Abstract
Mutations in the PARK2 gene cause hereditary Parkinson disease (PD). The PARK2 gene product, termed parkin, is an E3 ubiquitin ligase that mediates the transfer of ubiquitin onto diverse substrate proteins. Despite progress in defining the molecular properties and substrates of parkin, little is known about its physiological function. Here, we show that parkin regulates the function and stability of excitatory glutamatergic synapses. Postsynaptic expression of parkin dampens excitatory synaptic transmission and causes a marked loss of excitatory synapses onto hippocampal neurons. Conversely, knockdown of endogenous parkin or expression of PD-linked parkin mutants profoundly enhances synaptic efficacy and triggers a proliferation of glutamatergic synapses. This proliferation is associated with increased vulnerability to synaptic excitotoxicity. Thus, parkin negatively regulates the number and strength of excitatory synapses. Increased excitatory drive produced by disruption of parkin may contribute to the pathophysiology of PD.
Keywords: excitotoxicity, proteasome, synapse, glutamate
Parkinson disease (PD) is the most common neurodegenerative movement disorder (1, 2). Although classic clinical symptoms arise as a result of the loss of dopaminergic neurons of the substantia nigra, widespread neurological abnormalities are present in animal models of PD and in human disease (3–6). Heightened responsiveness to the excitatory neurotransmitter glutamate and associated excitotoxicity has been implicated in the pathogenesis of PD (7–9). However, the molecular mechanisms linking PD risk factors to altered excitability and excitotoxic vulnerability remain unclear.
Mutations responsible for rare hereditary forms of PD have been identified in several human genes (10, 11). Among these, PARK2 encodes a RING domain–containing E3 ubiquitin ligase that is widely expressed throughout the nervous system but whose cellular function is poorly understood (1, 12–17). Among the reported substrates of parkin are several proteins implicated in synaptic transmission, including CDCrel-1 (15), glycosylated α-synuclein, synphilin, synaptotagmin XI (18), Eps15 (19), and protein interacting with C kinase 1 (20). Parkin associates with PDZ scaffold proteins in the postsynaptic density (PSD) (21) and protects postmitotic neurons from glutamate receptor–mediated excitotoxicity (16, 22), suggesting a link between parkin and glutamatergic synapse function. Consistent with this notion, mice lacking parkin display both motor and cognitive behavioral deficits and altered excitability in the hippocampus and striatum (23, 24). We hypothesized that parkin may regulate the strength of excitatory synapses.
Results
Postsynaptic Parkin Dampens Excitatory Synaptic Transmission.
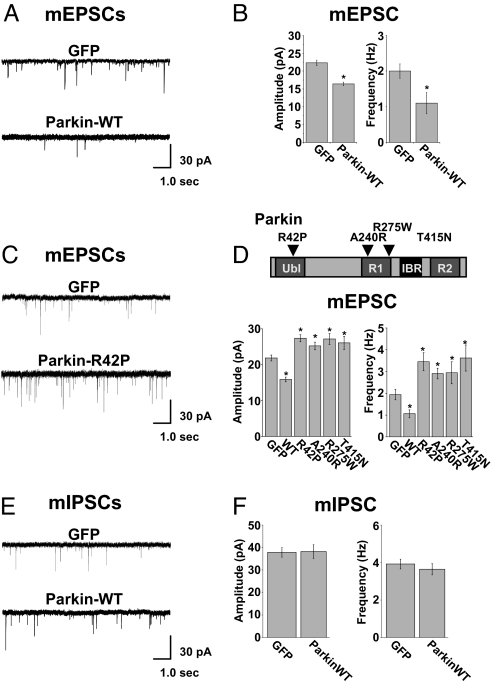
To determine the effects of postsynaptic parkin on synaptic transmission, GFP-tagged parkin (parkin-WT) was expressed in cultured rat hippocampal neurons, and miniature excitatory postsynaptic currents (mEPSCs) and miniature inhibitory postsynaptic currents (mIPSCs) were recorded using a whole-cell voltage clamp. Transfection efficiencies were typically <1% and autapses are rare in high-density cultures, ensuring that any observed effect of expressing parkin originates in the postsynaptic neuron. In neurons expressing parkin-WT, average mEPSC amplitudes and frequencies were significantly reduced compared with control GFP-transfected neurons (GFP, 22.3 ± 0.8 pA, 2.0 ± 0.2 Hz, n = 23; parkin-WT, 16.4 ± 0.5 pA, 1.1 ± 0.4 Hz, n = 18, *, P < 0.001. Fig. 1 A and B). Thus, elevating levels of postsynaptic parkin reduces the strength of excitatory synapses.
Fig. 1.
Parkin attenuates excitatory synaptic transmission. (A) Representative mEPSCs recorded from a control GFP-expressing hippocampal neuron (DIV21; Upper) and a neuron expressing GFP–parkin-WT (Lower). (B) Quantitative analysis of mEPSCs. Data represent mean ± SEM, n = 18, *, P < 0.001 by t test. (C) Examples of mEPSCs recorded from neurons expressing GFP (Upper) or the hereditary PD mutant parkin-R42P (Lower). (D) Quantitative analysis of mEPSCs. Data represent mean ± SEM of mEPSC amplitudes (Left) and frequencies (Right), n = 15, *, P < 0.001 by t test. Top, model of the domain structure of parkin (ubiquitin-like domain; in-between RING domain [IBR]), including disease-linked mutations examined in the current study. (E) Parkin has no effect on inhibitory synaptic transmission. mIPSCs recorded from neurons expressing GFP–control neuron (Top) and GFP–parkin-WT (Lower). (F) Quantitative analysis revealed no effect of parkin on the amplitude and frequency of mIPSCs relative to GFP-expressing control neurons.
At most excitatory synapses, postsynaptic currents consist of two components: a rapid component mediated by AMPA-type glutamate receptors and a slower component mediated by NMDA-type glutamate receptors. To determine whether parkin independently regulates AMPA receptor- or NMDA receptor–mediated excitatory synaptic currents, we recorded mEPSCs in Mg2+-free extracellular solution to prevent NMDA receptor block (25, 26). We measured peak currents (reflecting the contribution of AMPA receptors) and currents 25 msec after the peak (reflecting the contribution of NMDA receptors). A change in the NMDA-to-AMPA current ratio would indicate a selective effect of parkin on either the AMPA or NMDA component. However, we observed no significant difference in NMDA-to-AMPA mEPSC ratios between neurons expressing parkin-WT and GFP [supporting information (SI) Fig. S1A], despite a significant reduction in peak current in parkin-expressing cells (Fig. 1 B Left and Fig. S1A). Furthermore, scaling mEPSCs obtained from neurons expressing GFP or parkin-WT to the peak current amplitude revealed no difference in mEPSC rise times or decay kinetics (Fig. S1A Left). Specifically, average mEPSC decay kinetics, fit with two exponentials, were quantitatively indistinguishable between control and parkin-expressing cells (fast [τ1] and slow [τ2] decay time constants: control, τ1 = 6.7 ± 0.5 msec, τ2 = 78.5 ± 7.5 msec, n = 15; parkin, τ1 = 6.2 ± 0.3 msec, τ2 = 82.9 ± 8.1 msec, n = 15, P > 0.05, respectively). Double immunolabeling of hippocampal neurons for surface GluR1 AMPA receptors and the obligatory NMDA receptor subunit NR1 revealed no difference in AMPA/NMDA receptor fluorescence ratios at synapses on neurons expressing either GFP (0.99 ± 0.07 arbitrary fluorescence units, n = 12) or parkin-WT (0.92 ± 0.09 arbitrary fluorescence units, n = 11) (Fig. S1 B and C). The number of glutamatergic synapses lacking detectable GluR1 AMPA receptors (NMDA receptor–only synapses) was slightly decreased in neurons expressing parkin-WT relative to GFP control neurons (32.4% ± 3.6%, n = 11; and 39.9% ± 3.2%, n = 11, respectively; Fig. S1D). These results demonstrate that postsynaptic parkin coordinately down-regulates both AMPA receptor- and NMDA receptor–mediated excitatory synaptic currents, suggesting a generalized remodeling of the postsynaptic membrane at glutamatergic synapses.
Disease-Linked Parkin Mutants Increase mEPSC Amplitude and Frequency.
Mutations in the PARK2 gene are linked to hereditary PD (7, 27, 28). Given the reduction of excitatory synaptic transmission caused by elevating postsynaptic parkin (Fig. 1 A and B), we sought to test the effect of disease-linked parkin mutants. Postsynaptic expression of parkin variants bearing any of several point mutations known to cause hereditary PD (Fig. 1D Top) significantly and consistently increased both mEPSC amplitudes and frequencies (Fig. 1 C and D). For example, expression of parkin bearing an R42P mutation within the ubiquitin-like domain, which disrupts the association between parkin and the proteasome (1, 29), led to a significant increase in mEPSC amplitude and frequency (GFP: 21.8 ± 0.8 pA, 1.9 ± 0.3 Hz, n = 15; parkin-R42P: 27.3 ± 1.0 pA, 3.5 ± 0.4 Hz, n = 15; *, P < 0.001 for both amplitude and frequency, Fig. 1 C and D). Analysis of mEPSC amplitudes and frequencies in cells expressing parkin harboring mutations within or near the RING1 domain (A240R: 25.2 ± 1.1 pA and 2.9 ± 0.2 Hz, n = 17; R275W: 27.1 ± 1.5 pA and 2.9 ± 0.5 Hz, n = 18) or the RING2 domain (T415N: 26.1 ± 1.8 pA, 3.6 ± 0.6 Hz, n = 15), which are implicated in E2 binding and substrate recognition, respectively (1, 15, 30), also revealed significantly enhanced excitatory synaptic transmission compared with control neurons (amplitude *, P < 0.01, frequency *, P < 0.001 in all cases; Fig. 1E). The magnitude of the observed increase in mEPSC amplitudes (≈30%) and frequencies (≈80%) is similar to that reported for other well studied postsynaptic regulatory mechanisms (31, 32).
Parkin is a multi-domain protein containing an N-terminal ubiquitin-like domain and a C-terminal RING-IBR-RING triad responsible for E2 and substrate binding (10) (Fig. 1D Top). Disease-linked mutations occur in each of these domains (33), and different mutations produce different biochemical effects on parkin localization, solubility, ubiquitination properties, and inheritance phenotype (28, 34). Supporting an inhibitory effect of disease-linked parkin mutants in some cellular processes, we found that parkin mutants inhibited WT parkin-induced degradation of the putative parkin substrate CDCrel-1 (septin 5; Fig. S2).
Parkin Acts Postsynaptically and Selectively Regulates Excitatory Synapses.
We next tested the specificity of the postsynaptic effect of parkin on excitatory synaptic transmission. Using the uptake and release of the recycling styryl dye FM4–64 to monitor presynaptic function, we found that postsynaptic expression of parkin-WT or parkin mutants had no effect on depolarization-induced FM4–64 loading or on stimulus-dependent release of FM4–64 from boutons contacting parkin-expressing postsynaptic cells (Figs. S3A and B). The postsynaptic effect of parkin on synaptic transmission is thus not a result of a retrograde effect on transmitter release. We also tested whether the parkin-induced reduction in synaptic efficacy was selective for excitatory synapses by examining the effect of parkin-WT on mIPSCs. Whole-cell recordings revealed that, unlike for mEPSCs, the amplitudes and frequencies of mIPSCs were unaffected by parkin-WT (parkin-WT, 38.7 ± 2.2 pA, 3.8 ± 03. Hz, n = 19; GFP control, 37.8 ± 2.1 pA, 3.7 ± 0.5 Hz, n = 20; Fig. 1 E and F). Thus, parkin selectively decreases excitatory but not inhibitory synaptic transmission, thereby regulating the balance of excitation and inhibition.
Parkin Manipulation Alters Glutamatergic Synapse Number.
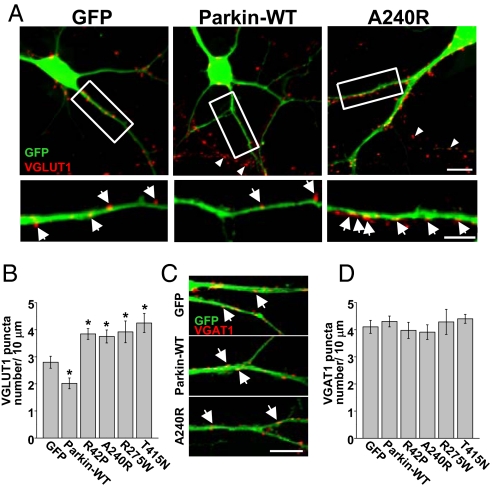
The decrease in mEPSC frequency caused by elevating postsynaptic parkin (Fig. 1 A–B), combined with a lack of effect on presynaptic function (Fig. S3 A and B) and preserved AMPA/NMDA ratios (Fig. S1 A and C), suggested a loss of glutamatergic synapses. To test this possibility, we examined whether postsynaptic expression of parkin influences the number of synaptic contacts. For this purpose, we used hippocampal neurons at 14 days in vitro (DIV), a period of active synapse formation and stabilization in culture. The number of excitatory glutamatergic presynaptic terminals contacting dendrites was analyzed by immunocytochemical staining for the vesicular glutamate transporter VGLUT1, which marks discrete sites representing presynaptic glutamatergic boutons, and for the PSD protein PSD-95, which labels postsynaptic sites. VGLUT1 labeling of control neurons decorated punctate sites along the dendrite and soma representing individual presynaptic terminals. Postsynaptic expression of parkin-WT resulted in a significant loss of contacting VGLUT-positive glutamatergic terminals (control, 2.5 ± 0.8 puncta per 10 μm, n = 18; parkin-WT, 1.7 ± 0.5 puncta per 10 μm, n = 18; *, P < 0.001; Fig. 2A Left and Middle and B). Parkin-WT also reduced the number of labeled PSD-95 puncta (puncta per 10 μm dendrite: control, 2.7 ± 0.4, n = 13; parkin-WT, 1.9 ± 0.4, n = 11; *, P = 0.01; Fig. S4). This reduction in VGLUT1 puncta coincided with a loss of functional presynaptic terminals as visualized by FM4–64 loading (Fig. S3C). Conversely, expression of PD-linked point mutants of parkin resulted in a robust increase in the number of contacting VGLUT-positive presynaptic terminals (R42P, 3.8 ± 0.2 puncta per 10 μm; A240R, 3.4 ± 0.5 puncta per 10 μm; R275W, 4.0 ± 0.4 puncta per 10 μm; T415N, 3.9 ± 0.9 puncta per 10 μm, n = 18; *, P < 0.01; Fig. 2 A and B), and likewise increased the number of PSD-95 puncta (puncta per 10 μm dendrite: R42P, 4.2 ± 0.5; A240R, 3.7 ± 0.4; R275W, 3.9 ± 0.6; T415N, 4.4 ± 0.7, n = 15; *, P < 0.01; Fig. S4 A and B) consistent with the increase in mEPSC frequency in neurons expressing these parkin mutants (Fig. 1 E and F).
Fig. 2.
Parkin promotes excitatory synapse loss. (A) Hippocampal neurons (DIV14) expressing GFP, GFP–parkin-WT, or the PD-linked parkin mutant GFP–parkin-A240R for 3 days were fixed and stained with anti-VGLUT1 antibody. Arrows indicate glutamatergic terminals contacting transfected dendrites. Arrowheads indicate glutamatergic terminals contacting neighboring untransfected dendrites in the same field. Boxed regions (Upper) are also magnified (Lower). (Scale bar, 10 μm, Upper; 5 μm, Lower.) (B) Quantitative analysis of the number of glutamatergic terminals contacting dendrites on neurons expressing GFP, GFP–parkin-WT, or PD-linked parkin mutants R42P, A240R, R275W, and T415N. Data represent mean ± SEM of VGLUT1 puncta per 10 μm dendrite, n = 18, *, P < 0.001 relative to GFP control by t test. (C) Parkin has no effect on the number of inhibitory synapses. GABAergic presynaptic terminals were visualized by immunocytochemical staining for the vesicular GABA transporter (VGAT). (Scale bar, 5 μm.) (D) Quantification of VGAT puncta number along dendrites of neurons expressed GFP, parkin-WT, or the indicated parkin mutants (n = 15 each). Data represent mean ± SEM, P > 0.05.
Unlike its effect on excitatory synapses, neither WT parkin nor PD-linked mutant parkin affected the density of inhibitory synapses visualized by staining for the vesicular GABA transporter (VGAT; puncta per 10 μm dendrite: GFP, 3.9 ± 0.2; parkin-WT, 4.1 ± 0.3; R42P, 4.0 ± 0.3; A240R, 3.8 ± 0.2; R275W, 4.3 ± 0.5; T415N, 4.3 ± 0.5, n = 15; Fig. 2 C and D;) and the inhibitory postsynaptic scaffolding protein gephyrin (puncta per 10 μm dendrite: GFP, 4.4 ± 0.4; parkin-WT, 4.2 ± 0.3; R42P, 4.5 ± 0.6; A240R, 3.9 ± 0.4; R275W, 4.2 ± 0.4; T415N, 4.3 ± 0.3 puncta per 10 μm, n = 13 neurons each; Fig. S5 A and B). These data are in agreement with the lack of effect of parkin on mIPSCs (Fig. 1 E and F), and show that elevating postsynaptic parkin causes a selective loss of excitatory synapses whereas disease-linked parkin mutants cause a proliferation of excitatory synapses.
Reduction of Endogenous Parkin Augments mEPSCs and Increases Excitatory Synapses.
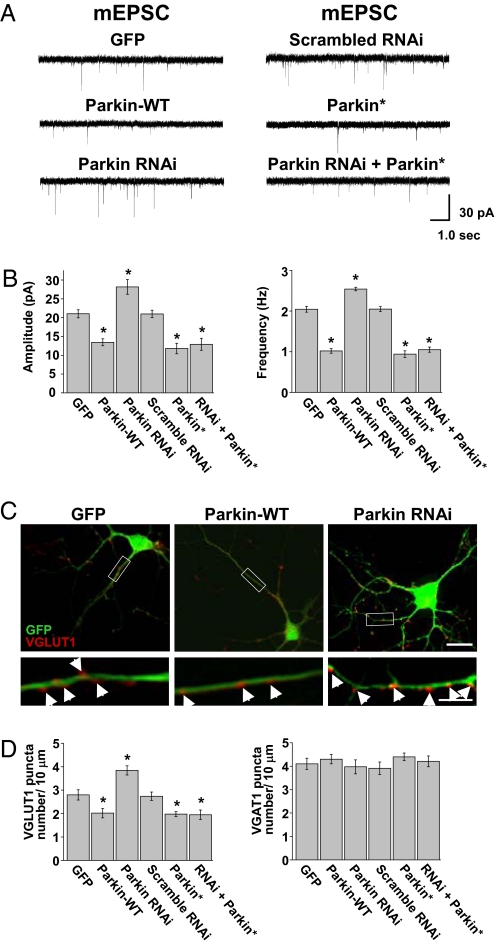
To examine the effect of endogenous parkin on excitatory synaptic transmission, we used an RNAi strategy. Two short 29-bp hairpins were generated against the rat PARK2 gene sequence (GenBank accession no. NM_020093) and cloned into GFP-expressing vectors allowing bicistronic expression of parkin shRNA and GFP. This approach produced a robust and reliable knockdown of parkin expression (Fig. S6). Whole-cell voltage-clamp recordings were then performed on hippocampal neurons (DIV14) expressing GFP (control), parkin-WT, or parkin shRNA. After 3 to 5 days, knockdown of endogenous parkin resulted in a significant increase in mEPSC amplitude and frequency compared with GFP control neurons (control, 21.0 ± 1.1 pA, 2.0 ± 0.1 Hz, n = 17; parkin shRNA, 28.2 ± 1.9 pA, 2.5 ± 0.04 Hz, n = 22; *, P < 0.01; Fig. 3 A and B). Importantly, the effect of parkin knockdown was completely reversed by coexpression of a parkin cDNA containing silent third codon mutations (parkin*) that rendered the parkin mRNA resistant to the specific shRNA sequence (Fig. 3 A and B). Specifically, when parkin* was expressed alone or in conjunction with parkin shRNA, mEPSC amplitudes and frequencies were reduced compared with control (parkin-WT, 13.5 ± 0.9 pA, 1.1 ± 0.1 Hz, n = 19; *, P < 0.001; parkin*, 11.8 ± 1.4 pA, 0.94 ± 0.1 Hz, n = 15; *, P < 0.01; shRNA + parkin*, 12.9 ± 1.6 pA, 1.1 ± 0.1 Hz, n = 14; *, P < 0.01; Fig. 3 A and B). Expression of a scrambled shRNA sequence failed to alter mEPSC amplitude or frequency relative to GFP-expressing control neurons (scrambled shRNA, 20.9 ± 0.9 pA, 2.1 ± 0.1 Hz, n = 17; Fig. 3 A and B). These findings demonstrate that loss of endogenous parkin increases excitatory synaptic transmission, similar to the effect observed upon expression of disease-linked parkin mutants (Fig. 1 C and D).
Fig. 3.
Knockdown of endogenous parkin results in enhanced excitatory transmission and a proliferation of glutamatergic synapses. (A) Representative mEPSC traces from hippocampal neurons expressing GFP, parkin-WT, parkin shRNA, scrambled shRNA, parkin*, and parkin shRNA + parkin*. (B) Quantitative analysis of mEPSC amplitudes and frequencies from neurons expressing the indicated constructs. Data represent mean ± SEM, n = 15, *, P < 0.01 relative to GFP control by t test. (C) Hippocampal neurons (DIV14) expressing GFP, GFP–parkin-WT, or parkin-shRNA for 5 days were fixed and stained with anti-VGLUT1 antibody. Boxed regions (Upper) are also magnified (Lower). (Scale bar, 10 μm [Upper] and 5 μm [Lower].) (D) Quantitative analysis of the number of glutamatergic (VGLUT1-positive, Left) and GABAergic (VGAT-positive, Right) terminals contacting dendrites on neurons expressing GFP, parkin-WT, parkin shRNA, scrambled shRNA, parkin*, and parkin shRNA + parkin*. Data represent mean ± SEM of puncta per 10 μm dendrite, P > 0.05.
To test whether the loss of endogenous parkin affected the number of contacting presynaptic terminals, we expressed parkin shRNA and performed immunocytochemical staining for excitatory (VGLUT1, PSD-95) and inhibitory (VGAT, gephyrin) synapses. Knockdown of endogenous parkin resulted in a significant increase in VGLUT1 and PSD-95 puncta compared with control cells, whereas expression of parkin-WT, parkin*, and shRNA + parkin* resulted in a marked reduction in VGLUT1-positive terminals (puncta per 10 μm dendrite: GFP, 2.8 ± 0.2, n = 14; parkin-WT, 2.0 ± 0.2, n = 16; *, P < 0.01; parkin shRNA, 3.8 ± 0.2, n = 19; *, P < 0.01; scrambled shRNA, 2.7 ± 0.2, n = 13; parkin*, 1.9 ± 0.1, n = 14; *, P < 0.01; shRNA + parkin*, 1.9 ± 0.2, n = 15; *, P < 0.01; Fig. 3 C and D Left) and PSD-95 puncta (puncta per 10 μm dendrite: GFP, 2.7 ± 0.4, n = 12; parkin-WT, 1.9 ± 0.3, n = 11; *, P < 0.01; R42P, 4.2 ± 0.5, n = 12; *, P < 0.01; A240R, 3.7 ± 0.4, n = 9; *, P < 0.01; R275W, 3.9 ± 0.6, n = 11; *, P < 0.01; T415N, 4.4 ± 0.7, n = 9; *, P < 0.07; parkin shRNA, 4.2 ± 0.6, n = 14; *, P < 0.01; parkin*, 1.7 ± 0.3, n = 11; *, P < 0.01; shRNA + parkin*, 1.6 ± 0.4, n = 9; *, P < 0.01; Fig. S4). In contrast, the number of contacting GABAergic inhibitory synapses was unaffected by increasing or decreasing parkin in postsynaptic cells as measured by VGAT staining (puncta per 10 μm dendrite: GFP, 4.1 ± 0.2, n = 11; parkin-WT, 4.3 ± 0.2, n = 13; parkin shRNA, 3.9 ± 0.3, n = 16; scrambled shRNA, 3.9 ± 0.3, n = 13; parkin*, 4.4 ± 0.2, n = 12; shRNA + parkin*, 4.2 ± 0.2, n = 12; Fig. 3D Right) and gephyrin staining (puncta per 10 μm dendrite: GFP, 4.4 ± 0.4, n = 10; parkin-WT, 4.2 ± 0.3, n = 12; R42P, 4.5 ± 0.6, n = 9; A240R, 4.5 ± 0.6, n = 13; R275W, 4.2 ± 0.5, n = 11; T415N, 4.3 ± 0.2, n = 11; parkin shRNA, 4. ± 0.5, n = 13; parkin*, 4.3 ± 0.4, n = 10; shRNA + parkin*, 3.8 ± 0.6, n = 10; Fig. S5). These results indicate that endogenous parkin expressed in postsynaptic neurons selectively limits or constrains the number of glutamatergic synapses.
Parkin Reduces Excitotoxicity by Synaptically Released Glutamate.
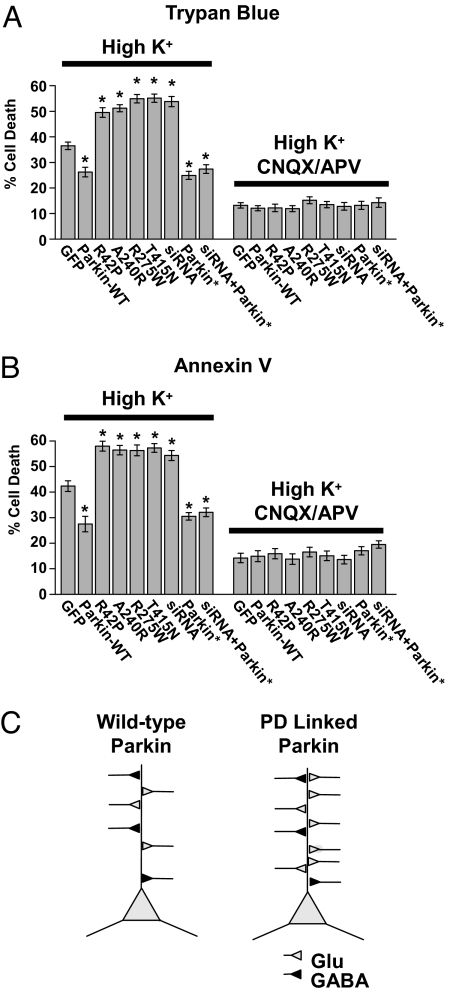
Glutamatergic transmission in many brain regions is altered in both animal models of PD and the human disease (6, 35), which may lead to undue excitation and neuronal degeneration through excitotoxicity (6–9). Given our findings of enhanced glutamatergic synaptic transmission and excitatory synapse proliferation in neurons expressing familial PD-linked mutants of parkin (Figs. 1 and 2) or upon parkin knockdown (Fig. 3), we hypothesized that defects in parkin could lead to increased excitotoxic vulnerability by augmenting excitatory synaptic transmission. To test this hypothesis, we measured the effect of expressing WT parkin, PD-linked mutant parkin, or parkin-shRNA on excitotoxic cell death induced by synaptically released glutamate. Glutamate release was triggered by a brief depolarization using a high K+ solution (90 mM, 5 min) (36). Cell death was assessed 4 to 6 h later by trypan blue dye staining (Fig. 4A) or annexin V labeling of externalized phosphatidylserine (Fig. 4B). To allow for sufficient cell counts, high-efficiency expression of parkin constructs in hippocampal neuron cultures was achieved by using lentivirus. In control neurons expressing GFP, brief depolarization by high K+ elicited significant cell death within 4 to 6 h (39% ± 3% trypan blue–positive cells, n = 5; Fig. 4A; 42.3% ± 2% annexin V–labeled cells, n = 6; Fig. 4B). Neurons expressing parkin-WT were resistant to excitotoxicity (23% ± 3% trypan blue–positive cells, n = 5; *, P < 0.001; 27.5% ± 3% annexin V–labeled cells, n = 6 coverslips; *, P < 0.01), consistent with the down-regulation of excitatory transmission mediated by parkin (Figs. 1 and 2). In contrast, expression of multiple disease-linked parkin mutants increased neuronal vulnerability to synaptic excitotoxicity (trypan blue–positive cells: parkin-R42P, 50% ± 2%, n = 6; *, P < 0.01; parkin-A240R, 50% ± 2%, n = 5; *, P < 0.01; parkin-R275W, 54.9% ± 2%, n = 5; *, P < 0.01; parkin-T415N, 52% ± 3%, n = 5; *, P < 0.01; Fig. 4A; annexin V–labeled cells: parkin-R42P, 58% ± 2%, n = 5; *, P < 0.01; parkin-A240R, 56% ± 2%, n = 5; *, P < 0.01; parkin-R275W, 56.3% ± 2%, n = 5; *, P < 0.01; parkin-T415N, 57% ± 2%, n = 6; *, P < 0.01; Fig. 4B). Similarly, shRNA knockdown of endogenous parkin increased neuronal vulnerability to synaptic excitotoxicity (54% ± 3% trypan blue–positive cells, n = 5; *, P < 0.01; 54% ± 2% annexin V–labeled cells, n = 6; *, P < 0.01; Fig. 4 A and B), whereas expression of the RNAi-resistant mutant recovered the parkin neuroprotective effect (parkin*, 25% ± 2% trypan blue–positive cells, n = 6; *, P < 0.01; 31% ± 3% annexin V–labeled cells, n = 6; *, P < 0.01; shRNA + parkin*, 27.4% ± 3% trypan blue–positive cells, n = 6; *, P < 0.01; 32.1% ± 2% annexin V–labeled cells, n = 6; *, P < 0.01; Fig. 4 A and B).
Fig. 4.
Disruption of parkin enhances neuronal vulnerability to excitotoxicity elicited by synaptically released glutamate. (A and B) Neurons expressing parkin-WT or parkin* exhibit reduced cell death whereas neurons expressing disease-linked parkin mutants (R42P, A240R, R275W, T415N) or parkin-shRNA (RNAi) exhibit enhanced cell death in response to a high K+ excitotoxic challenge. Data represent mean ± SEM of the number of cells labeled by trypan blue dye (A) or annexin V–Alexa 568 (B), both markers of cell death. Inclusion of the ionotropic glutamate receptor antagonists 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 25 μM) and D-AP5 (50 μM) blocked excitotoxicity, indicating a requirement for glutamatergic receptor activation. (C) Proposed model for synaptic enhancement by disease-linked mutations in parkin. Under normal physiological conditions, parkin restricts the number of excitatory synapses (Glu) and decreases synaptic strength. Mutations in parkin that cause hereditary PD or loss of WT parkin lead to an increase in the strength and number of excitatory synapses without affecting inhibitory synapses (GABA).
To confirm that the observed cell death was a result of synaptically released glutamate acting on postsynaptic receptors, we included glutamate receptor antagonists (25 μM CNQX, 50 μM D-AP5) during the high K+ depolarization. In the presence of glutamate receptor antagonists, cell death induced by high K+ was significantly reduced (percent trypan blue–positive cells: GFP, 12.2% ± 1%; parkin-WT, 11.1% ± 2%; parkin-R42P, 11.9% ± 2%; parkin-A240R, 11.4% ± 1%; parkin-R275W, 15.4% ± 2%; parkin-T415N, 12.2% ± 1%; parkin shRNA, 12.8% ± 2%; parkin*, 12.8% ± 2%; shRNA + parkin*, 14.3% ± 3%, n = 6; percent annexin V–positive cells: GFP, 14.2% ± 2%; parkin-WT, 11.1% ± 2%; parkin-R42P, 15.9% ± 2%; parkin-A240R, 11.9% ± 1%; parkin-R275W, 16.5% ± 2%; parkin-T415N, 13.5% ± 2%; parkin shRNA, 13.6% ± 2%; parkin*, 17.0% ± 2%; shRNA + parkin*, 19.5% ± 2%, n = 6; Fig. 4 A and B). These findings demonstrate that loss of endogenous parkin or expression of PD-linked parkin mutants renders neurons vulnerable to excitotoxicity by enhancing excitatory synaptic transmission.
Discussion
In the present study, we have demonstrated that the ubiquitin ligase parkin regulates the strength and abundance of excitatory synapses. When parkin is expressed postsynaptically, excitatory synaptic transmission is dampened and glutamatergic synaptic input is significantly reduced. In this regard, parkin acts to limit excitatory synaptic input. This decrease in synaptic strength and connectivity is opposed by disease-linked mutants of parkin or by loss of endogenous parkin. By refining or containing synaptic circuitry, the reduction of glutamatergic synapses by parkin provides a mechanism for adjusting excitation. The winnowing of excitatory synapses may be a developmental phenomenon or could reflect ongoing synaptic fine-tuning that continues into adult life. Indeed, parkin is expressed early in brain development and its expression is maintained into adulthood (14).
Although classically considered autosomal recessive, the genetics of parkin-linked PD is more complex than a simple model of recessive inheritance (11, 37). Parkin is a multi-domain protein and PD-linked mutations are found throughout the gene, including in the ubiquitin-like, RING1, and RING2 domains (28). These mutations in different functional domains can produce either loss of function or dominant inhibitory cellular phenotypes (15, 16, 34, 38, 39). Our observed dominant effect of mutant parkin on excitatory transmission was unexpected, as disease-linked mutations in parkin have been linked to recessively inherited juvenile PD, and suggested that parkin mutants exert gain of function effects on glutamatergic synapses. Here, we have demonstrated that the disease-linked parkin mutants R42P, A240R, R275W, and T415N produce a synaptic phenotype—proliferation of excitatory synapses—that mimics parkin loss of function and opposes the gain-of-function phenotype of decreased excitatory synapses upon parkin overexpression. Further, we have shown that the same parkin mutants can act in a dominant inhibitory fashion to prevent the degradation of the putative parkin substrate CDCrel-1 (septin 5) by coexpressed parkin-WT (Fig. S2). It is important to note that recessive inheritance patterns in juvenile PD associated with parkin mutations are defined based on classic signs and symptoms of nigrostriatal dopaminergic insufficiency, and such patterns of inheritance need not imply that the same mutations cause parkin loss of function in all cellular phenotypes (28, 34). Our results suggest a distinct role for parkin in glutamatergic synapse function and elimination that may occur in parallel with its regulation of dopaminergic neuron survival.
Consistent with a synaptic deficit, parkin-null mice show subtle deficiencies in non–motor-related behaviors including spacial learning and memory, as well as startle and anxiety responses (24, 40, 41). Interestingly, parkin-null mice show no abnormalities in gross brain morphology and no loss of dopaminergic neurons (23, 24, 40, 41). These results indicate possible compensation of parkin loss by developmental mechanisms that are not well understood. To avoid complications resulting from developmental compensation manifested in parkin-knockout mice, we acutely knocked down endogenous parkin using RNAi. Similar to overexpressing mutant parkin, reduction of endogenous parkin caused a proliferation of excitatory synapses (Fig. 3) and an increase in excitotoxic vulnerability (Fig. 4).
An intriguing aspect of the present study is that mutation or loss of parkin leads to the strengthening and proliferation of glutamatergic synapses, and the resulting unchecked excitatory drive increases neuronal vulnerability to synaptic excitotoxicity (Fig. 4). Previous studies identifying a neuroprotective effect of parkin proposed that parkin regulates downstream apoptotic signaling (16, 42, 43). Our results suggest that parkin reduces excitotoxic vulnerability at an additional earlier step by reducing overall excitatory synaptic input. The excitatory amino acid glutamate, acting as both a neurotoxin and a neurotransmitter, has long been posited to play a central role in the pathophysiology of PD (7–9). Interestingly, in rodent striatum, dopamine depletion produces an imbalance of glutamatergic synaptic input onto striatopallidal versus striatonigral medium spiny neurons (44). However, a direct mechanistic link between PD pathogenic factors and alterations in glutamatergic function has remained elusive. Our findings suggest that disease-linked mutations in parkin cause enhancement of excitatory synapses, which may contribute to neuronal loss and altered neural circuitry in PD.
Although the basic biochemical properties of parkin have been elucidated (7, 41), its normal cellular function is controversial (23, 24, 41). Recently, parkin has been implicated in several cellular pathways potentially involved in the regulation of synaptic strength and neuronal survival. Parkin interacts with Eps15, an adaptor protein involved in epidermal growth factor receptor endocytosis and trafficking (19). Parkin ubiquitination of Eps15 disrupts Eps15 epidermal growth factor binding, delaying receptor internalization and promoting phophatidylinositol-3 kinase–Akt signaling (19), thereby enhancing cell survival. Parkin also mono-ubiquitinates protein interacting with C-kinase (PICK) 1 (20), a protein known to regulate excitatory synaptic strength by the trafficking of AMPA receptors (45–49). In principle, the loss of excitatory synapses induced by parkin could be a result of direct ubiquitination of postsynaptic proteins (50), or to an indirect effect on downstream signaling. Complicating matters is the relatively diffuse distribution of parkin in axons, soma, dendrites, and spines (Fig. S6C and Fig. S7). Similarly, we observed no specific subcellular distribution of GFP-tagged parkin-WT or parkin mutants, whose signal was correspondingly diffuse throughout the neuron (Fig. 2 A and C and Figs. S4A and S5A; and data not shown for GFP-tagged parkin mutants R42P, R275W, and T415N). As with most ubiquitin ligases, the full range of substrates of parkin, and the validity of most reported substrates, is unclear. It will be important for future studies to determine which combination of the numerous identified and unidentified parkin substrates contribute to the parkin-dependent loss of excitatory synapses.
Methods
Cell Preparation and Transfection.
Hippocampal and cortical neurons from embryonic rat were cultured and transfected as previously described (50). For VGAT staining, neurons were obtained from newborn P0-P1 rats and cultured for 12 days. For electrophysiology, recordings were obtained 2 to 7 days after transfection. For immunocytochemistry, neurons were incubated for 3 days after transfection.
Immunocytochemistry and DNA Constructs.
Anti-VGLUT1 (Synaptic Systems; 1:2.000), anti-VGAT (Synaptic Systems; 1:2,000), anti-parkin (Abcam; 1:1,000), and anti–PSD-95 antibodies (Transduction Laboratories, 1:1,000) were used for immunocytochemistry. See SI Methods for details.
GFP-Parkin and GFP-parkin R275W were kindly provided by Edward Fon (Montreal, Canada) and Ted Dawson (Baltimore, MD). Mutations in GFP-parkin (R42P, T240R, T415N, and parkin*) were generated using a Stratagene site-directed mutagenesis kit according to the manufacturer's instructions. Parkin 253 and 635 shRNA constructs were generated against the rat PARK2 gene sequence (GenBank accession no. NM_020093) and cloned into the pRNAT-H1.3/Hygro vector (GenScript). The scrambled shRNA construct was cloned into the same pRNAT-H1.3/Hygro vector. See SI Methods for sequence details.
Electrophysiology.
Whole-cell voltage clamp recordings were performed on DIV17–24 rat hippocampal neurons cultured at high density on polylysine-coated glass coverslips. Glutamate receptors were blocked with 25 μM 6-cyano-7-nitroquinoxaline-2,3-dione and 50 μM D-AP5. See SI Methods for details.
Excitotoxicity Assays.
Neurons were infected at DIV14 or 15 with GFP, parkin-WT, mutant parkin (A240R, T415N), or parkin-shRNA lentiviral constructs. One week after infection, neurons were exposed to high potassium solution containing (in mM): 90 KCl, 31.5 NaCl, 2 CaCl2, 25 Hepes, and 30 glucose (pH 7.4). After 5 min exposure, cells were further incubated for 4 to 6 h in standard neurobasal medium (Gibco). Cell viability was examined using trypan blue dye exclusion or annexin-V–Alexa 568 fluorescence (Roche). Approximately 600 to 900 neurons were scored per coverslip. For additional information, including supplemental figures, see SI Methods.
Supplementary Material
Acknowledgments.
This work was supported by grants from the National Institutes of Health (M.D.E.). M.D.E. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802280105/DCSupplemental.
References
- 1.Shimura H, et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- 2.Samii A, Nutt JG, Ransom BR. Parkinson's disease. Lancet. 2004;363:1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- 3.Wichmann T, DeLong MR. Pathophysiology of Parkinson's disease: the MPTP primate model of the human disorder. Ann N Y Acad Sci. 2003;991:199–213. doi: 10.1111/j.1749-6632.2003.tb07477.x. [DOI] [PubMed] [Google Scholar]
- 4.Brown P. Oscillatory nature of human basal ganglia activity: Relationship to the pathophysiology of Parkinson's disease. Mov Disord. 2003;18:357–363. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- 5.Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends Neurosci. 2002;25:525–531. doi: 10.1016/s0166-2236(02)02235-x. [DOI] [PubMed] [Google Scholar]
- 6.Blandini F, Nappi G, Tassorelli C, Martignoni E. Functional changes of the basal ganglia circuitry in Parkinson's disease. Prog Neurobiol. 2000;62:63–88. doi: 10.1016/s0301-0082(99)00067-2. [DOI] [PubMed] [Google Scholar]
- 7.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 8.Beal MF. Excitotoxicity and nitric oxide in Parkinson's disease pathogenesis. Ann Neurol. 1998;44(suppl):S110–S114. doi: 10.1002/ana.410440716. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez MC, Obeso JA, Olanow CW. Subthalamic nucleus-mediated excitotoxicity in Parkinson's disease: a target for neuroprotection. Ann Neurol. 1998;44(suppl):S175–S188. doi: 10.1002/ana.410440726. [DOI] [PubMed] [Google Scholar]
- 10.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 11.Thomas B, Beal MF. Parkinson's disease. Human Mol Genet. 2007;16(R2):R183–R194. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- 12.Kitada T, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 13.Hattori N, Kobayashi H, Sasaki-Hatano Y, Sato K, Mizuno Y. Familial Parkinson's disease: A hint to elucidate the mechanisms of nigral degeneration. J Neurol. 2003;250(suppl 3):III2–III10. doi: 10.1007/s00415-003-1302-y. [DOI] [PubMed] [Google Scholar]
- 14.Wang M, et al. Developmental changes in the expression of parkin and UbcR7, a parkin-interacting and ubiquitin-conjugating enzyme, in rat brain. J Neurochem. 2001;77:1561–1568. doi: 10.1046/j.1471-4159.2001.00372.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, et al. Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci USA. 2000;97:13354–13359. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staropoli JF, et al. Parkin is a component of an SCF-like ubiquitin ligase complex and protects postmitotic neurons from kainate excitotoxicity. Neuron. 2003;37:735–749. doi: 10.1016/s0896-6273(03)00084-9. [DOI] [PubMed] [Google Scholar]
- 17.Imai Y, Takahashi R. How do Parkin mutations result in neurodegeneration? Curr Opin Neurobiol. 2004;14:384–389. doi: 10.1016/j.conb.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Huynh DP, Scoles DR, Nguyen D, Pulst SM. The autosomal recessive juvenile Parkinson disease gene product, parkin, interacts with and ubiquitinates synaptotagmin XI. Hum Mol Genet. 2003;12:2587–2597. doi: 10.1093/hmg/ddg269. [DOI] [PubMed] [Google Scholar]
- 19.Fallon L, et al. A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K-Akt signalling. Nat Cell Biol. 2006;8:834–842. doi: 10.1038/ncb1441. [DOI] [PubMed] [Google Scholar]
- 20.Joch M, et al. Parkin-mediated monoubiquitination of the PDZ protein PICK1 regulates the activity of acid-sensing ion channels. Mol Biol Cell. 2007;18:3105–3118. doi: 10.1091/mbc.E05-11-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fallon L, et al. Parkin and CASK/LIN-2 associate via a PDZ-mediated interaction and are co-localized in lipid rafts and postsynaptic densities in brain. J Biol Chem. 2002;277:486–491. doi: 10.1074/jbc.M109806200. [DOI] [PubMed] [Google Scholar]
- 22.Moszczynska A, et al. Parkin disrupts the alpha-synuclein/dopamine transporter interaction: consequences toward dopamine-induced toxicity. J Mol Neurosci. 2007;32:217–227. doi: 10.1007/s12031-007-0037-0. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg MS, et al. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- 24.Itier JM, et al. Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum Mol Genet. 2003;12:2277–2291. doi: 10.1093/hmg/ddg239. [DOI] [PubMed] [Google Scholar]
- 25.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 26.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 27.Giasson BI, Lee VM. Parkin and the molecular pathways of Parkinson's disease. Neuron. 2001;31:885–888. doi: 10.1016/s0896-6273(01)00439-1. [DOI] [PubMed] [Google Scholar]
- 28.Matsuda N, et al. Diverse effects of pathogenic mutations of Parkin that catalyze multiple monoubiquitylation in vitro. J Biol Chem. 2006;281:3204–3209. doi: 10.1074/jbc.M510393200. [DOI] [PubMed] [Google Scholar]
- 29.Sakata E, et al. Parkin binds the Rpn10 subunit of 26S proteasomes through its ubiquitin-like domain. EMBO Rep. 2003;4:301–306. doi: 10.1038/sj.embor.embor764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imai Y, Soda M, Takahashi R. Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J Biol Chem. 2000;275:35661–35664. doi: 10.1074/jbc.C000447200. [DOI] [PubMed] [Google Scholar]
- 31.El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- 32.Hennou S, et al. Homer-1a/Vesl-1S enhances hippocampal synaptic transmission. Eur J Neurosci. 2003;18:811–819. doi: 10.1046/j.1460-9568.2003.02812.x. [DOI] [PubMed] [Google Scholar]
- 33.Mata IF, Lockhart PJ, Farrer MJ. Parkin genetics: one model for Parkinson's disease. Hum Mol Genet. 2004;13:R127–R133. doi: 10.1093/hmg/ddh089. [DOI] [PubMed] [Google Scholar]
- 34.Sriram SR, et al. Familial-associated mutations differentially disrupt the solubility, localization, binding and ubiquitination properties of parkin. Hum Mol Genet. 2005;14:2571–2586. doi: 10.1093/hmg/ddi292. [DOI] [PubMed] [Google Scholar]
- 35.Obeso JA, et al. Pathophysiologic basis of surgery for Parkinson's disease. Neurology. 2000;55(suppl):S7–S12. [PubMed] [Google Scholar]
- 36.Crump FT, Dillman KS, Craig AM. cAMP-dependent protein kinase mediates activity-regulated synaptic targeting of NMDA receptors. J Neurosci. 2001;21:5079–5088. doi: 10.1523/JNEUROSCI.21-14-05079.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.West AB, Maidment NT. Genetics of parkin-linked disease. Hum Genet. 2004;114:327–336. doi: 10.1007/s00439-003-1074-6. [DOI] [PubMed] [Google Scholar]
- 38.Shimura H, et al. Ubiquitination of a new form of alpha-synuclein by parkin from human brain: implications for Parkinson's disease. Science. 2001;293:263–269. doi: 10.1126/science.1060627. [DOI] [PubMed] [Google Scholar]
- 39.Ren Y, Zhao J, Feng J. Parkin binds to alpha/beta tubulin and increases their ubiquitination and degradation. J Neurosci. 2003;23:3316–3324. doi: 10.1523/JNEUROSCI.23-08-03316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu XR, et al. Non-motor behavioural impairments in parkin-deficient mice. Eur J Neurosci. 2007;26:1902–1911. doi: 10.1111/j.1460-9568.2007.05812.x. [DOI] [PubMed] [Google Scholar]
- 41.Von Coelln R, et al. Loss of locus coeruleus neurons and reduced startle in parkin null mice. Proc Natl Acad Sci USA. 2004;101:10744–10749. doi: 10.1073/pnas.0401297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y, Nishimura I, Imai Y, Takahashi R, Lu B. Parkin suppresses dopaminergic neuron-selective neurotoxicity induced by Pael-R in Drosophila. Neuron. 2003;37:911–924. doi: 10.1016/s0896-6273(03)00143-0. [DOI] [PubMed] [Google Scholar]
- 43.Petrucelli L, et al. Parkin protects against the toxicity associated with mutant alpha-synuclein: Proteasome dysfunction selectively affects catecholaminergic neurons. Neuron. 2002;36:1007–1019. doi: 10.1016/s0896-6273(02)01125-x. [DOI] [PubMed] [Google Scholar]
- 44.Day M, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- 45.Lu W, Ziff EB. PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron. 2005;47:407–421. doi: 10.1016/j.neuron.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Lin DT, Huganir RL. PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. J Neurosci. 2007;27:13903–13908. doi: 10.1523/JNEUROSCI.1750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanley JG, Henley JM. PICK1 is a calcium-sensor for NMDA-induced AMPA receptor trafficking. EMBO J. 2005;24:3266–3278. doi: 10.1038/sj.emboj.7600801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daw MI, et al. PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses. Neuron. 2000;28:873–886. doi: 10.1016/s0896-6273(00)00160-4. [DOI] [PubMed] [Google Scholar]
- 49.Cao M, et al. PICK1-ICA69 heteromeric BAR domain complex regulates synaptic targeting and surface expression of AMPA receptors. J Neurosci. 2007;27:12945–12956. doi: 10.1523/JNEUROSCI.2040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.