Abstract
Purpose
To evaluate early changes in the central retinal response in HIV positive patients without infectious retinitis using multifocal electroretinography (mfERG).
Design
The study was a case control study.
Methods
We evaluated three cohorts - HIV negative controls and two groups of HIV positive patients separated according to their nadir CD4 counts (≥100 and <100 for a minimum of 6 months). MfERG first (FOK) and second order (SOK) kernels were analyzed separately by areas of rings, quadrants and individual hexagons for each cohort.
Results
Of 103 hexagon locations of FOK results there were no significant differences in amplitudes of P1 and N1 across the groups (0.05<p<0.50); although there was a trend for an overall reduction in the amplitudes. Similarly, latency N1 did not differ (0.28<p<0.95). There were significantly delayed latencies of P1 between cohorts across 103 hexagons in both kernels. SOK results also showed significant delay in latencies of P1 and a trend of reduced P1 amplitudes across studied locations among cohorts (0.24<p<0.08).
Conclusion
The results demonstrate widespread delay in latency in HIV positive patients, especially in those with prolonged low (below 100) CD4 nadir counts. These findings suggest early diffuse dysfunction of the inner retina resulted from severe HIV disease even in the era of HAART.
Introduction
As of December 2007 there are over 33 million people infected with human immunodeficiency virus (HIV) worldwide and more than 1.3 million live with HIV in North America alone 1. Since the introduction of highly active antiretroviral therapy (HAART) in 1996 the mortality and rate of severe systemic complications has significantly decreased among these patients. In particular, the incidence of infectious cytomegalovirus (CMV) retinitis has declined 75–85%, as compared to the pre-HAART era 2, 3. Due to immune recovery and an increase of CD4 counts induced by HAART, the incidence of immune recovery uveitis (IRU) and related visual loss has increased up to 63% in patients with regressed CMV 4,5,6. But even inclusive of IRU, the most common ocular manifestation (up to 50% of patients) 7 of HIV disease remains to be non-infectious HIV retinopathy characterized by cotton wool spots, intraretinal hemorrhages and microaneurysms. Although non-infectious HIV retinopathy usually is not associated with loss of visual acuity 8, the visual function in HIV positive patients may be disturbed even without any visible structural damage to the retina. It had been reported previously that visual field 9, 10, contrast sensitivity, color sensitivity 11, 12 and results of the electrophysiological tests 13,14, 15,16 are abnormal in HIV positive patients without any fundoscopic changes. It was also shown that these patients have significantly thinned retinal nerve fiber layer (RNFL) 17, 18.
We previously studied a small group of HIV positive patients without infectious retinitis using a mfERG 30Hz flicker stimulus to determine the outer retina function in these patients by analyzing the first order kernel (FOK). Our results did not show any consistent evidence of significant outer retina damage 19.
Multifocal ERG has been used to localize focal retinal damage occurring in numerous diseases and disorders 20,21,22,23,24,25,26,27 and has been proven to be a reliable diagnostic tool in clinical settings. Mulifocal ERG can measure the function of the outer retina as well as the inner retina. The first order kernel (FOK) reflects the average retinal response after a single flash (mean difference between response to bright and dark stimuli), and the second order kernel (SOK) represents the degree to which the retinal response is affected by an immediately preceding stimulus (dark or light). 28
Although the exact retinal origin of the SOK component is still not fully understood, most investigators agree that the second order kernel parameters reflect early changes in adaptive retinal mechanisms and inner retina cell function. 29, 30 The SOK of multifocal ERG has been shown to be sensitive to determining early retinal abnormalities in diseases involving mainly the inner retina, such as diabetes 22,23,31,32, glaucoma 20,33,34, 35,36 and Leber’s hereditary optic neuropathy 21.
It is not entirely clear if the visual loss in HIV positive patients without infectious retinitis is due to retinal or neural dysfunction. We hypothesized that since early visible retinal changes involve the inner retina (cotton wool spots, microaneurysms), it is most likely that the inner retina is the site of early functional sub-clinical changes leading to structural abnormalities seen later in the course of the disease. This is confirmed by visual field abnormalities showing loss of retinal nerve fiber layer (Goldbaum MH et al.. IOVS 2007;48: ARVO E-Abstract 704).
As we previously showed that the outer retina is not the main source of abnormalities in these patients 19, the current study is designed to determine the extent and localization of inner retina dysfunction. In our previous study we did not use the algorithm sensitive to determining inner retina function, therefore for this study we chose to use the double flash recording algorithm (SOK) of mfERG that allowed us to investigate the first and second order kernel components of mfERG responses simultaneously (SOK algorithm is described in Methods section in details). Our goal was to determine if there is a difference in FOK or SOK responses between HIV negative controls and HIV positive patients without infectious retinitis with low (below 100) or high (over 100) CD4 counts.
Methods
A total of 147 participants were tested in our study; 106 were chosen from a cohort of HIV positive patients seen at the Jacobs Retina Center at the University of California San Diego (UCSD) from May 2005 to September 2006; and 41 HIV negative participants (73 eyes) volunteered for participation in the control group. The Institutional Review Board at UCSD approved the study protocol and study procedures conformed to the Health Insurance Portability and Accountability Act (HIPAA) regulations and the Declaration of Helsinki for research involving human subjects.
The 106 HIV positive patients were divided into two groups according to their nadir CD4 counts. The high CD4 count group (H) included 50 patients (86 eyes) whose nadir CD4 count never dropped below 100 and the low CD4 count group (L) included 56 patients (85 eyes) whose nadir CD4 count was below 100 for a minimum of 6 months. Table 1 describes the patients’ characteristics for each of the three cohorts. There was no significant difference in age between the groups.
Table 1.
Participant Characteristics Stratified by Cohort
| HIV positive Low | HIV positive High | HIV negative | |
|---|---|---|---|
| nadir CD4 counts | nadir CD4 counts | Control Group | |
| (n=56/85 eyes) | (n=50/86 eyes) | (n=41/73 eyes) | |
| Age (years) | 46.1±9.7 | 48.2±8.2 | 41.4±14.1 |
| Gender: women | 7 (12.5%) | 4 (8%) | 17 (41.5%) |
| Mean visual acuity | − 0.015 (± 0.11) | − 0.05 (± 0.13) | −0.02 (± 0.12) |
| (ETDRS)* | [=20/19] | [=20/18] | [=20/19] |
| Mean IOP (mm Hg) | 13.2 ± 2.6 | 13.5 ± 2.6 | 12.9 ± 2.2 |
| Per-patient number of | |||
| eyes tested† | |||
| Both eyes | 52% (58 eyes) | 72% (72 eyes) | 78% (64 eyes) |
| Single eye | 48% (27 eyes) | 28% (14 eyes) | 22% (9 eyes) |
Note: Mean (SD) for continuous variables; frequency (percent) for discrete variables
Visual acuity data is presented in logMAR (±SD)[Snellen equivalent]
In control and High CD4 group one eye was tested for the reason of time and participant’s convenience; in Low CD4 count group only one eye was tested if patient had history of CMV retinitis, currently active CMV retinitis or other retinal disease.
We tested eyes without any visible retinal changes, except CWS due to non-infectious HIV retinopathy, as determined by ophthalmoscopy and fundus photography. Four of the eyes had non-infectious HIV retinopathy (CWS) at the time of exam and we did not exclude those eyes. Also, we did not exclude patients who has had a history of HIV retinopathy but at the time of exam did not have any apparent retinal changes. The presence of retinal diseases such as CMV retinitis, glaucoma, diabetes, AMD or abnormal ocular media in the tested eyes were carefully excluded. No participants had a refractive error of more than −4.0D or +2.5 D. All were able to maintain steady central fixation. We did not test eyes affected by CMV retinitis in HIV positive patients, and used only unaffected eyes in our analysis. We also tested only one eye in healthy volunteers according to their request.
Multifocal Electroretinogram Technique (mfERG)
Pupils were fully dilated with 1% tropicamide and 2.5% phenylephrine hydrochloride. After topical anesthesia with propacaine a Dawson-Trick-Litzkow thread electrode (DTL Plus Electrode, Diagnosys LLC, Lowell, MA) was placed under the lower lid of the tested eye and the contralateral eye was occluded. Two gold-cup electrodes were used for ground (attached to the forehead) and reference (attach to the temple) after cleaning of the skin with abrasive gel.
The stimuli were displayed on a CRT monitor (70Hz) with RETIscan software Version 3.20.15 (Roland Consult Elektrophysiologische Diagnostik Systeme, Wiesbaden, Germany) using a double flash paradigm to evoke a more pronounced SOK response. The room was darkened and the viewing distance was 28 cm (11inches) which allowed a viewing angle of approximately 28 degrees. Presbyopic participants used appropriate correction if necessary for clear visualization of the fixation target.
An array of 103 hexagons, scaled with eccentricity was displayed in the following sequence: 511×10 (H1DDDDH2H3DDD), where H is a light frame and D is a dark frame. The sample distance was 1.1 ms (901Hz) and the frame frequency was 70 Hz. The first order kernel was recorded as a response after a single H1 flash with a 66.4 ms interval of four dark frames allowing the retina to recover before H2 and an immediate H3 flash (double flash), which evoked the second order kernel response when the retina did not have sufficient time to recover from the preceding flash. Each hexagon was temporally modulated between black (<2 cd/m2) and white (200 cd/m2) with a contrast of 98%, according to a pseudorandom binary m-sequence with a base interval of approximately 16.6 ms. Each step of the m-sequence consisted of 5 frames in 83 ms lengths. Responses were band-pass filtered outside 10 to 100 Hz and amplified 100,000 times. Occasional artifacts, such as blinks during the recording, were eliminated by the RETIscan software and that part of the sequence was immediately repeated to record a new response free of artifacts.
A standard m-sequence was recorded over a 12 minutes per eye period and divided into 8 short segments (89 sec each) for patient comfort. The recording protocol was chosen according to the ISCEV guidelines for basic mfERG 37. To increase sensitivity to identify abnormalities in the amplitudes and latencies, we set the filters between 10–100Hz, instead of more commonly recommended 10–300 Hz, as this range of filtering was shown to be more sensitive to detect inner retinal responses in diabetes 39,40
The mean simultaneous response component for the first order kernel and second order kernel was recorded. Implicit times (latencies) and response densities - the amplitude relative to their respective areas (nV/deg2) of the first negative peak (N1) and the first positive peak (P1) were measured for both kernels. The N1 response amplitude was measured from the starting baseline to the base of the N1 trough; the P1 response amplitude was measured from the N1 trough to the peak P1. Averages of responses recorded during 8 cycles were calculated for each subject for 103 hexagons and analyzed with the RETIscan software. For all data obtained with the left eye the mfERG values were left-right flipped along the vertical axis so that they were comparable to the right eye data for statistical analysis.
Statistical Analysis
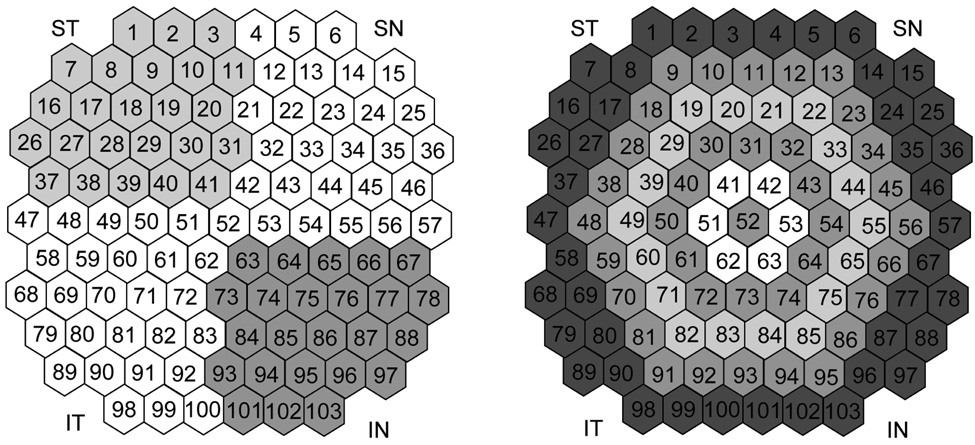
Analysis of responses was done by regional averages derived from concentric rings (6), quadrants (4) (Figure 1) and the whole field, as well as individually by each of 103 hexagons. FOK and SOK components were analyzed separately among the studied cohorts. Ring 1 (central hexagon): 3°, ring 2: 3°–6°, ring 3: 6°–10°, ring 4: 10°–15°, ring 5: 15°–22°, ring 6: 22°–28°. Quadrants: superior nasal (SN), inferior nasal (IN), inferior temporal (IT), superior temporal (ST).
Figure 1. Arrangement of the individual hexagons by rings and quadrants.
A. Superior nasal (SN), superior temporal (ST), inferior temporal (IT), inferior nasal (IN). Central hexagon (52) is excluded from any quadrant. For statistical analysis the quadrant distribution was flipped around the vertical axis for the left eyes.
B. Arrangement of concentric rings starting from central hexagon (52), which is the ring one and towards the periphery scaled with eccentricity to the ring six. Number in each hexagon represents individual hexagon numeration.
Analysis of variance was used to compare each of the FOK and SOK responses by areas across the three study cohorts. All averages were calculated per eye. All analyses were conducted at the 0.05 level and utilized SAS, version 10.0 (Cary, NC).
Results
First Order Kernel (FOK) response
In the analysis of FOK by rings and quadrants (Table 2), there was no difference in the amplitude N1 and P1, but there was a statistically significant delay in P1 latency in areas of rings 3 to 6 (tested retina of 6° degrees to 28° degrees from the fovea) (Table 2) in patients who had a history of prolonged low CD4 counts (0.0001<p<0.02). The analysis of FOK in average across the 103 hexagons and by quadrants showed significant delay in latency P1 across all 4 sectors in HIV positive patients as well (0.0001<p<0.03). The percent difference in mean P1 latency between controls and low CD4 group was on average 3 % (Table 4)
Table 2.
First Order Kernel (FOK) Responses by Areas between Study Cohorts
| Area | CD4 count | N1 amplitude | N1 latency (ms) | P1 amplitude | P1 latency (ms) |
|---|---|---|---|---|---|
| Sum | Low | 2.7 ± 2.2 | 18.0 ± 3.5 | 4.3 ± 3.6 | 37.0 ± 1.8 |
| High | 2.9 ± 2.0 | 18.0 ± 2.8 | 4.5 ± 4.5 | 37.0 ± 1.2 | |
| Control | 2.5 ± 1.6 | 18.0 ± 2.7 | 5.3 ± 3.4 | 35.0 ± 1.3 | |
| p-value | 0.35 | 0.95 | 0.18 | <0.0001* | |
| Quadrants† | |||||
| Low | 2.8 ± 2.1 | 19.0 ± 3.4 | 4.7 ± 3.9 | 37.0 ± 1.9 | |
| SN | High | 3.2 ± 2.3 | 19.0 ± 3.2 | 5.0 ± 4.4 | 36.0 ± 1.5 |
| Control | 2.8 ± 1.8 | 18.0 ± 2.4 | 5.8± 3.6 | 35.0 ± 1.7 | |
| p-value | 0.16 | 0.64 | 0.21 | 0.0002* | |
| IN | Low | 2.9 ± 2.3 | 18.0 ± 3.6 | 4.6 ± 3.8 | 36.0 ± 1.8 |
| High | 3.0 ± 2.1 | 18.0 ± 3.0 | 4.8 ± 4.5 | 36.0 ± 1.5 | |
| Control | 2.8 ± 1.7 | 18.0 ± 3.5 | 5.7 ± 3.6 | 35.0 ± 1.7 | |
| p-value | 0.50 | 0.93 | 0.13 | 0.0005* | |
| IT | Low | 2.9 ± 2.5 | 18.0 ± 2.8 | 4.7 ± 4.0 | 36.0 ± 2.6 |
| High | 2.9 ± 2.1 | 18.0 ± 2.3 | 4.8 ± 4.4 | 37.0 ± 1.4 | |
| Control | 2.7 ± 1.7 | 19.0 ± 2.3 | 5.6 ± 3.5 | 35.0 ± 1.7 | |
| p-value | 0.36 | 0.61 | 0.05* | <0.0001* | |
| ST | Low | 2.8 ± 2.3 | 18.0 ± 3.3 | 4.5 ± 3.8 | 37.0 ± 2.0 |
| High | 3.1 ± 2.2 | 18.0 ± 2.6 | 4.8 ± 4.6 | 37.0 ± 1.2 | |
| Control | 2.7 ± 1.9 | 18.0 ± 2.5 | 5.5 ± 3.6 | 36.0 ± 1.8 | |
| p-value | 0.24 | 0.74 | 0.15 | <0.0001* | |
| Rings | |||||
| 1 | Low | 16.0 ± 14.0 | 17.0 ± 3.9 | 31.0 ± 27.0 | 41.0 ± 5.1 |
| High | 20.0 ± 17.0 | 18.0 ± 3.3 | 35.0 ± 29.0 | 41.0 ± 4.4 | |
| Control | 19.0 ± 17.0 | 18.0 ± 3.1 | 40.0 ± 25.0 | 40.0 ± 4.1 | |
| p-value | 0.17 | 0.55 | 0.09 | 0.67 | |
| 2 | Low | 8.7 ± 7.2 | 18.0 ± 3.0 | 15.0 ± 13.0 | 39.0 ± 2.3 |
| High | 9.9 ± 7.3 | 17.0 ± 2.5 | 15.0 ± 13.0 | 38.0 ± 3.1 | |
| Control | 8.4 ± 5.3 | 17.0 ± 2.6 | 18.0 ± 10.0 | 38.0 ± 2.4 | |
| p-value | 0.20 | 0.28 | 0.26 | 0.11 | |
| 3 | Low | 5.4 ± 4.1 | 18.0 ± 2.7 | 9.5 ± 7.8 | 37.0 ± 2.4 |
| High | 6.0 ± 4.1 | 18.0 ± 3.1 | 9.7 ± 8.6 | 36.0 ± 2.0 | |
| Control | 5.3 ± 3.1 | 17.0 ± 2.5 | 11.1 ± 6.7 | 35.0 ± 1.7 | |
| p-value | 0.29 | 0.73 | 0.20 | 0.0002* | |
| 4 | Low | 4.0 ± 3.6 | 18.0 ± 3.5 | 6.4 ± 5.6 | 36.0 ± 2.2 |
| High | 4.1 ± 2.8 | 17.0 ± 3.1 | 6.2 ± 5.5 | 36.0 ± 1.7 | |
| Control | 3.5 ± 2.3 | 18.0 ± 2.7 | 7.6 ± 4.7 | 35.0 ± 1.6 | |
| p-value | 0.46 | 0.91 | 0.21 | 0.02* | |
| 5 | Low | 2.8 ± 2.4 | 17.0 ± 3.3 | 4.6 ± 4.1 | 36.0 ± 2.5 |
| High | 3.2 ± 2.3 | 18.0 ± 3.7 | 4.9 ± 4.5 | 36.0 ± 1.6 | |
| Control | 2.7 ± 1.9 | 18.0 ± 2.4 | 5.6 ± 3.4 | 35.0 ± 2.0 | |
| p-value | 0.21 | 0.74 | 0.20 | 0.0007* | |
| 6 | Low | 2.3 ± 1.9 | 18.0 ± 3.7 | 3.5 ± 3.0 | 37.0 ± 2.2 |
| High | 2.3 ± 1.7 | 18.0 ± 3.1 | 3.6 ± 3.3 | 37.0 ± 1.5 | |
| Control | 2.0 ± 1.4 | 18.0 ± 2.8 | 4.1 ± 2.7 | 35.0 ± 1.5 | |
| p-value | 0.47 | 0.92 | 0.27 | <0.0001* | |
All data are presented in mean ± SD; High CD4 group = nadir CD4 count ≥ 100
Low CD4= nadir CD4 count < 100 for a minimum of 6 months, control – HIV negative
p-value is statistically significant
Quadrants: SN- superior nasal; IN – inferior nasal; IT – inferior temporal; ST – superior temporal.
Table 4.
Mean Percent Difference in P1 Latency between Controls - HIV Low CD4 counts and Controls – High CD4 Counts
| FOK* | FOK | SOK* | SOK | |
|---|---|---|---|---|
| Control-High | Control-Low | Control-High | Control-Low | |
| Ring 1 | 2.2% | 2.9% | 0.1% | 1.5% |
| Ring 2 | 2.2% | 3.5% | 2.0% | 1.9% |
| Ring 3 | 2.9% | 4.3% | 2.8% | 3.6% |
| Ring 4 | 2.7% | 3.2% | 2.4% | 3.1% |
| Ring 5 | 3.8% | 3.2% | 3.5% | 3.4% |
| Ring 6 | 3.0% | 3.9% | 3.3% | 3.9% |
| Quadrant SN† | 3.6% | 3.8% | 3.4% | 3.6% |
| Quadrant IN | 2.0% | 2.9% | 3.7% | 4.0% |
| Quadrant IT | 3.1% | 3.4% | 3.4% | 3.6% |
| Quadrant ST | 2.9% | 4.0% | 5.1% | 4.5% |
| Sum | 3.1% | 3.8% | 3.1% | 3.7% |
FOK = first order kernel, SOK = second order kernel.
SN=superior nasal, IN= inferior nasal, IT =inferior temporal, ST =superior temporal.
High CD4 group = nadir CD4 count ≥ 100; Low CD4= nadir CD4 count < 100 for a minimum of 6 months.
Second Order kernel (SOK) response
The analysis of SOK by rings and quadrants (Figure 1) also showed statistically significant difference in the latencies, with delayed latencies (implicit times) in HIV positive patients compared to controls in the same areas as FOK abnormalities (Table 3). The distribution pattern of the statistically significant delays in implicit times of SOK is shown in Figure 2.
Table 3.
Second order kernel (SOK) responses by areas between study cohorts
| Area | CD4 count | N1 amplitude | N1 latency (ms) | P1 amplitude | P1 latency (ms) |
|---|---|---|---|---|---|
| Sum | Low | 0.93 ± 1.2 | 19.0 ± 3.4 | 3.8 ± 3.3 | 38.0 ± 1.8 |
| High | 0.97 ± 1.6 | 18.0 ± 2.4 | 4.3 ± 4.6 | 37.0 ± 1.3 | |
| Control | 1.0 ± 0.95 | 19.0 ± 3.4 | 5.1 ± 3.3 | 36.0 ± 1.4 | |
| p-value | 0.82 | 0.32 | 0.11 | 0.0001* | |
| Quadrants† | |||||
| SN | Low | 1.0 ± 1.3 | 20.0 ± 3.9 | 4.2 ± 3.8 | 38.0 ± 1.7 |
| High | 1.0 ± 1.6 | 20.0 ± 2.7 | 4.8 ± 5.1 | 38.0 ± 1.4 | |
| Control | 1.1 ± 1.0 | 19.0 ± 3.5 | 5.6 ± 3.7 | 37.0 ± 1.7 | |
| p-value | 0.85 | 0.32 | 0.13 | <0.0001* | |
| IN | Low | 0.93 ± 1.2 | 18.0 ± 4.2 | 3.9 ± 3.4 | 37.0 ± 1.8 |
| High | 0.88 ± 1.5 | 19.0 ± 2.8 | 4.3 ± 4.3 | 37.0 ± 2.0 | |
| Control | 1.2 ± 1.1 | 18.0 ± 3.5 | 5.2 ± 3.4 | 36.0 ± 1.6 | |
| p-value | 0.41 | 0.47 | 0.08 | <0.0001* | |
| IT | Low | 0.93 ± 1.3 | 19.0 ± 3.5 | 3.8 ± 3.3 | 38.0 ± 1.9 |
| High | 1.0 ± 1.7 | 19.0 ± 3.6 | 4.4 ± 4.7 | 38.0 ± 1.7 | |
| Control | 1.2 ± 1.1 | 17.0 ± 3.7 | 5.1 ± 3.3 | 36.0 ± 1.5 | |
| p-value | 0.49 | 0.10 | 0.13 | <0.0001* | |
| ST | Low | 0.98 ± 1.2 | 20.0 ± 3.5 | 4.0 ± 3.5 | 38.0 ± 2.3 |
| High | 1.0 ± 1.8 | 20.0 ± 2.9 | 4.5 ± 4.9 | 38.0 ± 1.7 | |
| Control | 1.1 ± 1.4 | 19.0 ± 3.6 | 5.3 ± 3.7 | 37.0 ± 2.7 | |
| p-value | 0.63 | 0.34 | 0.24 | <0.0001* | |
| Rings | |||||
| 1 | Low | 6.4 ± 8.1 | 18.0 ± 4.3 | 22.0 ± 19.0 | 40.0 ± 6.9 |
| High | 8.4 ± 14.0 | 19.0 ± 4.0 | 28.0 ± 30.0 | 39.0 ± 7.5 | |
| Control | 8.3 ± 8.0 | 18.0 ± 4.2 | 27.0 ± 19.0 | 39.0 ± 5.5 | |
| p-value | 0.29 | 0.27 | 0.13 | 0.76 | |
| 2 | Low | 3.2 ± 4.5 | 18.0 ± 2.7 | 12.0 ± 11.0 | 40.0 ± 3.4 |
| High | 2.9 ± 4.0 | 19.0 ± 2.9 | 12.0 ± 11.0 | 40.0 ± 2.7 | |
| Control | 3.9 ± 3.6 | 18.0 ± 2.9 | 15.0 ± 10.0 | 39.0 ± 2.1 | |
| p-value | 0.40 | 0.25 | 0.11 | 0.16 | |
| 3 | Low | 2.1 ± 2.7 | 19.0 ± 3.8 | 9.0 ± 7.6 | 38.0 ± 2.1 |
| High | 1.9 ± 3.5 | 18.0 ± 2.3 | 9.5 ± 9.7 | 38.0 ± 1.8 | |
| Control | 2.7 ± 2.5 | 18.0 ± 3.1 | 12.0 ± 7.8 | 37.0 ± 1.7 | |
| p-value | 0.41 | 0.08 | 0.12 | 0.0001* | |
| 4 | Low | 1.4 ± 2.1 | 19.0 ± 3.6 | 5.7 ± 5.4 | 37.0 ± 1.7 |
| High | 1.2 ± 2.4 | 18.0 ± 2.7 | 6.4 ± 6.4 | 37.0 ± 1.4 | |
| Control | 1.7 ± 1.6 | 18.0 ± 3.6 | 7.7 ± 5.0 | 36.0 ± 2.1 | |
| p-value | 0.59 | 0.44 | 0.08 | 0.0007* | |
| 5 | Low | 0.93 ± 1.2 | 18.0 ± 4.0 | 4.1 ± 3.7 | 37.0 ± 1.9 |
| High | 0.96 ± 1.8 | 19.0 ± 3.8 | 4.6 ± 4.8 | 37.0 ± 1.6 | |
| Control | 1.1 ± 1.0 | 18.0 ± 3.0 | 5.4 ± 3.7 | 36.0 ± 1.6 | |
| p-value | 0.70 | 0.22 | 0.14 | <0.0001* | |
| 6 | Low | 0.76 ± 0.89 | 20.0 ± 4.0 | 2.8 ± 2.5 | 38.0 ± 1.7 |
| High | 0.76 ± 1.2 | 19.0 ± 3.3 | 3.2 ± 3.4 | 38.0 ± 1.5 | |
| Control | 0.76 ± 0.71 | 20.0 ± 3.8 | 3.7 ± 2.4 | 36.0 ± 1.6 | |
| p-value | 0.86 | 0.41 | 0.14 | <0.0001* | |
All data are presented in mean ± SD; High CD4 group = nadir CD4 count ≥ 100
Low CD4= nadir CD4 count < 100 for a minimum of 6 months, control – HIV negative
p-value is statistically significant
Quadrants: SN- superior nasal; IN – inferior nasal; IT – inferior temporal; ST – superior temporal.
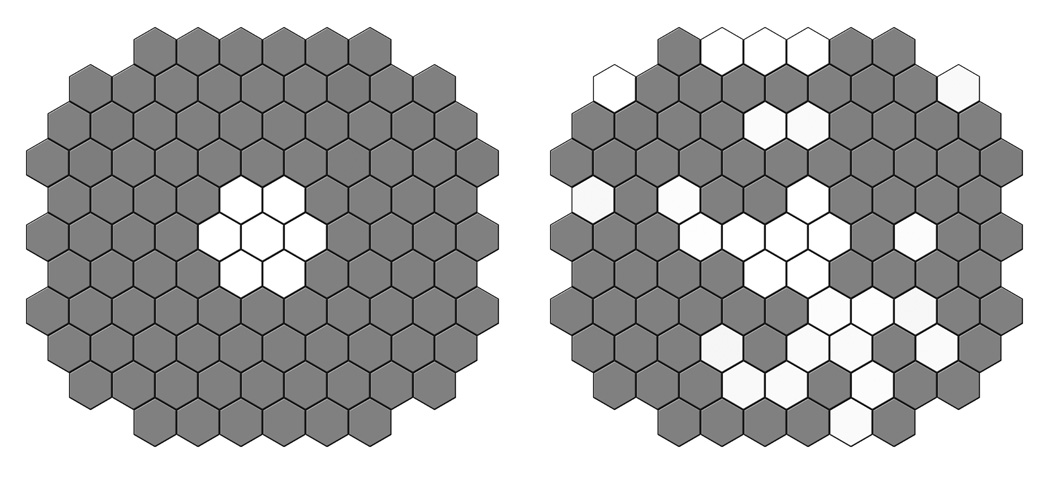
Figure 2. Distribution pattern of the average second order kernel implicit time delays in our group of HIV positive patients with history of low CD4 counts.
A. This figure represents distribution of the delays analyzed and averaged by rings; dark rings show the areas with statistically significant values (< 0.05) and white rings show non-significant delay in implicit times.
B. Distribution of statistically significant delay in implicit times analyzed by individual hexagon; dark hexagons represent statistically significant delay and white hexagons represent statistically non-significant delays.
The percent difference in latencies between controls (C) and High CD4 (H) was 3.1% and C and Low (L) was 3.7% (Table 4). In the macular area (rings 1–2) the percent difference was minimal – 1.5% between C and L. In the rings from 3 to 6 it varied from 3.4 to 3.9% between C and L. (Table 4).
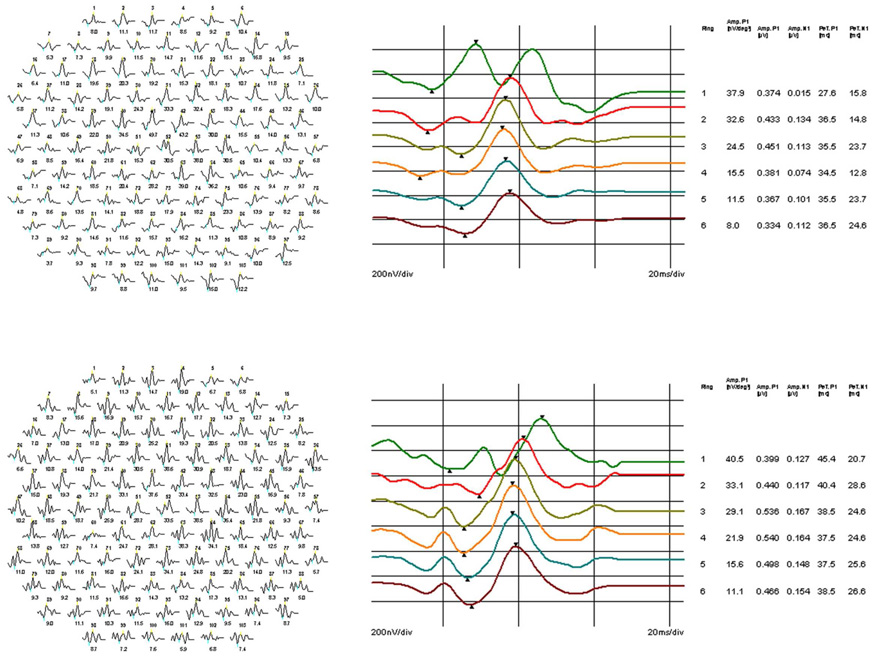
Although there was no statistically significant reduction in the amplitude of N1 and P1 between groups if analyzed by either kernel, we found a trend of P1 amplitude reduction in HIV positive patients across all areas in both kernels (Table 2–Table 3). Sample waveforms originated from individual hexagons as well as waveforms averaged by rings are shown in Figure 3 for the controls and low CD4 counts group of patients.
Figure 3. Example of second order kernel (SOK) waveforms from the HIV negative control and HIV positive patient with a history of low CD4 counts.
A. Example of SOK waveforms recorded from an HIV negative control; B. The waveforms averaged by rings from the same individual. C. Example of SOK waveforms from an HIV positive patient with a prolong history of low CD4 counts; D. The waveforms averaged by rings from the same patient. Note a delay of the average implicit time (latency) of P1 compared to the same response from the control patient.
Discussion
HIV is a complicated multi-systemic disease and the visual loss that these patients experience may be due to localized retinal and/or generalized neurological changes. It has been previously shown that patients with AIDS experience a lower axonal population with the extent and pattern of axonal loss suggesting that changes may be due to secondary damage of the inner retina (thining of RNFL) 17, 18 but also may reflect an AIDS-associated primary neuropathy 38. In the current study we focused on the detection of retinal abnormalities that may be present in early HIV disease and precede the infectious HIV retinopathy or the central vision loss. For this purpose we used multifocal electroretinography (mfERG) that has been shown to be a sensitive test to determine functional retinal abnormalities in diseases that mainly involve the inner retina, such as diabetes or glaucoma 20,22,23,33,34, 35. We did not exclude from our study those patients who have had history of prior HIV related background retinopathy such as cotton wool spots, non-infectious retinopathy or those who had these retinal changes at the time of the mfERG recording. We only excluded patients with infectious HIV retinopathy, such as CMV retinitis.
In our previous study 19 we primarily investigated the outer retina’s involvement in a limited cohort of HIV patients using 30Hz flicker algorithm of mfERG. We did not find any significant difference in the amplitudes of the FOK, but found 18 focal abnormal points with delayed FOK latencies, not following any particular pattern. Our conclusion was that the outer retina is not a major site of early retinal abnormalities in HIV patients.
The current study was designed to investigate the inner retina involvement with a different sequence of stimuli (double flash algorithm with longer periods between flashes), allowing us to analyze mfERG responses from both the outer and inner retina as well as identify more pronounced and consistent changes in the implicit times of both kernels. Also the cohort of participants in the current study was considerably larger than in our previous study. To increase sensitivity to identify abnormalities in the amplitudes and latencies, we set the filters between 10–100Hz, instead of more commonly recommended 10–300 Hz, as this range of filtering was shown to be more sensitive to detect inner retinal responses in diabetes 39,40.
The second order kernel (SOK) represents the degree to which the retinal response is affected by an immediately preceding stimulus. This response component thus reflects the effects of fast adaptive mechanisms in the retina. The findings of Palmowski et al 28 and Fortune et al 40 suggest that recovery of retinal sensitivity following a flash is abnormal in diabetes, even before the retinopathy is visible. Such pre-background diabetic retinopathy typically affects the inner retina: ganglion cells 41 and Muller cells 42.
Our results showed a high correlation between low CD4 count nadir and delayed implicit times found on both (first and second order) kernels. Also we found a trend of the P1 amplitudes reduction in both kernel responses, though this reduction was not statistically significant (Table 2–3). As previously shown the P1 latency of the FOK is delayed in early diabetes but the amplitude of the FOK is not necessarily reduced 23. We hypothesized that the mfERG changes in diabetes and early HIV disease may be similar as both diseases affect inner retina and microvasculature. It is important to remember that the SOK is not only affected by inner retinal function, but also by middle and outer retina. Despite these limitations, the changes that we observed by mfERG are confirmed by other functional (visual field) and structural tests (OCT, HRT, SLP). Therefore, this study shows that there is functional damage to the inner retina. Unfortunately, there is not an electrophysiological test that isolates the inner retinal elements form other retinal elements. If such a test were developed, our results suggest that this test might show much more function loss.
The greater sensitivity of implicit times compared to amplitude is primarily because of lower inter-subject variability; to reach statistical significance the relative deviation from “normal’ should be greater for amplitude than for implicit times 23. Therefore the latency delay is reflecting subtle functional changes that are not yet pronounced as structural changes resulting in markedly diminished amplitude and clinically relevant vision loss. The amplitude reduction is a more evident indicator of profound retinal cell damage; yet implicit time delay probably signifies a functional disturbance in communication between the retinal layers.
We want to note that in our study we used RETIscan and not the more widely used VERIS system, and even though these two systems are comparable in precision for detection of retinal abnormalities 43, our numerical results may show slightly higher amplitudes and slightly longer implicit times than the data obtained using the VERIS system.
It was previously reported that the implicit times (latencies) of mfERG tend to increase with increasing peripheral visual field defects in primary open-angle glaucoma 34. The difference between our study and the work by Hasegawa and colleagues 34 is that we separated the retina in concentric rings and Hasegawa and colleagues divided the retina into four quadrants. The magnitude of latency change observed in our study is similar to those obtained in glaucoma studies with visual field defects 34 and a relatively spared macula with both FOK and SOK (Table 2–3).
The difference in latencies between the HIV and control group showed a trend of increasing with eccentricity, which suggests that the inner retina damage in HIV patients differs from the periphery to the macula and seems to be more pronounced at the periphery. The explanation for these findings may be that the axonal loss in early HIV disease is most likely diffused, but is more detectable by mfERG on the periphery because of less redundancy of axons (RNFL thinning) towards the periphery. Pathological changes, such as microvascular compromise and ischemia, would be much more noticeable in the periphery than in the macular region 44. This phenomenon therefore does not necessarily imply that the HIV disease affects the peripheral retina first. The lack of foveal involvement may be due to more robust and redundant retinal elements, in particular ganglion cells, which also explains why clinically central vision is not affected in early HIV disease. All of our patients in both cohorts (low and high CD4 counts) had good central vision at the time of the testing (Table 1), which supports these findings and suggests that central retinal function is not yet affected by the inner retinal changes we detected with mfERG. It is our assumption that the inner retinal changes in HIV disease may precede the clinical loss of vision in these patients by several years. Longitudinal studies need to be performed to study this relationship.
It is also of interest to note that the majority of our HIV positive participants had been on HAART therapy for a considerable period of time prior to our testing, leading us to assume that this therapy alone may not protect against nor reverse the loss of retinal function in HIV disease. For this reason, pharmacological neuroprotection may be important along with systemic control of HIV disease itself to prevent vision loss in early stages of HIV disease. This will require further investigation with longer follow up and larger cohort of participants.
In conclusion, we propose that the functional vision abnormalities experienced by HIV positive patients without infectious retinopathy is most likely due to early diffuse changes in the inner retina. Our results support this hypothesis as we found statistically significant delays in the mfERG implicit times of both kernels. It is possible that these early changes may progress to a more clinically significant vision loss over time. Prospective studies using mfERG recordings in this same cohort of patients are underway.
Acknowledgements
Funding / Support: This research was supported in part by the National Eye Institute Core Grant EY03040 (SPA, LD), EY016323 (DUB) and NIH EY07366 (WRF), Research to Prevent Blindness, New York, NY (Dr. Freeman is the recipient of an RPB Physician Scientist award and departmental support to UCSD)
Financial disclosure: None of the authors has financial interest in this study
Contributions of authors: Design of the study (IAF, DUB, SPA, WRF); Conduct of the study (IAF, SPA, DUB, WRF); Collection (IAF, DUB); Management (IAF, DUB, WRF); Analysis (IAF, DUB, SPA, LD); Interpretation of the data (AAS, WRF); preparation (IAF, WRF); review (IAF, DUB, WRF); approval (IAF, DUB, SPA, LD, AAS, WRF)
Conformity. The UCSD Human Research Protection Program reviewed and approved this study. Informed consent was obtained from each participant after IRB approval for this study. The study adhered to the HIPAA requirements.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. [Retrieved on December 12, 2007];UNAIDS/WHO AIDS epidemic update: December 2007. www.unaids.org;
- 2.Jabs DA. AIDS and ophthalmology in 2004. Arch Ophthalmol. 2004;122:1040–1042. doi: 10.1001/archopht.122.7.1040. [DOI] [PubMed] [Google Scholar]
- 3.Jabs DA, Van Natta ML, Kempen HJ, et al. Characteristics of patients with cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol. 2002;133:48–61. doi: 10.1016/s0002-9394(01)01322-8. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg DE, Smithen LM, Angelilli A, Freeman WR. HIV-associated retinopathy in the HAART era. Retina. 2005;25:633–649. doi: 10.1097/00006982-200507000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Karavellas MP, Song M, Macdonald JC, Freeman WR. Long-term posterior and anterior segment complications of immune recovery uveitis associated with cytomegalovirus retinitis. Am J Ophthalmol. 2000;130:57–64. doi: 10.1016/s0002-9394(00)00528-6. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen QD, Kempen JH, Bolton SG, Dunn JP, Jabs DA. Immune recovery uveitis in patients with AIDS and cytomegalovirus retinitis after highly active antiretroviral therapy. Am J Ophthalmol. 2000;129:634–639. doi: 10.1016/s0002-9394(00)00356-1. [DOI] [PubMed] [Google Scholar]
- 7.Holland GN, Pepose JS, Pettit TH, Gottlieb MS, Yee RD, Foos RY. Acquired immune deficiency syndrome. Ocular manifestations. Ophthalmology. 1983;90:859–873. doi: 10.1016/s0161-6420(83)80009-8. [DOI] [PubMed] [Google Scholar]
- 8.Plummer DJ, Sample PA, Freeman WR. Visual dysfunction in HIV-positive patients without infectious retinopathy. AIDS Patient Care STDS. 1998;12:171–179. doi: 10.1089/apc.1998.12.171. [DOI] [PubMed] [Google Scholar]
- 9.Plummer DJ, Sample PA, Arevalo JF, et al. Visual field loss in HIV-positive patients without infectious retinopathy. Am J Ophthalmol. 1996;122:542–549. doi: 10.1016/s0002-9394(14)72115-4. [DOI] [PubMed] [Google Scholar]
- 10.Sample PA, Plummer DJ, Mueller AJ, et al. Pattern of early visual field loss in HIV-infected patients. Arch Ophthalmol. 1999;117:755–760. doi: 10.1001/archopht.117.6.755. [DOI] [PubMed] [Google Scholar]
- 11.Geier SA, Nöhmeier C, Lachenmayr BJ, Klauss V, Goebel FD. Deficits in perimetric performance in patients with symptomatic human immunodeficiency virus infection or acquired immunodeficiency syndrome. Am J Ophthalmol. 1995;119:335–344. doi: 10.1016/s0002-9394(14)71177-8. [DOI] [PubMed] [Google Scholar]
- 12.Quinceno JI, Capparelli E, Sadun AA, et al. Visual dysfunction without retinitis in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1992;113:8–13. doi: 10.1016/s0002-9394(14)75745-9. [DOI] [PubMed] [Google Scholar]
- 13.Iragui VJ, Kalmijn J, Plummer DJ, Sample PA, Trick GL, Freeman WR. Pattern electroretinograms and visual evoked potentials in HIV infection: evidence for asymptomatic retinal and postretinal impairment in the absence of infectious retinopathy. Neurology. 1996;47:1452–1456. doi: 10.1212/wnl.47.6.1452. [DOI] [PubMed] [Google Scholar]
- 14.Iragui VJ, Kalmijn J, Thai LJ, Grant I HNRC Group. Neurological dysfunction in asymptomatic HIV-1 infected men: evidence from evoked potentials. Electroencephalogr Clin Neurophysiol. 1994;92:1–10. doi: 10.1016/0168-5597(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 15.Keller SK, Schwarzkopf R, Schlüter S, Nieuwenhuis I, Schmidt B. Follow-up of HIV infected patients with reduced pattern ERG. Fortschr Ophthalmol. 1991;88:716–720. [PubMed] [Google Scholar]
- 16.Gellrich MM, Kade G, Gerling J, Bach M, Hansen LL. Pattern, flicker, and flash electroretinography in human immunodeficiency virus infection: a longitudinal study. Ger J Ophthalmol. 1996;5:16–22. [PubMed] [Google Scholar]
- 17.Kozak I, Bartsch DU, Cheng L, Kosobucki BR, Freeman WR. Objective analysis of retinal damage in HIV-positive patients in the HAART era using OCT. Am J Ophthalmol. 2005;139:295–301. doi: 10.1016/j.ajo.2004.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plummer DJ, Bartsch DU, Azen SP, Max S, Sadun AA, Freeman WR. Retinal nerve fiber layer evaluation in human immunodeficiency virus-positive patients. Am J Ophthalmol. 2001;131:216–222. doi: 10.1016/s0002-9394(00)00787-x. [DOI] [PubMed] [Google Scholar]
- 19.Falkenstein I, Kozak I, Kayikcioglu O, et al. Assessment of retinal function in patients with HIV without infectious retinitis by multifocal electroretinogram and automated perimetry. Retina. 2006;26:928–934. doi: 10.1097/01.iae.0000250009.60908.35. [DOI] [PubMed] [Google Scholar]
- 20.Hood DC, Greenstein VC, Holopigian K, et al. An attempt to detect glaucomatous damage to the inner retina with the multifocal ERG. Invest Ophthalmol Vis Sci. 2000;41:1570–1579. [PubMed] [Google Scholar]
- 21.Kurtenbach A, Leo-Kottler B, Zrenner E. Inner retina contributions to the multifocal electroretinogram: patients with Leber’s hereditary optic neuropathy (LHON). Multifocal ERG in patients with LHON. Doc Ophthalmol. 2004;108:231–240. doi: 10.1007/s10633-004-8676-8. [DOI] [PubMed] [Google Scholar]
- 22.Greenstein VC, Holopigian K, Hood DC, Seiple W, Carr RE. The nature and extent of retinal dysfunction associated with diabetic macular edema. Invest Ophthalmol Vis Sci. 2000;41:3643–3654. [PubMed] [Google Scholar]
- 23.Bearse MA, Adams AJ, Han Y, et al. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res. 2006;25:425–448. doi: 10.1016/j.preteyeres.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feigl B, Brown B, Lovie-Kitchin J, Swann P. The rod-mediated multifocal electroretinogram in aging and in early age-related maculopathy. Curr Eye Res. 2006;31:635–644. doi: 10.1080/02713680600762739. [DOI] [PubMed] [Google Scholar]
- 25.Holopigian K, Seiple W, Greenstein VC, Hood DC, Carr RE. Local cone and rod system function in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2001;42:779–788. [PubMed] [Google Scholar]
- 26.Luu CD, Lau AM, Lee SY. Multifocal electroretinogram in adults and children with myopia. Arch Ophthalmol. 2006;124:328–334. doi: 10.1001/archopht.124.3.328. [DOI] [PubMed] [Google Scholar]
- 27.Lai TY, Chan WM, Li H, Lai RY, Lam DS. Multifocal electroretinographic changes in patients receiving hydroxychloroquine therapy. Am J Ophthalmol. 2005;140:794–807. doi: 10.1016/j.ajo.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 28.Palmowski AM, Sutter EE, Bearse MA, Fung W. Mapping of retinal function in diabetic retinopathy using the multifocal electroretinogram. Invest Ophthalmol Vis Sci. 1997;38:2586–2596. [PubMed] [Google Scholar]
- 29.Hood DC. Assessing retinal function with multifocal technique. Prog Retin Eye Res. 2000;19:607–646. doi: 10.1016/s1350-9462(00)00013-6. [DOI] [PubMed] [Google Scholar]
- 30.Hood DC, Greenstein V, Frishman L, et al. Identifying inner retinal contributions to the human multifocal ERG. Vis Res. 1999;39:2285–2291. doi: 10.1016/s0042-6989(98)00296-x. [DOI] [PubMed] [Google Scholar]
- 31.Han Y, Adams AJ, Bearse MA, Schneck ME. Multifocal electroretinogram and short -wavelength automated perimetry measures in diabetic eyes with little or no retinopathy. Arch Ophthalmol. 2004;122:1809–1815. doi: 10.1001/archopht.122.12.1809. [DOI] [PubMed] [Google Scholar]
- 32.Han Y, Schneck ME, Bearse MA, et al. Formulation and evaluation of a predictive model to identify the sites of future diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004;45:4106–4112. doi: 10.1167/iovs.04-0405. [DOI] [PubMed] [Google Scholar]
- 33.Palmowski-Wolfe AM, Allgayer RJ, Vernaleken B, Schötzau A, Ruprecht KW. Slow-stimulated multifocal ERG in high- and normal-tension glaucoma. Doc Ophthalmol. 2006;112:157–168. doi: 10.1007/s10633-006-0007-9. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa S, Takagi M, Usui T, Takada R, Abe H. Waveform changes of the first-order multifocal electroretinogram in patients with glaucoma. Invest Ophthalmol Vis Sci. 2000;41:1597–1603. [PubMed] [Google Scholar]
- 35.Chan HL, Brown B. Multifocal ERG changes in glaucoma. Ophthalmic Physiol Opt. 1999;19:306–316. doi: 10.1046/j.1475-1313.1999.00439.x. [DOI] [PubMed] [Google Scholar]
- 36.Fortune B, Wang L, Bui BV, Cull G, Dong J, Cioffi GA. Local ganglion cell contributions to the macaque electroretinogram revealed by experimental nerve fiber layer bundle defect. Invest Ophthalmolol Vis Sci. 2003;44:4567–4579. doi: 10.1167/iovs.03-0200. [DOI] [PubMed] [Google Scholar]
- 37.Marmor MF, Hood DC, Keating D, Kondo M, Seeliger MW, Miyake Y. International Society for Clinical Electrophysiology of Vision Guidelines for basic multifocal electroretinography (mfERG) Doc Ophthalmol. 2003;106:105–115. doi: 10.1023/a:1022591317907. [DOI] [PubMed] [Google Scholar]
- 38.Tenhula WN, Xu SZ, Madigan MC, Heller K, Freeman WR, Sadun AA. Morphometric comparisons of optic nerve axon loss in acquired immunodeficiency syndrome. Am J Ophthalmol. 1992;113:14–20. doi: 10.1016/s0002-9394(14)75746-0. [DOI] [PubMed] [Google Scholar]
- 39.Han Y, Bearse MA, Schneck ME, Barez S, Jacobsen C, Adams AJ. Towards optimal filtering of “standard” multifocal electroretinogram (mfERG) recordings: findings in normal and diabetic subjects. Br J Ophthalmol. 2004;88:543–550. doi: 10.1136/bjo.2003.026625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fortune B, Schneck ME, Adams AJ. Multifocal electroretinogram delays reveal local retinal dysfunction in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 1999;40:2638–2651. [PubMed] [Google Scholar]
- 41.Meyer-Rüsenberg B, Pavlidis M, Stupp T, Thanos S. Pathological changes in human retinal ganglion cells associated with diabetic and hypertensive retinopathy. Graefes Arch Clin Exp Ophthalmol. 2007;245:1009–1018. doi: 10.1007/s00417-006-0489-x. [DOI] [PubMed] [Google Scholar]
- 42.Mizutani M, Gerhardinger C, Lorenzi M. Muller cell changes in human diabetic retinopathy. Diabetes. 1998;47:445–449. doi: 10.2337/diabetes.47.3.445. [DOI] [PubMed] [Google Scholar]
- 43.Bock M, Andrassi M, Belitsky L, Lorenz B. A comparison of two multifocal ERG systems. Doc Ophthalmol. 1998–1999;97:157–178. doi: 10.1023/a:1002093106520. [DOI] [PubMed] [Google Scholar]
- 44.Kim JW, Rizzo JF, Lessell S. Delayed visual decline in patients with “stable” optic neuropathy. Arch Ophthalmol. 2005;123:785–788. doi: 10.1001/archopht.123.6.785. [DOI] [PubMed] [Google Scholar]