Abstract
The thyroid hormone receptor (TR) has been proposed to regulate expression of target genes in the absence of triiodothyronine (T3) through the recruitment of the corepressors, NCoR and SMRT. Thus, NCoR and SMRT may play an essential role in thyroid hormone action, although this has never been tested in vivo. To accomplish this, we developed mice that express in the liver a mutant NCoR protein (L-NCoRΔID) that cannot interact with the TR. L-NCoRΔID mice appear grossly normal, however, when made hypothyroid the repression of many positively regulated T3-target genes is abrogated, demonstrating that NCoR plays a specific and sufficient role in repression by TR in the absence of T3. Remarkably, in the euthyroid state, expression of many T3-targets is also up-regulated in L-NCoRΔID mice, demonstrating that NCoR also determines the magnitude of the response to T3 in euthyroid animals. Although positive T3 targets were up-regulated in L-NCoRΔID mice in the hypo- and euthyroid state, there was little effect seen on negatively regulated T3 target genes. Thus, NCoR is a specific regulator of T3-action in vivo and mediates repression by the unliganded TR in hypothyroidism. Furthermore, NCoR appears to play a key role in determining the tissue-specific responses to similar levels of circulating T3. Interestingly, NCoR recruitment to LXR is also impaired in this model, leading to activation of LXR-target genes, further demonstrating that NCoR recruitment regulates multiple nuclear receptor signaling pathways.
Keywords: gene expression, thyroid hormone receptor
The nuclear receptor corepressor (NCoR) and the silencing mediator of retinoic acid and thyroid hormone receptors (SMRT) are key regulators of nuclear receptor signaling (1, 2). Among the first interaction partners of NCoR and SMRT identified were the thyroid hormone receptor (TR) isoforms. NCoR and SMRT have been postulated to mediate the ability of the TR to repress transcription of positively regulated T3-target genes in the absence of ligand (T3) by providing a platform for a multiprotein complex that mediates histone deacetylation (3–7). Because of this property the corepressors have been implicated in the pathophysiology of hypothyroidism and resistance to thyroid hormone (8, 9). This putative role of the corepressors in vivo is further substantiated by the fact that mice that lack all TR-isoforms are viable, while neonatal hypothyroidism is uniformly fatal in mice, consistent with a detrimental role of the unliganded TR bound to corepressors (10, 11). Despite the perceived roles of the corepressors in TR action, in vivo data are lacking as deletion of NCoR or SMRT is lethal late in embryogenesis, although transgenic overexpression of a NCoR inhibitor in liver did suggest a role for NCoR/SMRT in ligand-independent repression by the TR (12–14).
Both NCoR and SMRT are recruited to nuclear receptors via C-terminal receptor interacting domains (RIDs), which are characterized by the presence of an isoleucine rich motif termed a CoRNR box (15–17). Extensive analysis has disclosed the presence in both molecules of 3 RIDs that can be expressed alternatively in a tissue-specific manner (18–21). Despite their similarity the RIDs do not function equivalently. TR has been shown to preferably bind NCoR both in vitro and in mammalian cell lines (22, 23) via the most 5′ of its RIDs, N3, which is required for strong interactions with DNA- bound TR (18, 21, 24). Importantly, N3 must cooperate with another RID, preferably N2, to interact with a TR homodimer on DNA as each TR binds to 1 of the RIDs (18, 21–25).
Given the preference of NCoR for the TR, we took advantage of the role that the RIDs play in TR recruitment to develop an approach to test the role of NCoR in T3- action in the liver. Using this model we show that NCoR mediates both repression by the TR in the hypothyroid state and modulates the response to T3 in the euthyroid state on positively regulated T3-target genes. In addition, we show the key role that NCoR plays in LXR-signaling in the liver demonstrating the ability of this model to address the role of NCoR in other NR-signaling pathways in vivo.
Results
Generation and Characterization of L-NCoRΔID Mice.
We created a conditional NCoR allele by inserting loxP sites around the exons coding for the 2 most N-terminal RIDs, termed N3 and N2 [supporting information (SI) Fig. S1 A–C]. Upon Cre-mediated recombination, the targeted NCoR locus would encode a protein that contains only 1 RID–N1 (NCoRΔID) and thus would be unable to interact with the TR (Fig. 1A). Using targeted ES cells we generated NCoRlox−neo/+ animals, which were then crossed with mice ubiquitously expressing the flp recombinase from the Rosa26 locus to remove the Neo cassette.
Fig. 1.
Expression of the mutant NCoRΔID in L-NCoRΔID mice. (A) Schematic representation of the approach used to generate a mouse strain that expresses mutant NCoR lacking N3 and N2 RIDs in the liver (L-NCoRΔID). (B) Hepatic expression of NCoR mRNA in L-NCoRΔID and control mice as assessed by Q-PCR directed against the 5′ region of the mRNA. (C) Expression of NCoR mRNA in different tissues as assessed by Q-PCR directed against the 3′ region of the mRNA. mRNA levels are expressed as fold change compared with WT group. All values are expressed as mean± SEM. Significant differences compared with WT group: ***, P < 0.001. PGF-perigonadal fat pad. (D) Western blot analysis of NCoR in the liver in a variety of genotypes. RNA pol II is used as a loading control. (E) Co-IPs were performed on extracts from control and L-NCoRΔID livers by using 2 anti-TR antibodies (see SI Text). The protein complexes were resolved on NuPAGE Tris-acetate gel, and analyzed by Western blot using anti-NCoR antibody. The blots were scanned and quantified by using Image J software.
Given the paramount role the TRs play in the liver we developed mice that only expressed the NCoRΔID allele in hepatocytes by crossing NCoRlox/lox mice with an albumin-Cre transgenic strain (26). NCoRlox/lox-Cre (L-NCoRΔID) mice were born at the expected frequency and developed normally. Expression of the 5′ region of NCoR mRNA common to both NCoR and NCoRΔID was similar in the livers of WT, NCoRlox/lox and L-NCoRΔID mice (Fig. 1B). However, expression levels of the mRNA region encompassing N3 and N2 in L-NCoRΔID livers were <10% of those found in NCoRlox/lox and WT animals (Fig. 1C). We found no differences in the expression levels of this region in muscle, heart and adipose tissue of control and L-NCoRΔID mice confirming selective expression of NCoRΔID in the liver (Fig. 1C). This was confirmed by analysis of hepatic protein extracts which demonstrates that NCoRΔID is present in L-NCoRΔID mice and mice heterozygous for the NCoRΔID allele (L-NCoRΔID/+) express equal amounts of NCoR and NCoRΔID (Fig. 1D). Importantly, only full-length NCoR protein is present in the heart and muscle of L-NCoRΔID mice (Fig. S1D). Finally, there is no compensatory up-regulation of SMRT mRNA or protein levels in L-NCoRΔID mice (Fig. S1E), as was the case in a previous transgenic mouse model that overexpressed an NCoR inhibitor (14).
To demonstrate that NCoRΔID localized to the nucleus in a similar fashion to NCoR we performed immunocytochemistry on HepG2 cells transfected with plasmids expressing Flag-tagged NCoR or NCoRΔID, as well as immunohistochemical staining of control and L-NCoRΔID liver sections (Fig. S2 A and B). To confirm that NCoRΔID is defective in its ability to be recruited to the TR we used coimmunoprecipitation experiments using protein extracts from livers of hypothyroid NCoRlox/lox and L-NCoRΔID mice as well as 293T cells transfected with expression plasmids for NCoR, NCoRΔID and TRβ1. As shown in Fig. 1E and Fig. S3A TRβ1 strongly recruits WT NCoR both in vivo and in mammalian cells but cannot recruit NCoRΔID. This was confirmed with 2 separate antibodies that recognize TRβ1. Thus, L-NCoRΔID mice provide a unique in vivo model to explore the role of NCoR in TR action.
NCoR Regulates Expression of TR-Target Genes in the Liver.
To determine the role of NCoR in vivo we studied male and female WT, NCoRlox/lox and L-NCoRΔID mice at 9 weeks of age that were euthyroid, hypothyroid or hyperthyroid. Total T4 and total T3 levels in female mice in all states showed no difference among genotypes (Table S1). Male L-NCoRΔID mice had a slight decrease in T4 levels compared with NCoRlox/lox mice only, but otherwise showed no difference in thyroid hormone levels across genotypes.
To assess the role of the NCoRΔID on T3 action in the liver, we performed microarray analysis of hepatic gene expression. We identified 173 targets that were significantly repressed (representing positively regulated TR/T3-target genes) in hypothyroid control animals versus euthyroid control animals (Fig. 2A). Of these, 27 (16%) were significantly derepressed or activated in hypothyroid L-NCoRΔID mice and are shown in red in the upper left quadrant of Fig. 2A, with the heat map representing these genes shown in Fig. 2B. In contrast, 326 genes were activated in hypothyroidism (representing negatively regulated TR/T3-target genes) in control mice, and only 3 of these were significantly repressed (<1%) in hypothyroid L-NCoRΔID mice. These genes are shown in red in the right lower quadrant of Fig. 2A with the corresponding heat map in Fig. 2C. Thus, NCoR recruitment via N3 and N2 plays an important role in mediating ligand-independent repression of positive T3 targets in the liver, while its role in ligand-independent activation by the TR appears very limited. We also identified 39 genes whose expression was significantly altered in the euthyroid state between control and L-NCoRΔID mice. Of these 39 genes, 26 were activated in L-NCoRΔID mice and included known classic positive T3 targets such as thrsp, fasn and mod1 (group 1; Fig. 2D) suggesting that NCoR recruitment may play a role in transcriptional activation by T3. Interestingly, 13 genes were repressed in euthyroid L-NCoRΔID mice when compared with controls (group 2; Fig. 2D).
Fig. 2.
Microarray analysis of hepatic gene expression in euthyroid and hypothyroid lox/lox and L-NCoRΔID female mice (n = 3 per group). (A) Scatter plot representing genes that are differentially expressed in hypothyroid compared with euthyroid control animals. Genes that are expressed differently in hypothyroid L-NCoRΔID animals as compared with hypothyroid controls are shown in red. (B) Heat map showing expression levels of the genes that are down-regulated in the hypothyroid state and expressed differently in hypothyroid L-NCoRΔID and control animals. (C) Heat map showing expression levels of the genes that are up-regulated in the hypothyroid state and expressed differently in hypothyroid L-NCoRΔID and control animals. (D) Hierarchical clustering of the genes differentially expressed in euthyroid L-NCoRΔID mice compared with euthyroid lox/lox controls. All of the heat maps show color-coded expression levels (red, high expression; black, medium expression; and green, low expression). Each column represents data for 1 animal.
To validate these data we performed QPCR analysis on a number of genes identified in the array, and on other known hepatic T3 targets. The data presented here were obtained by using female hepatic mRNA but we have also analyzed expression of a number of these targets in male mice and obtained similar results (data not shown). Expression of dio1, bcl3, gpd2, idh3 and cyp27a was significantly repressed in control animals in the hypothyroid state and strongly derepressed in L-NCoRΔID animals by 2- to 3-fold. Expression of dio1, cyp27a and idh3 was also significantly activated in euthyroid L-NCoRΔID animals when examined in a greater number of animals than used in the microarray studies (Fig. 3A). Other classic T3 targets, thrsp, fasn and mod1, were not significantly repressed in control hypothyroid mice explaining why they were not present in the first set of genes in our microarray analysis. However, expression of each of these genes was significantly elevated (2- to 4-fold) in L- NCoRΔID mice in the hypothyroid state and exceeded their expression levels in euthyroid control mice. In addition, these targets were strongly activated in the euthyroid state confirming our microarray data and demonstrating the role of NCoR in mediating T3 sensitivity.
Fig. 3.
Changes in hepatic expression of TR target genes in L-NCoRΔID animals. (A) Expression of genes that are repressed in the hypothyroid state in control animals is derepressed in the livers of hypothyroid L-NCoRΔID animals. (B) Expression of positively regulated T3-target genes that are not significantly repressed in the hypothyroid state in control animals is activated in the livers of L-NCoRΔID mice in both the hypo and euthyroid state. (C) Expression of negatively regulated T3-target genes is not affected in L-NCoRΔID animals. n = 5–7 animals. mRNA levels are expressed as fold change compared with lox/lox chow group. All values are expressed as mean ± SEM. Significant differences compared with control mice on the same diet: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We next looked at the role of NCoR in the regulation of negative T3 targets including fbxo21, a target identified in our array analysis, and previously described targets gsta2 and st3gal4. In contrast to positive T3 targets, the regulation of these genes was not altered in hypothyroid L- NCoRΔID mice. Thus, NCoR appears to play little role in negative regulation in the liver (Fig. 3C).
Role of NCoR in TR-Mediated Regulation of Serum Cholesterol Levels.
Hypothyroidism is known to cause an elevation of serum cholesterol levels, through a rise in LDL cholesterol due to repression of cyp7a1 by unliganded TRβ1 (27). Surprisingly, despite significant derepression of cyp7a1 expression in hypothyroid (and euthyroid) L-NCoRΔID mice (Fig. 4A) there was only a small decrease in LDL-C levels and no change in total cholesterol levels between hypothyroid L-NCoRΔID and control animals (Fig. 4C and Table S2). In addition, hepatic cholesterol content was not changed (Fig. 4D). Since HMG-CoA reductase (hmgcr), a rate-limiting enzyme in cholesterol biosynthesis, is also known to be a T3-target we examined its expression in L-NCoRΔID mice and found that it is significantly elevated by close to 2-fold in both the hypothyroid and euthyroid states in L-NCoRΔID animals (Fig. 4B). Further examination of our array data demonstrates that expression of other enzymes involved in cholesterol biosynthesis such as sqle, mvd, pmvk and fdps is elevated in euthyroid and/or hypothyroid L-NCoRΔID animals. This increase in cholesterogenic enzyme mRNA levels likely leads to enhanced cholesterol synthesis and offsets the increase in cholesterol elimination mediated by elevated cyp7a1 expression.
Fig. 4.
Hypothyroidism-associated hypercholesterolemia is not reversed in L-NCoRΔID mice. (A and B) Hepatic cyp7a1 (A) and hmgcr (B) mRNA levels are elevated in euthyroid and hypothyroid L-NCoRΔID animals. mRNA levels are expressed as fold change compared with controls. (C) Serum cholesterol is significantly increased by hypothyroidism in all groups of animals, while hepatic cholesterol content is not changed (D). n = 5–7 per group. All values are expressed as mean ± SEM. Significant differences compared with control mice on the same diet: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Significant differences compared with the same genotype on chow: ###, P < 0.001.
NCoR Regulates Signaling by LXR in the Liver.
As our microarray analysis also showed significantly enhanced expression of a number of genes previously reported to be regulated by the LXR isoforms, we hypothesized that NCoRΔID might also be deficient in its ability to interact with LXRα, the principal isoform in the liver (28, 29). Indeed, co-IP experiments using extracts from euthyroid control and L-NCoRΔID mice show that LXR recruits NCoR well, while its interaction with NCoRΔID is substantially reduced (Fig. 5A). Similar results were also seen in 293T cells cotransfected with expression plasmids for LXRα, NCoRΔID and NCoR (Fig. S3B). Thus, the N3 and N2 RIDs are important for LXR–NCoR interaction. QPCR analysis of the expression of the LXR targets abca1, srebp1c, scd1 and pltp1 confirmed that expression of each was significantly activated in L-NCoRΔID mice, and induction of hypothyroidism did not change the level of enhancement (Fig. 5B). Despite activation of LXR targets in L-NCoRΔID mice, serum and hepatic triglyceride levels in the euthyroid and hypothyroid state were similar to controls but did trend higher (Fig. 5C).
Fig. 5.
NCoR modulates LXR signaling in the liver. (A) Co-IPs were performed on extracts from control and L-NCoRΔID livers by using an anti-LXR antibody that recognizes both LXR isoforms. The protein complexes were resolved on NuPAGE Tris-acetate gel, and analyzed by Western blot using anti-NCoR antibody. The blots were scanned and quantified by using Image J software. (B) mRNA expression levels of LXR target genes are elevated in the livers of L-NCoRΔID mice. mRNA levels are expressed as fold change compared with lox/lox chow controls. (C) Serum and liver triglyceride levels are not significantly increased in L-NCoRΔID animals. n = 5–7. All values are expressed as mean ± SEM. Significant differences compared with control mice on the same diet: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
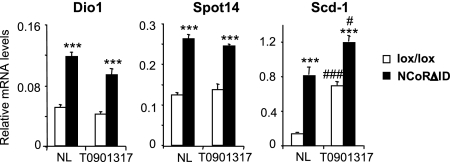
Because of the potential overlap between TR and LXR signaling pathways in the regulation of hepatic target genes such as thrsp, mod1 and fasn we wanted to ensure that the effect of NCoR loss was specific for the TR. To accomplish this we isolated primary hepatocytes from control and NCoRΔID mice and examined expression of these genes in the absence and presence of the LXR ligand T0901317. As shown in Fig. 6, expression of thrsp, dio1 and scd1 was increased in hepatocytes from L-NCoRΔID mice in the absence of ligand. In the presence of T0901317, which should dissociate NCoR form LXR, scd1 expression was significantly up-regulated in both control and L-NCoRΔID hepatocytes and the 7-fold derepression seen in NCoRΔID cells in the absence of ligand, had become much less pronounced. In contrast, T0901317 had no effect on thrsp or dio1 and derepression of these targets was maintained in L-NCoRΔID hepatocytes consistent with a specific role for the TR in mediating repression of these targets via NCoR.
Fig. 6.
NCoR specifically regulates TR-target genes. mRNA levels of TR and LXR target genes were determined from primary hepatocytes isolated from control and L-NCoRΔID animals cultured in the presence of 1 μM LXR ligand T0901317 where indicated. Relative mRNA levels are shown. All values are expressed as mean ± SEM. Significant differences compared with controls on the same treatment: ***, P < 0.001. Significant differences compared with the same genotype with no treatment: #, P < 0.05; ###, P < 0.001.
Discussion
Since their identification, NCoR and SMRT have been shown to play a key role in nuclear receptor signaling. In particular, it has been assumed that either or both are responsible for the potent effects of the unliganded TR. However, because global deletion of both NCoR and SMRT is embryonic lethal, this hypothesis has not been tested in vivo before this report (12, 13).
To analyze NCoR function in vivo, we took advantage of the unique role that RIDs play in its interaction with TR and other NRs. The NCoRΔID mutant protein lacks both the N3 and N2 RIDs and thus cannot be recruited to the TR. Importantly, the rest of the protein is produced, thereby preventing any detrimental effects to the animal. Thus, L-NCoRΔID mice provide a loss of function model to assess the role of NCoR in thyroid hormone action in vivo.
Herein, we demonstrate that NCoR mediates repression of positively regulated T3 target genes by the unliganded TR. Indeed, expression of dio1, bcl3, fasn, mod1, cyp27a, cyp7a and thrsp is significantly elevated in hypothyroid L-NCoRΔID mice as compared with hypothyroid control animals confirming the key role of NCoR in ligand-independent action by the TR. Importantly, the activation seen on some of these targets (fasn, mod1 and thrsp) is almost identical to that seen in hypothyroid TRβ−/− mice confirming that TR-mediated repression of these targets is due to NCoR (30). While the reversal of repression of dio1 is markedly less than the other targets, this is not unexpected as corepressors may not play a significant role in repression of this gene (31). Our data do not rule out a role for SMRT in mediating ligand-independent repression by the TR on certain targets, but clearly NCoR is sufficient to mediate this key function of the TR.
We also demonstrate that NCoR plays a key role in determining the response to T3 in the euthyroid state. Most positively regulated targets examined showed significantly enhanced mRNA expression in euthyroid L-NCoRΔID mice. This is consistent with data demonstrating that only 50% of hepatic TRs are bound to T3 in the euthyroid state (32). Thus, in the absence of recruited NCoR, total TR-responsive gene expression is enhanced as has been seen previously in vitro (33). Furthermore, these data strongly suggest that levels of NCoR can determine individual responses to set levels of T3 and could explain why tissue-specific responses to identical levels of T3 can vary in individuals. Interestingly, the response of certain targets is also enhanced in the hyperthyroid state but to a lesser extent than in the euthyroid state. It is possible that this reflects the persistence of unliganded TRs, even in the setting of excess ligand. However, in certain cases, such as in the regulation of fasn, this may also be explained by cross-talk with other NR signaling pathways such as LXR (34–36).
We also examined the role of NCoR in the regulation of expression of genes known to be negatively regulated by T3 (37, 38). It has been speculated that corepressors may be paradoxically responsible for ligand-independent activation on negative targets (14, 39). If this were the case, the activation of TR-targets in hypothyroidism would be reversed in L-NCoRΔID mice. While we identified >300 genes that were activated in hypothyroid control mice, only 3 of these were significantly deactivated or repressed in hypothyroid L-NCoRΔID animals. Analysis of expression of 1 of them, fbxo21, confirmed it to be a negative target, but showed that it was activated equally well in hypothyroid L-NCoRΔID and control mice. Analysis of other well-known negative T3-targets in the liver showed that the loss of N3 and N2 in NCoR has no influence on their expression, although it remains possible that NCoRΔID can still interact with the TR on negative TREs. Thus, recruitment of NCoR through N3 and N2 appears to play a preferential role on positively regulated targets. The mechanism by which the majority of genes are activated by the TR in the hypothyroid state remains unclear.
Given that the rise in serum cholesterol levels seen in hypothyroidism have been shown to be mediated by the action of unliganded TRβ in the liver, we expected hypothyroid L-NCoRΔID mice to be resistant to the development of hypercholesterolemia. Despite derepression of cyp7a expression, serum cholesterol levels were similar in hypothyroid control and L-NCoRΔID mice and LDL-C levels were only slightly reduced. This is likely explained by the elevated mRNA expression of hmgcr and other enzymes in the cholesterol biosynthetic pathway in hypothyroid L-NCoRΔID mice. Indeed, deletion of hdac3 from the liver also activates similar genes in the cholesterol synthesis pathway demonstrating that the recruitment of HDAC3 by NCoR plays a key role in regulating cholesterol biosynthesis (40).
In addition to its role in TR action, NCoR also regulates the action of LXR isoforms (29, 41). Our data demonstrate that recruitment of NCoR to LXR requires 2 NCoR RIDs in vivo. Furthermore, known LXR targets are clearly activated in L-NCoRΔID mice. A role for NCoR in LXR signaling is further supported by the finding that the derepression of scd1 seen in L-NCoRΔID primary hepatocytes as compared with control hepatocytes is almost lost in the presence of LXR ligand, consistent with dismissal of NCoR and recruitment of coactivators.
Overall the results of our microarray analysis show activation of TR and LXR signaling pathways that are both known to positively regulate expression of lipogenic enzymes and enzymes involved in cholesterol catabolism, and share a number of target genes. While our data do not exclude the possibility that other nuclear receptor signaling pathways might be affected, it clearly demonstrates that NCoR plays an important role in TR-signaling in vivo.
In summary, we have generated mice that express only in the liver a modified NCoR protein that is defective in its ability to interact with TR and LXR. Using these mice we demonstrate that NCoR specifically regulates repression of positively regulated TR-target genes in the hypothyroid state. NCoR also modulates expression of positive TR targets in the euthyroid state and regulates the response to set levels of T3. In contrast, NCoR appears to play little or no role in negative regulation by T3 in the liver. NCoR recruitment also regulates expression of LXR targets in the liver. Taken together these observations suggest that nuclear receptor action on target genes could be altered in vivo by the relative amount of NCoR present. This implies in particular that thyroid status of a particular tissue would depended on both tissue-specific NCoR and T3 levels.
Materials and Methods
Generation of NCoRΔID Mice.
The targeting vector was constructed in the pZErO-1 plasmid (Invitrogen) by using a BAC clone containing a fragment of mouse NCoR gene derived from the 129S6 mouse strain. All cloning manipulations were performed by using homologous recombination in EL250 and EL350 Escherichia coli strains as described by Lee et al. (42). For more details and breeding strategies used to obtain L-NCoRΔID mice see SI Text.
Animal Experiments.
All experiments described in SI Text were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee.
Blood Chemistry and Hormonal Analysis.
Total T4 and T3 levels were measured by solid-phase RIA (Coat-a-Count; Diagnostic Products) in 25 and 50 μl of serum, respectively. Enzymatic-colorimetric assays for total cholesterol, triglycerides and alkaline phosphatase activity were purchased form Stanbio Laboratory. Number and size of lipoprotein particles were measured by NMR spectroscopy, and the amount of HDL cholesterol was calculated based on these results by LipoScience.
Liver Triglycerides and Cholesterol.
The lipids were extracted by the method of Folch with modifications as described in SI Text (43).
Coimmunoprecipitation and Western Blot Analysis.
Coimmunoprecipitations (Co-IP) were performed as described in SI Text using protein extracts from livers of lox/lox and L-NCoRΔID euthyroid or hypothyroid mice or 293T cells transfected with expression plasmids for murine full-length and ΔID NCoR, human TRβ1 and human LXRα as indicated (44).
Immunocytochemistry and Immunohistochemistry.
Immunostaining of HepG2 cells transfected with either Flag-tagged NCoR or Flag-tagged NCoRΔID expression plasmid, and frozen liver sections from lox/lox and L-NCoRΔID mice was performed as described in SI Text.
Gene Expression Profiling.
Three female mice from the NCoRlox/lox and L-NCoRΔID euthyroid and hypothyroid groups described above were chosen for microarray analysis. Isolated mRNA was further purified with the RNeasy Mini kit per manufacturer's instructions (Qiagen). Total RNA (200 ng) was then used for GeneChip analysis. Preparation of terminal-labeled cDNA, hybridization to genome-wide murine Gene Level 1.0 ST GeneChips (Affymetrix) and scanning of the arrays were carried out according to manufacturer's protocols (https://www.affymetrix.com).
Real-Time Quantitative (Q)PCR.
Total RNA extraction and real-time QPCR were performed by using standard techniques as described in SI Text.
Bioinformatic Analysis.
RMA Signal extraction, normalization and filtering was performed as described (www.bioconductor.org/) (45, 46). All calculations were performed in “R.” Heat maps were generated by using EPCLUST (http://ep.ebi.ac.uk/EP/EPCLUST/). The details are described in SI Text. Complete GeneChip datasets are available online as GEO entry GSE10001 (www.ncbi.nlm.nih.gov/projects/geo/query/acc.cgi?acc=GSE10001).
Primary Hepatocytes.
Hepatocytes were isolated from 8- to 10-week-old mice by using a 2-step collagenase perfusion procedure as described in SI Text. The cells were cultured in RPMI medium 1640 supplemented with 10% steroid-depleted FBS (HyClone), 100 nM dexamethasone, 10 μg/ml insulin and 1 μM LXR ligand T090137 (Sigma) or DMSO. After 16–18 h hepatocytes were harvested and RNA was prepared for Q-PCR analysis as above. N = 3 per treatment group.
Supplementary Material
Acknowledgments.
We thank Evan Rosen for helpful discussions and the Gene Manipulation Facility of the Mental Retardation and Developmental Disabilities Research Center at Children's Hospital in Boston [National Institutes of Health (NIH) Grant P30-HD18655] for ES cell work. This work was supported by NIH Grants DK056123 and DK078090 (to A.N.H.) and T32 DK07516 (to I.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804604105/DCSupplemental.
References
- 1.Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 2.Horlein AJ, et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 3.Alland L, et al. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49–55. doi: 10.1038/387049a0. [DOI] [PubMed] [Google Scholar]
- 4.Guenther MG, Barak O, Lazar MA. The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol Cell Biol. 2001;21:6091–6101. doi: 10.1128/MCB.21.18.6091-6101.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinzel T, et al. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature. 1997;387:43–48. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- 6.Nagy L, et al. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997;89:373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 7.Yoon HG, et al. Purification and functional characterization of the human N-CoR complex: The roles of HDAC3, TBL1 and TBLR1. EMBO J. 2003;22:1336–1346. doi: 10.1093/emboj/cdg120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flamant F, et al. Congenital hypothyroid Pax8(−/−) mutant mice can be rescued by inactivating the TRalpha gene. Mol Endocrinol. 2002;16:24–32. doi: 10.1210/mend.16.1.0766. [DOI] [PubMed] [Google Scholar]
- 9.Tagami T, Jameson JL. Nuclear corepressors enhance the dominant negative activity of mutant receptors that cause resistance to thyroid hormone. Endocrinology. 1998;139:640–650. doi: 10.1210/endo.139.2.5742. [DOI] [PubMed] [Google Scholar]
- 10.Gauthier K, et al. Different functions for the thyroid hormone receptors TRalpha and TRbeta in the control of thyroid hormone production and post-natal development. EMBO J. 1999;18:623–631. doi: 10.1093/emboj/18.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gothe S, et al. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes Dev. 1999;13:1329–1341. doi: 10.1101/gad.13.10.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermanson O, Jepsen K, Rosenfeld MG. N-CoR controls differentiation of neural stem cells into astrocytes. Nature. 2002;419:934–939. doi: 10.1038/nature01156. [DOI] [PubMed] [Google Scholar]
- 13.Jepsen K, et al. SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature. 2007;450:415–419. doi: 10.1038/nature06270. [DOI] [PubMed] [Google Scholar]
- 14.Feng X, Jiang Y, Meltzer P, Yen PM. Transgenic targeting of a dominant negative corepressor to liver blocks basal repression by thyroid hormone receptor and increases cell proliferation. J Biol Chem. 2001;276:15066–15072. doi: 10.1074/jbc.m011027200. [DOI] [PubMed] [Google Scholar]
- 15.Hu X, Lazar MA. The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature. 1999;402:93–96. doi: 10.1038/47069. [DOI] [PubMed] [Google Scholar]
- 16.Nagy L, et al. Mechanism of corepressor binding and release from nuclear hormone receptors. Genes Dev. 1999;13:3209–3216. doi: 10.1101/gad.13.24.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perissi V, et al. Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev. 1999;13:3198–3208. doi: 10.1101/gad.13.24.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen RN, et al. The specificity of interactions between nuclear hormone receptors and corepressors is mediated by distinct amino acid sequences within the interacting domains. Mol Endocrinol. 2001;15:1049–1061. doi: 10.1210/mend.15.7.0669. [DOI] [PubMed] [Google Scholar]
- 19.Malartre M, Short S, Sharpe C. Alternative splicing generates multiple SMRT transcripts encoding conserved repressor domains linked to variable transcription factor interaction domains. Nucleic Acids Res. 2004;32:4676–4686. doi: 10.1093/nar/gkh786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Short S, Malartre M, Sharpe C. SMRT has tissue-specific isoform profiles that include a form containing one CoRNR box. Biochem Biophys Res Commun. 2005;334:845–852. doi: 10.1016/j.bbrc.2005.06.175. [DOI] [PubMed] [Google Scholar]
- 21.Webb P, et al. The nuclear receptor corepressor (N-CoR) contains three isoleucine motifs (I/LXXII) that serve as receptor interaction domains (IDs) Mol Endocrinol. 2000;14:1976–1985. doi: 10.1210/mend.14.12.0566. [DOI] [PubMed] [Google Scholar]
- 22.Cohen RN, Putney A, Wondisford FE, Hollenberg AN. The nuclear corepressors recognize distinct nuclear receptor complexes. Mol Endocrinol. 2000;14:900–914. doi: 10.1210/mend.14.6.0474. [DOI] [PubMed] [Google Scholar]
- 23.Jonas BA, Varlakhanova N, Hayakawa F, Goodson M, Privalsky ML. Response of SMRT (silencing mediator of retinoic acid and thyroid hormone receptor) and N-CoR (nuclear receptor corepressor) corepressors to mitogen-activated protein kinase kinase kinase cascades is determined by alternative mRNA splicing. Mol Endocrinol. 2007;21:1924–1939. doi: 10.1210/me.2007-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makowski A, Brzostek S, Cohen RN, Hollenberg AN. Determination of nuclear receptor corepressor interactions with the thyroid hormone receptor. Mol Endocrinol. 2003;17:273–286. doi: 10.1210/me.2002-0310. [DOI] [PubMed] [Google Scholar]
- 25.Zamir I, Zhang J, Lazar MA. Stochiometric and steric principles governing repression by nuclear hormone receptors. Genes Dev. 1997;11:835–846. doi: 10.1101/gad.11.7.835. [DOI] [PubMed] [Google Scholar]
- 26.Postic C, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 27.Gullberg H, Rudling M, Forrest D, Angelin B, Vennstrom B. Thyroid hormone receptor beta-deficient mice show complete loss of the normal cholesterol 7alpha-hydroxylase (CYP7A) response to thyroid hormone but display enhanced resistance to dietary cholesterol. Mol Endocrinol. 2000;14:1739–1749. doi: 10.1210/mend.14.11.0548. [DOI] [PubMed] [Google Scholar]
- 28.Peet DJ, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 29.Wagner BL, et al. Promoter-specific roles for liver X receptor/corepressor complexes in the regulation of ABCA1 and SREBP1 gene expression. Mol Cell Biol. 2003;23:5780–5789. doi: 10.1128/MCB.23.16.5780-5789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flores-Morales A, et al. Patterns of liver gene expression governed by Trbeta. Mol Endocrinol. 2002;16:1257–1268. doi: 10.1210/mend.16.6.0846. [DOI] [PubMed] [Google Scholar]
- 31.Amma LL, Campos-Barros A, Wang Z, Vennstrom B, Forrest D. Distinct tissue-specific roles for thyroid hormone receptors beta and alpha1 in regulation of type 1 deiodinase expression. Mol Endocrinol. 2001;15:467–475. doi: 10.1210/mend.15.3.0605. [DOI] [PubMed] [Google Scholar]
- 32.Oppenheimer JH, Schwartz HL, Surks MI. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: Liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology. 1974;95:897–903. doi: 10.1210/endo-95-3-897. [DOI] [PubMed] [Google Scholar]
- 33.Sohn YC, et al. Dynamic inhibition of nuclear receptor activation by corepressor binding. Mol Endocrinol. 2003;17:366–372. doi: 10.1210/me.2002-0150. [DOI] [PubMed] [Google Scholar]
- 34.Blennemann B, Leahy P, Kim TS, Freake HC. Tissue-specific regulation of lipogenic mRNAs by thyroid hormone. Mol Cell Endocrinol. 1995;110:1–8. doi: 10.1016/0303-7207(95)03509-6. [DOI] [PubMed] [Google Scholar]
- 35.Joseph SB, et al. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J Biol Chem. 2002;277:11019–11025. doi: 10.1074/jbc.M111041200. [DOI] [PubMed] [Google Scholar]
- 36.Pawar A, Botolin D, Mangelsdorf DJ, Jump DB. The role of liver X receptor-alpha in the fatty acid regulation of hepatic gene expression. J Biol Chem. 2003;278:40736–40743. doi: 10.1074/jbc.M307973200. [DOI] [PubMed] [Google Scholar]
- 37.Feng X, Jiang Y, Meltzer P, Yen PM. Thyroid hormone regulation of hepatic genes in vivo detected by complementary DNA microarray. Mol Endocrinol. 2000;14:947–955. doi: 10.1210/mend.14.7.0470. [DOI] [PubMed] [Google Scholar]
- 38.Sadow PM, et al. Specificity of thyroid hormone receptor subtype and steroid receptor coactivator-1 on thyroid hormone action. Am J Physiol E. 2003;284:36–46. doi: 10.1152/ajpendo.00226.2002. [DOI] [PubMed] [Google Scholar]
- 39.Tagami T, Park Y, Jameson JL. Mechanisms that mediate negative regulation of the thyroid-stimulating hormone alpha gene by the thyroid hormone receptor. J Biol Chem. 1999;274:22345–22353. doi: 10.1074/jbc.274.32.22345. [DOI] [PubMed] [Google Scholar]
- 40.Knutson SK, et al. Liver-specific deletion of histone deacetylase 3 disrupts metabolic transcriptional networks. EMBO J. 2008;27:1017–1028. doi: 10.1038/emboj.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghisletti S, et al. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee EC, et al. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- 43.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 44.Cohen RN, Wondisford FE, Hollenberg AN. Two separate NCoR interacting domains mediate corepressor action on thyroid hormone response elements. Mol Endocrinol. 1998;12:1567–1581. doi: 10.1210/mend.12.10.0188. [DOI] [PubMed] [Google Scholar]
- 45.Bilban M, et al. Deregulated expression of fat and muscle genes in B-cell chronic lymphocytic leukemia with high lipoprotein lipase expression. Leukemia. 2006;20:1080–1088. doi: 10.1038/sj.leu.2404220. [DOI] [PubMed] [Google Scholar]
- 46.Irizarry RA, et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.