Abstract
Effective immunization against hepatitis C virus (HCV) infections is likely to require the induction of both robust T and B cell immunity. Although neutralizing antibodies may play an important role in control of infection, there is little understanding of the structure of the HCV envelope glycoproteins and how they interact with such antibodies. An additional challenge for vaccine design is the genetic diversity of HCV and the rapid evolution of viral quasispecies that escape antibody-mediated neutralization. We used a cell culture-infectious, chimeric HCV with the structural proteins of genotype 1a virus to identify envelope residues contributing to the epitope recognized by a broadly neutralizing, murine monoclonal antibody, AP33. By repetitive rounds of neutralization followed by amplification, we selected a population of viral escape mutants that resist stringent neutralization with AP33 and no longer bind the antibody. Two amino acid substitutions, widely separated in the linear sequence of the E2 envelope protein (N415Y and E655G), were identified by sequencing of cloned cDNA and shown by reverse genetics analysis to contribute jointly to the AP33 resistance phenotype. The N415Y mutation substantially lowered virus fitness, most likely because of a defect in viral entry, but did not reduce binding of soluble CD81 to immobilized HCV-pseudotyped retrovirus particles. The in vitro selection of an HCV escape mutant recapitulates the ongoing evolution of antigenic variants that contributes to viral persistence in humans and reveals information concerning the conformational structure of the AP33 epitope, its role in viral replication, and constraints on its molecular evolution.
Keywords: antibody, immunity, vaccine, viral envelope
Infections with hepatitis C virus (HCV) persist for years in most patients, and can lead to chronic, sometimes life-threatening liver disease. Despite almost two decades of research, no vaccine is available to prevent infection with this virus (1). Efforts to develop vaccines have been stymied by the high degree of genetic diversity among HCV strains, the lack of readily available animal models, and the absence of clearly established in vitro correlates of protective immunity. Multiple lines of evidence suggest that CD4+ and CD8+ T-cell responses, although critical for control of acute infection, are not sufficient to prevent long-term persistence (2). Other evidence suggests that neutralizing antibodies may modify infection but are not capable of preventing it (3). In part, the inability of T- and B-cell immunity to prevent progression to viral persistence may arise from the continuing evolution of new HCV quasispecies that escape these host responses (4–7). Although clearly a challenge for vaccine development, the current paradigm for effective immunization calls for induction of both robust T- and B-cell responses. However, the design of immunogens capable of eliciting broadly reactive neutralizing antibodies to this highly diverse virus will likely require a much better understanding of the antigenic structure of HCV.
Unfortunately, there are no crystallographic or cryoelectron microscopic reconstructions of HCV to guide such studies. Only a rudimentary understanding exists of structure-function relationships within the two glycoproteins that comprise the HCV envelope. Moreover, until recently, studies of virus neutralization have not been possible in vitro because of the lack of viruses that could be propagated in cell culture, resulting in the use of surrogate assays using HCV-pseudotyped retroviruses (HCVpp). Nonetheless, a hypervariable region (HVR-1) near the N terminus of the second envelope glycoprotein (E2) has been shown to be a major determinant of isolate-specific neutralizing antibodies (8, 9). These antibodies provide little protection against infection, as the HVR-1 sequence continuously evolves in response to pressure exerted by HVR-1-specific neutralizing antibodies (6, 9). More broadly neutralizing antibodies are usually directed against conformational epitopes in E2 (10–14). Cross-competition analyses, coupled with alanine scanning studies of recombinant antigens, have delineated at least three immunogenic clusters of overlapping epitopes bound by functionally distinct groups of monoclonal antibodies (mAbs) isolated from infected humans (15). One cluster, designated domain A, is bound by non-neutralizing antibodies, whereas two other clusters, domains B and C, bind distinctly different groups of neutralizing mAbs (16, 17). Antibodies to domain B may be particularly important, as some are capable of broadly neutralizing multiple genotypes of HCV (17–19). Domain B is comprised of a cluster of discontinuous, conformation-dependent epitopes containing multiple residues involved in E2 binding to CD81, an important co-receptor for virus. Thus, antibodies to domain B may neutralize virus by blocking this critical step in viral entry.
Although the sequence spanning residues 523–540 in E2 contributes to conformational epitopes within domain B, cross-competition and alanine replacement studies suggest that residues 396–424, immediately downstream of HVR-1, are also involved (17, 18). Importantly, immunization of mice and rats with recombinant E2 also elicits mAbs that recognize this E2 segment (20–22). One such mAb, AP33, is of particular interest because it demonstrates broad and potent, cross-genotypic neutralizing activity. Prior studies using synthetic peptides, recombinant antigens, and HCVpp suggest that AP33 binds a highly conserved, linear epitope spanning residues 413–420 (22, 23). Here, we describe the use of a cell culture-infectious, inter-genotypic chimeric HCV with genotype 1a structural proteins (24, 25) to further define the AP33 epitope by analyzing mutants selected for the ability to escape neutralization by this mAb. Despite its broad neutralizing activity against most naturally occurring viruses, we show that it is possible to select stable, antibody-resistant viral variants in cell culture. Our results recapitulate the ongoing evolution of antigenic variants that contributes to viral persistence in humans and reveal information concerning the structure of the AP33 epitope as well as constraints on its molecular evolution.
Results
In Vitro Selection of a Neutralization-Escape Mutant.
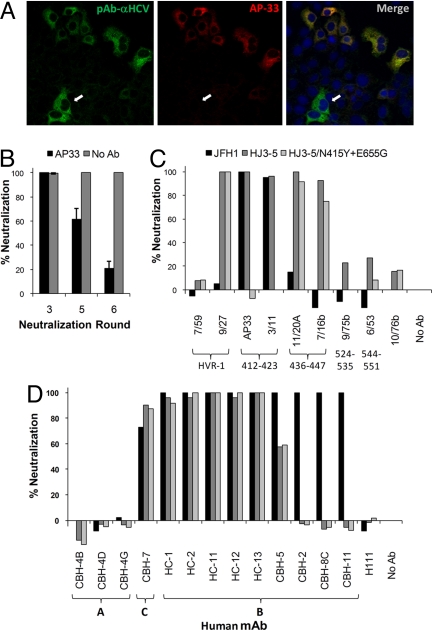
To select HCV mutants resistant to neutralization by the mAb, AP33, we subjected virus to repetitive rounds of antibody-mediated neutralization followed by amplification of the surviving virus by passage of infected cells in the absence of antibody (for summary of selection process, see [Supporting Information (SI) Fig. S1], provided online on the PNAS web site). After three to six cell passages, virus was harvested from supernatant fluids and, after titration by fluorescent focus unit (FFU) assay, subjected to the next round of neutralization. By the third round, two-color immunofluorescence staining using AP33 and human polyclonal anti-HCV as primary antibodies revealed a small number of cells infected with virus that failed to bind AP33 but that were readily labeled with polyclonal antibody (Fig. 1A). By the fifth neutralization round, a significant non-neutralizable virus fraction was detectable in an FFU-reduction neutralization assay (Fig. 1B). This increased to ≈80% after the sixth round of neutralization, at which point flow cytometry using two-color labeling confirmed the existence of two antigenically distinct, infected cell populations (data not shown).
Fig. 1.
In vitro selection of an HCV-neutralizing antibody escape mutant. (A) Dual antibody staining of cells infected with virus after the third round of neutralization with AP33. Arrows indicate cells staining only with polyclonal anti-HCV (green) but not AP33 (red). (B) AP33-selected viruses from the third, fifth, and sixth rounds of neutralization were tested for neutralization with AP33 (100 μg/ml) by FFU-reduction assay. (C) The N415Y and E655G mutations identified in E2 from AP33-resistant virus were recreated in the infectious pHJ3–5 plasmid DNA, and RNA transcribed from it transfected into Huh7 cells to generate virus with defined amino acid substitutions. The wt JFH1, HJ3–5, and the HJ3–5/N415G+E655G viruses were tested for neutralization against AP33 (100 μg/ml) and a panel of rat mAbs (>10 μg/ml) in the FFU-reduction assay. Shown below each mAb are the E2 residues to which it has been shown to bind in previous peptide assays. HVR-1, hypervariable domain 1. 10/76b is an irrelevant control mAb. (D) Similar neutralization assays of wt and mutant viruses with a panel of human mAbs (100 μg/ml). Below each mAb is the E2 domain to which it has been mapped in previous antibody competition studies (15, 17). H-111 is directed against a linear epitope in E1 and was included as a control. Bars represent the same viruses as in (C).
RNA from the sixth-round virus harvest was isolated, and the E1 and E2 envelope protein coding sequence was amplified by reverse transcription-polymerase chain reaction (RT-PCR) and molecularly cloned. In 22 of 23 individual clones sequenced, point mutations resulted in two single amino acid substitutions within E2: N415Y and E655G. These mutations were not found in virus passaged in parallel without AP33 neutralization. Although N415 is within a domain identified previously as comprising the AP33 epitope 413–420 (22, 23), E655 is distant in the linear E2 sequence and has not been implicated previously in recognition of HCV by AP33. To validate the role of these mutations in conferring neutralization escape, they were engineered both singly and together into pHJ3–5 plasmid DNA, and the cognate viruses rescued after RNA transfection of naive cells. The dual mutant, HJ3–5/N415Y+E655G (Figs. 1C and 2A) and single mutant, HJ3–5/N415Y (Fig. 2A) were highly resistant to neutralization by high concentrations of AP33 (Figs. 1C and 2A), whereas HJ3–5/E655Y demonstrated only partial resistance to neutralization by low concentrations of AP33 (Fig. 2A). Importantly, the addition of the E655G mutation significantly and reproducibly enhanced resistance of the N415Y mutant to high concentrations of AP33 (6.3 μg/ml and greater, Fig. 2A), confirming its contribution to the escape phenotype. These results demonstrate the usefulness of this selection protocol for identifying envelope protein residues important for antibody-mediated neutralization of HCV.
Fig. 2.
Neutralization assays with HCVcc or HCVpp containing mutations in E2 conferring resistance to AP33 neutralization. (A and B) The N415Y and E655G mutations were inserted into pHJ3–5 plasmid DNA singly or in combination to generate virus with defined amino acid substitutions. N415Y (open triangles), E655G (open squares), and N415Y+E655G (open circles) mutants and wt (filled diamonds) virus were analyzed for resistance to neutralization by various concentrations of (A) AP33 or (B) HC-1 mAbs by FFU reduction assay. Error bars indicate range in replicate experiments. (C and D) HCVpp containing either wt H77c envelope protein (filled diamonds) or N415Y (open triangles), E655G (open squares), and N415Y+E655G (open circles) mutants were tested for resistance to neutralization by various concentrations of (C) AP33 or (D) HC-1 mAbs. Neutralization is expressed as percent reduction (±SD) in luciferase activity.
Resistance Profiling of the AP33 Escape Mutant.
To assess the breadth of the neutralization resistance engendered by the N415Y and E655G mutations, we determined the capacity of each of a panel of rat mAbs (26) to neutralize the N415Y+E655G mutant. These results were compared with neutralization assays using the parental HJ3–5 virus, as well as the genotype 2a JFH1 virus (Fig. 1C). Three rat mAbs (9/75b, 6/53, and 7/59) failed to neutralize a significant fraction of any virus, whereas three others (9/27, 11/20A, and 7/16b) neutralized the genotype 1a HJ3–5 virus but not JFH1 virus. Interestingly, the N415Y and E655G mutations did not confer resistance against any of these genotype-specific mAbs. In contrast, the combination of these mutations conferred complete resistance to mAb 3/11, which was capable of neutralizing both wild-type (wt) genotype 1a and 2a viruses (Fig. 1C). More detailed analysis of this antibody revealed that N415Y was the primary cause of this neutralization resistance, although E655G caused a slight reduction in neutralization by 3/11 (Fig. S2). These data are consistent with prior studies showing that 3/11 and AP33 recognize similar, yet distinct, epitopes (22, 27).
We also evaluated the ability of these viruses to be neutralized by each of a panel of human mAbs derived from patients with chronic HCV infection that recognize distinct antigenic domains on the HCV envelope that have been mapped by alanine scanning. HC-1, −2, -11, -12, -13, and CBH-5 recognize residues comprising “domain B” and neutralize both genotype 1a and 2a virus, whereas CBH-8C and -11 recognize overlapping epitopes but neutralize only the 2a virus (23, 28). CBH-7 recognizes an epitope in domain C that does not overlap those bound by domain B antibodies and that is capable of neutralizing both genotypes. At a concentration of 100 μg/ml, each of these mAbs, as well as several others in domain A that do not neutralize either 1a or 2a virus, showed comparably strong, weak, or nonexistent neutralizing activity against HJ3–5/N415Y+ E655G and its HJ3–5 parent (Fig. 1D). Neutralization by one of the domain B mAbs, HC-1, was examined in further detail over a range of antibody concentrations. These results suggested that the E655G mutation might cause a slight decrease in neutralization (Fig. 2B). Overall, however, the data suggest that these human mAbs recognize epitopes that are functionally distinct from the AP33 binding site.
To compare these results with prior studies of HCV neutralization epitopes that have used HCVpp as a surrogate for HCV, we produced HCVpp bearing the genotype 1a H77 E1 and E2 proteins with or without the N415Y and E655G mutations. These results generally mirrored those obtained in the FFU-reduction assay (as evident when comparing Figs. 2A and 2B with 2C and 2D), revealing that N415Y causes a high level of escape from AP33 but no resistance to HC-1 neutralization (Figs. 2C and 2D). Although the HCVpp neutralization assay revealed partial resistance of the E655G mutant to low concentrations of AP33, N415Y alone was capable of causing 100% escape, in contrast to the results obtained with the cell culture-infectious HJ3–5/N415Y virus (as can be noted when comparing Figs. 2C and 2A). These results thus suggest that infectious virus neutralization assays may be superior to the surrogate HCVpp assay for detecting subtle differences in neutralization epitopes.
Mechanism of Neutralization Escape.
The two-color labeling of cells infected with virus during the escape mutant selection process (Fig. 1A) suggested that the N415Y and E655G mutations may confer resistance to AP33 by ablating binding of the mAb. To formally prove this, we measured the binding of AP33 (and as a control the human mAb, HC-1) to HCVpp isolated by centrifugation onto a sucrose-cushion (Figs. 3A and 3B). These assays were carried out in solid phase, with HCVpp bound to lectin-coated microtiter plates, and bound mAbs detected by an alkaline phosphatase-conjugated secondary antibody (see Materials and Methods). Results were normalized according to the HCVpp abundance in each preparation, which was determined by measuring the binding of a non-neutralizing human mAb (H-111) that recognizes a linear epitope within E1 (29). These studies revealed complete loss of AP33 binding to the N415Y and N415Y+E655G mutants (Fig. 3A). This was confirmed by multicolor labeling of cells infected with wt or mutant virus (Fig. S3). In contrast, AP33 binding to HCVpp was not reduced by the E655G mutation alone (Fig. 3A). Although the human mAb HC-1 bound to each of the mutants, binding was significantly reduced by N415Y and was reduced even further by the combination of N415Y and E655G (Fig. 3B). This was a surprising finding, inasmuch as these mutations had little if any effect on HC-1 neutralization of either virus or HCVpp (Fig. 2B and 2D). These results suggest that the AP33 and HC-1 epitopes either share some contact residues in common or that the AP33 escape mutations to some extent globally alter the E2 conformation.
Fig. 3.
ELISA analysis of mAb binding to HCVpp containing AP33-selected escape mutations. HCVpp with wt E2 (filled diamonds) or E2 containing N415Y (open triangles), E655G (open sqaures), and N415Y+E655G (open circles) mutations were captured by immobilized GNA and assessed for their ability to bind various concentrations of (A) AP33 or (B) HC-1 mAbs. Results were normalized to binding values obtained with H-111, which recognizes a linear epitope in E1.
Fitness of AP33 Escape Mutants.
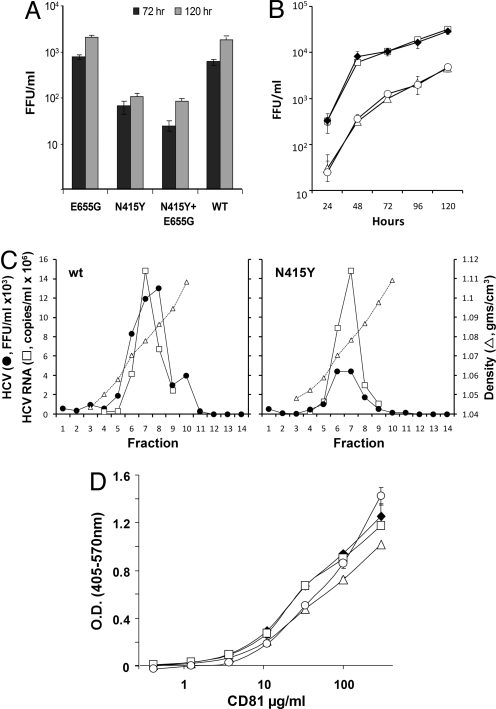
Unlike many other anti-HCV mAbs, AP33 possesses broad neutralizing activity against many different HCV genotypes. Given our ability to select for neutralization resistance in vitro, it is perhaps surprising that viruses capable of escaping AP33 neutralization have not emerged naturally in the course of HCV evolution. The absence of natural resistance to AP33 suggests that its epitope may not be immunogenic in vivo, possibly because it is masked by other antibodies or other components of the envelope (see Discussion). Alternatively, it is possible that the epitope might be conserved to preserve essential virion functions, and that escape mutations within it could confer significant loss of fitness. To assess this latter possibility, we infected cells with the mutant and wt HJ3–5 viruses at a low multiplicity of infection (MOI = 0.01), and determined the infectious virus yield 72 and 120 hours later. These results revealed a 10–20-fold reduction in yield of the N415Y or N415Y+E655G mutants compared with HJ3–5 virus (Fig. 4A), suggesting that the N415Y mutation imposes a substantial cost on fitness. Similar reductions in infectious virus yields were observed after transfection of cells with RNAs containing the N415Y mutation, compared with cells transfected with wt HJ3–5 or the single mutant E655G RNA (Fig. 4B). Replication of the mutant viral RNAs was not impaired, as intracellular RNA abundances were similar during the first 48 hours after transfection (Fig. S4). These results thus demonstrate that the N415Y mutation causes a significant loss of viral fitness.
Fig. 4.
The N415Y mutation confers loss of replication fitness, but does not alter affinity for CD81. (A). Virus yield assays. Wt single or double mutant virus stocks were inoculated onto FT3-7 cells at an MOI of 0.01. Supernatant fluids were collected at 72 hours and 120 hours and the titer of infectious virus assessed by FFU assay. (B) Infectious virus yields produced by RNA-transfected cells. Supernatant fluids were collected for FFU assay at 24, 48, 72, 96, and 120 hours after RNA transfection: wt HJ3–5 (filled diamonds), N415Y (filled triangles), E655G (open squares), and N415Y+E655G (open circles). (C) Rate-zonal gradient analysis of wt HJ3–5 and N415Y viruses. Peak fractions from gradients loaded with each virus were tested for infectious virus (filled circles) by FFU assay and for viral RNA (open squares) by quantitative real-time RT-PCR. Results for the N415Y virus were normalized to wt RNA abundance in the peak fraction 7. (D). Binding of soluble CD81 to purified wt or mutant HCVpp captured on a solid-phase support. Symbols are as in (B). Results were normalized to binding values for the H-111 mAb.
To assess whether the reduction in viral fitness caused by the N415Y mutation involves either impaired viral entry or a defect in release of virus, we isolated wt and N415Y viruses by rate-zonal ultracentrifugation in sucrose density gradients, and we compared virus infectivity (in FFU) and viral RNA abundance (by quantitative real-time RT-PCR) in peak gradient fractions. These results revealed that the specific infectivity of the N415Y mutant was less than one-third that of the wt virus (0.46 ± 0.02 vs. 1.6 ± 0.67 FFU/103 RNA copies) (Fig. 4C). Taken together with the results described above, these data suggest that the N415Y mutant is likely to be defective in some aspect of viral entry. Because previous studies suggest that both the AP33 and 3/11 mAbs may interfere with the ability of E2 to bind CD81 (20, 26), we considered the possibility that the N415Y mutation reduces the affinity of virus for this cellular co-receptor. However, we found no more than a slight reduction in binding of recombinant soluble CD81 to immobilized HCVpp carrying the N415Y and/or E655G mutations (kd = 1.2 mmol/l for HCVpp/N415Y+E655G vs. 0.83 mmol/l for wt HCVpp) and no measurable reduction for either single mutant (Fig. 4D). As the loss of fitness was equivalent for HJ3–5/N415Y and HJ3/N415Y+E655G (Fig. 4A and 4B), these results suggest that it is not due to loss of CD81 binding. Consistent with this, we also noted no reduction in the ability of anti-CD81 to block infection with any of the mutant viruses (data not shown). For additional information see Fig. S5.
Discussion
The role of B-cell immunity in resolution of acute HCV infection and prevention of reinfection remains incompletely defined. Neutralizing antibodies are typically delayed in appearance in acute HCV infection, generally do not confer sterile immunity, and are usually present in chronically infected persons (30–32). Nonetheless, substantial data suggest that virus-specific antibodies exert a level of control over HCV infection. Virus replication was blocked in experimentally challenged chimpanzees by prior in vitro neutralization with antibodies directed to HVR-1 or pooled Ig prepared from infected individuals (8, 33). Although human mAbs-recognizing epitopes within domain B protect transgenic mice with chimeric human livers against infection with heterologous HCV (18), infection was only delayed, and not prevented, by passive transfer of hepatitis C immune globulin to animals before challenge (3). Some, but not all, studies also suggest that the rapid induction of high titer neutralizing antibodies may be associated with viral clearance during acute HCV infection (34, 35). Thus, it may be possible to develop effective therapeutic or preventive B-cell immunization strategies. However, the development of effective B-cell immunogens will be challenging and will likely demand a better understanding of the antigenic structures on the surface of the virion that elicit and bind neutralizing antibodies.
In this study, we used a chimeric HCV (HJ3–5 virus), with structural proteins derived from genotype 1a H77 virus and robust capacity to replicate in Huh7 cells (24, 25), to select variants that resist neutralization to AP33, a murine mAb that broadly neutralizes HCVpp with envelope proteins derived from multiple HCV genotypes (36). Previous studies have shown that AP33 recognizes a broadly conserved linear sequence spanning residues 413–420 in the E2 protein (H77 virus) (20–22, 36). Alanine substitutions at L413, N415, G418, and W420 of E2 result in greater than 75% reduction of E2 binding by AP33 (22). In addition, Owsianka et al. (36) reported a naturally occurring genotype 5 E2 protein which is not recognized by AP33 and contains substitutions of residues 415–418; HCVpp bearing this E2 protein were not neutralized by AP33. Consistent with these data, the N415Y mutation found in our AP33 escape mutant blocks the ability of AP33 to bind and neutralize cell culture-derived HCV (Fig. 2A). However, several previous studies, including one showing that the binding of AP33 to an E2 peptide is not reduced by an N415A substitution, suggest that the AP33 epitope is at least partly conformational (21, 22). Importantly, we found that an E655G mutation also contributes to the AP33 escape phenotype (Fig. 2A), suggesting that the cognate epitope is in fact discontinuous. This finding both provides novel insight into the folded structure of E2, and also potentially explains these earlier observations. Residue 655 is either Glu or Asp in most HCV strains. Other amino acids are occasionally present at this position, but G655 occurs in only eight of 627 unique E2 sequences available in the Los Alamos HCV Database.
A hallmark of HCV infection is continuing evolution of the virus in vivo, with ongoing selection of quasispecies that resist neutralizing antibodies produced by the immune response. Why, then, has the AP33 epitope remained so conserved among different HCV genotypes, in clear contrast to the relatively rapid selection of a highly resistant escape mutant, as demonstrated here? One answer may lie in the low prevalence of antibodies that resemble AP33 (recognized by peptide capture and competition enzyme-linked immunosorbent assay [ELISA] experiments), which are found in less than 2.5% of sera from both acute-resolved and chronic HCV infections (21). This suggests that the AP33 epitope is poorly immunogenic, possibly because it is shielded by glycans. Residue N417 is a highly conserved glycosylation site, present in all HCV genotypes (32). Neutralization by the closely related rat mAb, 3/11 (22), is enhanced by mutations knocking out N-linked glycosylation at residues 417, 532, and 645, suggesting that these residues may be in close proximity to the 3/11 (and thus AP33) epitope (32). The enhanced neutralization associated with ablation of glycosylation at N645 is particularly interesting, as it is only 10 residues distant from E655, which our data suggest contributes to AP33 recognition (Fig. 2A). The elimination of glycosylation at all three of these residues also enhances neutralization of HCVpp by the soluble extracellular domain of CD81 (32). This is consistent with the ability of AP33 to prevent binding of E2 to CD81 (20, 22), and the possibility that AP33 may neutralize infectivity by disrupting the interaction of HCV with the CD81 co-receptor.
It is also possible that the AP33 epitope is immunogenic but masked by antibodies that bind close to it. This is suggested by studies showing that immunoglobulins prepared from multiple anti-HCV-positive donors contain neutralizing antibodies that bind a peptide spanning residues 412–419 of E2, but that the binding of these antibodies to virus is blocked by non-neutralizing antibodies directed at a neighboring epitope (residues 434–446), with resulting inhibition of neutralization activity (31). Yet another possibility is that the epitope may be masked by lipoproteins associated with virus produced within the liver (37). Such virus differs significantly in its buoyant density, and probably in its lipid content, from virus produced in cell culture (38), and it is possible that the antigenic nature of cell culture-derived virus (as well as pseudotyped HCVpp) may differ from that of the native HCV particle produced in vivo.
Our data indicate that substitutions within the AP33 epitope result in a significant loss of replication fitness (Fig. 4A and 4B), suggesting that this may contribute to the conservation of the involved residues. The underlying cause of this loss of fitness is uncertain. The N415Y mutation lowered the specific infectivity of virus without reducing the efficiency of RNA replication (Figs. 4C and S4), suggesting a defect in viral entry. However, it did not reduce the binding of soluble CD81 to (Fig. 4D), despite the facts that both AP33 and 3/11 interfere with the binding of E2 to CD81 (20, 26) and that the closely positioned W420 residue is important for CD81 binding by HCVpp (26, 39). The N415Y mutation may reduce affinity for an alternative cellular receptor or may impose constraints on conformational changes required for viral maturation or cellular entry. Further investigation will be needed to distinguish between these possibilities.
The approach that we have taken here to mapping the neutralization epitopes of HCV mimics the natural evolution of escape mutants in vivo and has several advantages over methods used previously, such as alanine replacement analysis, phage display, and peptide scanning. This is particularly true for the identification of conformationally dependent epitopes formed by residues that are widely separated within the linear sequence of E2 but that are brought into proximity by the folding of the envelope proteins. Broader use of this experimental approach should inform efforts at rational vaccine design.
Materials and Methods
Virus.
HJ3–5 virus (previously designated H-[NS2/NS3]-J/Y361H/Q1251L) (24), is an intergenotypic chimeric virus produced by replacing the core-NS2 segment of the JFH-1 virus genome (40) with the comparable segment of the genotype 1a H77 virus. Virus stocks were produced in Huh7/FT3-7 cells. Viral titers were determined by FFU assay in Huh-7.5 cells, as described elsewhere (41).
Cells.
Huh-7.5 cells (a generous gift from Charles Rice) and Huh7/FT3-7 cells were cultured as described previously (24). 293T cells were obtained from the American Type Culture Collection (CRL-11268) and cultured as previously described (16).
Antibodies.
The murine AP33 mAb (36) and rat mAbs 3/11, 10/76b, 7/16b, 9/75b, 6/53, 11/20A, 7/59, and 9/27 have been described (26, 42). Human mAbs, CBH-2, CBH-5, CBH-8C, CBH-11, CBH-4B, CBH-4D, CBH-4G, CBH-7, HC-1, HC-2, HC-11, HC-12, HC-13, and H111 were generated from peripheral blood B cells collected from HCV-infected patients, as described elsewhere (11, 17). Anti-core C7–50 mAb was from Affinity BioReagents (Golden, CO), and anti-CD81 JS-81 mAb from BD Pharmingen (San Diego, CA).
In Vitro Virus Neutralization Assays.
Virus neutralization was assessed by an FFU-reduction neutralization assay carried out as described previously. Additional details are provided in the SI Materials and Methods, provided online as SI on the PNAS web site.
Selection of Antibody-Resistant Neutralization Escape Mutants.
HJ3–5 virus (105 FFU) was neutralized with AP33 (100 μg/ml) for 1 hour at 37 °C, then inoculated onto 1 × 105 Huh7/FT3-7 cells seeded 24 hours previously into a 24-well plate. Control cells were inoculated in parallel with mock-neutralized virus. The cultures were placed in a 5% CO2 environment at 37 °C for 24 hours, re-fed with 500 μL of fresh medium, and then re-incubated for an additional 48 hours. The cells were subsequently passed at 3- to 4-day intervals by trypsinization and reseeding with a 1:3 to 1:4 split into progressively larger culture vessels, to allow for amplification of the small amount of virus surviving neutralization. At selected passages, cells were examined by two-color confocal immunofluorescence microscopy for the presence of AP33-binding mutants. After three to six passages, supernatant culture fluids were collected, the infectious virus titer determined by FFU assay (43), and surviving virus subjected to a second round of AP33-mediated neutralization, followed by amplification of surviving virus in naive cells. These steps were repeated through multiple rounds of neutralization followed by amplification (Fig. S1).
Confocal Immunofluorescence Microscopy.
Cells cultured in eight-well chamber slides (BD Biosciences, Bedford, MA) were washed with PBS and fixed with 4% paraformaldehyde in PBS at room temperature for 30 minutes, permeabilized with Triton X-100 (0.2%) for 15 minutes, and blocked with 10% normal goat serum at room temperature for 1 hour. Slides were incubated with a mixture of human anti-HCV-positive serum (1:50) and AP33 (1:200) as primary antibodies for 1 hour, and followed by fluorescein isothiocyanate (FITC)-labeled anti-human Ig and goat anti-mouse Ig conjugated to Alexa 594 as secondary antibodies for 1 hour at room temperature. Slides were washed, counterstained with DAPI (Invitrogen, Carlsbad, CA), and mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA), then sealed and examined with a Zeiss LSM 510 laser scanning confocal microscope within the UTMB Infectious Diseases and Toxicology Optical Imaging Core.
Viral Sequence Analysis.
Total RNA was extracted from virus present in culture supernatant fluids using the QIAamp Viral RNA kit (Qiagen, Valenica, CA). The E1 and E2 region of the HCV genome was subjected to first-strand cDNA synthesis with SuperScript III reverse transcriptase (Invitrogen), followed by PCR amplification using the Expand High Fidelity PCR System (Roche Applied Sciences, Indianapolis, IN) with the following primers: E1-SE (GGAACCTTCCTGGTTGCTCTTTCTCTATCTTCC) and E2-AS (ACCACGCAAAGCAGAAGAACACGAGGAAGG). The amplified DNAs were cloned into the TOPO TA vector (Invitrogen), and plasmid DNAs from individual bacterial colonies were sequenced in the UTMB Molecular Biology Core Facility.
HCV-Pseudotyped Retroviral Particles.
HCVpp (genotype 1a) were produced as described elsewhere (44). Culture supernatant fluids (30 ml) containing HCVpp were passed through a 0.45-μm filter and centrifuged onto a 20% sucrose cushion in a Beckman Coulter SW28 rotor at 25,000 rpm for 2 hours at 4 °C. For further purification, the pellet was resuspended in 150 μl of NTE buffer (150 mmol/l NaCl, 1 mmol/l ethylenediaminetetraacetate (EDTA), 10 mmol/l Tris-HCl, pH 7.4), then layered onto a 20–60% sucrose gradient and centrifuged to equilibrium at 36,000 rpm for 18 hours at 4 °C in a Beckman Coulter SW55Ti rotor. Fractions (250-μl) collected from the top of the gradient were tested for E1E2 by immunoblotting and HCVpp infectivity by luciferase assay (see SI Methods and Materials). Fractions 10, 11, and 12, containing purified HCVpp, were pooled for subsequent studies.
Supplementary Materials and Methods.
Additional details are provided online as SI on the PNAS web site.
Supplementary Material
Acknowledgments.
We thank Yuqiong Liang and Yinghong Ma for excellent technical support. This work was supported by grants from the National Institutes of Health, U19-AI40035 (S.M.L.) and RO1-HL079381 (S.K.H.F.); the Medical Research Council (A.H.P. and J.A.M.); and the Wellcome Trust (J.A.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809879105/DCSupplemental.
References
- 1.Houghton M, Abrignani S. Prospects for a vaccine against the hepatitis C virus. Nature. 2005;436:961–966. doi: 10.1038/nature04081. [DOI] [PubMed] [Google Scholar]
- 2.Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–952. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 3.Krawczynski K, et al. Effect of immune globulin on the prevention of experimental hepatitis C virus infection. J Infect Dis. 1996;173:822–828. doi: 10.1093/infdis/173.4.822. [DOI] [PubMed] [Google Scholar]
- 4.Bowen DG, Walker CM. Mutational escape from CD8+ T cell immunity: HCV evolution, from chimpanzees to man. J Exp Med. 2005;201:1709–1714. doi: 10.1084/jem.20050808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato N, et al. Humoral immune response to hypervariable region 1 of the putative envelope glycoprotein (gp70) of hepatitis C virus. J Virol. 1993;67:3923–3930. doi: 10.1128/jvi.67.7.3923-3930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Hahn T, et al. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology. 2007;132:667–678. doi: 10.1053/j.gastro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Cox AL, et al. Cellular immune selection with hepatitis C virus persistence in humans. J Exp Med. 2005;201:1741–1752. doi: 10.1084/jem.20050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farci P, et al. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc Natl Acad Sci USA. 1996;93:15394–15399. doi: 10.1073/pnas.93.26.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimizu YK, et al. Neutralizing antibodies against hepatitis C virus and the emergence of neutralization escape mutant viruses. J Virol. 1994;68:1494–1500. doi: 10.1128/jvi.68.3.1494-1500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allander T, et al. Recombinant human monoclonal antibodies against different conformational epitopes of the E2 envelope glycoprotein of hepatitis C virus that inhibit its interaction with CD81. J Gen Virol. 2000;81:2451–2459. doi: 10.1099/0022-1317-81-10-2451. [DOI] [PubMed] [Google Scholar]
- 11.Hadlock KG, et al. Human monoclonal antibodies that inhibit binding of hepatitis C virus E2 protein to CD81 and recognize conserved conformational epitopes. J Virol. 2000;74:10407–10416. doi: 10.1128/jvi.74.22.10407-10416.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bugli F, et al. Mapping B-cell epitopes of hepatitis C virus E2 glycoprotein using human monoclonal antibodies from phage display libraries. J Virol. 2001;75:9986–9990. doi: 10.1128/JVI.75.20.9986-9990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishii K, et al. High titers of antibodies inhibiting the binding of envelope to human cells correlate with natural resolution of chronic hepatitis C. Hepatology. 1998;28:1117–1120. doi: 10.1002/hep.510280429. [DOI] [PubMed] [Google Scholar]
- 14.Habersetzer F, et al. Characterization of human monoclonal antibodies specific to the hepatitis C virus glycoprotein E2 with in vitro binding neutralization properties. Virology. 1998;249:32–41. doi: 10.1006/viro.1998.9202. [DOI] [PubMed] [Google Scholar]
- 15.Keck ZY, et al. Hepatitis C virus E2 has three immunogenic domains containing conformational epitopes with distinct properties and biological functions. J Virol. 2004;78:9224–9232. doi: 10.1128/JVI.78.17.9224-9232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keck ZY, et al. Analysis of a highly flexible conformational immunogenic domain a in hepatitis C virus E2. J Virol. 2005;79:13199–13208. doi: 10.1128/JVI.79.21.13199-13208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keck ZY, et al. Definition of a conserved immunodominant domain on HCV E2 glycoprotein by neutralizing human monoclonal antibodies. J Virol. 2008;9:9. doi: 10.1128/JVI.02475-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Law M, et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med. 2008;14:25–27. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 19.Johansson DX, et al. Human combinatorial libraries yield rare antibodies that broadly neutralize hepatitis C virus. Proc Natl Acad Sci USA. 2007;104:16269–16274. doi: 10.1073/pnas.0705522104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owsianka A, Clayton RF, Loomis-Price LD, McKeating JA, Patel AH. Functional analysis of hepatitis C virus E2 glycoproteins and virus-like particles reveals structural dissimilarities between different forms of E2. J Gen Virol. 2001;82:1877–1883. doi: 10.1099/0022-1317-82-8-1877. [DOI] [PubMed] [Google Scholar]
- 21.Tarr AW, et al. Determination of the human antibody response to the epitope defined by the hepatitis C virus-neutralizing monoclonal antibody AP33. J Gen Virol. 2007;88:2991–3001. doi: 10.1099/vir.0.83065-0. [DOI] [PubMed] [Google Scholar]
- 22.Tarr AW, et al. Characterization of the hepatitis C virus E2 epitope defined by the broadly neutralizing monoclonal antibody AP33. Hepatology. 2006;43:592–601. doi: 10.1002/hep.21088. [DOI] [PubMed] [Google Scholar]
- 23.Owsianka AM, et al. Broadly neutralizing human monoclonal antibodies to the hepatitis C virus E2 glycoprotein. J Gen Virol. 2008;89:653–659. doi: 10.1099/vir.0.83386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yi M, Ma Y, Yates J, Lemon SM. Compensatory mutations in E1, p7, NS2 and NS3 enhance yields of cell culture-infectious inter-genotypic chimeric hepatitis C virus. J Virol. 2007;81:629–638. doi: 10.1128/JVI.01890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Y, Yates J, Liang Y, Lemon SM, Yi M. NS3 helicase domains involved in infectious intracellular hepatitis C virus particle assembly. J Virol. 2008;82:7624–7639. doi: 10.1128/JVI.00724-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flint M, et al. Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. J Virol. 1999;73:6235–6244. doi: 10.1128/jvi.73.8.6235-6244.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKeating JA, et al. Diverse hepatitis C virus glycoproteins mediate viral infection in a CD81-dependent manner. J Virol. 2004;78:8496–8505. doi: 10.1128/JVI.78.16.8496-8505.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keck ZY, et al. Immunogenic and functional organization of hepatitis C virus (HCV) glycoprotein E2 on infectious HCV virions. J Virol. 2007;81:1043–1047. doi: 10.1128/JVI.01710-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keck ZY, et al. Human monoclonal antibody to hepatitis C virus E1 glycoprotein that blocks virus attachment and viral infectivity. J Virol. 2004;78:7257–7263. doi: 10.1128/JVI.78.13.7257-7263.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Netski DM, et al. Humoral immune response in acute hepatitis C virus infection. Clin Infect Dis. 2005;41:667–675. doi: 10.1086/432478. [DOI] [PubMed] [Google Scholar]
- 31.Zhang P, et al. Hepatitis C virus epitope-specific neutralizing antibodies in Igs prepared from human plasma. Proc Natl Acad Sci USA. 2007;104:8449–8454. doi: 10.1073/pnas.0703039104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helle F, et al. The neutralizing activity of anti-hepatitis C virus antibodies is modulated by specific glycans on the E2 envelope protein. J Virol. 2007;81:8101–8111. doi: 10.1128/JVI.00127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu MY, et al. Neutralizing antibodies to hepatitis C virus (HCV) in immune globulins derived from anti-HCV-positive plasma. Proc Natl Acad Sci USA. 2004;101:7705–7710. doi: 10.1073/pnas.0402458101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pestka JM, et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci USA. 2007;104:6025–6030. doi: 10.1073/pnas.0607026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan DE, et al. Discordant role of CD4 T-cell response relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology. 2007;132:654–666. doi: 10.1053/j.gastro.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 36.Owsianka A, et al. Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein. J Virol. 2005;79:11095–11104. doi: 10.1128/JVI.79.17.11095-11104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen SU, et al. Association between hepatitis C virus and very-low-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients. J Virol. 2006;80:2418–2428. doi: 10.1128/JVI.80.5.2418-2428.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindenbach BD, et al. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proc Natl Acad Sci USA. 2006;103:3805–3809. doi: 10.1073/pnas.0511218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owsianka AM, et al. Identification of conserved residues in the E2 envelope glycoprotein of the hepatitis C virus that are critical for CD81 binding. J Virol. 2006;80:8695–8704. doi: 10.1128/JVI.00271-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wakita T, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blight KJ, McKeating JA, Rice CM. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol. 2002;76:13001–13014. doi: 10.1128/JVI.76.24.13001-13014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu M, et al. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc Natl Acad Sci USA. 2003;100:7271–7276. doi: 10.1073/pnas.0832180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi M, Villanueva RA, Thomas DL, Wakita T, Lemon SM. Production of infectious genotype 1a hepatitis C virus (Hutchinson strain) in cultured human hepatoma cells. Proc Natl Acad Sci USA. 2006;103:2310–2315. doi: 10.1073/pnas.0510727103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartosch B, Dubuisson J, Cosset FL. Infectious hepatitis C virus pseudo-particles containing functional E1–E2 envelope protein complexes. J Exp Med. 2003;197:633–642. doi: 10.1084/jem.20021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.