Abstract
Objective:
It is believed that off-pump coronary artery bypass grafting (OPCAB) leads to hypercoagulability, but efforts to document such a state have been unrevealing. We hypothesized that OPCAB increases the risk of developing a regional hypercoagulable state.
Methods:
Blood was obtained from the aorta and coronary sinus (CS) after CABG performed off- (N=69) or on-pump (N=35) to determine the transcardiac gradients of F1.2 (thrombin production), XIIa (coagulation activation), myoglobin (ischemia) and IL-6, IL-8 using ELISA and platelet-derived microparticles using FACS. Platelet function was measured using aggregometry. Regional myocardial pH and SVG flow were recorded intraoperatively. SVG biopsies were analyzed for endothelial integrity (EI) using immunohistochemistry and graft patency was determined by predischarge CT angiography.
Results:
Compared with on-pump, OPCAB provoked significantly higher transcardiac F1.2 (117±200 v. 31±38%), FXII-a (14±29 v. 2±4%), microparticles (14±−9.5% v. 6.4±−4.1%), IL-6 (119±183 v. 28±39%), and a trend toward increased IL-8 (67±94 v. 24±46%, P = 0.077). Myoglobin release after OPCAB, also greater than on-pump CABG (54±89 v. 8±14%, P < 0.01), correlated with regional pH change (R=−0.96, P < 0.0001), and F1.2 release (R=0.55, P = 0.0002). In contrast, systemic changes in these markers were all less after OPCAB. SVG flow was significantly reduced in OPCAB (39.4 versus 66.5 mL/min, P = 0.0002), but EI and graft patency rates were the same.
Conclusions:
Through the use of transcardiac assays, we illustrated that regional coagulation was enhanced after off- compared with on-pump CABG. If the findings of this pilot study are confirmed, OPCAB may require additional antithrombotic therapies to respond to this local hypercoagulable state.
Keywords: Off-pump coronary artery bypass graft surgery, Ischemia, Thrombin, Saphenous vein graft
Enthusiasm for off pump coronary artery bypass (OPCAB) has been dampened by initial retrospective1,2 and more recent prospective3 trials that raise concerns about the early patency rate for saphenous vein grafts (SVG). Traditional on-pump surgery has established a low incidence of postoperative thrombotic events4 that is believed to be mediated in part by an unidentified anticoagulating effect of cardiopulmonary bypass (CPB).5 By circumventing CPB, OPCAB is widely believed to be associated with a “hypercoagulable state” akin to that described after general surgical procedures.6
Sporadic adoption of clopidogrel use after OPCAB7 reflects the tentative belief that the risk of SVG thrombosis in these patients is not adequately addressed by aspirin monotherapy. If a hypercoagulable state could be reliably identified, this information could be used to focus an aggressive antithrombotic strategy on high-risk cases, limiting bleeding risk in the population as a whole. However, there is currently no widely accepted technique for defining this state. Theoretically, the most integrated method for defining the risk of thrombosis would be to directly image preocclusive clot within the SVG. While this is not possible clinically, the development of thrombus within the graft may be extrapolated via an analysis of coronary sinus (CS) blood. Clot is initiated by the local production of thrombin which leads to the release of prothrombin fragment F1.2. Blood flow through the SVG carries F1.2 to the CS where it can be measured. Similar regional changes in coagulability have been documented in blood downstream of vascular injury in animal models and after intracoronary stenting.8,9 The purpose of this study was to investigate whether OPCAB is associated with a regional hypercoagulable state relative to on-pump CABG using this CS analysis.
METHODS
Patient enrollment and data management
Following IRB approval (protocol #0303108), all subjects provided informed consent before enrollment. From November 2002 until December 2005, 520 patients were screened and 133 patients excluded from the study due to: creatinine >2.0mg/dl (n=80), refusal of consent (n=32), and inability to obtain valid consent due to patient condition or emergent surgery (n=21). After enrollment, CT angiogram was not obtained in 52 patients because: heart rate>100 bpm or creatinine >2.0 mg/dl (n=28), patient withdrawal of consent (n=12), lost to follow-up (n=10), and patient death without an autopsy to confirm bypass graft patency (n=2). Patient group assignment was done in a nonrandomized fashion based on surgeon preference. This analysis is limited to the subset of off- (N=69) and on-pump (N=35) patients in which the complete complement of postoperative blood samples were obtained.
Surgical technique
Four different surgeons enrolled patients. After median sternotomy, the left internal thoracic artery was used in all patients; the saphenous vein was harvested using an endoscopic (VasoView5, Guidant Systems, Inc., Minneapolis, MN) or open approach, based on anatomic considerations. Conduits were stored in heparinized saline after harvest. For OPCAB, heparin was given at a dose calculated to obtain an ACT >300 seconds by the Hepcon instrument (Medtronic, Inc., Minneapolis, MN) and every 30 minutes to maintain the heparin level at >2 IU/ml. For on-pump CABG, the doses were increased to an ACT >480 seconds and heparin level >2.8 IU/ml. Proximal anastomoses were performed using a partial occluding aortic clamp. All distal anastomoses during OPCAB were facilitated by suction-based exposure and stablizing devices (Octopus 4.3, Medtronic, Inc., Minneapolis, MN) without intracoronary shunts. Heparin was reversed by a protamine dose calculated by the Hepcon device. Preoperative aspirin (325 mg p.o. qd) was continued and restarted within 6 hours of surgery as the sole antithrombotic agent. Shed mediastinal blood was collected intraoperatively in both groups using a cellsaving device (Cobe BRAT 2; Cobe Cardiovascular, Arvada, Colorado), processed, and retransfused.
Intraoperative blood flow analysis
Blood flow and flow waveform were measured in each graft using transit time ultrasound (Transonic, Inc, Ithaca, NY). Waveforms were analyzed for pulsatility index (PI=maximum-minimum/mean blood flow) and percentage diastolic flow using digital data acquisition software (WinDaq, DATAQ Instruments, Inc, Dayton, OH). Grafts with flow <10 mL/min and PI>5 despite anastomotic revision were excluded from analysis (N=2).
Blood sample collection
Blood samples, collected in tubes containing 3.2% citrate, were obtained preoperatively from the arterial line. Postoperatively, 30 minutes after heparin reversal, blood was obtained from the systemic arterial line and CS. Additional systemic blood was collected on postoperative days 1, 3, and 30. Platelet-poor plasma was obtained by rapid centrifugation and stored at −80 C°.
Myocardial Markers
Systemic blood samples were analyzed for troponin-I (Tn-I) at baseline, POD1, and POD3. Myoglobin release was determined by comparing arterial versus CS levels in the blood samples obtained 30 minutes after protamine. Tn-I and myoglobin were both assessed using an ELISA (Life Diagnostics, Inc., West Chester, PA). Regional (ie, anterior and posterior ventricular wall) changes in pH were monitored in real time using the Khuri pH monitoring system (Terumo Corp, Tokyo, Japan).
Assays for Coagulation
Thrombin formation was evaluated by assaying preoperative, postoperative systemic, and CS samples for the presence of prothrombin fragment 1.2 (F1.2) (Dade Behring, Marburg, Germany). An additional measure of the intrinsic coagulation cascade was evaluated by assaying FXII-a (American Diagnostica, Inc., Stamford, CT).
Assays for Platelet Function
Thrombelastography
(TEG, Hemoscope, Niles, IL): Maximum amplitude (MA) of the TEG trace was measured after 30 seconds of activation by kaolin or tissue factor (0.0001% Innovin, Dade-Behring, Marburg, Germany).
Whole blood aggregometry
(Chronolog, Hawerton, PA): Impedance changes (Ω) were assessed at 6 minutes following the addition of 1μg/ml and 5μg/ml collagen. ASA-R was defined as: %Ωlow/high=Ω1 mcg collagen / Ω5 mcg collagen·100>50%, according to prior reports.10
11-dehydro-TXB2 levels
Platelet poor serum was assayed for 11-dehydro-TXB2 levels using ELISA (Assay Designs Inc, Ann Arbor, MI). ASA-R was defined as >25% increase in serum levels relative to baseline.11
Whole blood flow cytometry
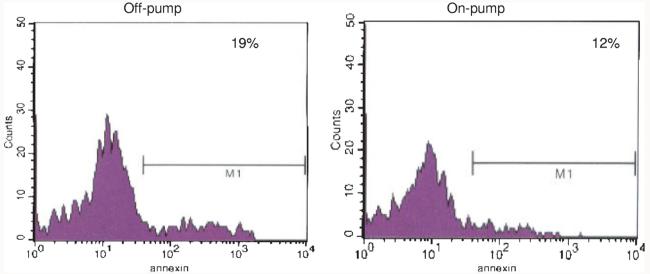
(Becton-Dickinson FACScan, Franklin Lakes, NJ): In an unselected subset of patients, citrated blood samples were diluted with modified Tyrode Buffer containing 5mM GPRP (Centerchem Inc., Norwalk, CT) and then incubated with antibodies against CD41-FITC (Serotec, Raleigh, NC) and AnnexinV-APC (BD Biosciences, San Jose, CA). After 20 minutes incubation, the samples were fixed with 1% paraformaldehyde and stored at 4°C until analysis within 72 hours by FACS. Both, forward scatter and sideward light scatter were set at logarithmic gain, and platelet derived microparticles were identified. The percentage of annexin-positive microparticle events compared with total annexin-positive, CD41 positive events was calculated as described12 (Fig. 1).
FIGURE 1.
To quantify platelet derived microparticles, debris from the coronary sinus that was gated as less than 1 μm in size by flow cytometry was sorted for the constitutive platelet marker, CD41, and the activation marker, annexin V. These representative histograms illustrate that a higher proportion of the subcellular debris from the coronary sinus of patients after OPCAB was derived from activated platelets compared with a smaller proportion in the sample obtained after on-pump CABG.
Assays for Inflammation
Preoperative, postoperative systemic, and CS plasma samples were analyzed for interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-α (TNF-α) using ELISA (Bender MedSystems, Vienna, Austria). Soluble ICAM (sICAM), and sVCAM were also assessed by ELISA (R&D Systems, Minneapolis, MN).
Endothelial Integrity
Surplus segments from each bypass conduit were stored in balanced salt solution at room temperature for 30-60 minutes, embedded in cutting compound (Tissue-Tek O.C.T., Redding, CA) and then frozen in liquid nitrogen. Four separate 5 μm thick sections were assessed for the expression of an EC marker, CD31 (R&D System, Inc., Minneapolis, MN) and tissue factor (United States Biologic, Swampscott, MA) using immunohistochemistry. The vessel circumference positive for CD31 was calculated in each section using image analysis software (Bioquant Nova Prime, Nashville, TN) and EC integrity defined by the average %CD31 staining for all sections analyzed.10
Graft Patency
Bypass graft patency was determined by blinded review (CW) of a 16 detector row, CT angiography (CTA) scan (420 milliseconds rotation, 100-150 mL contrast agent IV at 5 mL/s) using retrospective ECG gating. Patency was defined as any flow through the entire graft regardless of the presence of stenosis. The graft was classified as nonpatent if a stump was seen or if there was no contrast in an area known by operative report to contain a graft, a recently validated definition of postoperative bypass graft failure.10
Statistics
The primary end point of this study was the difference in the F1.2 gradient for off- versus on-pump CABG. Preliminary data obtained in these two groups showed that there was a three-fold difference in the CS versus aortic gradient of F1.2 (90% versus 30% transcardiac change in F1.2). Assuming a SD of 20%, we estimated that the minimum number of patients needed to demonstrate a significant difference between groups for 80% power and P = 0.05 was 15 per group, as derived from the power calculator at http://calculators.stat.ucla.edu/powercalc/.
The secondary end point of this study was to investigate the mechanism of a regional hypercoagulable state by comparing the transcardiac gradients of inflammatory, ischemic, and coagulation markers between the two surgical techniques. The on- and off-pump groups were compared by means of the unpaired Student t test for continuous variables and the Fisher exact test for categorical variables. Correlations were examined using linear regression for parametric data. Data points categorized as outliers were excluded as described at http://www.graphpad.com/quick-calcs/Grubbs1.cfm (n=2). Statistical analysis was performed using the InStat statistical package with the assistance of a biostatistician.
RESULTS
Patient characteristics were similar between groups with the exception of age, which was higher in OPCAB, and weight and the proportion of diabetes, which was higher in on-pump (Table 1).
TABLE 1.
Patient Characteristics
| OPCAB | On-pump | P value | ||
|---|---|---|---|---|
| Height (cm) | 166±21 | 171±21 | 0.38 | |
| Weight (kg) | 85±25 | 96±20 | 0.02 | |
| Gender (M) | 67% | 82 | 0.15 | |
| Age (yr) | 67±10 | 60±9 | 0.003 | |
| Active Smoking | 30% | 32% | 0.82 | |
| Hypertension (>90 and/or >140) | 84% | 85% | >0.99 | |
| Peripheral Vascular Disease | 17% | 6% | 0.21 | |
| Previous Myocardial Infarction | 50% | 41% | 0.52 | |
| Previous Stroke | 3% | 12% | 0.18 | |
| Diabetes | 33% | 56% | 0.03 | |
| COPD | 14% | 9% | 0.53 | |
| Previous TIA | 6% | 0% | 0.24 | |
| History of Carotid Artery Disease | 19% | 15% | 0.53 | |
| Hypercholesterolemia | 87% | 97% | 0.15 | |
| Previous Heart Surgery | 5% | 12% | 0.24 | |
| Medications At Surgery | Beta blocker | 83% | 91% | 0.37 |
| Intravenous nitrates | 19% | 21% | >0.99 | |
| Intravenous heparin | 48% | 47% | >0.99 | |
| Aspirin | 95% | 97% | >0.99 | |
| ACE inhibitor | 54% | 48% | 0.67 | |
| Clopidogrel | 23% | 24% | >0.99 |
Myocardial Necrosis and Ischemia
Periods of coronary occlusion during OPCAB were associated with varying degrees of regional myocardial acidosis as defined by real time pH monitoring (mean pH drop 0.07±0.06, n=10) (Fig. 2). The magnitude of pH drop during native vessel occlusion correlated strongly with the transcardiac gradient of myoglobin (R = −0.96, P < 0.0001, n = 10) (Fig. 3). Although cold, cardioplegic arrest during on-pump CABG was associated with more consistent changes in pH (mean pH drop 0.15±0.04, N=6), the transcardiac release of myoglobin was significantly less than OPCAB (8±14%, n=12, v. 54±89%, n=34, P = 0.006) and there was no relationship to the amount of myoglobin release. The area under the curves for Tn-I release over the 3-day postoperative period were not significantly different between groups (1.14±1.78 v. 1.0±1.68 ng·day/ml, P = NS). Also, the degree of transcardiac change of myoglobin noted postoperatively did not predict later release of Tn-I.
FIGURE 2.
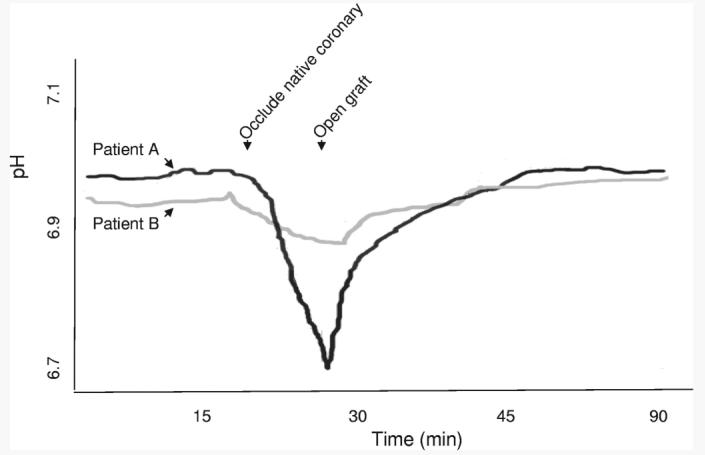
Regional ischemia in OPCAB was assessed by changes in intramyocardial pH measured with a real time pH monitor (Terumo Cardiovascular). During native vessel occlusion required for the distal anastomosis, pH declined in the region of the occluded coronary to varying degrees. In these representative examples, acidosis developed in the anterior wall over the 10-minute period of LAD occlusion with a pH change of 0.2 in Patient A. Perhaps due to more developed collateral circulation, only a modest change in anterior wall pH (ie, <0.05) was noted during coronary occlusion in Patient B.
FIGURE 3.
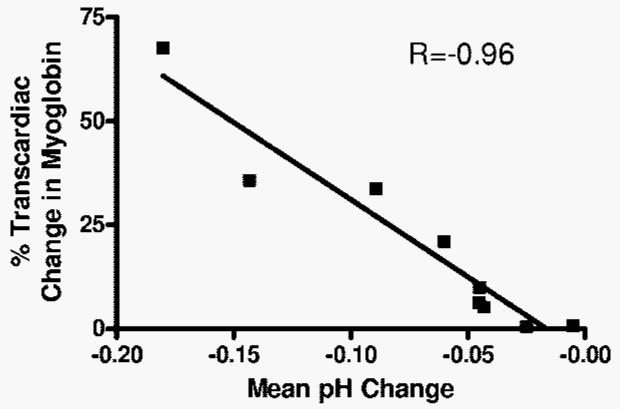
Simultaneous blood draws were obtained from the aorta and coronary sinus at 30 minutes after protamine administration and assayed for myoglobin for patients undergoing OPCAB. This transcardiac gradient of myoglobin showed a strong inverse correlation with the maximal degree of regional pH change that developed during native vessel occlusion (R=-0.96, P < 0.0001).
Regional versus Systemic Hypercoagulability
The analysis of CS blood at the completion of CABG provided three lines of evidence to suggest a regional hyper-coagulable state after OPCAB (Fig. 4): statistically significant increases in transcardiac F1.2 (117±200% change, n=35 v. 31±38%, n=13, P = 0.02), transcardiac FXII-a (14±29% change, n=30 v. 2±4%, n=9, P = 0.048) and platelet derived microparticles (14.7±9.5% annexin positive microparticles/all annexin positive CD41 events, n=8, v. 6.4±4.1%, n=5, P = 0.05). Myoglobin release after OPCAB showed a strong correlation with the transcardiac gradient of F1.2 (R=0.55, P = 0.0002). In contrast, analyses of systemic blood samples showed an increase in all three of these markers after on- compared with off-pump CABG, which reached statistical significance for F1.2 (283±426%, n=69 v. 645±965%, n=35, P = 0.04). We found no statistically significant difference in platelet function between the groups at any measured time point (Table 2). The incidence of ASA resistance, measured by the agreement between aggregometry and serum 11-dehydro-TXB2 criteria, was also not significantly different between the two groups at any time point (data not shown).
FIGURE 4.
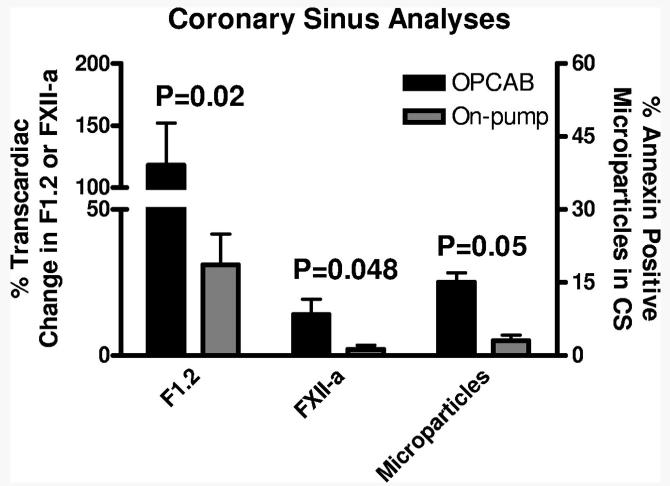
Trancardiac F1.2 and FXII-a gradients and the proportion of annexin positive microparticles released from the heart were determined by obtaining CS samples at the completion of CABG. Compared with on-pump CABG, CS samples obtained after OPCAB showed more thrombin formation (F1.2), increased activity of the intrinsic coagulation cascade (FXII-a) and greater development of microparticles.
TABLE 2.
Platelet Function
| POSTOP | OPCAB | On- pump |
P value |
|
|---|---|---|---|---|
| TEG with Kaolin (MA) | 58±9 | 56±9 | NS | |
| TEG with Tissue Factor (MA) | 64±9 | 62±9 | NS | |
| WBA with 1μg/ml Collagen (Ohms) |
5.5±3.6 | 4.5±3.1 | NS | |
| WBA with 5μg/ml Collagen (Ohms) |
10.1±5.6 | 9.3±5.1 | NS | |
| POD1 | ||||
| TEG with Kaolin (MA) | 60±7 | 60±8 | NS | |
| TEG with Tissue Factor (MA) | 65±9 | 59±13 | NS | |
| WBA with 1μg/ml Collagen (Ohms) |
7±3.9 | 7.7±3.9 | NS | |
| WBA with 5μg/ml Collagen (Ohms) |
13.5±4.8 | 13.3±5.3 | NS | |
| POD3 | ||||
| TEG with Kaolin (MA) | 68±7 | 66±7 | NS | |
| TEG with Tissue Factor (MA) | 73±6 | 69±12 | NS | |
| WBA with 1μg/ml Collagen (Ohms) |
8.1±4 | 7.9±4.1 | NS | |
| WBA with 5μg/ml Collagen (Ohms) |
13.5±4.4 | 11.9±4.1 | NS |
Inflammation
OPCAB was associated with significantly elevated transcardiac gradients of IL-6 (119±183%, n=31 v. 28±39%, n=11, P = 0.014) and a trend toward elevated IL-8 (67±94%, n=28, v. 24±46%, n=11, P = 0.077) (Fig. 5). Similar to the coagulation data, systemic analyses showed a trend toward reduced levels of IL-6 (99±315, n=21 v. 328±602, n=9, P = 0.152) and no statistical difference in levels of IL-8 in OPCAB compared with on pump CABG. TNF-α, sVCAM, and sICAM showed no statistical difference between OPCAB and on pump CABG using either systemic or CS blood samples.
FIGURE 5.
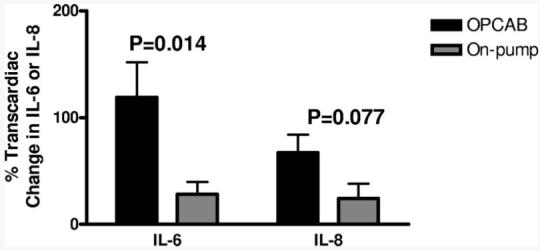
The change in IL-6 and IL-8 was determined after CABG by analyzing simultaneously drawn samples from the aorta and coronary sinus using ELISA. When compared with on-pump CABG, patients undergoing OPCAB showed higher transcardiac gradients of IL-6 and IL-8.
Graft Quality
SVG flow was significantly lower in OPCAB compared with on pump CABG (36.3±13.1 versus 64.0±28.9 mL/min, P = 0.003) but there were no significant differences in the fraction of diastolic flow (49±14% versus 59±15%, P = NS) or pulsatile index (2.6±0.9 versus 2.8±1.8, P = NS) noted between the 2 groups.
Percent luminal EI (50.3±34% versus 40.7±30.3% P = NS) and the graft failure rate (N=69/35 12% versus 17%, P = NS) were also not statistically different between the 2 groups.
DISCUSSION
A burst in thrombin formation that leads to intense platelet activation is a prerequisite for thrombosis. The analysis of F1.2 and platelet derived microparticles are established techniques for quantifying the formation and activity of thrombin in vivo. Because thrombin formation is tightly controlled in time and place, analyses of systemic blood samples often fail to reflect events taking place in local vascular beds. Prior investigators have analyzed the CS blood as a more sensitive means of defining a regional hypercoagulability within the upstream coronary circulation.9,13 In this study, we used this approach to illustrate that OPCAB was associated with a regional hypercoagulable state that was not found following on-pump CABG and not detectable using systemic analyses. Similar to other reports, an analysis of thrombin markers solely from systemic (ie, peripheral arterial or venous) blood would have led us to the contrary conclusion that coagulation is more activated by on-pump CABG. Our current practice is to use aspirin monotherapy for thrombosis prophylaxis after either on- or off-pump CABG. If these preliminary findings are confirmed by ongoing prospective studies, our data suggest that antithrombotic strategies after OPCAB need to be revised to respond to this hypercoagulable tendency.
Saphenous vein graft (SVG) closure after coronary artery bypass grafting (CABG) is a poorly understood problem that is often blamed on technical defects, particularly during OPCAB. Contemporary flow measurement technologies enable revision and/or exclusion of defective grafts from analysis. This has allowed our group to elucidate two independent risk factors for graft failure that are unrelated to anastomotic technique: endothelial cell (EC) disruption in the SVG and postoperative aspirin resistance (ASA-R).10 Particularly in the setting of abnormal intraluminal blood flow, platelets, and coagulation factors are known to react to injured and thrombogenic vascular tissue by generating thrombin. The fact that EC disruption and ASA-R were similar between the two surgical groups suggests that OPCAB may have an independent influence on the development of regional hypercoagulability. However, the assignment of patients was not randomized, which resulted in clinical differences between groups that could have influenced thrombin formation (eg, age and incidence of diabetes).14 This pilot study was not appropriately powered to perform multivariate analysis to confirm an independent influence of OPCAB. Therefore, our conclusions should be considered hypothesis pending confirmation in ongoing prospective analyses with these more powerful statistical methods.
A plausible mechanism by which OPCAB may induce hypercoagulability is via the obligatory periods of regional warm ischemia that accompany the distal anastomoses. Animal models have demonstrated an induction of thrombin production during periods of warm ischemia as little as 15 minutes.15,16 Likely depending on the collateral coronary circulation, a similar period of coronary occlusion in our cohort resulted in changes in regional intramyocardial pH that varied widely between patients. The degree of pH change noted directly correlated with the transcardiac release of myoglobin, a low-molecular-weight protein that is known to leak from myocytes in the absence of necrosis.17 Surprisingly, on-pump CABG resulted in more profound intramyocardial acidosis during asystolic arrest but was not associated with myoglobin release. This difference, possibly related to the reduced myocardial temperature during cold cardioplegic arrest,18 was important because the transcardiac change in myoglobin after OPCAB showed a direct correlation with thrombin generation. Additionally, several inflammatory markers that have been linked to thrombotic events19 also showed an increased gradient across the heart after OPCAB. Reperfusion after warm ischemia was associated with the induction of the intrinsic cascade (ie, FXII-a) in our study and in others20, which is suggested to be an essential component of thrombosis.21 Despite this prothrombotic microenvironment, postoperative SVG patency and troponin I levels were equal between on- and off-pump groups.
The main limitation of this study was the small number of analyses done per group. As a result, there were too few data points to perform a meaningful multivariate analysis to confirm that OPCAB was an independent predictor of regional hypercoagulability. Further enrollment in this ongoing study will provide enough patients to perform this valuable statistical technique. Because this study was performed only at a single center, generalizability to other centers may be limited by institution specific practices such as the aggressive use of the cell saver system during OPCAB. Because of the nonrandomized assignment of patients to on- versus off-pump CABG, we noted significant difference in three variables: age, which was higher in OPCAB; weight and the prevalence of diabetes, which were both higher in on-pump CABG. This disproportion is most likely because of the decision-making algorithm used by the surgeons at our center22 and therefore is an integral part of each procedure. Finally, bypass graft flow was lower within SVG grafted off-pump. While less optimal anastomoses performed during OPCAB may play a role, we feel that changes in the vascular reactivity of the coronary bed due to ischemic stunning are more likely to be responsible.
In the present study, we showed that a regional hyper-coagulable state associated with OPCAB is revealed by analyzing thrombin production and activity in the coronary sinus. While brief periods of warm ischemia during OPCAB may be involved, reduced SVG flow, increased inflammation, and higher proportion of elderly patients also likely contribute importantly to the complex calculus describing the thrombotic microenvironment within the SVG induced by OPCAB. If these findings are confirmed in ongoing studies, they would support the use of additional antithrombotic therapies to appropriately respond to this phenomenon after OPCAB.
Acknowledgments
R.S.P. is supported by a Scientist Development Grant from the American Heart Association (0435318N), and the Office of Naval Research. Supplies for the completion of this work were donated by Haemoscope, Inc.
REFERENCES
- 1.Kim K, Lim C, Lee C, et al. Off-pump coronary artery bypass may decrease the patency of saphenous vein grafts. Ann Thor Surg. 2001;72:S1033–1037. doi: 10.1016/s0003-4975(01)02946-0. [DOI] [PubMed] [Google Scholar]
- 2.Gundry S, Romano M, Shattuck OH, et al. Seven-year follow-up of coronary artery bypasses performed with and without cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1998;115:1273–1278. doi: 10.1016/S0022-5223(98)70209-0. [DOI] [PubMed] [Google Scholar]
- 3.Khan NE, De Souza A, Mister R, et al. A randomized comparison of off-pump and on-pump multivessel coronary-artery bypass surgery. N Engl J Med. 2004;350:21–8. doi: 10.1056/NEJMoa031282. [DOI] [PubMed] [Google Scholar]
- 4.Goldman S, Zadina K, Moritz T, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004;44:2149–56. doi: 10.1016/j.jacc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 5.MacGillivray TE, Vlahakes GJ. Patency and the pump–the risks and benefits of off-pump CABG. N Engl J Med. 2004 Jan 1;350:3–4. doi: 10.1056/NEJMp038185. [DOI] [PubMed] [Google Scholar]
- 6.White RH, Henderson MC. Risk factors for venous thromboembolism after total hip and knee replacement surgery. Curr Opin Pulm Med. 2002;8:365–71. doi: 10.1097/00063198-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 7.D'Ancona G, Donias HW, Karamanoukian RL, et al. OPCAB therapy survey: off-pump clopidogrel, aspirin or both therapy survey. Heart Surg Forum. 2001;4:354–8. [PubMed] [Google Scholar]
- 8.Gurbel PA, Serebruany VL, Komiathy SF, et al. Pretreatment with an inhibitor of mac-1 alters regional and systemic platelet function during ischemia-reperfusion in swine. Pharmacology. 1996 Aug;53:79–86. doi: 10.1159/000139418. [DOI] [PubMed] [Google Scholar]
- 9.Scharn DM. Activation of platelets in blood perfusing angioplasty damaged coronary arteries. Cardiovasc Surg. 2002;10:566. doi: 10.1161/01.atv.12.12.1475. [DOI] [PubMed] [Google Scholar]
- 10.Poston RS, Gu J, Brown JM, et al. Endothelial injury and acquired aspirin resistance as promoters of regional thrombin formation and early vein graft failure after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2006 Jan;131:122–30. doi: 10.1016/j.jtcvs.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 11.Hart RG, Leonard AD, Talbert RL, et al. Aspirin dosage and thromboxane synthesis in patients with vascular disease. Pharmacotherapy. 2003;23:579–84. doi: 10.1592/phco.23.5.579.32206. [DOI] [PubMed] [Google Scholar]
- 12.Nieuwland R, Berckmans RJ, Rotteveel-Eijkman RC, et al. Cell-derived microparticles generated in patients during cardiopulmonary bypass are highly procoagulant. Circulation. 1997;96:3534–3541. doi: 10.1161/01.cir.96.10.3534. [DOI] [PubMed] [Google Scholar]
- 13.Laskey WK, Zawacki K, Lim R, Herzog W. Effects of local heparin administration on coronary thrombin activity during percutaneous transluminal coronary angioplasty. Catheter Cardiovasc Interv. 1999 Sep;48:84–8. doi: 10.1002/(sici)1522-726x(199909)48:1<84::aid-ccd17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 14.Hemker HC, Al Dieri R. Age-dependency of thrombin generation. Thromb Haemost. 2006 May;95:756–7. doi: 10.1160/th06-03-0162. [DOI] [PubMed] [Google Scholar]
- 15.Chong AJ, Pohlman TH, Hampton CR, et al. Tissue factor and thrombin mediate myocardial ischemia-reperfusion injury. Ann Thorac Surg. 2003 Feb;75:S649–55. doi: 10.1016/s0003-4975(02)04691-x. [DOI] [PubMed] [Google Scholar]
- 16.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 2. Circulation. 2001 Dec 18;104:3158–67. doi: 10.1161/hc5001.100039. [DOI] [PubMed] [Google Scholar]
- 17.Roberts R, Fromm RE. Management of acute coronary syndromes based on risk stratification by biochemical markers: an idea whose time has come. Circulation. 1998 Nov 3;98:1831–3. doi: 10.1161/01.cir.98.18.1831. [DOI] [PubMed] [Google Scholar]
- 18.Wolberg AS, Meng ZH, Monroe DM, 3rd, Hoffman M. A systematic evaluation of the effect of temperature on coagulation enzyme activity and platelet function. J Trauma. 2004 Jun;56:1221–8. doi: 10.1097/01.ta.0000064328.97941.fc. [DOI] [PubMed] [Google Scholar]
- 19.Wagner DD. New links between inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2006 Jul;25:1321–4. doi: 10.1161/01.ATV.0000166521.90532.44. [DOI] [PubMed] [Google Scholar]
- 20.Merlini PA, Cugno M, Rossi ML, et al. Activation of the contact system and inflammation after thrombolytic therapy in patients with acute myocardial infarction. Am J Cardiol. 2004 Apr 1;93:822–5. doi: 10.1016/j.amjcard.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Renne T, Pozgajova M, Gruner S, et al. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2995 Jul;202:271–81. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown JM, Poston RS, Gammie JS, et al. Off-pump versus on-pump coronary artery bypass grafting in consecutive patients: decision-making algorithm and outcomes. Ann Thorac Surg. 2006 Feb;81:555–61. doi: 10.1016/j.athoracsur.2005.06.081. [DOI] [PubMed] [Google Scholar]