Abstract
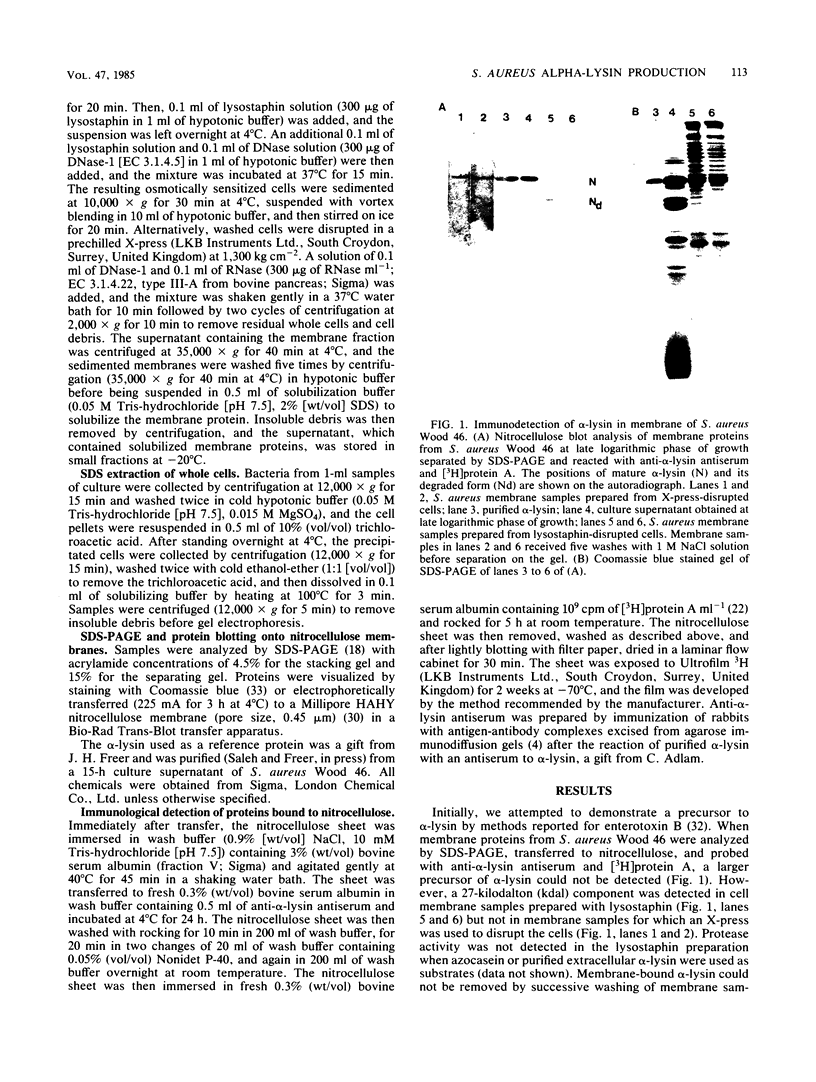
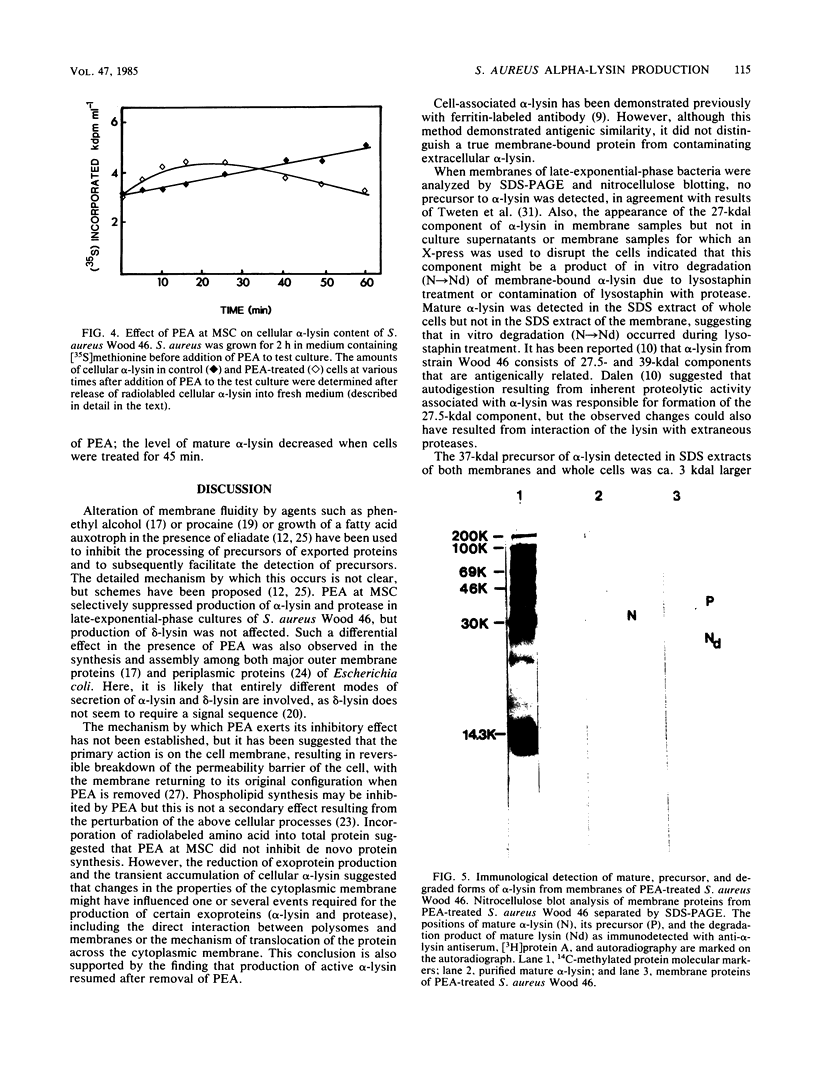
Phenethyl alcohol, at the maximum concentration which did not inhibit growth (0.3% [vol/vol]), inhibited the production of alpha-lysin and exoproteases but not that of delta-lysin in Staphylococcus aureus Wood 46. The inhibition of alpha-lysin was reversible, and transient accumulation of cell-associated alpha-lysin occurred in the presence of PEA. A precursor of alpha-lysin ca. 3,000 daltons larger than extracellular alpha-lysin was immunologically detected in the sodium dodecyl sulfate extracts of membranes and whole cells of phenethyl alcohol-treated S. aureus cultures. Also, a degraded form of alpha-lysin was detected in membranes prepared from cells lysed by lysostaphin but not in membranes from cells lysed with an X-press.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenbern R. A. Extreme sensitivity of staphylococcal enterotoxin B and C production to inhibition by cerulenin. Antimicrob Agents Chemother. 1977 May;11(5):906–908. doi: 10.1128/aac.11.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNHEIMER A. W., SCHWARTZ L. L. Isolation and composition of staphylococcal alpha toxin. J Gen Microbiol. 1963 Mar;30:455–468. doi: 10.1099/00221287-30-3-455. [DOI] [PubMed] [Google Scholar]
- Birkbeck T. H., Stephen J. Immunochemical isolation of vaccinia-virus antigens. Immunochemistry. 1971 Nov;8(11):1029–1039. doi: 10.1016/0019-2791(71)90491-5. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L. P., Birkbeck T. H. Assay of staphylococcal delta-haemolysin with fish erythrocytes. J Med Microbiol. 1978 Aug;11(3):303–313. doi: 10.1099/00222615-11-3-303. [DOI] [PubMed] [Google Scholar]
- Colacicco G., Buckelew A. R., Jr Lipid monolayers: influence of lipid film and urea on the surface activity of staphylococcal alpha-toxin. Lipids. 1971 Aug;6(8):546–553. doi: 10.1007/BF02531134. [DOI] [PubMed] [Google Scholar]
- Coulter J. R., Mukherjee T. M. Electron microscopic localization of alpha toxin within the staphylococcal cell by ferritin-labeled antibody. Infect Immun. 1971 Nov;4(5):650–655. doi: 10.1128/iai.4.5.650-655.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalen A. B. Proteolytic degradation of staphylococcal alpha-toxin. Acta Pathol Microbiol Scand B. 1976 Oct;84B(5):309–314. doi: 10.1111/j.1699-0463.1976.tb01943.x. [DOI] [PubMed] [Google Scholar]
- Davis B. D., Tai P. C. The mechanism of protein secretion across membranes. Nature. 1980 Jan 31;283(5746):433–438. doi: 10.1038/283433a0. [DOI] [PubMed] [Google Scholar]
- DiRienzo J. M., Inouye M. Lipid fluidity-dependent biosynthesis and assembly of the outer membrane proteins of E. coli. Cell. 1979 May;17(1):155–161. doi: 10.1016/0092-8674(79)90303-9. [DOI] [PubMed] [Google Scholar]
- Duncan J. L., Cho G. J. Production of staphylococcal alpha toxin. I. Relationship between cell growth and toxin formation. Infect Immun. 1971 Oct;4(4):456–461. doi: 10.1128/iai.4.4.456-461.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman Y., Rottem S., Citri N. Preferential suppression of normal exoenzyme formation by membrane-modifying agents. J Bacteriol. 1980 Mar;141(3):1435–1438. doi: 10.1128/jb.141.3.1435-1438.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer J. H., Arbuthnott J. P., Bernheimer A. W. Interaction of staphylococcal alpha-toxin with artificial and natural membranes. J Bacteriol. 1968 Mar;95(3):1153–1168. doi: 10.1128/jb.95.3.1153-1168.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer J. H., Arbuthnott J. P. Toxins of Staphylococcus aureus. Pharmacol Ther. 1982;19(1):55–106. doi: 10.1016/0163-7258(82)90042-0. [DOI] [PubMed] [Google Scholar]
- Halegoua S., Inouye M. Translocation and assembly of outer membrance proteins of Escherichia coli. Selective accumulation of precursors and novel assembly intermediates caused by phenethyl alcohol. J Mol Biol. 1979 May 5;130(1):39–61. doi: 10.1016/0022-2836(79)90551-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazdunski C., Baty D., Pagès J. M. Procaine, a local anesthetic interacting with the cell membrane, inhibits the processing of precursor forms of periplasmic proteins in Escherichia coli. Eur J Biochem. 1979 May 2;96(1):49–57. doi: 10.1111/j.1432-1033.1979.tb13012.x. [DOI] [PubMed] [Google Scholar]
- Lee K. Y., Birkbeck T. H. In vitro synthesis of the delta-lysin of Staphylococcus aureus. Infect Immun. 1984 May;44(2):434–438. doi: 10.1128/iai.44.2.434-438.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller G. H. Protein labelling with 3H-NSP (N-succinimidyl-[2,3-3H]propionate). J Cell Sci. 1980 Jun;43:319–328. doi: 10.1242/jcs.43.1.319. [DOI] [PubMed] [Google Scholar]
- Nunn W. D., Tropp B. E. Effects of phenethyl alcohol on phospholipid metabolism in Escherichia coli. J Bacteriol. 1972 Jan;109(1):162–168. doi: 10.1128/jb.109.1.162-168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagès J. M., Piovant M., Varenne S., Lazdunski C. Mechanistic aspects of the transfer of nascent periplasmic proteins across the cytoplasmic membrane in Escherichia coli. Eur J Biochem. 1978 May 16;86(2):589–602. doi: 10.1111/j.1432-1033.1978.tb12343.x. [DOI] [PubMed] [Google Scholar]
- Rogolsky M. Nonenteric toxins of Staphylococcus aureus. Microbiol Rev. 1979 Sep;43(3):320–360. doi: 10.1128/mr.43.3.320-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Wendt L. Mechanism of action of phenethyl alcohol: breakdown of the cellular permeability barrier. J Bacteriol. 1967 Feb;93(2):560–566. doi: 10.1128/jb.93.2.560-566.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore T. S., Popkin T. J., Cole R. M. The separation and isolation of plasma membranes and mesosomal vesicles from Staphylococcus aureus. Prep Biochem. 1971;1(3):233–248. doi: 10.1080/00327487108081942. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweten R. K., Christianson K. K., Iandolo J. J. Transport and processing of staphylococcal alpha-toxin. J Bacteriol. 1983 Nov;156(2):524–528. doi: 10.1128/jb.156.2.524-528.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweten R. K., Iandolo J. J. Purification and partial characterization of a putative precursor to staphylococcal enterotoxin B. Infect Immun. 1981 Dec;34(3):900–907. doi: 10.1128/iai.34.3.900-907.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yoshikawa M., Matsuda F., Naka M., Murofushi E., Tsunematsu Y. Pleiotropic alteration of activities of several toxins and enzymes in mutants of Staphylococcus aureus. J Bacteriol. 1974 Jul;119(1):117–122. doi: 10.1128/jb.119.1.117-122.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]





