Abstract
It has previously been observed that during isometric dorsiflexion exercise, the time course of T2-weighted signal intensity (SI) changes is spatially heterogeneous. The purpose of this study was to test the hypothesis that this spatially heterogeneity would increase at higher contraction intensities. Eight subjects performed 90s isometric dorsiflexion contractions at 30 and 60% of maximum voluntary contraction (MVC) while T2-weighted (TR/TE=4000/35ms) images were acquired. SI was measured before, during, and after the contractions in regions of interest (ROIs) in the extensor digitorium longus (EDL) muscle and the deep and superficial compartments of the tibialis anterior (D-TA and S-TA, respectively). For all ROIs at 30% MVC, SI changes were similar. The maximum post-contraction SI was greater than the SI during exercise. At 60% MVC, SI changes during contraction were greater in the S-TA than in the DTA and EDL. For the EDL and D-TA, the maximum post-contraction SI was greater than those during exercise. For the S-TA, the maximum post-contraction change was greater than the changes at t=8, 20, and 56 s, but not the end-exercise value. We conclude that spatial heterogeneity increases during more intense dorsiflexion contractions, possibly reflecting regional differences in perfusion or neural activation of the muscle.
Keywords: human, isometric, perfusion, neural activation, T2
INTRODUCTION
An increasingly common finding from imaging studies of in vivo muscle function is that many of the physiological, biochemical, and mechanical properties associated with exercise are spatially heterogeneous. For example, in the triceps surae muscle group, the mechanical strain characteristics (1–4), pennation angle changes (5), and hemodynamic and metabolic responses (5,6) during contraction are all spatially non-uniform. These spatial heterogeneities may result from locally varying structural features (such as pennation angles (7) or non-uniform tendon material properties), histological features (such as regional variations in muscle fiber type or capillary density), patterns of neural activation (8)), and/or some combination of these.
The dorsiflexors, which are the target of the present investigation, have not been studied extensively using imaging techniques capable of absolutely quantifying physiological variables such as blood flow. However, a recent 31P MRS study indicated that during sustained isometric contractions at 30% of maximum voluntary contraction (MVC), the pH changes experienced by the primary dorsiflexor (the tibialis anterior (TA) muscle) are spatially heterogeneous (9). In part, this pH heterogeneity was related to the presence of oxidative and glycolytic muscle fiber types. The heterogeneity may have also been related to differences in the compliance of the tissue of muscle fiber origin, as the fibers originating on the tibia experienced greater pH changes than those originating on the fascia of the anterior compartment. This was proposed to affect the pH change through a relatively greater restriction of blood flow in the deep portion of the muscle, producing an increased reliance on anaerobic glycolysis.
Muscle functional magnetic resonance imaging (mfMRI) exploits the increases in the transverse relaxation time constant (T2) that occur during exercise. The T2 change of exercise occurs secondarily to increased rates of cellular energy metabolism (10–12) and changes in blood volume and oxygenation (13,14). The metabolic basis of T2 changes lies in the intracellular accumulation of osmolytes such as inorganic phosphate and lactate, which draw water into the cell along osmotic pressure gradients (10,12). Intracellular T2 increases may further depend on the acidification caused by the accumulation of certain glycolytic intermediates and end-products, especially lactic acid (10,15). The metabolic factors produce a large magnitude, but slowly evolving, increase in the intracellular T2. In contrast to the large T2 increases related to metabolism, blood oxygenation-level dependent (BOLD) contrast makes a small, rapid, and negative contribution to the T2 change of exercise (13).
In typical analyses of mfMRI data, regions of interest are specified in images obtained following whole-body exercise (16,17) or a series of contractions of an individual group of muscles (8), revealing patterns of signal intensity change that generally agree with electromyographic measures of motor activation (8). While post-exercise T2-weighted SI changes are useful as a non-invasive indication of the spatial pattern of muscle activation during a previous bout of exercise, it has also been shown that post-exercise T2-weighted SI changes may not fully capture the spatially heterogeneous functional properties of exercising muscles (18). For such applications, it may be necessary to examine the time course of SI changes, obtained during the exercise bout itself (18,19). For instance, regional variations in the mfMRI SI time course obtained during sustained isometric dorsiflexion exercise at 40% of MVC have been observed in the anterior compartment muscles that may reflect heterogeneity in the metabolic, hemodynamic, and/or mechanical responses to neural activation (18).
In this work, we examine the T2-weighted SI changes in the anterior compartment of the leg in response to sustained isometric dorsiflexion at 30 and 60% of MVC, testing the hypothesis that the spatial heterogeneity in the T2-weighted SI response would be greater at higher contraction intensities than at low contraction intensities. The results were consistent with this hypothesis and suggest that differences in neural activation, perfusion, or both exist within the TA muscle and that they are greater during moderate than low intensity contractions. A paper describing other aspects of this experiment has previously been published (13).
METHODS
Subjects
The experimental procedures described below were approved by the Vanderbilt University Institutional Review Board. Eight subjects (4 male, 4 female), each with no known history of cardiovascular, endocrine, metabolic, neurological, or neuromuscular disorders, participated in the study. The subjects had mean (standard error of the mean, SEM) age=22.5 (0.4) yrs, height=177.2 (5.0) cm, and mass=73.3 (6.2) kg. After receiving an explanation of the potential risks and benefits of participation in the study, the subjects provided written informed consent to participate.
Experimental Protocol
The subjects reported to the lab for orientation and testing sessions. During the orientation session, health and MRI screening procedures were performed, informed consent was received, the MVC force was measured, and the contraction protocol was rehearsed. To measure the MVC force, the subject performed two or more 3 second isometric dorsiflexion contractions. After accounting for the baseline force offset, the greatest force produced during each contraction was recorded. Contractions were performed until the maximum forces recorded during two contractions were within 5% of each other; the higher of the two values was then recorded as the MVC force. The subject then rehearsed the contraction protocol, using feedback from a computer screen to maintain a desired submaximal force level (see below).
During the testing sessions, the subject performed sustained isometric dorsiflexion contractions at either 30 or 60% of MVC (one per day) while mfMRI data were acquired (see below). The order of the contraction intensities was randomized. The subjects were instructed not to consume caffeine or use tobacco during the six hours prior to a test session and not to use alcohol or perform moderate or heavy physical activity for at least 24 hours prior to each session. To ensure compliance with these instructions, the subjects completed a survey of their diet and lifestyle activities covering this period, and a test session was postponed in the event of noncompliance.
Isometric Contractions
Contraction Protocol
All exercise involved isometric dorsiflexion of the subject’s self-reported dominant foot. The subject lay supine with his/her foot strapped into a home-built isometric exercise device. During the orientation session, the exercise device was bolted to a grid plate attached to an exercise table; for test sessions, the device was bolted to a similar grid plate mounted on the patient bed of the imager. The ankle angle was 90° and the knee was supported by a bolster and slightly flexed (angle of leg flexion ~7.5°). The foot was firmly strapped to the exercise device using 3.8 cm wide nylon straps; to limit the involvement of the toe extensors, the straps were placed across the foot proximal to the base of the fifth digit. To eliminate hydrostatic effects on the circulation, the heart was at approximately the same level as the TA muscle. The imaging session began with the acquisition of scout and anatomical images. Following these, the subjects performed one 90 s isometric dorsiflexion contraction at either 30 or 60% of MVC. The subjects were verbally encouraged to maintain the target force throughout the contraction.
Force Data Acquisition and Analysis
The force acquisition system has been described in detail previously (20,21). Briefly, the isometric dorsiflexion device included an Interface Force (Interface Force, Scottsdale AZ) Model SSM-AJ-500 load cell, the signals from which were amplified and conditioned using a bridge amplifier (Model SGA/A, Interface Force) and then transmitted to an analog-to-digital conversion card (Model 6036E, National Instruments, Austin TX) via a serial connector box (Model SCB-68, National Instruments). Custom data acquisition software, written in LabVIEW 7.1 (National Instruments), was used to collect force data at 1 kHz and provide real-time, 20 Hz visual feedback to the subjects in the form of a simulated LED panel on a computer screen (during the orientation session) or on a pair of MRI-compatible LCD goggles (for MRI testing sessions). The baseline force offset was measured immediately prior to the contraction and accounted for when calculating relative force production. The force data from the submaximal contractions were filtered at 25 Hz using a low-pass, fifth-order Butterworth filter. To correspond to the MRI data analysis described below, the average force production was calculated during 4 s windows centered on contraction durations of 8 s, 20 s, 56 s, and 88 s.
MRI Data Acquisition and Analysis
MRI data were obtained on a 3T Philips Intera Achieva MR imager/spectrometer. After positioning the foot in the exercise device, the subject contracted the dorsiflexors. During the contraction, the maximum cross-sectional area of the TA muscle was identified visually and by palpation. A 6-channel sensitivity encoding (SENSE) RF torso coil was then positioned around both legs. The subject was advanced into the magnet such that the area of interest was at the magnet’s isocenter. The imaging procedures began with the acquisition of 3-plane gradient-echo scout images, used to verify to the location of the TA’s maximum girth. If necessary, the patient bed was moved so that this location was nearer the magnet isocenter. Then a high resolution, T1-weighted anatomical image was obtained using a fast spin-echo sequence and slice thickness=7.5 mm, field of view=18×18 cm, acquired matrix=256×256 (reconstructed matrix=512×512), repetition time (TR)/echo time (TE)=500/18.6 ms, echo-train length=3, and number of excitations (NEX)=2. A volume of interest was specified within the imaged portion of the leg and used for second-order shimming. Finally, mfMRI data were obtained using a T2-weighted spin-echo echo-planar imaging sequence for 20 s before, during, and 60 s after each contraction. The functional images were obtained using the same geometric parameters as the anatomical image, but using acquired matrix=64×64 (reconstructed matrix=128×128), a fat-frequency selective saturation (SPIR) pulse applied immediately before the excitation pulse, NEX=1, 60.3% k-space sampling, and TR/TE=4000/35 ms.
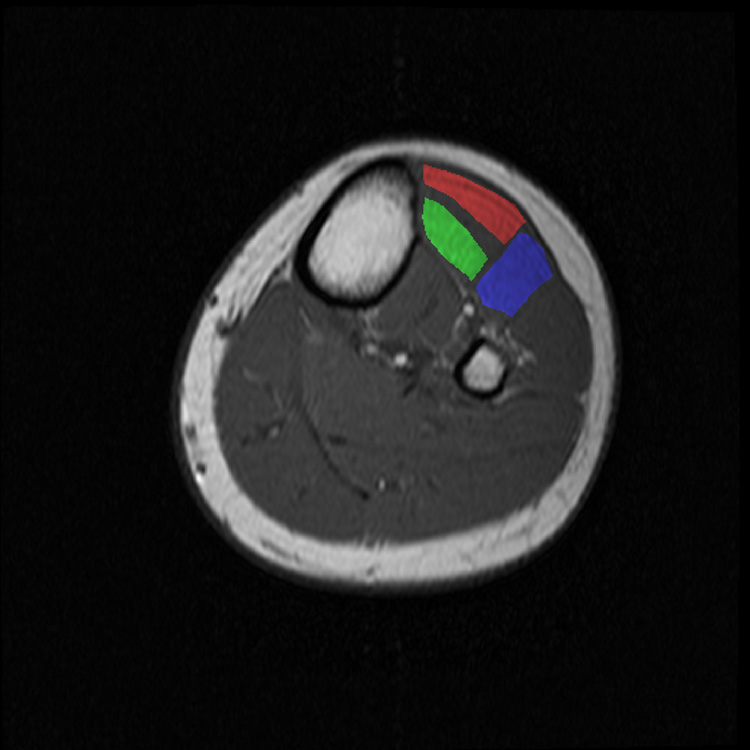
Image analysis was conducted using Matlab v. 7.0.1 (The Mathworks, Natick MA). In the functional images, three ROIs were specified: one around the deep compartment of the TA muscle (D-TA), one around the superficial compartment of the TA muscle (S-TA), and one in the extensor digitorum longus (EDL) muscle (Figure 1). Resolved vessels were excluded from the ROIs, and reproducible anatomical features (bone, subcutaneous fat, and tendons/aponeuroses) were used to define the ROI boundaries. For each ROI, the mean pre-contraction SI values were calculated and the subsequent values were normalized to this value. If necessary, the ROI position was manually adjusted to account for subject motion during contraction. For statistical purposes, the SI values in the images acquired at exercise durations (t) of 8, 20, 56, and 88 s were considered of particular interest. The t = 8 and t = 20 s time points correspond respectively to the times at which the initial rise and the nadir of the early dip in SI occur. The SI at end-exercise (t = 88 s) was used to characterize the maximum SI change undergone during the contraction, and the midpoint of the early dip and end-exercise times (t = 56 s) was used to characterize the early portion of the secondary rise in SI. Also, the peak post-contraction SI value was recorded for each ROI.
Figure 1.
High-resolution, T1 weighted anatomical with ROI definitions overlaid. The red, green, and blue ROIs represent the S-TA, D-TA, and EDL, respectively.
Statistical Analysis
Statistical analyses were performed using SPSS v. 15 (SPSS, Chicago IL). Descriptive data include the mean and SE. The data were analyzed using the General Linear Model (GLM; Compartment×Intensity×Duration). All factors in the GLM were within-subjects. Statistical comparisons were considered significant at p<0.05.
RESULTS
Force Production
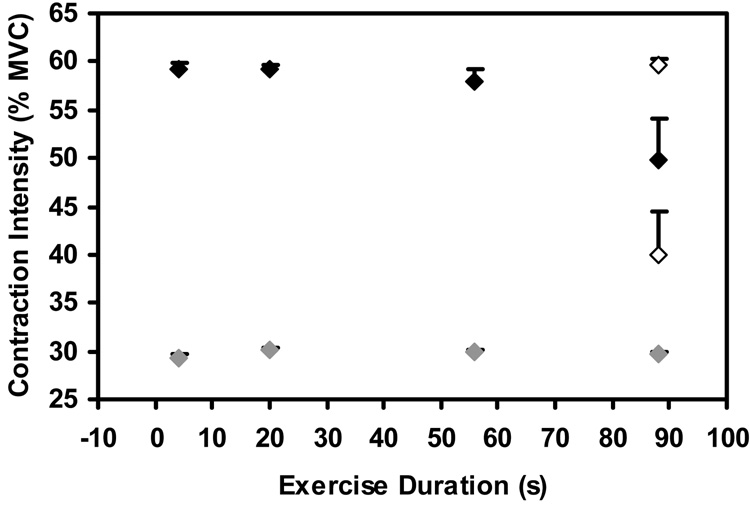
The mean MVC force was 298.6 (SEM 26.8) N. During the sustained contractions at 30% MVC, the subjects were able to maintain the target force level at all exercise durations (p=0.997; Figure 2). During the 60% MVC contractions, the four strongest subjects were unable to maintain the target force at the end of the exercise bout (mean contraction intensity = 40.0; SEM = 4.4 %), while the other four subjects were able to maintain the target force (mean contraction intensity = 59.6; SEM = 0.8 %). Overall, there were no significant changes in force production during the 60% MVC contractions (p=0.307).
Figure 2.
Contraction intensity during 4 s windows centered on exercise durations of 8, 20, 56, and 88 s during the 30% and 60% MVC tasks. Mean, SEM is shown. No significant changes were observed for the group across exercise durations. However, four subjects underwent considerable fatigue (mean intensity at end-exercise=40.0% MVC) and four subjects were able to maintain the target force at end-exercise (mean intensity at end-exercise=59.6% MVC). The responses of these two groups of subjects are shown as the open symbols at t=88 s.
mfMRI Data
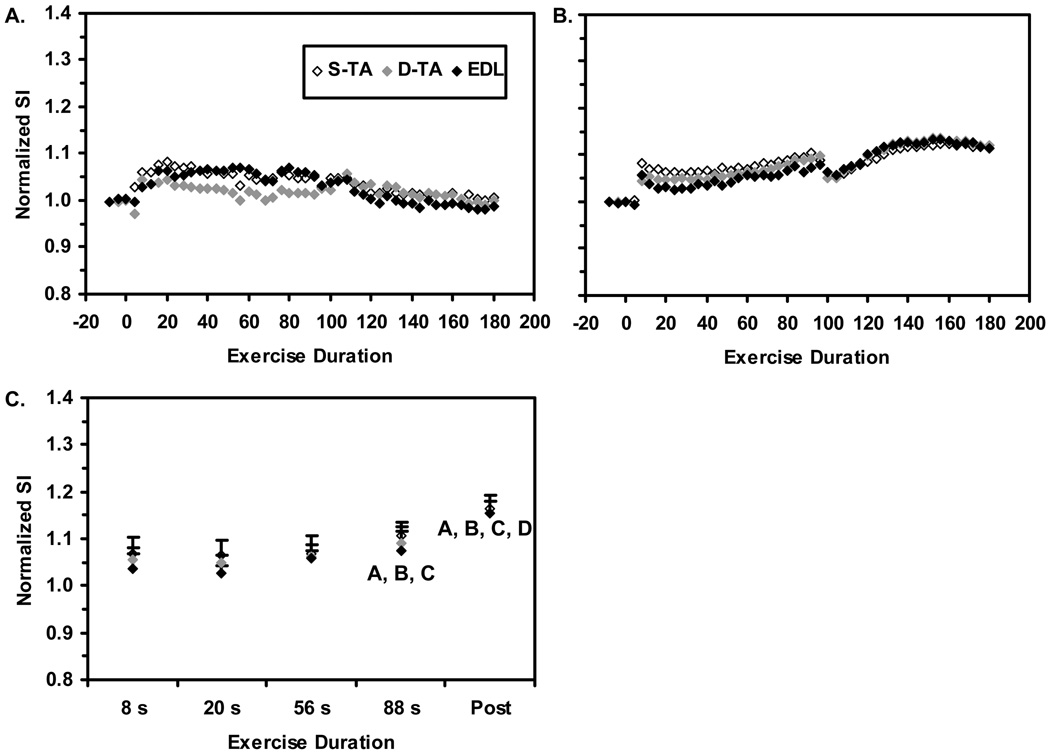
Sample data illustrating the SI changes in the D-TA, S-TA, and EDL ROIs at 30% of MVC are shown in Figure 3A; the mean SI time courses for all 8 subjects are shown in Figure 2B. For clarity, error bars are not shown. In Figure 3C, the results from the GLM are shown. The Duration main effect was significant (p<0.0005). Analysis of the 95% confidence intervals revealed that mean SI value at t=88 s was significantly greater than those at all preceding time points analyzed. Also, the mean post-contraction maximum SI value was greater than the SI values at all preceding time points analyzed. Neither the Compartment main effect nor the Compartment×Duration interaction was significant (p=0.22 and 0.268, respectively), indicating that there were no systematic differences in the SI responses to 30% MVC contractions among the three ROIs.
Figure 3. T2-weighted SI responses to 30% MVC contractions.
A. Sample data from one subject. B. Group-mean data for all subjects; for clarity, error bars are not shown. C. Group-mean data used in the discrete analysis. Error bars represent the SEM. The Duration main effect was significant, and so the symbols (A, B, C, D) represent significant differences between the indicated group mean SI value and the mean value at the t=8, 20, 56, and 88 s exercise durations, respectively. Neither the Compartment main effect nor the Compartment×Duration interaction were significant, and so contrasts indicated by the Duration effect apply equally to all compartments.
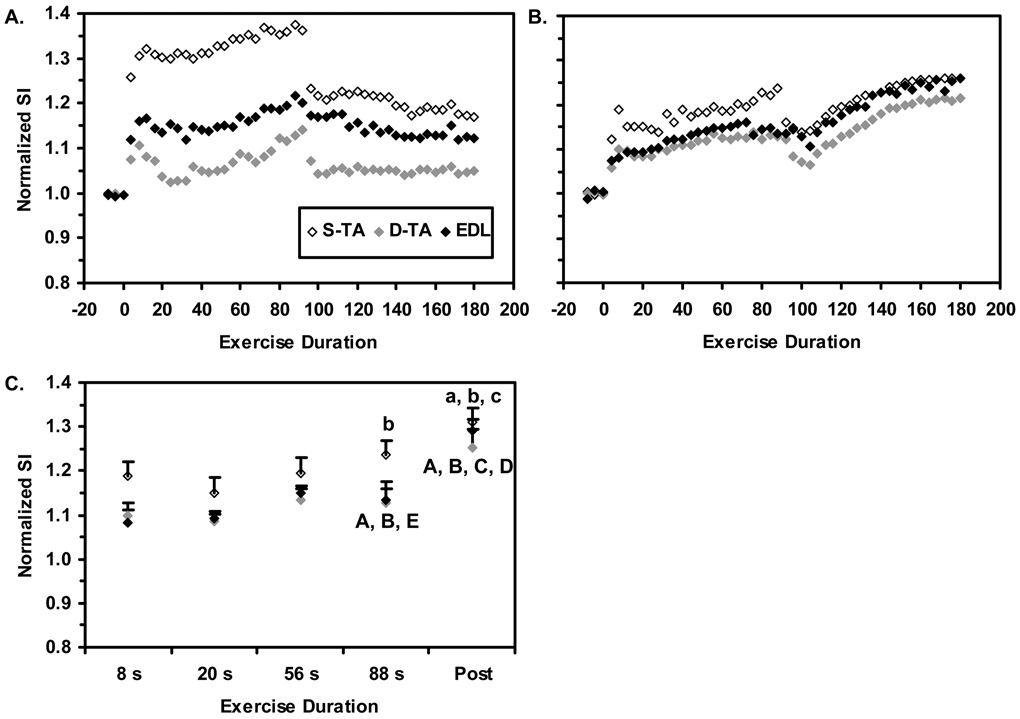
Sample data from the D-TA, S-TA, and EDL ROIs during and after the 60% MVC contractions are shown in Figure 3A; the mean SI time courses are shown in Figure 3B. In Figure 3C, the results from the GLM are shown. The Duration main effect was significant (p<0.0005). Also, the three panels of Figure 4 illustrate a consistent relative ordering of the compartmental SI changes (significant Compartment main effect, p=0.019), with the SI changes in the S-TA being greater than those in the D-TA. Finally, the Compartment×Duration interaction was significant (p=0.022). In the S-TA, the mean SI value at t=88 s was significantly greater than the value at t=20 s. Notably, the maximum-post-contraction SI value was greater than the mean SI values at t=8, 20, and 56 s, but not the value at t=88 s. In the D-TA, the mean SI value at t=88 s was significantly greater than the value at t=8 and t=20 s and significantly lower than the maximum post-contraction SI value. Also, the mean maximum post-contraction SI value was greater than all preceding time points analyzed. An identical pattern was noted in the EDL. The maximum post-contraction values for the three compartments did not differ from each other.
Figure 4. T2-weighted SI responses to 60% MVC contractions.
A. Sample data, for the same subject whose data were illustrated in Figure 3A. B. Group-mean data for all subjects; for clarity, error bars are not shown. C. Group-mean data used in the discrete analysis. Error bars represent the SEM. The Duration and Compartment main effects and the Compartment×Duration interaction were significant, indicating that the SI responses in the S-TA differed from those of the EDL and D-TA ROIs. The symbols (A, B, C, D, E) represent significant differences between the indicated group mean EDL and D-TA SI values and the corresponding value at the t=8, 20, 56, 88 s, and post-contraction exercise durations, respectively. The symbols (a, b, c) represent significant differences between the indicated group mean S-TA SI value and the values at the t=8, 20, and 56 s exercise durations, respectively.
DISCUSSION
The principal finding of this study was that spatial heterogeneity exists in the mfMRI SI time courses obtained from the anterior compartment muscles during sustained, 60% MVC isometric dorsiflexion exercise, with larger SI changes occurring in the S-TA than in the D-TA and the EDL. Qualitatively, this finding is similar to that of Akima et al. (19) in their study of intermittent isotonic dorsiflexion exercise and to that of our previous study of sustained isometric dorsiflexion at 40% MVC (18). Moreover, this study demonstrates that this spatial heterogeneity is exercise intensity-dependent, because it exists during contractions at 60% of MVC but not during contractions at 30% of MVC. Finally, the findings confirm those of our previous study, in which we observed heterogeneity in the mfMRI SI time course during exercise, but not following exercise (18).
Given the underlying theoretical basis for the mfMRI SI time course (14), these findings suggest metabolic and/or hemodynamic heterogeneity across the anterior compartment during moderate intensity exercise. This heterogeneity may reflect different levels of neural activation between the muscle compartments or from different metabolic and hemodynamic responses to similar levels of neural activation. As we consider these possibilities, we focus our attention on the heterogeneity in the TA muscle, because this muscle is the main dorsiflexor and there are sufficient fiber type differences between the TA and EDL muscles (22) as to preclude a direct comparison of the T2-weighted SI responses.
Preferential Recruitment of the TA's Superficial Compartment during Moderate Intensity Exercise
This explanation requires that two assumptions be valid. The first is that there is separate neural control of the D-TA and S-TA, for which support exists from a study of cadaveric muscle by Wolf and Kim (23). The second necessary assumption is that the metabolic and hemodynamic determinants of the T2 change respond similarly to equally intense activation of the S-TA and D-TA. Given the demonstrated importance of muscle fiber type to the T2 change of exercise (12), it is noteworthy that the Type I/Type II fiber composition of the D-TA and STA is similar (22). This suggests that the metabolic component of the T2-weighted SI change would be similar for similar levels of neural activation. Also, we presume that since the fiber type composition is similar, the capillary density would also be similar in both muscle compartments (although we were not able to find data in the literature either to support or refute this presumption). Modeling data that we have previously published suggest that extravascular BOLD effects are small at 3T (13), and so any differences in capillary orientation with respect to the B0 field in the two compartments would have small effects (and perhaps immeasurably so). In summary, the necessary histological conditions exist for there to be similar T2 changes in response to equal levels of neural activation.
The larger T2-weighted SI change in the S-TA at 60% MVC would then represent a preferential recruitment of this compartment. This conclusion is consistent with the generally direct relationship between the T2 change and exercise intensity previously observed (17,24,25) and with the good correlations between electromyographic measures of muscle activation and T2 changes (26,27). A preferential recruitment of the S-TA during moderate intensity contractions might exist for the following reason. We have noticed in unpublished findings from other studies that the volume of the S-TA is smaller than that of the D-TA. Also, at the muscle's maximum cross-sectional area, the pennation angles and fascicle lengths in the two compartments are similar at rest (28,29). The volume difference would therefore create a difference in the physiological cross-sectional areas of the two compartments, with the S-TA having a lower absolute force production capability than the D-TA. If both compartments were equally activated, a force imbalance would result, creating shear strain along the TA's central aponeurosis. This effect would be small during low-intensity contractions, and so it would not be necessary for the nervous system to activate the two compartments differentially. At 60% MVC, however, the hypothesized greater activation level of the S-TA would account for the difference in physiological cross-sectional area and reduce shear strain along the aponeurosis. Consistent with this, the pennation angle and fascicle length changes in the S-TA and the D-TA during contraction are similar (28).
Perfusion Restriction in the Deep Compartment of the TA Muscle
Despite the teleological sensibility of the differential recruitment hypothesis, the data are also open to a considerably different interpretation. A key structural difference between the S-TA and the D-TA is that the fibers of the S-TA originate on the fascia overlying the anterior compartment, while those of the D-TA originate on the tibia. Although the anterior compartment fascia is less compliant than other muscle fascial coverings (contributing to the etiology of compartment syndrome), it is still considerably more compliant than the tibia. One consequence of this structural difference is that a relatively higher level of intramuscular pressure would develop in the D-TA than in the S-TA. The upper limit of intramuscular pressures during contraction of the TA muscle is ~200 mmHg (30), and those pressures above systolic blood pressure will occlude arterial flow. Elevated intramuscular pressures, such as those during compartment syndrome, reduce blood flow and elevate levels of the end-products of anaerobic metabolism (31).
At first inspection, this would seem to result in an increased T2 response for a given level of neural activation. For instance, the osmotically driven water entry into cells that is responsible for much of the T2 change (10,12) would be enhanced with higher metabolite accumulation. The assumptions underlying this statement are that 1) the muscle cell membrane is a perfect osmometer (32) and 2) the cells have access to an essentially infinite reservoir of fluid in the vascular system. When both of these conditions exist, water will move along the osmotic pressure gradient generated by lactate and inorganic phosphate accumulation and maintain the intracellular osmolarity at its normal value, without affecting the water concentration in the infinite reservoir. However, in a flow-restricted condition such as that expected to exist in the DTA, the infinite reservoir condition would not be met and osmotically determined T2 changes would be correspondingly reduced. Consistent with this, in exercising muscles in which blood flow is restricted by proximal arterial ligation, muscle volume changes and T2 responses do not become maximal until flow is restored (12). Therefore, perfusion restriction in the D-TA could result in a smaller SI change in this compartment than in the S-TA, even when activation and metabolite levels in the two compartments are similar.
Which Explanation is Correct?
Given the many and interrelated factors that determine the T2 change of exercise, there is insufficient information in the data to reject one of the hypotheses outright (and in fact the hypotheses are not mutually incompatible). However, for three reasons the post-contraction responses are generally more consistent with the perfusion restriction hypothesis than the differential recruitment hypothesis. First, when electromyogaphy data indicate that differences in neural activation do exist among muscles (27) or within a muscle during different contraction types and intensities (26), the post-contraction T2 value is sufficiently sensitive to detect this. However, there were no differences in the maximum post-contraction SI values among the three ROIs at either exercise intensity. Also, the maximum SI value in the D-TA following 60% MVC contractions was significantly larger than the end-exercise value, suggesting a prior perfusion restriction in a manner similar to the Prior et al. arterial ligation study (12). Houtman et al. attributed their observation of spatial heterogeneity in pH in the TA muscle to this mechanism as well (9). In contrast, the statistical similarity of the end-and post-exercise SI values for the STA ROI at 60% MVC suggests that perfusion to this compartment was sufficient to allow unrestricted water movement during the contraction. Finally, the larger pH changes in the deep portion of the TA muscle reported by Houtman et al (9) are more consistent with perfusion restriction in the D-TA than with preferential recruitment of the S-TA.
Summary and Conclusions
In this study, we set out to determine the extent to which the spatially heterogeneous T2 responses that we have previously observed in the anterior compartment depend on exercise intensity. Taking the present observations at 30 and 60% MVC and those of our previous study at 40% MVC (18) into account together, we conclude spatial heterogeneity in T2 does not exist or is below the level of detection during sustained isometric contractions performed at 30% MVC, but does exist during contractions at 40 and 60% of MVC. The physiological origin for the spatial heterogeneity – differential recruitment, spatially dependent perfusion restrictions, or both – cannot be definitively stated. However, the data are generally more consistent with the hypothesis that there is a spatially dependent pattern to intramuscular pressure generation during isometric contractions of the TA muscle, producing a greater level of perfusion restriction in the deep portion of the muscle. These findings, in concert with previous findings in the TA muscle (9, 18, 19) and from the triceps surae group (1–6), emphasize that the assumption of homogeneous mechanical and physiological responses to muscle contraction within individual muscles made in many biomechanical and metabolic studies needs to be carefully examined. Moreover, the questions raised in this study about the origin of the T2-weighted SI heterogeneity indicate that the assumption that T2 changes reflect neural activation in a simple and direct manner needs validation on an individual muscle/exercise paradigm basis as well.
ACKNOWLEDGMENTS
We thank Robin Avison, RT(N, MR), CNMT and Donna Butler, RT(R, MR) for their assistance with data collection.
Grant support: NIH/NIAMS R01 AR050101, NIH/NCRR M01 RR00095
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Finni T, Hodgson JA, Lai AM, Edgerton VR, Sinha S. Nonuniform strain of human soleus aponeurosis-tendon complex during submaximal voluntary contractions in vivo. J Appl Physiol. 2003;95:829–837. doi: 10.1152/japplphysiol.00775.2002. [DOI] [PubMed] [Google Scholar]
- 2.Finni T, Hodgson JA, Lai AM, Edgerton VR, Sinha S. Mapping of movement in the isometrically contracting human soleus muscle reveals details of its structural and functional complexity. J Appl Physiol. 2003;95:2128–2133. doi: 10.1152/japplphysiol.00596.2003. [DOI] [PubMed] [Google Scholar]
- 3.Hodgson JA, Finni T, Lai AM, Edgerton VR, Sinha S. Influence of structure on the tissue dynamics of the human soleus muscle observed in MRI studies during isometric contractions. J Morphol. 2006;267:584–601. doi: 10.1002/jmor.10421. [DOI] [PubMed] [Google Scholar]
- 4.Lee HD, Finni T, Hodgson JA, Lai AM, Edgerton VR, Sinha S. Soleus aponeurosis strain distribution following chronic unloading in humans: an in vivo MR phase-contrast study. J Appl Physiol. 2006;100:2004–2011. doi: 10.1152/japplphysiol.01085.2005. [DOI] [PubMed] [Google Scholar]
- 5.Miura H, McCully K, Hong L, Nioka S, Chance B. Regional difference of muscle oxygen saturation and blood volume during exercise determined by near infrared imaging device. Jpn J Physiol. 2001;51:599–606. doi: 10.2170/jjphysiol.51.599. [DOI] [PubMed] [Google Scholar]
- 6.Richardson RS, Haseler LJ, Nygren AT, Bluml S, Frank LR. Local perfusion and metabolic demand during exercise: a noninvasive MRI method of assessment. J Appl Physiol. 2001;91:1845–1853. doi: 10.1152/jappl.2001.91.4.1845. [DOI] [PubMed] [Google Scholar]
- 7.Agur AM, Ng-Thow-Hing V, Ball KA, Fiume E, McKee NH. Documentation and three-dimensional modelling of human soleus muscle architecture. Clin Anat. 2003;16:285–293. doi: 10.1002/ca.10112. [DOI] [PubMed] [Google Scholar]
- 8.Price TB, McCauley TR, Duleba AJ, Wilkens KL, Gore JC. Changes in magnetic resonance transverse relaxation times of two muscles following standardized exercise. Med Sci Sports Exerc. 1995;27:1421–1429. [PubMed] [Google Scholar]
- 9.Houtman CJ, Heerschap A, Zwarts MJ, Stegeman DF. pH heterogeneity in tibial anterior muscle during isometric activity studied by 31P-NMR spectroscopy. J Appl Physiol. 2001;91:191–200. doi: 10.1152/jappl.2001.91.1.191. [DOI] [PubMed] [Google Scholar]
- 10.Damon BM, Gregory CD, Hall KL, Stark HJ, Gulani V, Dawson MJ. Intracellular acidification and volume increases explain R2 decreases in exercising muscle. Magn Reson Med. 2002;47:14–23. doi: 10.1002/mrm.10043. [DOI] [PubMed] [Google Scholar]
- 11.Ploutz-Snyder LL, Nyren S, Cooper TG, Potchen EJ, Meyer RA. Different effects of exercise and edema on T2 relaxation in skeletal muscle. Magn Reson Med. 1997;37:676–682. doi: 10.1002/mrm.1910370509. [DOI] [PubMed] [Google Scholar]
- 12.Prior BM, Ploutz-Snyder LL, Cooper TG, Meyer RA. Fiber type and metabolic dependence of T2 increases in stimulated rat muscles. J Appl Physiol. 2001;90:615–623. doi: 10.1152/jappl.2001.90.2.615. [DOI] [PubMed] [Google Scholar]
- 13.Damon B, Wadington M, Hornberger J, Lansdown D. Absolute and relative contributions of BOLD effects to the muscle functional MRI signal intensity time course: Effect of exercise intensity. Magn Reson Med. 2007 doi: 10.1002/mrm.21319. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damon BM, Gore JC. Physiological basis of muscle functional MRI: predictions using a computer model. J Appl Physiol. 2005;98:264–273. doi: 10.1152/japplphysiol.00369.2004. [DOI] [PubMed] [Google Scholar]
- 15.Fung BM, Puon PS. Nuclear magnetic resonance transverse relaxation in muscle water. Biophys J. 1981;33:27–37. doi: 10.1016/S0006-3495(81)84870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hug F, Bendahan D, Le Fur Y, Cozzone PJ, Grelot L. Heterogeneity of muscle recruitment pattern during pedaling in professional road cyclists: a magnetic resonance imaging and electromyography study. Eur J Appl Physiol. 2004;92:334–342. doi: 10.1007/s00421-004-1096-3. [DOI] [PubMed] [Google Scholar]
- 17.Reid RW, Foley JM, Jayaraman RC, Prior BM, Meyer RA. Effect of aerobic capacity on the T2 increase in exercised skeletal muscle. J Appl Physiol. 2001;90:897–902. doi: 10.1152/jappl.2001.90.3.897. [DOI] [PubMed] [Google Scholar]
- 18.Damon BM, Wigmore DM, Ding Z, Gore JC, Kent-Braun JA. Cluster analysis of muscle functional MRI data. J Appl Physiol. 2003;95:1287–1296. doi: 10.1152/japplphysiol.00178.2003. [DOI] [PubMed] [Google Scholar]
- 19.Akima H, Ito M, Yoshikawa H, Fukunaga T. Recruitment plasticity of neuromuscular compartments in exercised tibialis anterior using echo-planar magnetic resonance imaging in humans. Neurosci Lett. 2000;296:133–136. doi: 10.1016/s0304-3940(00)01644-x. [DOI] [PubMed] [Google Scholar]
- 20.Damon BM, Hornberger JL, Wadington MC, Lansdown DA, Kent-Braun JA. Dual gradient-echo MRI of post-contraction changes in skeletal muscle blood volume and oxygenation. Magn Reson Med. 2007;47:670–679. doi: 10.1002/mrm.21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maguire MA, Weaver TW, Damon BM. Delayed blood reoxygenation following maximum voluntary contraction. Medicine and Science in Sports and Exercise. 2007;39:257–267. doi: 10.1249/01.mss.0000246990.25858.47. [DOI] [PubMed] [Google Scholar]
- 22.Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973;18:111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- 23.Wolf SL, Kim JH. Morphological analysis of the human tibialis anterior and medial gastrocnemius muscles. Acta Anat (Basel) 1997;158:287–295. doi: 10.1159/000147942. [DOI] [PubMed] [Google Scholar]
- 24.Fisher MJ, Meyer RA, Adams GR, Foley JM, Potchen EJ. Direct relationship between proton T2 and exercise intensity in skeletal muscle MR images. Invest Radiol. 1990;25:480–485. doi: 10.1097/00004424-199005000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Jenner G, Foley JM, Cooper TG, Potchen EJ, Meyer RA. Changes in magnetic resonance images of muscle depend on exercise intensity and duration, not work. J Appl Physiol. 1994;76:2119–2124. doi: 10.1152/jappl.1994.76.5.2119. [DOI] [PubMed] [Google Scholar]
- 26.Adams GR, Duvoisin MR, Dudley GA. Magnetic resonance imaging and electromyography as indexes of muscle function. J Appl Physiol. 1992;73:1578–1583. doi: 10.1152/jappl.1992.73.4.1578. [DOI] [PubMed] [Google Scholar]
- 27.Price TB, Kamen G, Damon BM, Knight CA, Applegate B, Gore JC, Eward K, Signorile JF. Comparison of MRI with EMG to study muscle activity associated with dynamic plantar flexion. Magn Reson Imaging. 2003;21:853–861. doi: 10.1016/s0730-725x(03)00183-8. [DOI] [PubMed] [Google Scholar]
- 28.Maganaris CN, Baltzopoulos V. Predictability of in vivo changes in pennation angle of human tibialis anterior muscle from rest to maximum isometric dorsiflexion. Eur J Appl Physiol Occup Physiol. 1999;79:294–297. doi: 10.1007/s004210050510. [DOI] [PubMed] [Google Scholar]
- 29.Lansdown DA, Ding Z, Wadington M, Hornberger JL, Damon BM. Quantitative diffusion tensor MRI-based fiber tracking of human skeletal muscle. J Appl Physiol. 2007;103:673–681. doi: 10.1152/japplphysiol.00290.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sejersted OM, Hargens AR, Kardel KR, Blom P, Jensen O, Hermansen L. Intramuscular fluid pressure during isometric contraction of human skeletal muscle. J Appl Physiol. 1984;56:287–295. doi: 10.1152/jappl.1984.56.2.287. [DOI] [PubMed] [Google Scholar]
- 31.Qvarfordt P, Christenson JT, Eklof B, Ohlin P, Saltin B. Intramuscular pressure, muscle blood flow, and skeletal muscle metabolism in chronic anterior tibial compartment syndrome. Clin Orthop Relat Res. 1983;179:284–290. [PubMed] [Google Scholar]
- 32.Dydynska M, Wilkie DR. The osmotic properties of striated muscle fibers in hypertonic solutions. J Physiol. 1963;169:312–329. doi: 10.1113/jphysiol.1963.sp007258. [DOI] [PMC free article] [PubMed] [Google Scholar]