Abstract
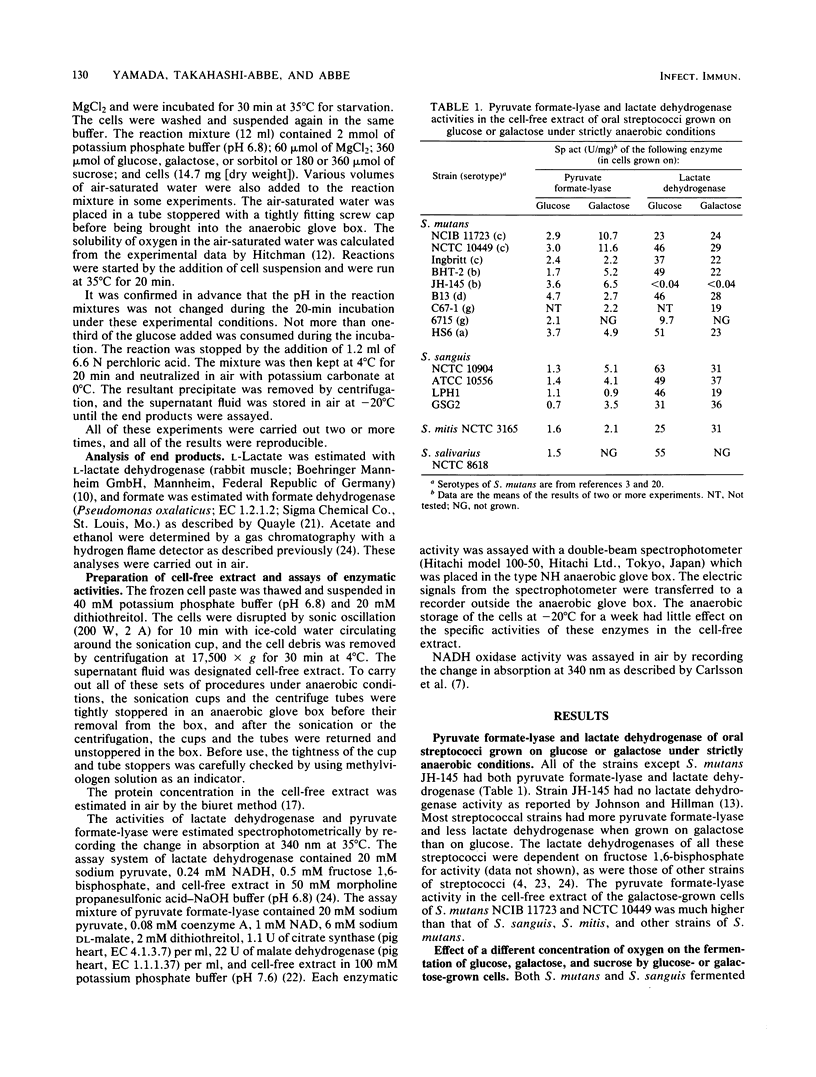
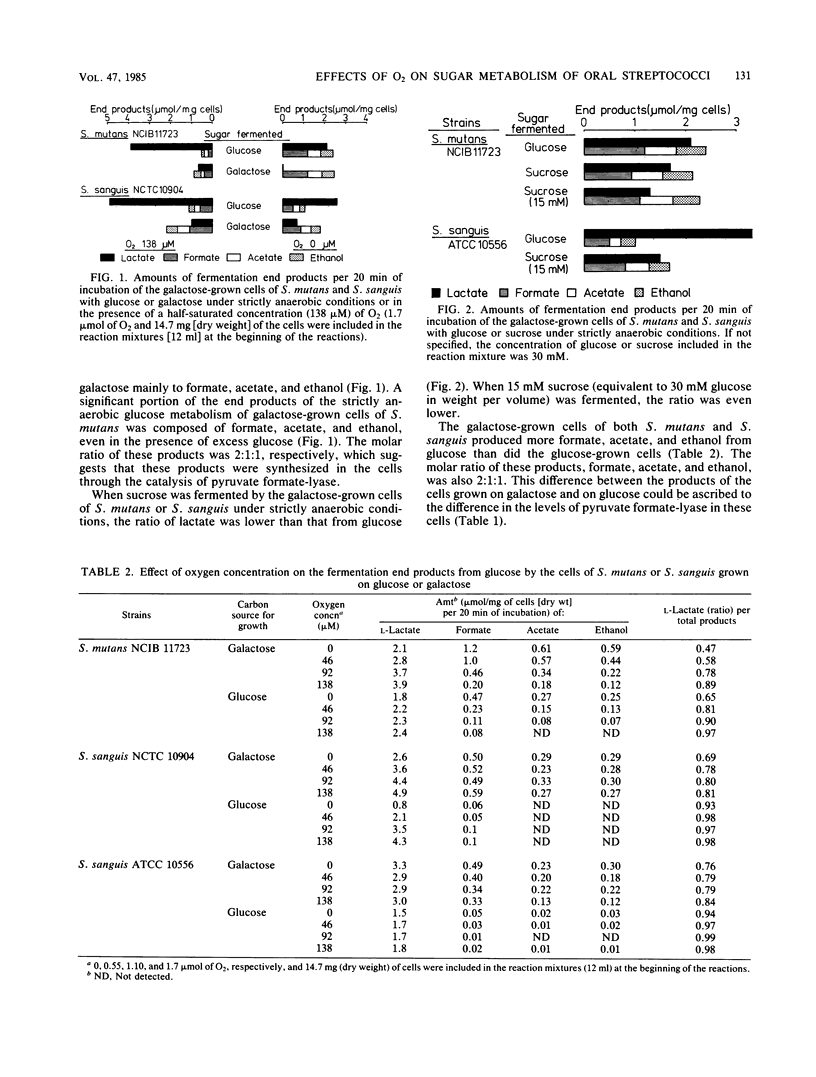
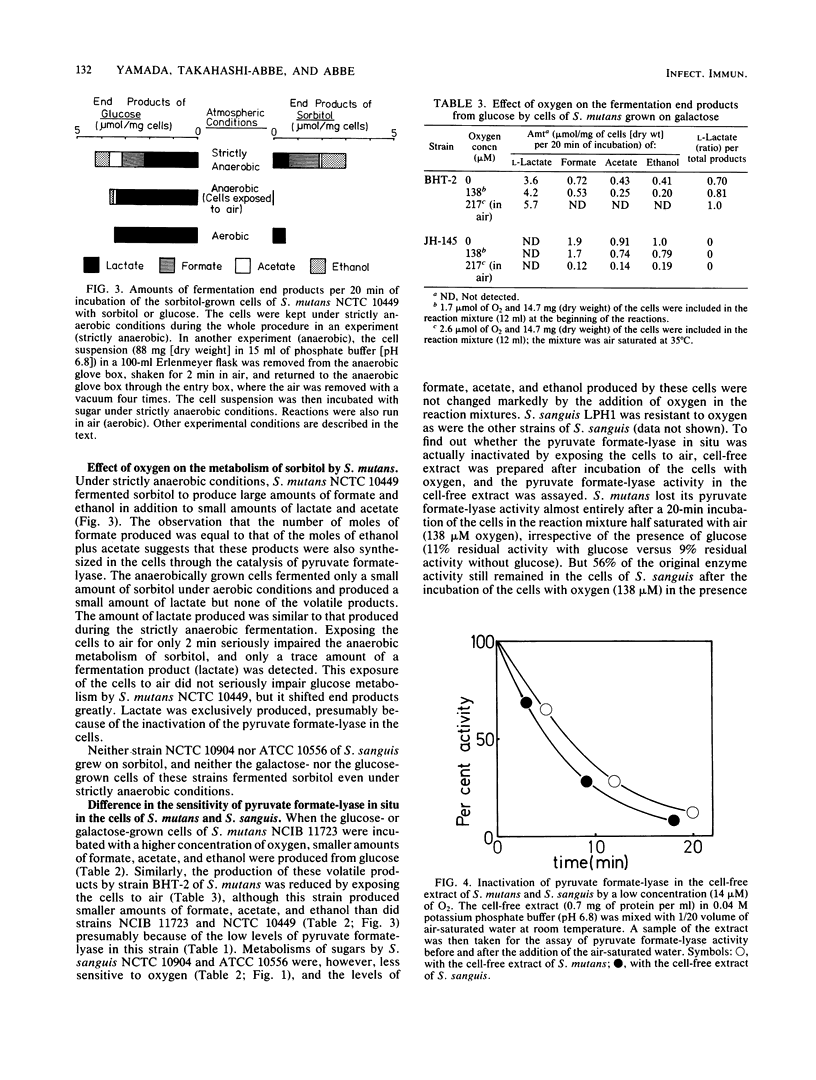
The strictly anaerobic metabolism of sugar in strains of Streptococcus mutans and Streptococcus sanguis was studied because deep layers of dental plaque are strictly anaerobic. Galactose-grown cells of these streptococcal strains had higher pyruvate formate-lyase activity than did glucose-grown cells. Among these strains, two strains of S. mutans had a significantly higher pyruvate formate-lyase activity than did the others. This enzyme is extremely sensitive to oxygen, and even in situ the enzyme was inactivated by exposure of the cells to air. Lactate was less than 50% of the total end product of the strictly anaerobic incubation of the galactose-grown cells of S. mutans with excess glucose, and a significant amount of formate, acetate, and ethanol was produced through the catalysis of pyruvate formate-lyase. But the cells exclusively produced lactate when exposed to air for 2 min before the anaerobic incubation. The metabolism of sorbitol by S. mutans was seriously impaired by the exposure of the cells to oxygen, and the metabolic rate was reduced to less than 1/20 of that found under strictly anaerobic conditions because of the inactivation of pyruvate formate-lyase. S. sanguis produced a smaller amount of the volatile products from glucose than did S. mutans because of the low level of pyruvate formate-lyase. However, the pyruvate formate-lyase in situ in S. sanguis was less sensitive to oxygen than was that in S. mutans. Because of this low sensitivity, S. sanguis metabolized glucose more rapidly under aerobic conditions, whereas the rates of the aerobic and anaerobic metabolism of glucose by S. mutans were similar, which suggests that S. mutans rather than S. sanguis can sustain the rapid sugar metabolism in the deep layers of dental plaque.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbe K., Takahashi S., Yamada T. Involvement of oxygen-sensitive pyruvate formate-lyase in mixed-acid fermentation by Streptococcus mutans under strictly anaerobic conditions. J Bacteriol. 1982 Oct;152(1):175–182. doi: 10.1128/jb.152.1.175-182.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbe K., Yamada T. Purification and properties of pyruvate kinase from Streptococcus mutans. J Bacteriol. 1982 Jan;149(1):299–305. doi: 10.1128/jb.149.1.299-305.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Brown A. T., Wittenberger C. L. Fructose-1,6-diphosphate-dependent lactate dehydrogenase from a cariogenic streptococcus: purification and regulatory properties. J Bacteriol. 1972 May;110(2):604–615. doi: 10.1128/jb.110.2.604-615.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J. A numerical taxonomic study of human oral streptococci. Odontol Revy. 1968;19(2):137–160. [PubMed] [Google Scholar]
- Carlsson J., Grahnén H., Jonsson G., Wikner S. Establishment of Streptococcus sanguis in the mouths of infants. Arch Oral Biol. 1970 Dec;15(12):1143–1148. doi: 10.1016/0003-9969(70)90005-1. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Iwami Y., Yamada T. Hydrogen peroxide excretion by oral streptococci and effect of lactoperoxidase-thiocyanate-hydrogen peroxide. Infect Immun. 1983 Apr;40(1):70–80. doi: 10.1128/iai.40.1.70-80.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerlad R. J. Dental caries research in gnotobiotic animals. Caries Res. 1968;2(2):139–146. doi: 10.1159/000259552. [DOI] [PubMed] [Google Scholar]
- Hillman J. D. Lactate dehydrogenase mutants of Streptococcus mutans: isolation and preliminary characterization. Infect Immun. 1978 Jul;21(1):206–212. doi: 10.1128/iai.21.1.206-212.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. P., Hillman J. D. Competitive properties of lactate dehydrogenase mutants of the oral bacterium Streptococcus mutans in the rat. Arch Oral Biol. 1982;27(6):513–516. doi: 10.1016/0003-9969(82)90093-0. [DOI] [PubMed] [Google Scholar]
- Katayama T., Suzuki T., Okada S. Clinical observation of dental plaque maturation. Application of oxidation-reduction indicator dyes. J Periodontol. 1975 Oct;46(10):610–613. doi: 10.1902/jop.1975.46.10.610. [DOI] [PubMed] [Google Scholar]
- Kenney E. B., Ash M. M., Jr Oxidation reduction potential of developing plaque, periodontal pockets and gingival sulci. J Periodontol. 1969 Nov;40(11):630–633. doi: 10.1902/jop.1969.40.11.630. [DOI] [PubMed] [Google Scholar]
- Krasse B. Human streptococci and experimental caries in hamsters. Arch Oral Biol. 1966 Apr;11(4):429–436. doi: 10.1016/0003-9969(66)90107-5. [DOI] [PubMed] [Google Scholar]
- Linzer R., Slade H. D. Purification and characterization of Streptococcus mutans group d cell wall polysaccharide antigen. Infect Immun. 1974 Aug;10(2):361–368. doi: 10.1128/iai.10.2.361-368.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikx F. H., Van der Hoeven J. S. Symbiosis of Streptococcus mutans and Veillonella alcalescens in mixed continuous cultures. Arch Oral Biol. 1975 Jul;20(7):407–410. doi: 10.1016/0003-9969(75)90224-1. [DOI] [PubMed] [Google Scholar]
- Perch B., Kjems E., Ravn T. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):357–370. doi: 10.1111/j.1699-0463.1974.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Abbe K., Yamada T. Purification of pyruvate formate-lyase from Streptococcus mutans and its regulatory properties. J Bacteriol. 1982 Mar;149(3):1034–1040. doi: 10.1128/jb.149.3.1034-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN M. J. FRUCTOSE-1,6-DIPHOSPHATE REQUIREMENT OF STREPTOCOCCAL LACTIC DEHYDROGENASES. Science. 1964 Nov 6;146(3645):775–777. doi: 10.1126/science.146.3645.775. [DOI] [PubMed] [Google Scholar]
- Yamada T., Carlsson J. Regulation of lactate dehydrogenase and change of fermentation products in streptococci. J Bacteriol. 1975 Oct;124(1):55–61. doi: 10.1128/jb.124.1.55-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


