essential for motility, angiogenesis, and recruitment of CAG (i.e., HSP-90, VEGF-A, VEGF-R1, SDF-1α, and SCF) even when the cells were exposed to high glucose concentrations (Fig. 2B). The stimulating effect of hydroxylase inhibitors on HIF target genes repressed by hyperglycemia can be observed even in hypoxia as illustrated for VEGF Fig. S2.
Fig. 2.
Inhibition of HIF hydroxylases can reverse the glucose inhibition of HIF-1α. (A) Immunoblots detecting HIF-1α in mouse db/db skin fibroblasts show destabilization of HIF-1α in high glucose concentrations (30 mM). This effect is overcome by treatment with hydroxylase inhibitors (DMOG [2 mM] or DFX [100 μM]). (B) Hydroxylase inhibitors induce expression of HIF-1α target genes essential for wound healing (DMOG [2 mM] or DFX [100 μM]) even in the presence of high glucose levels (*, P < 0.05, treatment vs. control). (No, normoxia (21% O2); Hy, hypoxia (1% O2); G, glucose.)
Hyperglycemia Has Repressive Effects on HIF-1α in Diabetic Wounds.
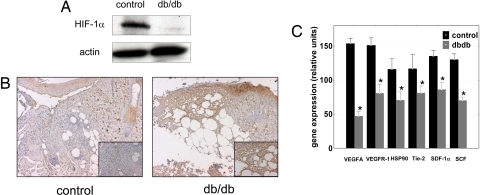
The negative regulatory effect of hyperglycemia on HIF-1α stability and function was confirmed in diabetic wounds of the db/db mice. HIF-1α expression was much lower in db/db wounds than in control heterozygote mice (Fig. 3A), despite more pronounced hypoxia in db/db wounds (as evaluated by binding of the hypoxia dye pimonidazole hydrochloride; Fig. 3B). Moreover HIF-1 target genes essential for wound healing cell motility (i.e., HSP-90), angiogenesis (i.e., VEGFA and VEGF-R1), and recruitment of CAG (i.e., SDF-1α, SCF, and Tie-2) were also repressed in the diabetic wounds, as compared with the wounds in normoglycemic heterozygote mice (Fig. 3C).
Fig. 3.
HIF-1α function is negatively regulated in diabetic wounds. (A) Wounds in diabetic mice are more hypoxic than in normoglycemic control mice as evaluated by pimonidazole adduct formation; note the detailed granulation tissue (Inset). (B) Immunoblot detecting HIF-1α in a whole-cell extract from the wound of diabetic or normoglycemic mice. (C) The expression of HIF target genes involved in wound healing is down-regulated in wounds of db/db mice (*, P < 0.05 expression in db/db mice vs. normoglycemic litter-mates).
Local Inhibition of HIF Hydroxylases Markedly Improves the Defective Healing Process in Diabetic Animals.
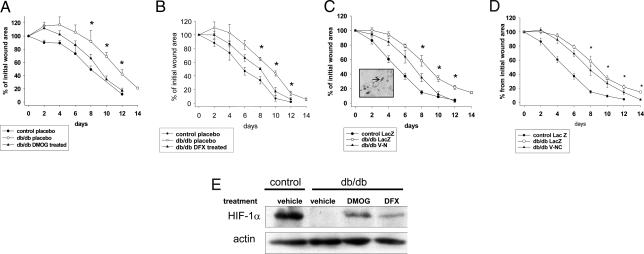
To examine the pathophysiological consequence of hyperglycemia-induced impaired regulation of HIF-1 levels and function in diabetic wound healing, we locally applied hydroxylase inhibitors in the db/db mouse wound healing model. In agreement with the results in primary mouse skin fibroblasts, both DMOG and DFX treatment improved the healing process in db/db mice (Fig. 4 A and B) despite the presence of persistent chronic hyperglycemia (placebo, 517.2 ± 12.5 mg/dl; DFX, 502.2 ± 6.6 mg/dl; DMOG, 547 ± 45 mg/dl) without interfering with the animal's weight (placebo, 46.8 ± 3.6 g; DFX, 48.8 ± 3.2 g; DMOG, 49.2 ± 5.6 g).
Fig. 4.
Local stabilization and activation of HIF-1α by hydroxylase inhibitors or direct transfer of stabilized HIF improve wound healing in diabetic mice. (Top) The healing rate of full-thickness wounds in db/db mice is promoted by local treatment with DMOG (2 mM; A1), DFX (1 mM; B1) or by adenovirus-transferred of stable forms of HIF-1α (V-N; C1), (V-NC; D1) compared with placebo or empty virus (LacZ), respectively (values are means ± SEM; *P < 0.05 db/db treated vs. db/db placebo or LacZ). C1, Inset: virus expression at the edge of the wound as revealed by β-galactosidase staining. (Lower) Representative examples showing wound healing in db/db mice treated as in Upper. (D). Immunoblot detecting HIF-1α in whole-cell protein extracts from wounds. Note that inhibition of hydroxylase activity by local treatment with either DMOG (2 mM) or DFX (1 mM) overcomes hyperglycemia-dependent negative regulation of HIF-1α.
HIF Stabilization Is Critical for Improving Defective Wound Healing in Diabetic Mice.
It is an important question whether the wound healing-promoting effects observed after treatment with the hydroxylase inhibitors were mediated through HIF. Consistent with the model that HIF is the molecular target of these substances, we observed stabilization of HIF-1α in the diabetic wounds after local treatment with hydroxylase inhibitors (Fig. 4E). To obtain direct evidence we performed gain-of-function studies by adenovirus-mediated expression of constitutively stable forms of HIF-1α (V-N and V-NC) in which both the critical proline residues have been substituted with alanines (Fig. S2). In the V-NC construct CTAD was additionally substituted with the potent VP16 TAD. The adenoviral transfer was identified mainly at the wound edge in the granulation tissue (Fig. 4C Inset) and had the same positive effect on wound healing in diabetic animals as pharmacological inhibition of hydroxylases, confirming that activation of HIF is critical to reverse negative effects of hyperglycemia on the wound healing process (Fig. 4C). Even though it is sensitive in vitro to the repressing effects of glucose, CTAD did not appear to be essential for wound healing, as its constitutive activation in V-NC did not have any additional effect compared with the double proline-mutated HIF construct (V-N; Fig. 4D). Consequently, we pursued our analysis using only the double proline-mutated construct.
Stabilization of HIF-1α Activates Several Processes Involved in Wound Healing.
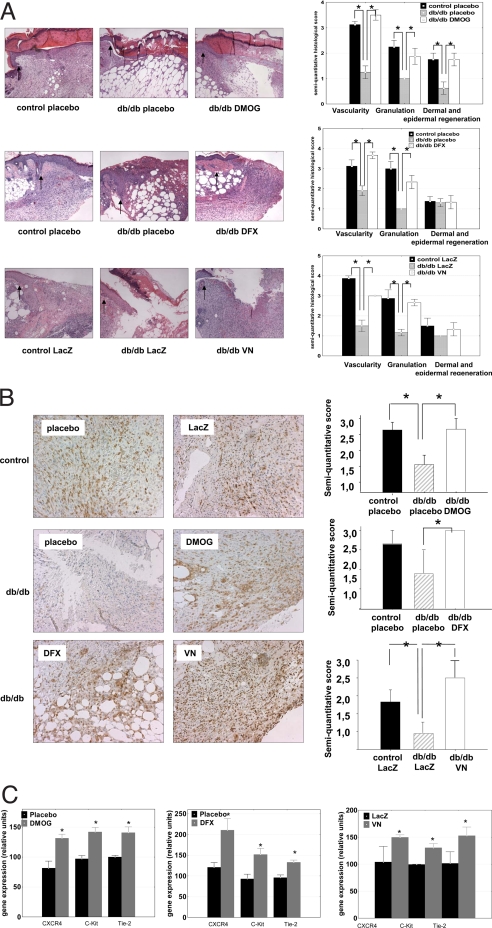
A histological analysis revealed that treatment with either hydroxylase inhibitor (DMOG or DFX) or inoculation of the stable V-N HIF-1α adenovirus construct was followed by an improvement in all of the essential steps of wound healing. The db/db mice exhibit a delayed wound healing rate as a consequence of a decrease in granulation tissue formation and neovascularization (20). The diabetic wounds in which HIF-1α was either pharmacologically stabilized or overexpressed in a stable form showed a thick, well structured granulation tissue similar to the granulation tissue observed in the non-diabetic mice (Fig. 5A). A significant improvement of the total number of vessels, as evaluated either by classical histology or by staining with GS-1 lectin, was also demonstrated following either strategy to improve HIF function (Fig. 5 A and B). Both these strategies also lead to induction of HIF target genes with critical roles in cell motility (i.e., HSP-90) (15) or genes encoding angiogenic cytokines involved both in vessel development or recruitment of CAG (i.e., VEGF-A, VEGF-R1, SDF-1α, SCF) (22) (Fig. S4), in excellent agreement with the histological changes observed. However, strategies for CAG mobilization have clinical relevance if they are followed by an increase CAG homing at the wound level. Recruitment of angiogenic cells to the wounds was indirectly evaluated by measuring the levels of the cytokine receptor expression typically present on these cells. This approach circumvents the technical problem to detect the small numbers of angiogenic cells usually present in tissues (22). In our wound healing model, HIF-1α stabilization was followed by an increase at the wound level of these cytokine receptors (i.e., CXCR4, C-Kit, Tie-2), indicating the local homing of endothelial precursor cells in the wound area (Fig. 5C).
Fig. 5.
Stabilization and activation of HIF-1α in diabetic wounds is followed by activation of granulation angiogenesis and recruitment of CAG. (A) Hematoxylin and eosin staining shows improvement in the granulation tissue and visualization of the wounds in db/db mice locally treated with DMOG (2 mM) or DFX (1 mM) or by adenovirus-mediated expression of a stable HIF-1α (V-N; original magnification ×25). (Right) Semiquantitative evaluation for granulation, angiogenesis, and dermal and epidermal regeneration. Graphs represent means ± SEM. Vehicle-treated or empty virus treated (LacZ) normoglycemic heterozygous db mice (black bars), vehicle-treated or empty virus treated (LacZ) homozygous diabetic db/db mice (gray bars), and DMOG, DFX, or HIF V-N treated homozygous db mice (white bars) are shown (*, P < 0.05). (B) Left: vascular density in wounds evaluated by Griffonia simplicifolia 1 (GS-1) lectin staining is increased in the db/db diabetic animals after treatment with DMOG (2 mM), DFX (1 mM), or V-N. (Right) The semiquantitative evaluation of vessel density evaluated by GS-1 lectin staining. Vehicle-treated or LacZ-treated heterozygous normoglycemic mice (black bars), vehicle-treated or Lac Z-treated homozygous diabetic mice (gray bars), and DMOG, DFX, or V-N-treated homozygous diabetic mice (white bars) are shown. Graphs represent mean ± SEM. (*, P < 0.05). (C) Local treatment with DMOG, DFX, or V-N in wounds of diabetic mice increases mRNA expression of cytokine receptors typically present on CAG (*, P < 0.05 treated vs. placebo or LacZ).
Discussion
We describe here the multilevel interaction between hyperglycemia and HIF function. First of all, hyperglycemia interferes with HIF-1α stability in hypoxia through a VHL-dependent mechanism. However, VHL is not induced by hyperglycemia (Fig. 1B), suggesting that hyperglycemia can make HIF-1α more sensitive to VHL machinery. Besides the canonical oxygen-dependent binding of VHL to HIF-1α after proline hydroxylation (6, 7), some other residues have been advocated to be important for promoting VHL-dependent ubiquitination (23, 24). Furthermore, SUMOylation of HIF-1α has recently been shown to have an important role in VHL-dependent degradation of HIF (25). Even though the mechanism of hyperglycemia-induced HIF-1 destabilization is still unclear, here we provide strong evidence that it can be satisfactorily overcome by blocking the prolyl hydroxylation.
Hyperglycemia interferes not only with HIF stability but also with its transactivation by modulating both transactivation domains. However, in vivo adenovirus delivery experiments show that the V-NC construct had no additive effects compared with the V-N construct, strongly suggesting that CTAD repression is not critical for wound healing. Interestingly, a central pathogenic role for the NTAD without any additional regulatory effect from the CTAD has also recently been suggested for HIF-2α-driven renal carcinogenesis (26). An alternative explanation for lack of superiority of V-NC compared with VN is the squelching effect (27) of a highly active transactivation activity classically described for VP16. Our observation is highly relevant for future development of hydroxylase inhibitors as potential therapy. They should be able to discriminate between hydroxylases responsible for CTAD- versus NTAD-specific transcriptional activation, i.e., PHDs versus factor inhibiting HIF, and not only between collagen hydroxylases and HIF hydroxylases.
We demonstrated that several HIF-regulated genes essential for different mechanisms activated in wound healing (i.e., migration, recruitment of CAG, and angiogenesis) were repressed in diabetic wounds. These findings underscore the central role of hyperglycemia-induced HIF destabilization in typical diabetic defects of tissue regeneration. Delayed expression of VEGF-A (28) and a low expression of SDF-1α have already been observed in diabetic wounds (29), and both mechanisms have been suggested to be good candidates for therapeutic interventions. We show here that repression of HIF is the common causative link between hyperglycemia and suppression of VEGF-A and SDF-1 expression, thereby allowing the proposal of a potentially more effective therapy.
Bearing in mind the essential role of reactive oxygen species in the pathogenesis of chronic complications of diabetes (30), we expected that DFX, which also exhibits some antioxidant properties (31), would have a better effect on wound healing. However, DMOG was at least equally efficient as DFX— if not more effective—in promoting wound healing (both used as the highest efficient doses as titrated in pilot experiments). This could possibly be attributed to the known antiproliferative effects of DFX (32), which we also observed in vitro (data not shown).
Several processes critical for wound healing were activated by local stabilization of HIF in diabetic wounds. The positive effect on angiogenesis was not followed by edema, which is in agreement with the phenotype of transgenic mice overexpressing HIF (33) and in contrast to the mice overexpressing VEGF (34) in the skin. Lack of homing of CAG has been suggested to be an important factor in delayed wound healing in diabetes (29), but the intimate mechanisms for it have not been established. Here we provide evidence that hyperglycemia-induced repression of the HIF-1α underlies this defect, and by stabilizing HIF-1 it is possible to increase the homing of the endogenous CAG into the wound area.
In conclusion, we show here that hyperglycemia-induced destabilization and inhibition of HIF-1α is a central pathogenic mechanism for delayed wound healing in diabetes. This represents a clinically relevant situation in which repression of HIF-1α is linked with a disease. Pharmacological and gene transfer-based stabilization of HIF-1α were able to reverse this defect, pointing out for the need to develop specific inhibitors for PHDs as treatment of diabetic wounds.
Methods
Cell culture reagents, medium and supplements (DMEM, RPMI, FCS) were from Gibco. DMOG from Frontier Scientific, rabbit anti HIF-1α from Novus Bioscience, secondary peroxidase-linked anti-rabbit IgG from Amersham Biosciences, anti-β-galactosidase from Abcam, isolectin B4 from Vector Laboratories, and Hypoxiprobe kit from Natural-Amersham Pharmacia. DFX and, if not stated otherwise, all other chemicals were purchased from Sigma.
Cell Culture.
Primary mouse skin fibroblast cultures were established using the skin explant technique as previously published (35). The cells were maintained in a humidified atmosphere with 5% CO2 at 37 °C DMEM (5.5 mM glucose) supplemented with 2 mM L-glutamine, 100 IU/ml penicillin and streptomycin, and 10% heat-inactivated FBS. Only cells between passages 4 and 9 were used.
The human SKRC-7 cell line, originating from renal carcinoma from a patient with point mutated VHL, was kindly provided by E. Oosterwijk (Nijmegen, The Netherlands) and maintained as described (36).
3T3 cells and human dermal fibroblasts (Promocell) were maintained like primary mouse fibroblasts.
Adenovirus Preparation.
Adenoviruses expressing full-length mouse HIF-1α either stabilized against degradation by point mutations (P402A/P563A) only (V-N) or additionally with C-terminally truncated and VP16 replaced (V-NC) were cloned by homologous recombination into an E1- and partially E3-deleted serotype 5 adenovirus plasmid. Viruses were produced in 293 cells (37). Viruses were tested to be free of microbiological contaminants, mycoplasma, and endotoxin. Biological activity of the virus constructs was confirmed by an in vitro assay. LacZ adenovirus was used as a control (37).
Immunoblot Analysis.
Primary mouse skin fibroblasts grown for 48 h in normal (5.5 mM) or high glucose concentrations (30 mM) were exposed for additional 6 h to either normoxia (21% O2), hypoxia (1% O2), DMOG (2 mM), or DFX (100 μM) as specified. SKRC-7 cells were exposed for 24 h to different glucose concentrations (5.5 mM or 30 mM) in normoxia.
Proteins were extracted and analyzed by Western blot as described (17). Proteins from the wounds were extracted using 2-mm Zirconia beads (Biospec Products).
Reporter Gene Assay.
3T3 cells were transfected with 500 ng of a GAL4-driven luciferase reporter gene plasmid and 10 ng of NTAD residues (pFLAG-GAL4-mHIF1α-[531–584]) or CTAD residues (pFLAG-GAL4-mHIF1α-[772–822]) using Lipofectamine 2000 (Invitrogen) in 60-mm plates, following the instructions of the manufacturer. After 12 h, the cells were trypsinized and transferred in 12-well plates. After another 12 h, the cells were exposed to different glucose concentrations (5.5 mM or 30 mM) for 48 h and to different O2 tensions for the last 24 h. The luciferase activity was determined (BioThema) in the cell extract and expressed on the total protein concentration.
VHL siRNA.
Human dermal fibroblasts were transiently transfected with 200 pmol per well of either pVHL-siRNA (Hs_VHL_5 HP validated siRNA SI02664550 for gene pVHL) or scrambled siRNA from Qiagen using HiPerFect. transfection reagent (Qiagen). After 48 h, cells were exposed to different glucose concentrations (5.5 mM and 30 mM) for another 48 h when the RNA was prepared as described.
Animals and Experimental Protocol.
C57BL/KsJm/Leptdb (db_/db_) mice (Stock 000662) and their normoglycemic heterozygous litter-mates were obtained from Charles River, housed five per cage in a 12-h light/dark cycle at 22 °C and provided ad libitum with standard laboratory food and water. The animals were caged individually for 1 week, handled daily, and then wounded as described in Wound Model. The experimental procedure was approved by the North Stockholm Ethical Committee for Care and Use of Laboratory Animals.
Wound Model.
Following blood glucose control, general anesthesia was performed with 3% isoflurane (Abbott). The hair of the back was shaved with an electric clipper followed by a depilatory cream. The skin was rinsed with alcohol and two full-thickness wounds extending through the panniculus carnosus were made on the dorsum on each side of midline, using a 6-mm biopsy punch. A transparent dressing (Tegaderm; 3M) was applied to cover the wounds after topical application of 100 μl of DMOG (2 mM), DFX (1 mM), or vehicle alone. When specified, a viral suspension was injected intradermally into the wound edges using a 30-gauge needle. Four injections of 20 μl per wound were administrated (109 pfu/ml) containing either HIF-1 V-N, HIF-1 V-NC, or LacZ-expressing adenoviruses. Following the surgical procedure, the animals were individually housed. During the first 2 days, the animals received s.c. buprenorphine (0.03 mg/kg) twice a day for relief of any possible distress caused by the procedure. In the experiments aimed to analyze histology, mRNA, or protein expression, the wounds were harvested at 7 days after surgery (≈50% closed).
Treatment with freshly made 100 μl DMOG (2 mM), DFX (1 mM), or control was applied through the dressing using a 30-gauge needle every other day. Viruses (HIF-VN, HIF-V-NC, LacZ) were inoculated once at the beginning of the experiment. Each treatment was evaluated in 10 animals per group.
Wound Analysis.
Digital photographs were recorded at the day of surgery and every other day after wounding. A circular reference was placed alongside to permit correction for the distance between the camera and the animals. The wound area was calculated in pixels with ImageJ 1.32 software (National Institutes of Health), corrected for the area of the reference circle and expressed as percentage of the original area.
Tissue Preparation and Histological Analysis.
After fixation in formalin, the samples were embedded in paraffin and sectioned (5 μm). For histological evaluation, sections were deparaffinized and rehydrated followed by hematoxylin and eosin staining. All slides were then evaluated by light microscopy by two independent observers unaware of the identity of the biopsy, using a semiquantitative score to evaluate vascularity, granulation, and dermal and epidermal regeneration as previously described (38) and internally validated in our laboratory. We used four-point scales to evaluate vascularity (1, severely altered angiogenesis with one or two vessels per site and endothelial edema, thrombosis, and/or hemorrhage; 2, moderately altered angiogenesis with three to four vessels per site, moderate edema and hemorrhage, but absence of thrombosis; 3, mildly altered angiogenesis with five to six vessels per site, moderate edema, but absence of thrombosis and hemorrhage; and 4, normal angiogenesis with more than seven vessels per site with only mild edema but absence of thrombosis and hemorrhage) and granulation tissue formation (1, thin granulation layer; 2, moderate granulation layer; 3, thick granulation layer; and 4, very thick granulation layer) and a three-point scale to evaluate dermal and epidermal regeneration (1, little regeneration; 2, moderate regeneration; and 3, complete regeneration).
Immunohistochemistry Staining and Evaluation.
We evaluated microvessel density by semiquantitative, double-blind analysis of the specific binding of GS-1 isolectin B4 to microvascular structures using a four-point scale (0, no positive vessels; 1, low number of positive vessels; 2, moderate number of positive vessels; and 3, high number of positive vessels). Isolectin B4 binding was performed using biotinylated isolectin B4 (diluted 1:25).
Expression of the adenovirus-mediated transfer of β-galactosidase was evaluated by immunohistochemistry using anti-β-galactosidase antibody (1:500) from Abcam. Matched IgG isotype controls were included for each marker.
The hypoxia level within the wounds was evaluated using the Hypoxiprobe kit (Natural-Amersham Pharmacia) following the instructions of the manufacturer.
Quantitative RT-PCR.
Total RNA was isolated from cells using Pure link Micro-to-Midi Total RNA Purification Kit (Invitrogen) and from skin using an RNeasy Fibrous Tissue Mini Kit (Qiagen). cDNA was obtained by reverse-transcribing total RNA with SuperScript III and first-strand synthesis Supermix for quantitative RT-PCR according to the manufacturer's recommended protocol (Invitrogen). The primers (see SI Text) were selected from Harvard University primer bank (primers 1–4) (http://pga.mgh.harvard.edu/primerbank/), used as previously published (primers 5–9) (22), or designed by using the Primer 3 program (http://frodo.wi.mit.edu; primers 10–12).
Real-time PCR was performed in an Applied Biosystems 7300 unit using Platinum SYBR Green quantitative PCR Supermix-UDG with ROX reference dye (Invitrogen). After incubation for 2 min at 50 °C and 2 min at 95 °C, a two-step cycling protocol (15 s at 94 °C, 30 s at 60 °C) was used for 40 cycles. The melting curve analysis was done using the program supplied by Applied Biosystems. The quality of the quantitative PCR run was determined by standard curves and melting curve analysis. The amplification products were verified by sequencing.
Statistic Analysis.
Differences between groups were computed using one-way analysis or two-way repeated-measures analysis of variance as appropriate, with the Bonferroni post-hoc test. A P value <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments.
We thank the Family Erling Persson Foundation, Juvenile Diabetes Research Foundation, von Kantzow Foundation, Thurings Foundation, Åke Wiberg Foundation, Swedish Diabetes Association, and Swedish Research Council for financial support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805230105/DCSupplemental.
References
- 1.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–1724. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 2.Dinh TL, Veves A. Treatment of diabetic ulcers. Dermatol Ther. 2006;19:348–355. doi: 10.1111/j.1529-8019.2006.00093.x. [DOI] [PubMed] [Google Scholar]
- 3.Edmonds ME. The diabetic foot, 2003. Diabetes Metab Res Rev. 2004;20(suppl 1):S9–S12. doi: 10.1002/dmrr.458. [DOI] [PubMed] [Google Scholar]
- 4.Tandara AA, Mustoe TA. Oxygen in wound healing–more than a nutrient. World J Surg. 2004;28:294–300. doi: 10.1007/s00268-003-7400-2. [DOI] [PubMed] [Google Scholar]
- 5.Knighton DR, Silver IA, Hunt TK. Regulation of wound-healing angiogenesis-effect of oxygen gradients and inspired oxygen concentration. Surgery. 1981;90:262–270. [PubMed] [Google Scholar]
- 6.Jaakkola P, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 7.Ivan M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 8.Kallio PJ, Wilson WJ, O'Brien S, Makino Y, Poellinger L. Regulation of the hypoxia-inducible transcription factor 1alpha by the ubiquitin-proteasome pathway. J BiolChem. 1999;274:6519–6525. doi: 10.1074/jbc.274.10.6519. [DOI] [PubMed] [Google Scholar]
- 9.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 10.Epstein AC, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL. Life with oxygen. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- 12.Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 13.Elson DA, Ryan HE, Snow JW, Johnson R, Arbeit JM. Coordinate up-regulation of hypoxia inducible factor (HIF)-1alpha and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing. Cancer Res. 2000;60:6189–6195. [PubMed] [Google Scholar]
- 14.Kelly BD, et al. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 15.Li W, et al. Extracellular heat shock protein-90alpha: linking hypoxia to skin cell motility and wound healing. EMBO J. 2007;26:1221–1233. doi: 10.1038/sj.emboj.7601579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceradini DJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 17.Catrina SB, Okamoto K, Pereira T, Brismar K, Poellinger L. Hyperglycemia regulates hypoxia-inducible factor-1alpha protein stability and function. Diabetes. 2004;53:3226–3232. doi: 10.2337/diabetes.53.12.3226. [DOI] [PubMed] [Google Scholar]
- 18.Fadini GP, et al. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats. Diabetologia. 2006;49:3075–3084. doi: 10.1007/s00125-006-0401-6. [DOI] [PubMed] [Google Scholar]
- 19.Gao W, et al. High glucose concentrations alter hypoxia-induced control of vascular smooth muscle cell growth via a HIF-1alpha-dependent pathway. J Mol Cell Cardiol. 2006 doi: 10.1016/j.yjmcc.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Michaels J, et al. db/db mice exhibit severe wound-healing impairments compared with other murine diabetic strains in a silicone-splinted excisional wound model. Wound Repair Regen. 2007;15:665–670. doi: 10.1111/j.1524-475X.2007.00273.x. [DOI] [PubMed] [Google Scholar]
- 21.Hirota K, Semenza GL. Regulation of hypoxia-inducible factor 1 by prolyl and asparaginyl hydroxylases. Biochem Biophys Res Commun. 2005;338:610–616. doi: 10.1016/j.bbrc.2005.08.193. [DOI] [PubMed] [Google Scholar]
- 22.Bosch-Marce M, et al. Effects of Aging and Hypoxia-Inducible Factor-1 Activity on Angiogenic Cell Mobilization and Recovery of Perfusion Following Limb Ischemia. Circ Res. 2007 doi: 10.1161/CIRCRESAHA.107.153346. [DOI] [PubMed] [Google Scholar]
- 23.Jeong JW, et al. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell. 2002;111:709–720. doi: 10.1016/s0092-8674(02)01085-1. [DOI] [PubMed] [Google Scholar]
- 24.Pereira T, Zheng X, Ruas JL, Tanimoto K, Poellinger L. Identification of residues critical for regulation of protein stability and the transactivation function of the hypoxia-inducible factor-1alpha by the von Hippel-Lindau tumor suppressor gene product. J Biol Chem. 2003;278:6816–6823. doi: 10.1074/jbc.M209297200. [DOI] [PubMed] [Google Scholar]
- 25.Cheng J, Kang X, Zhang S, Yeh ET. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell. 2007;131:584–595. doi: 10.1016/j.cell.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan Q, Bartz S, Mao M, Li L, Kaelin WG., Jr The hypoxia-inducible factor 2alpha N-terminal and C-terminal transactivation domains cooperate to promote renal tumorigenesis in vivo. Mol Cell Biol. 2007;27:2092–2102. doi: 10.1128/MCB.01514-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill G, Ptashne M. Negative effect of the transcriptional activator GAL4. Nature. 1988;334:721–724. doi: 10.1038/334721a0. [DOI] [PubMed] [Google Scholar]
- 28.Frank S, et al. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J BiolChem. 1995;270:12607–12613. doi: 10.1074/jbc.270.21.12607. [DOI] [PubMed] [Google Scholar]
- 29.Gallagher KA, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 31.Emerit J, Beaumont C, Trivin F. Iron metabolism, free radicals, and oxidative injury. Biomed Pharmacother. 2001;55:333–339. doi: 10.1016/s0753-3322(01)00068-3. [DOI] [PubMed] [Google Scholar]
- 32.Dayani PN, Bishop MC, Black K, Zeltzer PM. Desferoxamine (DFO)–mediated iron chelation: rationale for a novel approach to therapy for brain cancer. J Neurooncol. 2004;67:367–377. doi: 10.1023/b:neon.0000024238.21349.37. [DOI] [PubMed] [Google Scholar]
- 33.Elson DA, et al. Induction of hypervascularity without leakage or inflammation in transgenic mice overexpressing hypoxia-inducible factor-1alpha. Genes Dev. 2001;15:2520–2532. doi: 10.1101/gad.914801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thurston G, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 35.Hehenberger K, Heilborn JD, Brismar K, Hansson A. Inhibited proliferation of fibroblasts derived from chronic diabetic wounds and normal dermal fibroblasts treated with high glucose is associated with increased formation of l-lactate. Wound Repair Regen. 1998;6:135–141. doi: 10.1046/j.1524-475x.1998.60207.x. [DOI] [PubMed] [Google Scholar]
- 36.Grabmaier K, et al. Strict regulation of CAIX(G250/MN) by HIF-1alpha in clear cell renal cell carcinoma. Oncogene. 2004;23:5624–5631. doi: 10.1038/sj.onc.1207764. [DOI] [PubMed] [Google Scholar]
- 37.Laitinen M, et al. Adenovirus-mediated gene transfer to lower limb artery of patients with chronic critical leg ischemia. Hum Gene Ther. 1998;9:1481–1486. doi: 10.1089/hum.1998.9.10-1481. [DOI] [PubMed] [Google Scholar]
- 38.Galeano M, et al. Adeno-associated viral vector-mediated human vascular endothelial growth factor gene transfer stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetologia. 2003;46:546–555. doi: 10.1007/s00125-003-1064-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.