Abstract
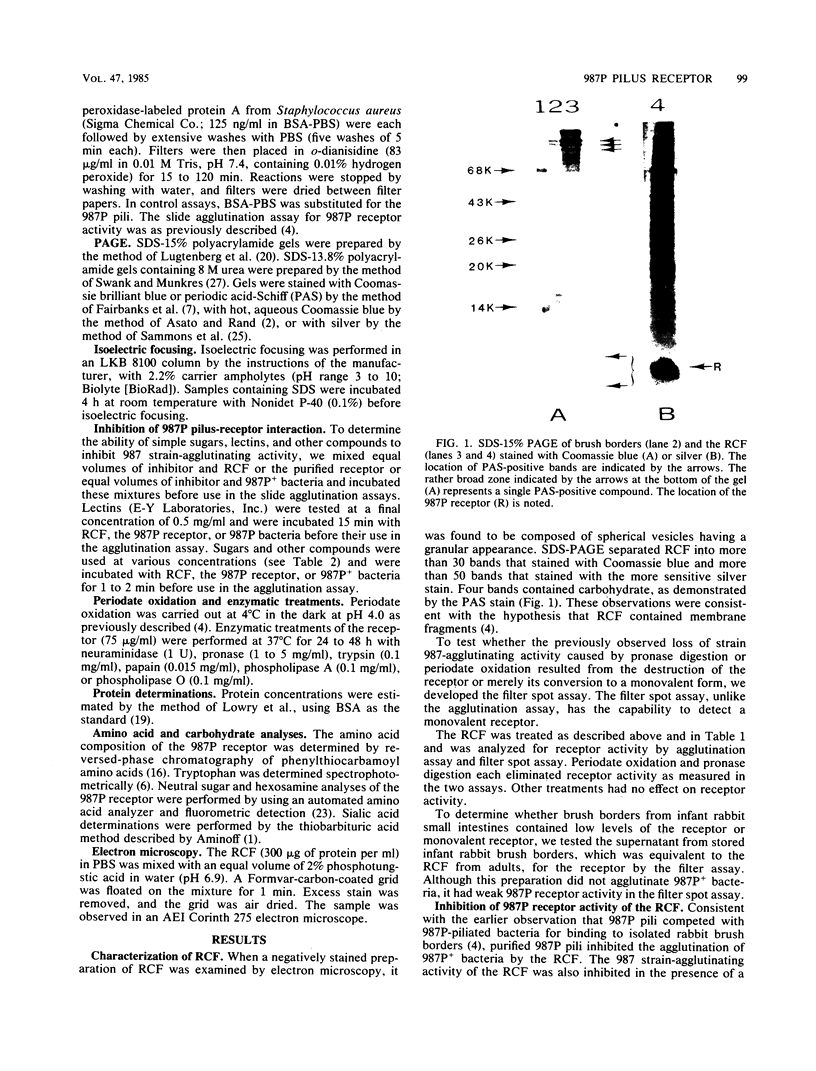
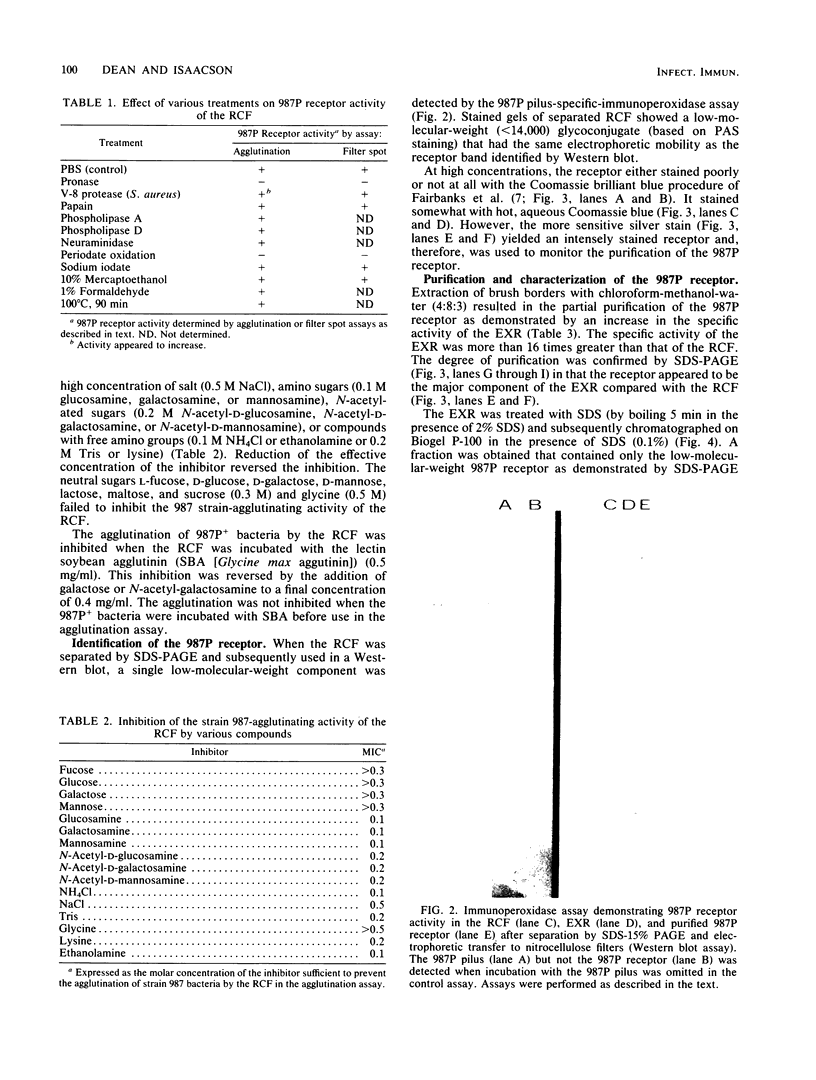
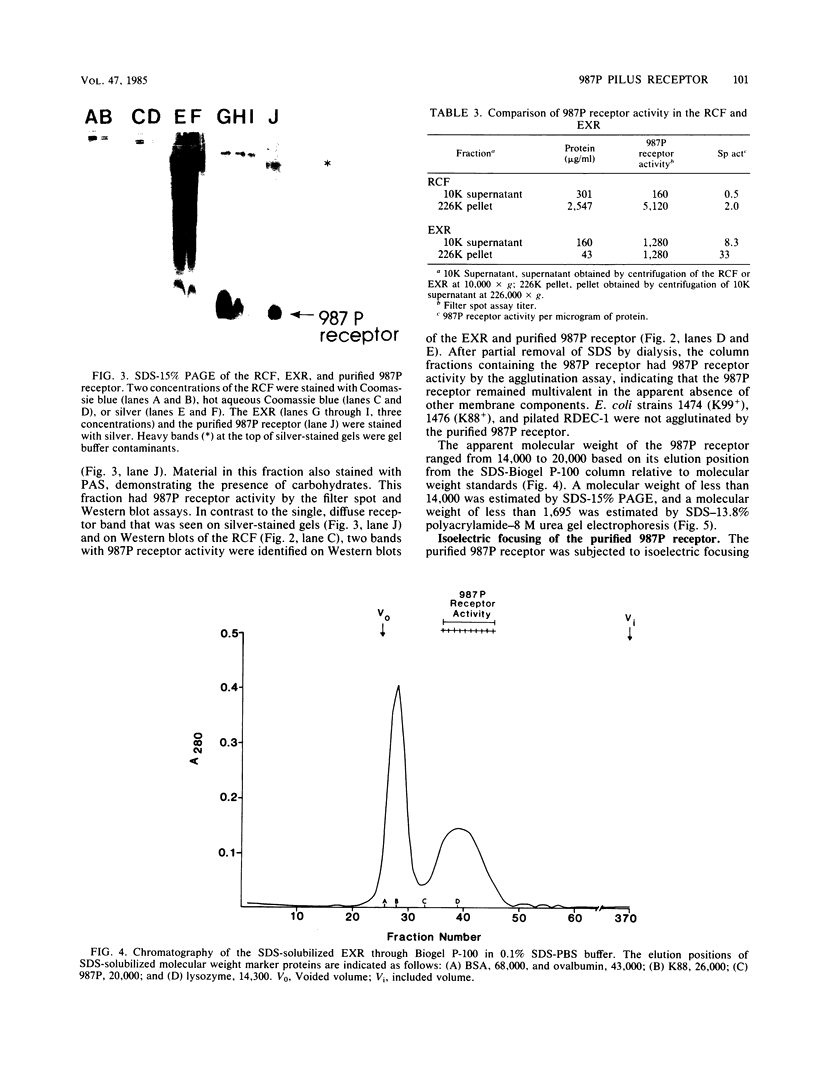
A receptor-containing fraction that caused visible aggregation of piliated Escherichia coli strain 987 bacteria was released into solution when brush borders from adult rabbit small intestines were stored. The receptor-containing fraction was separated into more than 50 bands by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, 4 of which contained carbohydrate as demonstrated by staining with periodic acid-Schiff. Receptor activity in the receptor-containing fraction was associated with a low-molecular-weight (less than 14,000) glycoprotein as measured by Western blots. Extraction of brush borders with chloroform-methanol-water (4:8:3) resulted in increased pilus 987P receptor activity in the aqueous extract. Gel filtration chromatography of sodium dodecyl sulfate-solubilized aqueous extract yielded a fraction with 987P receptor activity that contained only the low-molecular-weight glycoprotein. The purified 987P receptor contained 81% carbohydrate and 19% amino acids by weight. Isoelectric focusing separated the 987P receptor receptor into 3 peaks corresponding to isoelectric points of 2.2, 3.8, and 4.1. Each of the peaks contained 987P receptor activity. The 987P receptor activity was sensitive to periodate oxidation and to digestion with pronase. The strain 987-agglutinating activity of the receptor was inhibited by amino sugars and their N-acetylated derivatives, by compounds having a free amino group, by high concentrations of salt, and by lectins that recognize D-galactose or L-fucose.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asato N., Rand A. G., Jr Resolution of prorennin and rennin by polyacrylamide gel electrophoresis. Anal Biochem. 1971 Nov;44(1):32–41. doi: 10.1016/0003-2697(71)90342-3. [DOI] [PubMed] [Google Scholar]
- Dean E. A., Isaacson R. E. In vitro adhesion of piliated Escherichia coli to small intestinal villous epithelial cells from rabbits and the identification of a soluble 987P pilus receptor-containing fraction. Infect Immun. 1982 Jun;36(3):1192–1198. doi: 10.1128/iai.36.3.1192-1198.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fletcher M. A., Brunschwig J. P., Lo H., Caldwell K. E., Lo T. M. Biochemical and morphological properties of bovine erythrocyte membrane glycoproteins. J Cell Biochem. 1982;19(2):157–170. doi: 10.1002/jcb.240190206. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M., Jr Glycoproteins of cell surfaces. A comparative study of three different cell surfaces of the rat. J Biol Chem. 1971 Oct 25;246(20):6339–6346. [PubMed] [Google Scholar]
- Isaacson R. E. K99 surface antigen of Escherichia coli: purification and partial characterization. Infect Immun. 1977 Jan;15(1):272–279. doi: 10.1128/iai.15.1.272-279.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Richter P. Escherichia coli 987P pilus: purification and partial characterization. J Bacteriol. 1981 May;146(2):784–789. doi: 10.1128/jb.146.2.784-789.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidroni G., Spiro M. J., Spiro R. G. Studies on thyroid cell surface glycoproteins: isolation of plasma membranes and characterization of carbohydrate units. Arch Biochem Biophys. 1980 Aug;203(1):151–160. doi: 10.1016/0003-9861(80)90163-0. [DOI] [PubMed] [Google Scholar]
- Koop D. R., Morgan E. T., Tarr G. E., Coon M. J. Purification and characterization of a unique isozyme of cytochrome P-450 from liver microsomes of ethanol-treated rabbits. J Biol Chem. 1982 Jul 25;257(14):8472–8480. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leach B. S., Collawn J. F., Jr, Fish W. W. Behavior of glycopolypeptides with empirical molecular weight estimation methods. 1. In sodium dodecyl sulfate. Biochemistry. 1980 Dec 9;19(25):5734–5741. doi: 10.1021/bi00566a011. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- MORAWIECKI A. DISSOCIATION OF M- AND N-GROUP MUCOPROTEINS INTO SUBUNITS IN DETERGENT SOLUTIONS. Biochim Biophys Acta. 1964 Nov 1;83:339–347. doi: 10.1016/0926-6526(64)90012-6. [DOI] [PubMed] [Google Scholar]
- Moon H. W., Isaacson R. E., Pohlenz J. Mechanisms of association of enteropathogenic Escherichia coli with intestinal epithelium. Am J Clin Nutr. 1979 Jan;32(1):119–127. doi: 10.1093/ajcn/32.1.119. [DOI] [PubMed] [Google Scholar]
- Perini F., Peters B. P. Fluorometric analysis of amino sugars and derivatized neutral sugars. Anal Biochem. 1982 Jul 1;123(2):357–363. doi: 10.1016/0003-2697(82)90458-4. [DOI] [PubMed] [Google Scholar]
- Reinwald E., Rautenberg P., Risse H. J. Purification of the variant antigens of Trypanosoma congolense: a new approach to the isolation of glycoproteins. Biochim Biophys Acta. 1981 Mar 27;668(1):119–131. doi: 10.1016/0005-2795(81)90155-0. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins: their biochemistry, biology and role in human disease (first of two parts). N Engl J Med. 1969 Oct 30;281(18):991–contd. doi: 10.1056/NEJM196910302811806. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- le Maire M., Rivas E., Møller J. V. Use of gel chromatography for determination of size and molecular weight of proteins: further caution. Anal Biochem. 1980 Jul 15;106(1):12–21. doi: 10.1016/0003-2697(80)90112-8. [DOI] [PubMed] [Google Scholar]