Summary
The synthetic L1 retrotransposon, ORFeus, is useful for probing mechanisms of L1 retrotransposition in vivo and for genome-wide mouse mutagenesis because of its high level of activity. To achieve controlled activation of ORFeus in mice, we constructed ORFeusLSL, in which ORFeus coding sequences were separated from the promoter by a loxP-β-geo-stop-loxP (LSL) cassette, and derived transgenic mouse lines containing single-copy ORFeusLSL. We observed tissue-specific ORFeus activation by crossing ORFeusLSL to various Cre-expressing lines, specifically in the germ line or the pancreas, providing definite evidence that all host factors and machinery required posttranscriptionally for L1 retrotransposition are available in somatic tissues in living animals. Notably, the single-copy ORFeus transgene is about threefold more active per copy than a previously described multicopy ORFeus transgene in the germ line and even more active somatically. This conditional transgenic ORFeus mouse model should allow further exploration of posttranscriptional cellular requirements for L1 retrotransposition and facilitate the development of ORFeus mouse lines suitable for in vivo mutagenesis. genesis 46:373–383, 2008.
Keywords: LINE-1, retrotransposon, transgenic mouse, Cre/lox system, conditional, copy number
INTRODUCTION
Long interspersed elements type 1 (LINE-1 or L1) are the most abundant transposable elements in both human and mouse genomes. For example, the mouse genome contains 3600,000 copies of L1, with 33,000 full-length, potentially active copies (Goodier et al., 2001). Full-length L1s are 6–7 kb, encompassing an internal promoter in the 5′ untranslated region (5′UTR), two nonoverlapping open reading frames (ORF1 and ORF2), and a weak polyadenylation signal in the 3′ UTR (Ostertag and Kazazian, 2001). The expression of full-length L1 transcripts and proteins are mostly confined to gonadal tissues (Branciforte and Martin, 1994; Ergun et al., 2004; Trelogan and Martin, 1995); this pattern of expression is consistent with the evolutionary pressure for L1 to successfully increase its copy number. L1 replicates in the genome by retrotransposition, a copy-and-paste mechanism involving reverse transcription of an RNA intermediate. The retrotransposition activity of a cloned L1 element can be tested ex vivo in cell culture by incorporating a retrotransposition indicator cassette in its 3′UTR (Moran et al., 1996). Native human L1 isolates are more active in such assays as compared to native mouse L1s (Goodier et al., 2001; Naas et al., 1998), but a synonymously recoded, synthetic mouse L1 (termed ORFeus) is the most active L1 so far reported (Han and Boeke, 2004). By way of retrotransposition, L1 affects the host genome in various ways and constitutes a major force in driving mammalian genome evolution (Kazazian, 2004). Thus, attempts have been made in establishing mouse models of L1 to further our understanding on L1 biology in vivo, and to develop suitable tools for genome-wide mouse mutagenesis. Several native human L1 elements have been introduced into the mouse genome by transgenesis with modest activity (Babushok et al., 2006; Muotri et al., 2005; Ostertag et al., 2002; Prak et al., 2003). In contrast, the synthetic retrotransposon ORFeus is highly active in both somatic tissues and the mouse germ line when expressed from a constitutive heterologous promoter, lending great promise for mouse mutagenesis and in vivo L1 studies (An et al., 2006).
A conditional L1 system is highly desirable for these applications. For example, to be used as an efficient insertional mutagen in mice, a transposon is usually equipped with a gene trapping cassette so that, after transposition, the endogenous transcriptional unit where the transposed copy is inserted will be rendered inactive and the site of integration can be easily identified by the transposon tag (Carlson and Largaespada, 2005). A challenge for developing an L1-based mutagenesis system is that having an unregulated potent gene-trapping cassette will invariably affect the fitness of the host animal once the retrotransposition frequency exceeds some threshold. In fact, we have observed an unusually high rate of embryonic lethality and failure to produce by surrogate mothers during microinjection of ORFeus transgenes equipped with gene-trapping cassettes (W. An, E. Davis, K. O’Donnell, M. Davisson, M. Wiles, J. Kulik, J. Boeke, unpublished data). Even if an active line is established after extensive screening, it will be genetically unstable, and difficult to maintain due to ongoing retrotransposition. Retroviral and DNA transposon-based systems overcome such problems by separating the cis and trans functions of the transposable element into a binary system (Carlson and Largaespada, 2005; Miller, 1997). However, it is challenging to apply this approach to L1 as it retrotransposes preferentially in cis; L1 proteins expressed from one vector cannot efficiently mobilize a passenger RNA expressed in trans (Esnault et al., 2000; Wei et al., 2001). The underlying mechanism for cis preference is not fully understood but L1 proteins are found to colocalize with the encoding L1 RNA in cytoplasmic ribonucleoprotein particles, which are proposed retrotransposition intermediates (Boeke, 1997; Hohjoh and Singer, 1996; Kulpa and Moran, 2005, 2006; Martin, 1991). Obviously, the cis preference of L1 retrotransposition exerts a restriction on ORFeus vector design and requires use of a single vector encoding both L1 proteins and containing any desired utility element such as a retrotransposition indicator cassette and/or gene-trapping cassette.
To explore the possibility of regulating an ORFeus transgene in vivo, we exploited Cre/loxP. Cre is a site-specific recombinase from bacteriophage P1 that mediates recombination at a pair of conserved recognition sequences (loxP) (Sauer, 1998). Cre is often used in conditional transgenesis (Nagy, 2000), in which the promoter and the coding region of the transgene is separated by a floxed transcriptional ‘‘stop’’ sequence (usually consisting of strong tandem polyadenylation signals), which blocks the formation of transcripts for the downstream transgene unless the stop sequence is removed by Cre-mediated excision. In one variation on this scheme (Lobe et al., 1999) the triple-polyadenylation stop sequence is preceded by a β-geo coding sequence (Friedrich and Soriano, 1991), enabling efficient screening for overexpression in ES cells prior to ‘‘investing’’ in a given line. Here, we adopted the Z/AP strategy in new ORFeusLSL transgenic mouse lines, and established single-copy ORFeusLSL mouse lines that are tissue specifically activatable via Cre-mediated excision.
RESULTS
Construction of Transgenic ORFeusLSL Mouse Lines
Our objectives for regulated ORFeus transgenic mouse lines are twofold: First, we would like to suppress ORFeus activity in founder animals and subsequently activate it in a spatiotemporally controlled manner in the progeny; Second, we prefer a system that enables us to screen for integration loci compatible with overexpression before committing to a specific ES cell line as the basis of a new line of mice. To this end, we incorporated the Z/AP design (Lobe et al., 1999) by grafting the loxP-β-geo-stop-loxP (LSL) cassette described for that transgene between the CAG promoter (Niwa et al., 1991) and ORFeus coding sequences (Han and Boeke, 2004); this construct is termed ORFeusLSL (Fig. 1a). In principle, before introducing Cre, ORFeus will not be expressed; once the LSL cassette is excised, ORFeusLSL becomes an active ORFeusL transgene (Fig. 1b), and fully retrotransposition-competent (Fig. 1c). We transfected ORFeusLSL into C57BL/6J ES cells (Koentgen et al., 1993), and derived founder mice from three ES cell clones (1B9, 1E2 and 2G6) with high-level lacZ expression and one copy of ORFeusLSL as determined by Southern blotting (Fig. S1). Quantitative RT-PCR suggested in-significant β-geo transcript variation among the three cell lines. Therefore, we focused our subsequent studies on line 2G6 except as noted.
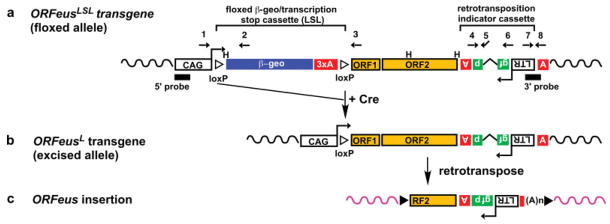
FIG. 1.
Construction of ORFeusLSL mouse lines. (a) Schematic representation of ORFeusLSL transgene. The ORFeusLSL transgene consists of the following sequence elements from 5′ to 3′: a composite CMV IE enhancer/modified chicken β-actin promoter (CAG; Niwa et al., 1991); a floxed β-geo/stop cassette comprising a β-galactosidase/neomycin phosphotransferase fusion gene (β-geo; Friedrich and Soriano, 1991) and triple tandem copies of SV40 late polyadenylation signal (3xpA; Lobe et al., 1999); ORFeus ORF1 and ORF2 (Han and Boeke, 2004); a gfp-based retrotransposition indicator cassette with its own promoter (inverted LTR) and polyadenylation signal (boxed inverted letter A); and β-globin polyadenylation signal (boxed upright letter A). Note the gfp ORF is antisense relative to ORFeus transcription direction and is interrupted by an intron. The splicing of this intron from the ORFeus transcript restores an intact gfp coding sequence, a feature that is diagnostic for retrotransposition events. Approximate locations of genotyping PCR primers (numbered arrowheads) and Southern blotting probes are indicated H, HindIII sites. (b) The predicted structure of the ORFeus transgene following Cre-mediated excision of the floxed β-geo/stop cassette. (c) A schematic of an insertion by ORFeus retrotransposition.
We used PCR to genotype founders and backcrossed progeny. To monitor Cre-mediated excision of the β-geo/ stop cassette in ORFeusLSL transgene, we devised two PCR assays using three primers (Fig. 1a). A positive signal for a PCR reaction named ‘‘floxed’’ (primers 1 and 2) indicates presence of ORFeusLSL; a positive signal for the PCR reaction ‘‘excised’’ (using primers 1 and 3) indicates a recombined allele (ORFeusL). To monitor retrotransposition activity, we employed a third PCR reaction ‘‘intron’’ using intron-flanking primers in the retrotrans-position indicator cassette (Fig. 1a, primers 4 and 6). Donor transgene amplification of ORFeusLSL (Fig. 1a) or ORFeusL (Fig. 1b) should generate a 1,370-bp band, whereas ORFeus retrotranspositions (Fig. 1c) lack the intronic sequence and present a 470-bp band. A fourth PCR reaction ‘‘3′ end’’ (primers 7 and 8) amplifies the 3′ end of ORFeus, and confirms the presence of donor transgene and/or retrotransposition events. Finally, the PCR reaction ‘‘cre’’ amplifies the Cre transgene and the PCR reaction ‘‘Hprt’’ amplifies mouse hypoxanthine guanine phosphoribosyl transferase, an endogenous control for genomic DNA (gDNA). As expected, gDNA from mice heterozygous for ORFeusLSL tested negative for the intronless signal in the PCR reaction intron, showing that the β-geo/stop cassette effectively blocks the transcription of ORFeus and renders it inactive (for example, see parental animal H616 in Fig. 2b).
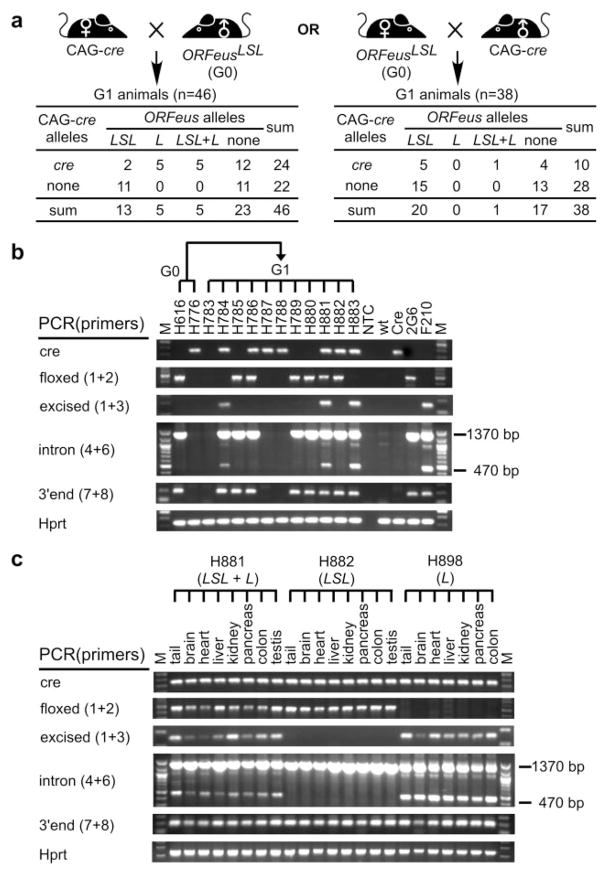
FIG. 2.
Activation of ORFeusLSL by ubiquitous CAG-cre. (a) Reciprocal breeding scheme and summary of G1 progeny genotypes. G1 mice are tabulated according to the genotyping PCR results for the CAG-cre transgene (cre or none) and the ORFeusLSL transgene. The status of the latter is defined as floxed ORFeusLSL allele (LSL), excised ORFeus allele (L), mosaic (LSL1L), or none. (b) PCR genotyping G1 animals by tail biopsy. Results for G1 progeny from breeding between a female CAG-cre mouse (H776) and a male ORFeusLSL mouse (H616) are shown along with both parents. Detailed description on individual PCR can be found in the text. Relevant ORFeusLSL primers are shown in parentheses. NTC, no template control; wt, wild-type C57BL/6 sample; Cre, cre transgene control; 2G6, a sample containing floxed ORFeusLSL allele; F210, a sample from F210 mouse line (An et al., 2006), which has the excised form of ORFeus (ORFeusL) in a multi-copy concatemer; M, 100-bp DNA molecular weight ladder (New England Biolabs). (c) Genotyping tissues of three animals with distinct ORFeus alleles. The animals were selected according to the initial genotyping results with tail biopsy, and were dissected for indicated tissues. All animals were positive for the CAG-cre transgene and their ORFeus allelic statuses are indicated. Tail samples were included for comparison.
Ubiquitous Activation of Donor ORFeusLSL Transgene by CAG-cre
To demonstrate whether ORFeus is activated by Cre-mediated excision in ORFeusLSL mouse lines, we turned to a ubiquitously expressed Cre mouse line, CAG-cre (Sakai and Miyazaki, 1997). The Cre in this mouse line like ORFeusLSL itself is regulated by a CAG promoter. Thus, CAG-cre should permit ORFeus activation in the broadest spectrum of tissue types as the promoters for both Cre and target transgene have the maximal overlap of tissue specificity (Nagy, 2000). This CAG-cre mouse line is reported to possess a maternal effect: target transgenes in progeny mice from heterozygous female CAG-cre parents undergo complete excision of the floxed sequence even in the absence of inheritance of CAG-cre, presumably reflecting the presence of Cre transcripts in oocytes prior to completion of the first meiotic division and subsequent partitioning of Cre RNA/protein to resulting oocytes. In contrast, excision is only seen in Cre-transgene containing progeny mice from male CAG-cre parents (Sakai and Miyazaki, 1997). We therefore set up reciprocal breedings between our heterozygous ORFeusLSL mice (designated G0) and heterozygous CAG-cre animals (Fig. 2a). G1 progeny were born from female (N = 46) and male (N = 38) CAG-cre parents, and subsequently genotyped for Cre-mediated excision events and retrotransposition (Fig. 2b). To our surprise, no maternal effect was observed as excision was detected exclusively in the G1 progeny carrying the CAG-cre transgene from either CAG-cre positive dam or sire. For doubly heterozygous G1 progeny of CAG-cre females, 5/12 displayed complete excision in the tail tissue examined (Fig. 2b, animals H784 and H883), 5/12 displayed a mosaic excision pattern, indicated by the presence of both floxed and excised alleles in the same tissue (Fig. 2b, animal H881), whereas the remaining 2/12 showed no sign of excision (Fig. 2b, animal H786). Among the progeny of CAG-cre males, Cre-mediated excision was detected in only 1/6 of doubly heterozygous G1 progeny (summarized in Fig. 2a); this individual was also mosaic for ORFeusLSL and ORFeusL alleles. Hence, the observed excision rate in doubly heterozygous G1 progeny was also much lower than that in the initial study which reported excision in 100% of such animals without mosaicism (Sakai and Miyazaki, 1997). Similar results were obtained from reciprocal crosses between CAG-cre and a different ORFeusLSL mouse line (line 1E2) (Fig. S2).
We next examined whether the excised ORFeusL transgene was active in those G1 progeny that suffered Cre-mediated LSL excision. With intron PCR, we detected intronless signal in the tail tissue of all animals that have undergone either complete (Fig. 2b, animals H784 and H883) or mosaic LSL excision (Fig. 2b, H881), confirming that retrotransposition occurs in cells with an activated copy of ORFeus.
We initially examined Cre-mediated excision of the ORFeusLSL transgene and subsequent activation of the ORFeus transgene in tail biopsies only. To further explore the pattern of Cre-mediated excision in our ORFeusLSL transgene, we selected three doubly heterozygous G1 animals representing different states of ORFeus alleles (‘‘LSL’’ only, ‘‘L’’ only, and mixed) deduced from genotyping results on tail biopsies, and inspected a panel of tissues from each of these mice. H881 was mosaic for ORFeusLSL and ORFeusL alleles, H882 was positive only for ORFeusLSL, and H898 was positive only for ORFeusL; all carried the CAG-cre transgene (Fig. 2c). Interestingly, all other tissues examined had identical results for the status of the ORFeus transgene and retro-transposition activity as in the tail for each animal, despite the fact that all three animals carried the CAG-cre transgene (Fig. 2c), suggesting that the tail genotype predicts the status of other tissues in the same animal. This consistency of excision in all tissues of the animal suggests that Cre expression levels are set at the whole animal level by the incoming gametic copy of CAG-cre. The implication is that the transgene comes in one of at least two states (off or switching on and off) and those states are maintained throughout the development of the animal. The status of the three classes of animals can be simply determined by tail genotyping.
Tissue-Specific Activation of ORFeusLSL in Somatic Tissues by Pdx1-cre
Having demonstrated that ORFeusLSL transgene can be activated in a broad spectrum of tissues by CAG-cre, next we sought to test whether ORFeusLSL could be activated in a more restrictive, tissue-specific manner by crossing to Pdx1-cre (Hingorani et al., 2003). The cre transgene in this line is regulated by the promoter for the mouse pancreatic and duodenal homeobox 1 gene (Pdx1), which directs Cre expression specifically in the pancreas, and to a lesser extent, in the stomach and duodenum (Hingorani et al., 2003). We bred heterozygous ORFeusLSL mice to heterozygous Pdx1-cre animals, and examined a panel of six tissues from three doubly heterozygous ORFeusLSL;Pdx1-cre mice (see Fig. 3). As expected, Cre-mediated excision could be detected in 3/3 pancreatic samples, 3/3 stomach samples, 2/3 duodenal samples, but none of several other tissues examined (Fig. 3, PCR excised), confirming the previously reported tissue specificity. To detect tissue-specific insertion events, we employed the PCR intron assay to detect ret-rotransposed copies of the activated ORFeusL as in Figure 2b. Only the 1,370-bp intron-containing signal was detected in all tissues using the standard assay (not shown). We hypothesized that the inability of detecting the 470-bp intronless signal by the intron-flanking PCR might reflect a low abundance of insertions relative to the donor element in this tissue. The Pdx1-cre line we used reportedly displays mosaic expression in the pancreas (Hingorani et al., 2003). As such, we expected only a fraction of the pancreatic cells to undergo Cre-mediated excision and activation of the ORFeusLSL transgene. To enhance detection sensitivity for retrotransposition events, we devised a seminested PCR strategy in which the intron-flanking PCR product was diluted and amplified in a subsequent PCR (with primers 4 and 5 as in Fig. 1a) using a splicing junction-spanning primer (primer 5), which preferentially amplifies the spliced product. Retrotransposition signals were detected in 3/3 pancreatic samples, 1/3 stomach and 1/3 duodenal samples but not in any other tissues examined (Fig. 3, PCR ‘‘intron nested’’). Consistent with the prevalence of excised allele of ORFeus, all positive samples for the 470-bp band were also positive for Cre-mediated excision events (Fig. 3, PCR excised). ORFeus retrotransposition in these tissues was independently confirmed by the recovery of several insertions using inverse PCR (iPCR) (Table S1). Thus L1 machinery is intact in somatic cells in which L1 is not normally expressed.
FIG. 3.
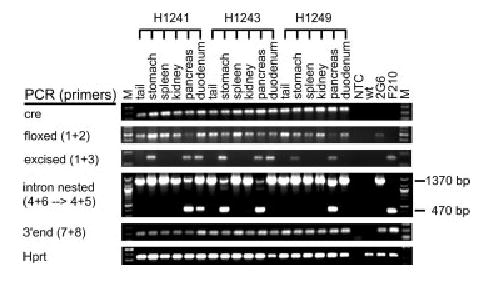
Tissue-specific ORFeusLSL activation by Pdx1-cre. Selected tissues from three doubly heterozygous ORFeusLSL; Pdx1-cre mice were genotyped by PCR for cre transgene (cre), Cre-mediated excision (floxed and excised), retrotransposition (intron nested), 3′ end of ORFeusLSL transgene (3′ end) and endogenous Hprt gene (Hprt). All PCR reactions were performed as in Figure 2b with the exception for detecting retrotransposition activity where a seminested PCR strategy was used.
Germ Cell Specific Activation of ORFeusLSL by Oocyte and Spermatid-Specific Cre
The CAG-cre mouse line has been used to produce animals with complete excision of the floxed target sequence throughout an animal’s body (Sakai and Miyazaki, 1997), but our results indicate that the efficiency is relatively low: 5/12 for doubly heterozygous ORFeusLSL; CAG-cre G1 animals from female CAG-cre parents and 0/6 for those from male CAG-cre parents. An alternative strategy for achieving uniform excision is to use Cre-expressing mouse lines under the regulation of germ line specific promoters. To this end, we first tested an oocyte-specific Cre-expressing mouse line, Zp3-cre, in which Cre expression is controlled by the mouse zona pellucida glycoprotein 3 (Zp3) promoter; expression is restricted to growing oocytes (de Vries et al., 2000). We bred ORFeusLSL females to Zp3-cre males, backcrossed doubly heterozygous ORFeusLSL;Zp3-cre G1 females to C57BL/6 males, and then genotyped the G2 progeny for Cre-mediated excision and retrotransposition (Fig. 4a). Genotyping results for a litter of eight G2 animals are shown along with their G1 maternal parent (Fig. 4b). As expected, no Cre-mediated excision was observed in the tail biopsy of the doubly heterozygous female G1 parent (Fig. 4b, animal H648) as Zp3-cre expression is restricted to oocytes of such females; excised ORFeusL transgene was detected in four G2 animals derived from this female, all had undergone complete LSL excision. Notably, three of these four animals showed complete excision even though they themselves do not carry Zp3-cre (Fig. 4b, animals H698, H702, and H704). Similar analysis was extended to 68 G2 mice from such breedings (summarized in Fig. 4a). Among 36 mice that inherited ORFeusLSL, 18 were positive for Zp3-cre and had undergone complete LSL excision; the remaining 18 were negative for Zp3-cre. Among these, 14/18 had complete LSL excision, but 4/18 lacked excision. Complete excision in the absence of Zp3-cre transgene is not surprising, as data from an independently derived Zp3-cre mouse line suggest that Zp3-cre is expressed early in oocyte maturation, prior to the completion of meiosis I, and Cre produced in the growing oocyte is sufficient to mediate recombination of the target gene after fertilization (Lewandoski et al., 1997). Overall, our data indicate that Zp3-cre is fairly efficient, as 89% (32/36) of target-containing G2 progeny of doubly heterozygous females underwent complete excision. Further analysis on selected tissues from G2 mice that carried either floxed or excised alleles of the ORFeusLSL transgene confirms the absence of mosaic excision, and indicates that the genotyping result of the tail tissue is diagnostic of other tissues from the same animal (Fig. S3). Similar results were obtained with a male germ-line specific transgene Prm1-cre (O’Gorman et al., 1997) (Fig. S4).
FIG. 4.
Activation of ORFeusLSL in female germ line by oocyte-specific Zp3-cre. (a) Breeding scheme and summary of progeny genotypes. (b) Representative genotyping PCR results on tail biopsy for G1, G2, and G3 animals. Symbols used are identical to Figure 2b.
Comparison of Retrotransposition Activity Between Single-Copy and Multiple-Copy ORFeus Transgenes
We have demonstrated that Cre/loxP system can be used to control ORFeus activation in a tissue-specific manner by using a readily available repository of Cre animals. Such floxed ORFeusLSL mouse lines can be easily propagated, as no ORFeus expression or activity will interfere with the well being of the animals before Cre-mediated excision. However, an immediate concern for this Cre/loxP-based conditional ORFeusLSL transgenic system is the relative level of retrotransposition activity from these single-copy transgenes as opposed to an ORFeus mouse line carrying a multicopy ORFeus transgene (An et al., 2006), the unit copy of which is structurally identical to the activated single-copy ORFeusL in this study, including the single loxP site. To address this question, we compared the retrotransposition activity of single-copy and multicopy ORFeus transgenes by iPCR profiling.
We have previously established that when independent replicative iPCR reactions are performed on the same gDNA sample after restriction and ligation, the number and migration positions of DNA bands vary as the result of stochastic amplification of a complex pool of insertions contained in the sample (An et al., 2006). However, it is not known whether the average number of bands per iPCR reaction for each sample represents the relative abundance of insertion events from sample to sample. To test this directly, we created a dilution series of donor-containing samples from our previous multicopy ORFeus mouse line F210 and carried out iPCR reactions on each dilution (Fig. 5a). The results from this experiment indicate that the mean number of bands from independent PCRs mirrors the abundance of insertions in the gDNA preparation, and thus can be used to assess the relative level of retrotransposition activity between different mouse lines. Using the same iPCR technique, we surveyed several ORFeusL-containing G2 progeny of ORFeusLSL;Zp3-cre animals (Fig. 5b, animals H686, H698, H701, H702) and N2 animals from previously described multicopy ORFeus line F210 (Fig. 5b, animals B645 and B649). Among G2 individuals carrying activated single-copy ORFeusL, we observed relatively broader variation on the average number of iPCR bands per animal as compared to N2 animals from line F210. Overall, the level of retrotransposition activity in somatic tissues for animals carrying single-copy ORFeusL (11 bands/lane) is roughly 65% that of animals from multi-copy line F210 (17 bands/lane) if the number of iPCR bands is used as a proxy for retrotransposition activity. In fact, ORFeusL insertions can be readily identified from these G2 animals (Table S1).
FIG. 5.

Comparison of ORFeus activity between single-copy trans-gene and multiple-copy transgene. (a) iPCR-based insertion profiling on a dilution series of a multi-copy ORFeus-containing sample. A donor-containing sample from line F210 (An et al., 2006) was diluted at indicated dilution factor with wild-type (wt) mouse gDNA samples, and subjected to iPCR amplification. An undiluted F210-derived sample and a wt sample were used as controls. (b) Four G2 samples parented by doubly heterozygous ORFeusLSL; Zp3-cre female G1 mouse were analyzed by iPCR and compared to two independent samples from line F210. The number of discernable bands in each lane is counted and the average among triplicate PCRs for each sample is indicated at the bottom of the gel.
To explore the utility of this Cre/loxP-based single-copy ORFeusLSL system for germ line applications, we further evaluated the germ line insertion frequency of this single-copy ORFeus transgene. Germ line insertions are defined as retrotransposition events that occur in germ cells of an animal containing an activated allele of the ORFeusLSL transgene. Accordingly, a germ line insertion can be present in ORFeusLSL-containing animals if the ORFeus transgene has been activated in their transgenic parent’s germ cells; alternatively, it can be detected in donorless progeny animals if the retrotrans-position event occurs prior to meiosis II in the corresponding germ cell and subsequently segregate away from the donor transgene. To calculate germ line insertion frequency, we considered germ line insertions only in donorless animals so that somatic insertions that occurred in donor-containing animals would not confound the analysis. We bred G2 mice containing an activated ORFeusL allele to wild-type C57BL/6 mice (Fig. 4a), and genotyped 161 G3 animals. Among these mice, 78 were donor-negative (Fig. 4b, animals H866, H868, H869, H871, and H872), and 9% (7/78) had insertion signals by intron PCR (Fig. 4b, animal H872). This is equivalent to an average germ line insertion frequency of 9.4% per animal. In contrast, the germ line insertion frequency is 33% for line F210 which had 310 copies of donor transgene (An et al., 2006), averaging 3.3% per unit copy. Therefore, our single-copy ORFeusL mouse line is 3threefold more active per copy in the germ line than mouse line F210.
DISCUSSION
We have established a conditional L1 system in which the ORFeus transgene is kept totally inactive in mice but can be activated in specific tissues using different Cre mouse lines. The tissue specificity of ORFeus activation is jointly determined by the promoter specificity of the target ORFeusLSL transgene and that of the Cre transgene. The use of a ubiquitous promoter to drive the expression of ORFeus transgene offers a significant advantage over an alternative design with a more restrictive promoter, as the activation of our ORFeus transgene will be determined solely by the expression pattern of the Cre transgene (Nagy, 2000). Thus, once a single line is made it has the potential to be utilized in a broader range of studies by exploiting the ever-expanding repository of Cre mouse lines (http://nagy.mshri.on.ca/cre/). This compares favorably with reinvesting time and resources in constructing individual ORFeus mouse lines controlled by distinct tissue-specific promoters. Indeed, we have demonstrated the activation of ORFeusLSL trans-gene globally by ubiquitous CAG-cre (Sakai and Miyazaki, 1997), in defined tissue types by pancreas-specific Pdx1-cre (Hingorani et al., 2003), and in female and male germ lines by oocyte-specific Zp3-cre (de Vries et al., 2000) and spermatid-specific Prm1-cre (O’Gorman et al., 1997), respectively. Overall, the tissue specificity is consistent with the known promoter specificity of the Cre transgene. In contrast, prior to crossing to Cre-expressing mice, we detected no excision of the LSL cassette even with a highly sensitive PCR based assay. Thus the Cre/loxP switch allows one to maintain the transgene free of ORFeus expression and retrotransposition, i.e. in a genetically stable form. As part of the Z/AP-like design of Cre/loxP configuration, the transgene also incorporates a selection cassette, conferring yet an additional advantage in that active mouse ES cell lines can be selected/evaluated before committing to make a mouse line.
A remarkable feature of our conditional ORFeusLSL system is that it employs a single-copy L1 transgene. All previous efforts on making L1 transgenics used standard transgenesis (An et al., 2006; Babushok et al., 2006; Muotri et al., 2005; Ostertag et al., 2002; Prak et al., 2003), which normally results in a multicopy concatemer of donor transgenes (Palmiter and Brinster, 1986). Such multicopy donor concatemers are not compatible with the Cre/loxP approach as sequences between two distal loxP sites are subjected to Cre-mediated excision, leaving behind transgenes at reduced copy numbers (Garrick et al., 1998; Lakso et al., 1996). Although L1 possesses a multiplicative copy-and-paste mode of replication and a single copy of L1 can theoretically spawn an unlimited number of insertions, our initial concern was whether the reduced copy number in the ORFeusLSL transgene would adversely affect its retrotransposition frequency. To this end, we compared both somatic and germ line retrotransposition frequencies between the activated single-copy ORFeusL and our previously reported 310 copy ORFeus concatemer donor (An et al., 2006), the unit copy of which is structurally identical to the activated single-copy ORFeusL in this study. The single copy line is 65% as active as the multicopy line in somatic tissues and 28% that of the multicopy line in the germ line. However, on a per-copy basis, the single-copy line is about threefold more active than the multicopy line in the germ line and about 6.5-fold more active somatically. A plausible explanation for the high activity of the single-copy line is that the ORFeusLSL transgene was integrated at a highly active genomic locus as the result of lacZ screening in ES cells. An alternative yet complementary explanation is that the high level of activity might reflect a potential intrinsic advantage of a single-copy transgene over multicopy transgene arrays as the latter tend to be the target for repeat-induced gene silencing (Garrick et al., 1998). In this regard, a direct comparison of single-copy and multi-copy ORFeus transgenes at identical genomic loci would be highly desirable. Regardless the underlying mechanisms, our results demonstrate that ORFeus can be very effective in retrotransposition when present in even a single copy in the mouse genome.
The efficiency of Cre-mediated recombination is affected by several factors other than the promoter driving Cre expression (Sauer, 1998; Schmidt-Supprian and Rajewsky, 2007). Over the course of this study, we encountered lower-than-expected excision efficiency for several Cre mouse lines tested. For example, the CAG-cre mouse line was reported to result in complete excision of the target transgene in all G1 progeny of female CAG-cre parents regardless of their CAG-cre status (Sakai and Miyazaki, 1997); but we observed mosaic excision in a significant proportion of doubly heterozygous G1 animals and no excision in G1 animals derived from female CAG-cre heterozygotes that did not inherit CAG-cre. We also observed mosaic excision of ORFeusLSL transgene using a separate Cre-expressing mouse line under the regulation of human CMV minimal promoter (CMVmini-cre (Schwenk et al., 1995); not shown). In addition, we found that two germ line specific Cre mouse lines Zp3-cre (de Vries et al., 2000) and Prm1-cre (O’Gorman et al., 1997), although resulting in complete excision, were not 100% efficient in the context of our construct. We initially hypothesized that the unexpected excision patterns observed here could reflect chromatin-related differential accessibility of the target loxP locus (Baubonis and Sauer, 1993; Vooijs et al., 2001). Therefore, we set out to test CAG-cre and CMVmini-cre mouse lines on two independent ORFeusLSL target lines (2G6 and 1E2) that had identical transgene sequence at different genomic loci (Fig. S2 and data not shown). The seemingly identical results obtained from such experiments favor an alternative explanation, i.e. some target sequence-specific effects. The excision efficiency by a different site-specific recombinase, FLP, is known to be adversely affected by an increasing distance between its target sites (Ringrose et al., 1999). The distance between loxP sites in ORFeusLSL is 4.9 kb, at least 2.5-fold longer than that in target transgenes tested by the original reports for these Cre mouse lines (de Vries et al., 2000; O’Gorman et al., 1997; Sakai and Miyazaki, 1997; Schwenk et al., 1995). Thus, our data are consistent with inefficient excision by Cre recombinase on more distantly positioned loxP sites. Overall, among the Cre lines tested, Zp3-cre was the most efficient in achieving complete excision of floxed target sequence in all tissues; CAG-cre, although less efficient, has the advantage of generating complete excision in a single breeding cycle.
On a different note, our study presents potential evidence that adds to the increasing literature for Cre-induced toxicity (Schmidt-Supprian and Rajewsky, 2007). Such toxicity may explain the apparently low prevalence of CAG-cre transgene among G1 progeny of heterozygous CAG-cre male parents derived from two independent ORFeusLSL lines:10/38 for line 2G6 (Fig. 2a; P < 0.01) and 14/47 for line 1E2 (Fig. S2; P < 0.01). It appears that this Cre-mediated effect is independent of the presence of exogenous loxP sites as approximately half of CAG-cre positive G1 progeny carried the target ORFeus transgene. In addition, the segregation of the CAG-cre transgene in G1 progeny from heterozygous CAG-cre female parents conformed to a Mendelian ratio, suggesting this is an effect on the male germ line. The underlying mechanisms of this effect are a subject for future investigation. Nevertheless, it is noteworthy that the paternal genome may be more susceptible to Cre-mediated actions than the maternal counterpart as paternal DNA is uniquely remodeled from a nucleosome-based to a protamine-based chromatin during spermatogenesis (Kimmins and Sassone-Corsi, 2005). It has been documented that chronic expression of Cre recombinase in postmeiotic spermatids from the Prm1 promoter results in pronounced chromosome rearrangements and complete male sterility (Schmidt et al., 2000).
Our Cre/loxP-based conditional ORFeusLSL transgene presents great opportunities for probing L1 biology in vivo. Endogenous human and mouse L1 protein products are predominantly detected in germ cells and rarely in somatic tissues except for certain types of gonadal somatic cells (Branciforte and Martin, 1994; Ergun et al., 2004; Trelogan and Martin, 1995). Scores of disease-causing L1 retrotransposition events have been reported (Ostertag and Kazazian, 2001) but the developmental timing of L1 retrotransposition can be deduced only for two such events: in one case, it apparently originated during maternal meiosis I (Brouha et al., 2002), and in another during early embryogenesis resulting in somatic and germ line mosaicism (van den Hurk et al., 2007). Additional in vivo evidence for L1 retrotransposition in somatic tissues is scarce. There is a single case report on a somatic retrotransposition event from a colorectal cancer patient (Miki et al., 1992). Recent transgenic studies using human L1s under the regulation of endogenous L1 promoters suggest they can retrotranspose not only in mouse germ cells (Muotri et al., 2005; Ostertag et al., 2002) but also in the brain (Muotri et al., 2005), presumably as the result of transcriptional activation of the L1 transgene in these cellular compartments. Driven by a constitutive CAG promoter, our conditional ORFeusLSL system is not designed to address the developmental timing of L1 expression, but it is perfectly suited for dissecting cellular requirements for retrotransposition. In fact, using Pdx1-cre, our study demonstrates that all of the host factors and machinery required for L1 retrotransposition are intact and available in several somatic tissues (pancreas, duodenum and stomach) in which transposition normally does not occur in the context of a living animal, a question that could not be asked previously. We anticipate further experiments could provide more insights into mechanisms controlling L1 activity in vivo, especially when coupled with inducible Cre-expressing mouse lines. For example, it has been debated whether L1 retrotransposition can occur in nondividing cells. Previous attempts were performed in cell culture with both immortalized cancer cells and primary cells by either transfection or by adenoviral infection (Kubo et al., 2006; Shi et al., 2007), reaching conflicting conclusions. Findings from these studies can be validated by using ORFeusLSL mice as an in vivo model, which naturally provide a wide range of both actively dividing and terminally differentiated cell types. Particularly, the effect of the degree of differentiation on retrotransposition may be studied by examining the epidermis, which is composed of multiple stratified layers of increasingly differentiated keratinocytes. An excellent example of using inducible Cre recombinase in studying dynamic Cre-mediated excision and expression of a reporter gene in epidermis has been previously reported (Brocard et al., 1997).
Taken together, by coupling Cre/loxP with ORFeus retrotransposon, we have demonstrated a conditional L1 transgenic system that can be regulated in a tissue-specific fashion by crossing to a wide variety of readily available Cre mouse lines. The tight control of L1 activity by Cre-mediated excision should prove instrumental not only in deriving potent gene trap-equipped ORFeus lines for use in genome-wide mutagenesis studies but also for probing L1 functions in vivo, especially in conjunction with inducible Cre recombinase.
METHODS
Plasmids
The ORFeusLSL transgene contains the following sequence elements: CAG promoter and loxP-β-geo-stop-loxP sequences from pQX107 (a gift from Jeremy Nathans), ORFeus coding sequences from pBSsmL1 (Han and Boeke, 2004), a modified gfp-based retrotransposition indicator cassette from pRSVGFPuvINT, and β-globin polyadenylation signal from pQX107. To make pRSVGFPuvINT, the Rous Sarcoma Virus promoter was PCR amplified from pREP10 (Invitrogen) and cloned into the EcoRV site of pBluescriptSK(2) (Stratagene) to make pBSRSV; a GFPuvINT fragement was converted from the plasmid pBSKS-EGFP-INT (Ostertag et al., 2000) by a series of mutagenesis/fusion PCRs, digested with BamHI/EcoRV and cloned into the corresponding sites of pBluescriptKS(2) to generate pBSGFPuvINT; the RSV promoter from pBSRSV was removed with NheI/AvrII and cloned into the corresponding sites of pBSGFPuvINT to make pRSVGFPuvINT. To make pBSsmL1glob, the β-globin polyadenylation signal was PCR amplified from the plasmid pQX107, digested with BamHI/EcoRI, and cloned into the corresponding sites of pBSsmL1 to produce pBSsmL1glob. The RSVGFPu-vINT cassette from pRSVGFPuvINT was removed with BamHI/EcoRV and cloned into the BamHI/HpaI sites of pBSsmL1glob to make pBSsmL1GFPuv. The CAG promoter and LSL cassette from pQX107 was removed with NotI/XmnI, blunted with Klenow fragment, and cloned into the BstZ17I sites of pBSsmL1GFPuv to make ORFeusLSL, which was sequencingly verified in its entirety.
Transgenic Mice
C57BL/6J Bruce4 embryonic stem cells (Koentgen et al., 1993) were electroporated with NotI-linearized ORFeusLSL transgenic construct and selected with 200 lg/ml G418. G418-resistant ES clones were screened for β-galactosidase (lacZ) expression by X-gal staining and the transgene copy number was determined by Southern blotting of HindIII-digested gDNA with 5′ and 3′ probes. Primers for generating these probes are listed in Table S1. Three independent ES clones with single copy trans-genes and high levels of lacZ expression were selected from 3500 ES cell clones and injected into BALB/c blastocysts, and the resulting chimeric males were mated to C57BL/6J females. Founders were identified by Southern blot and maintained as heterozygotes. All Cre mouse lines used in this study have been reported previously by various groups: CAG-cre mice (Sakai and Miyazaki, 1997) were provided by Charles Hawkins; CMV-Cre (Schwenk et al., 1995) and Pdx1-cre mice (Hingorani et al., 2003) were maintained by Frank Koentgen, and Anirban Maitra, respectively; Zp3-cre (de Vries et al., 2000) and Prm1-cre mice (O’Gorman et al., 1997) were acquired from Jackson Laboratories (Maine, USA). Protocols for the use of mice were approved by Institutional Animal Care and Use Committee.
Genotyping PCR and iPCR
All primers used in this study are listed in Table S2. Genotyping PCR reactions were performed as previously described (An et al., 2006) except for PCR intron nested, which entails a second round PCR with primers 4 and 5 for 25 cycles by using 1/25 of the amplification product from PCR intron as DNA template. The 3′ genomic junctions of ORFeus insertions were recovered from doubly heterozygous ORFeusLSL; Pdx1-cre G1 animals by a nested iPCR protocol, which includes a second round amplification for 25 cycles by using 1/25 of the first round iPCR reaction of 35 cycles as previously described (An et al., 2006).
Supplementary Material
This article contains supplementary material available via the Internet at http://www.interscience.wiley.com/jpages/1526-954X/suppmat.
Acknowledgments
We thank Jeremy Nathans for helpful discussions and Charles Hawkins for providing the CAG-cre mice.
Abbreviations
- gDNA
genomic DNA
- iPCR
inverse PCR
- L1
long interspersed element type 1
- LSL
loxP-β-geo-stop-loxP
Footnotes
Contract grant sponsor: Life Sciences Research Foundation (Affymetrix Postdoctoral Fellowship); National Institutes of Health.
LITERATURE CITED
- An W, Han JS, Wheelan SJ, Davis ES, Coombes CE, Ye P, Triplett C, Boeke JD. Active retrotransposition by a synthetic L1 element in mice. Proc Natl Acad Sci USA. 2006;103:18662–18667. doi: 10.1073/pnas.0605300103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babushok DV, Ostertag EM, Courtney CE, Choi JM, Kazazian HH., Jr L1 integration in a transgenic mouse model. Genome Res. 2006;16:240–250. doi: 10.1101/gr.4571606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baubonis W, Sauer B. Genomic targeting with purified Cre recombinase. Nucleic Acids Res. 1993;21:2025–2029. doi: 10.1093/nar/21.9.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke JD. LINEs and Alus—The polyA connection. Nat Genet. 1997;16:6–7. doi: 10.1038/ng0597-6. [DOI] [PubMed] [Google Scholar]
- Branciforte D, Martin SL. Developmental and cell type specificity of LINE-1 expression in mouse testis: Implications for transposition. Mol Cell Biol. 1994;14:2584–2592. doi: 10.1128/mcb.14.4.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocard J, Warot X, Wendling O, Messaddeq N, Vonesch JL, Chambon P, Metzger D. Spatio-temporally controlled site-specific somatic mutagenesis in the mouse. Proc Natl Acad Sci USA. 1997;94:14559–14563. doi: 10.1073/pnas.94.26.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouha B, Meischl C, Ostertag E, de Boer M, Zhang Y, Neijens H, Roos D, Kazazian HH., Jr Evidence consistent with human L1 retro-transposition in maternal meiosis I. Am J Hum Genet. 2002;71:327–336. doi: 10.1086/341722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CM, Largaespada DA. Insertional mutagenesis in mice: New perspectives and tools. Nat Rev Genet. 2005;6:568–580. doi: 10.1038/nrg1638. [DOI] [PubMed] [Google Scholar]
- de Vries WN, Binns LT, Fancher KS, Dean J, Moore R, Kemler R, Knowles BB. Expression of Cre recombinase in mouse oocytes: A means to study maternal effect genes. Genesis. 2000;26:110–112. [PubMed] [Google Scholar]
- Ergun S, Buschmann C, Heukeshoven J, Dammann K, Schnieders F, Lauke H, Chalajour F, Kilic N, Stratling WH, Schumann GG. Cell type-specific expression of LINE-1 open reading frames 1 and 2 in fetal and adult human tissues. J Biol Chem. 2004;279:27753–27763. doi: 10.1074/jbc.M312985200. [DOI] [PubMed] [Google Scholar]
- Esnault C, Maestre J, Heidmann T. Human LINE retrotransposons generate processed pseudogenes. Nat Genet. 2000;24:363–367. doi: 10.1038/74184. [DOI] [PubMed] [Google Scholar]
- Friedrich G, Soriano P. Promoter traps in embryonic stem cells: A genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- Garrick D, Fiering S, Martin DI, Whitelaw E. Repeat-induced gene silencing in mammals. Nat Genet. 1998;18:56–59. doi: 10.1038/ng0198-56. [DOI] [PubMed] [Google Scholar]
- Goodier JL, Ostertag EM, Du K, Kazazian HH., Jr A novel active L1 retrotransposon subfamily in the mouse. Genome Res. 2001;11:1677–1685. doi: 10.1101/gr.198301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Boeke JD. A highly active synthetic mammalian retrotransposon. Nature. 2004;429:314–318. doi: 10.1038/nature02535. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- Hohjoh H, Singer MF. Cytoplasmic ribonucleoprotein complexes containing human LINE-1 protein and RNA. EMBO J. 1996;15:630–639. [PMC free article] [PubMed] [Google Scholar]
- Kazazian HH., Jr Mobile elements: Drivers of genome evolution. Science. 2004;303:1626–1632. doi: 10.1126/science.1089670. [DOI] [PubMed] [Google Scholar]
- Kimmins S, Sassone-Corsi P. Chromatin remodelling and epigenetic features of germ cells. Nature. 2005;434:583–589. doi: 10.1038/nature03368. [DOI] [PubMed] [Google Scholar]
- Koentgen F, Suss G, Stewart C, Steinmetz M, Bluethmann H. Targeted disruption of the MHC class II Aa gene in C57BL/6 mice. Int Immunol. 1993;5:957–964. doi: 10.1093/intimm/5.8.957. [DOI] [PubMed] [Google Scholar]
- Kubo S, Seleme MC, Soifer HS, Perez JL, Moran JV, Kazazian HH, Jr, Kasahara N. L1 retrotransposition in nondividing and primary human somatic cells. Proc Natl Acad Sci USA. 2006;103:8036–8041. doi: 10.1073/pnas.0601954103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulpa DA, Moran JV. Ribonucleoprotein particle formation is necessary but not sufficient for LINE-1 retrotransposition. Hum Mol Genet. 2005;14:3237–3248. doi: 10.1093/hmg/ddi354. [DOI] [PubMed] [Google Scholar]
- Kulpa DA, Moran JV. Cis-preferential LINE-1 reverse transcriptase activity in ribonucleoprotein particles. Nat Struct Mol Biol. 2006;13:655–660. doi: 10.1038/nsmb1107. [DOI] [PubMed] [Google Scholar]
- Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandoski M, Wassarman KM, Martin GR. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr Biol. 1997;7:148–151. doi: 10.1016/s0960-9822(06)00059-5. [DOI] [PubMed] [Google Scholar]
- Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. Z/AP, a double reporter for cre-mediated recombination. Dev Biol. 1999;208:281–292. doi: 10.1006/dbio.1999.9209. [DOI] [PubMed] [Google Scholar]
- Martin SL. Ribonucleoprotein particles with LINE-1 RNA in mouse embryonal carcinoma cells. Mol Cell Biol. 1991;11:4804–4807. doi: 10.1128/mcb.11.9.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki Y, Nishisho I, Horii A, Miyoshi Y, Utsunomiya J, Kinzler KW, Vogelstein B, Nakamura Y. Disruption of the APC gene by a retrotransposal insertion of L1 sequence in a colon cancer. Cancer Res. 1992;52:643–645. [PubMed] [Google Scholar]
- Miller AD. Development and application of retroviral vectors. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses Plainview. New York: Cold Spring Harbor Laboratory Press; 1997. pp. 437–473. [PubMed] [Google Scholar]
- Moran JV, Holmes SE, Naas TP, DeBerardinis RJ, Boeke JD, Kazazian HH., Jr High frequency retrotransposition in cultured mammalian cells. Cell. 1996;87:917–927. doi: 10.1016/s0092-8674(00)81998-4. [DOI] [PubMed] [Google Scholar]
- Muotri AR, Chu VT, Marchetto MC, Deng W, Moran JV, Gage FH. Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition. Nature. 2005;435:903–910. doi: 10.1038/nature03663. [DOI] [PubMed] [Google Scholar]
- Naas TP, DeBerardinis RJ, Moran JV, Ostertag EM, Kingsmore SF, Seldin MF, Hayashizaki Y, Martin SL, Kazazian HH. An actively retrotransposing, novel subfamily of mouse L1 elements. EMBO J. 1998;17:590–597. doi: 10.1093/emboj/17.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A. Cre recombinase: The universal reagent for genome tailoring. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- O’Gorman S, Dagenais NA, Qian M, Marchuk Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc Natl Acad Sci USA. 1997;94:14602–14607. doi: 10.1073/pnas.94.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostertag EM, DeBerardinis RJ, Goodier JL, Zhang Y, Yang N, Gerton GL, Kazazian HH., Jr A mouse model of human L1 retrotransposition. Nat Genet. 2002;32:655–660. doi: 10.1038/ng1022. [DOI] [PubMed] [Google Scholar]
- Ostertag EM, Kazazian HH., Jr Biology of mammalian L1 retrotransposons. Annu Rev Genet. 2001;35:501–538. doi: 10.1146/annurev.genet.35.102401.091032. [DOI] [PubMed] [Google Scholar]
- Ostertag EM, Prak ET, DeBerardinis RJ, Moran JV, Kazazian HH., Jr Determination of L1 retrotransposition kinetics in cultured cells. Nucleic Acids Res. 2000;28:1418–1423. doi: 10.1093/nar/28.6.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Brinster RL. Germline transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prak ET, Dodson AW, Farkash EA, Kazazian HH., Jr Tracking an embryonic L1 retrotransposition event. Proc Natl Acad Sci USA. 2003;100:1832–1837. doi: 10.1073/pnas.0337627100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringrose L, Chabanis S, Angrand PO, Woodroofe C, Stewart AF. Quantitative comparison of DNA looping in vitro and in vivo: Chromatin increases effective DNA flexibility at short distances. EMBO J. 1999;18:6630–6641. doi: 10.1093/emboj/18.23.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Miyazaki J. A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem Biophys Res Commun. 1997;237:318–324. doi: 10.1006/bbrc.1997.7111. [DOI] [PubMed] [Google Scholar]
- Sauer B. Inducible gene targeting in mice using the Cre/lox system. Methods. 1998;14:381–392. doi: 10.1006/meth.1998.0593. [DOI] [PubMed] [Google Scholar]
- Schmidt EE, Taylor DS, Prigge JR, Barnett S, Capecchi MR. Illegitimate Cre-dependent chromosome rearrangements in transgenic mouse spermatids. Proc Natl Acad Sci USA. 2000;97:13702–13707. doi: 10.1073/pnas.240471297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol. 2007;8:665–668. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Seluanov A, Gorbunova V. Cell divisions are required for L1 retrotransposition. Mol Cell Biol. 2007;27:1264–1270. doi: 10.1128/MCB.01888-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelogan SA, Martin SL. Tightly regulated, developmentally specific expression of the first open reading frame from LINE-1 during mouse embryogenesis. Proc Natl Acad Sci USA. 1995;92:1520–1524. doi: 10.1073/pnas.92.5.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hurk JA, Meij IC, del Carmen Seleme M, Kano H, Nikopoulos K, Hoefsloot LH, Sistermans EA, de Wijs IJ, Mukhopadhyay A, Plomp AS, de Jong PT, Kazazian HH, Cremers FP. L1 retrotransposition can occur early in human embryonic development. Hum Mol Genet. 2007;16:1587–1592. doi: 10.1093/hmg/ddm108. [DOI] [PubMed] [Google Scholar]
- Vooijs M, Jonkers J, Berns A. A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Rep. 2001;2:292–297. doi: 10.1093/embo-reports/kve064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Gilbert N, Ooi SL, Lawler JF, Ostertag EM, Kazazian HH, Boeke JD, Moran JV. Human L1 retrotransposition: cis preference versus trans complementation. Mol Cell Biol. 2001;21:1429–1439. doi: 10.1128/MCB.21.4.1429-1439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains supplementary material available via the Internet at http://www.interscience.wiley.com/jpages/1526-954X/suppmat.