Abstract
Objectives
To evaluate ventricular function and the occurrence of HF among persons with MI meeting only troponin criteria compared to persons meeting CK-MB criteria.
Background
The 2000 ACC/ESC MI definition enabled identification of MIs meeting only troponin-based criteria. Data on ventricular function and HF among these are lacking.
Methods
Between 11-2002 and 05-2006, we prospectively identified 835 MIs in the community using standardized criteria including cardiac pain, ECG and biomarkers. Troponin and CK-MB were prospectively measured in all; each patient was classified according to the criteria met.
Results
We performed echocardiograms (median of 1 day post-MI) in 482 patients (age 68 ± 15 years; 45% women); 363 patients met CK-MB criteria while 119 met only troponin criteria. The latter had lower wall motion score index (1.3 ± 0.4 vs 1.5 ± 0.5 for CK-MB; p <0.01). Diastolic dysfunction was similar in both groups. After one year of follow up, 142 patients developed post-MI HF. Patients meeting only troponin criteria had a lower risk of HF after adjustment for age, sex, comorbidity (HR 0.56, 95% CI 0.37, 0.85, p<0.01), which persisted after further adjustments for systolic or diastolic function.
Conclusions
In the community, the prospective application of the new MI definition identifies patients meeting only troponin criteria with better systolic function than cases meeting CK-MB criteria. Such MIs have a lower risk of subsequent HF. These findings are important for risk stratification in clinical practice.
Keywords: Community, Myocardial infarction, Heart failure, Prognosis
The American College of Cardiology/European Society of Cardiology (ACC/ESC) diagnostic criteria for myocardial infarction (MI) combines ischemic symptoms with changes in the electrocardiogram and with biochemical markers of myocardial necrosis, emphasizing the use of troponins which are more sensitive and specific than other biomarkers on detecting myocardial injury (1, 2). Troponin allows the detection of small amounts of tissue injury that would have gone undetected by creatinine kinase and its MB fraction (CK-MB) (1–3). This biomarker change was expected to have two key consequences: to increase the number of MIs and to shift the clinical spectrum of the disease (4). Indeed, the implementation of the new definition in the community resulted in a large increase in the number of cases of MI (5). Less is known about implications on the clinical spectrum of the disease. Cases identified as MI by the new definition are lacking data on left ventricular systolic and diastolic function. Further, while mortality among cases meeting only troponin-based criteria is lower than mortality among cases meeting CK-MB criteria (5), the occurrence of HF among these is not known.
Despite the controversy (4, 6) that surrounded the publication of the new criteria, few studies have prospectively evaluated its impact. Indeed, most of the current knowledge is based on convenience samples and case series that used different assays and diagnostic criteria that were often incompletely standardized. In the present study, we sought to specifically evaluate parameters of left ventricular function among patients with MI and measure the occurrence of post-MI heart failure (HF) to assess differences between cases meeting troponin-based criteria and cases meeting both troponin and CK-MB criteria.
Methods
Study population
Olmsted County, Minnesota, is relatively isolated from other urban centers, and nearly all medical care in virtually every specialty is delivered to residents by few providers, which include the Mayo Clinic and its affiliated hospitals, the Olmsted Medical Center and its affiliated community hospital, local nursing homes, and a few private practitioners. Each provider in the community uses a unit medical record in which details of care for a patient, regardless of setting, are available in one place. The records are easily retrievable because Mayo Clinic maintains extensive indices that through the Rochester Epidemiology Project are extended to the records of other health care providers in the county, resulting in the linkage of all medical records from all sources of care through a centralized system (7). This provides a unique infrastructure to analyze disease outcomes. Of particular importance for the present study is the availability of information on all episodes of HF including those confined to the outpatient setting.
Ascertainment of MI
All Olmsted County residents hospitalized between November 2002 and May 2006 presenting with a troponin T value greater than or equal to 0.03 ng/mL were prospectively identified within twelve hours of the blood draw through the electronic files of the Department of Laboratory Medicine. Consent was sought from patients or the next of kin (if a patient could not grant consent) to perform a transthoracic echocardiogram and to measure CK-MB in unused serum initially stored for additional clinical need. If serum was not available, an additional blood sample was drawn in connection with a clinically indicated draw whenever possible. Cases underwent serial troponin measurements as part of clinical practice, which did not change during the study period. Troponin was measured at baseline and after symptom onset as recommended (1, 2, 8), and dynamic changes were used to diagnose MI (5). Peak creatine kinase ratios were used as previously described (9). Troponin T and CK-MB were measured using a sandwich electrochemiluminescence immunoassay on the Elecsys 2010® (Roche Diagnostic Corp; Indianapolis, Indiana). Biomarkers were measured in the laboratories of the Department of Laboratory Medicine and Pathology, which is certified by the Clinical Laboratory Improvement Act of 1988 and the College of American Pathologists, with all quality control procedures in place.
Three electrocardiograms per episode (first after arrival, last before discharge, and first one after third hospital day) (10) were coded using the Minnesota Code Modular ECG Analysis System (11). Cases were classified using published recommendations based on a combination of cardiac pain, biomarkers, and Minnesota coding of electrocardiograms.(7, 11) The reliability of this methodology is excellent.(12)
Clinical data
Killip class was assessed within 24 hours of admission. Comorbidity was measured by the Charlson Index (12). Clinical diagnoses were used to ascertain hypertension, diabetes mellitus, hyperlipidemia, family history of coronary disease (defined as coronary disease in first line male descendants less than 55 years of age and in first line female descendants less than 65 years of age), and smoking.
Echocardiography
In Olmsted County, all echocardiograms are performed at the Mayo Clinic Echocardiography Laboratory. M-Mode, bi-dimensional, Doppler and Doppler tissue imaging were performed according to the guidelines of the American Society of Echocardiography (13). Digital echocardiography data containing a minimum of 3 consecutive beats (5 in atrial fibrillation) were acquired and transferred to a server for storage and archiving (Prosolv Echo Management System, Problem Solving Concepts, Camel, Ind). All echocardiograms were performed at rest early during the index hospitalization and not repeated during follow-up.(14)
Left ventricular ejection fraction (EF) was measured by previously validated methods, including M-mode or bi-dimensional echocardiography using the Quinones formula from the parasternal views (15), by the quantitative bidimensional biplane volumetric Simpson method from 4 and 2 chambers views (13), and bi-dimensional estimate method from multiple echocardiographic views (15, 16). EF values were averaged when multiple measurements were performed. As recommended (17), preserved systolic function was defined as an EF greater than or equal to 50%. Left ventricular end-diastolic diameter, interventricular septal and posterior wall thickness were measured by M-mode or bidimensional echocardiography from the parasternal views at end-diastole as recommended by the American Society of Echocardiography, and they were used to calculate left ventricular mass, which was indexed to the body surface area (13).
Diastolic function was assessed by a previously-described approach(18, 19) which integrates Doppler measurements of the mitral inflow and Doppler tissue imaging of the mitral annulus using the medial annulus velocity (20). This approach enabled classification of diastolic function which was categorized as normal, mild (impaired relaxation without evidence of increased filling pressures), moderate (impaired relaxation with moderate elevation of filling pressures) or severe (advanced reduction in compliance). This classification was previously validated and published (19). Isolated diastolic dysfunction was defined as diastolic dysfunction with EF greater than or equal to 50%.
Follow-up
The complete (inpatient and outpatient) medical record for each participant was reviewed by abstractors unaware of biomarker values. HF, including both in and out-patient events, was validated using the Framingham criteria(21) the reliability of which has been published for Olmsted County studies (22).
Statistical analysis
Continuous variables were summarized as means ± standard deviations and categorical variables as percents. Variables with skewed distributions were presented as medians (25th– 75th percentiles). Chi square tests were used to examine the association between type of MI and categorical variables. Rank sum tests were used for continuous variables. Cox proportional hazards regression models were constructed to estimate the unadjusted and covariate-adjusted hazard ratio (HR) and 95% confidence interval (CI) for HF associated with the type of MI. The frequency of missing values was less than 10% except for left ventricular mass which was missing in 22% of the cases. The Institutional Review Board approved the study.
Results
Between November 2002 and May 2006, 835 incident MIs (246 meeting only troponin-based criteria and 589 also meeting CK-MB criteria) occurred in Olmsted County. Among those, 482 underwent echocardiogram prior to hospital discharge and constitute the study population. The mean age of the participants was 68 ± 15 years and 45% were women
Among the participants, 363 (75%) met CK-MB-based criteria and 119 (25%) met only troponin-based criteria. Several key characteristics differed in the two biomarker groups (Table 1). Patients meeting only troponin-based criteria were older, more likely to be women, to have hypertension, greater comorbidities, and non ST elevation MI. They were less likely to present with chest pain, have an anterior MI, or Q-waves on their electrocardiograms than patients meeting CK-MB criteria. Patients diagnosed only by troponin had lower troponin level than those diagnosed by CK-MB.
Table 1.
Clinical characteristics stratified by the type of biomarker used for MI diagnosis
| Overall (N=482) | Troponin T only (N=119) | CKMB (N=363) | P value | |
|---|---|---|---|---|
| Age, mean ± sd | 68 ± 15 | 71 ± 16 | 66 ± 15 | <0.01 |
| Women, % | 45 | 53 | 42 | 0.04 |
| Presence of cardiac pain, % | 77 | 57 | 83 | <0.01 |
| Killip class >1, % | 30 | 25 | 31 | 0.22 |
| Hypertension, % | 68 | 77 | 66 | 0.02 |
| Hyperlipidemia, % | 59 | 56 | 60 | 0.54 |
| Diabetes, % | 22 | 24 | 22 | 0.69 |
| Current smoker, % | 21 | 15 | 23 | 0.07 |
| Familial CAD, % | 16 | 13 | 17 | 0.29 |
| Comorbidity Index | ||||
| 0, % | 34 | 26 | 37 | |
| 1–2, % | 35 | 32 | 36 | <0.01 |
| 3+, % | 31 | 42 | 27 | |
| Electrocardiogram | ||||
| Anterior location, % | 42 | 34 | 45 | 0.03 |
| Non ST segment elevation infarction, % | 76 | 94 | 70 | <0.01 |
| Presence of Q waves, % | 60 | 42 | 65 | <0.01 |
| Biomarkers | ||||
| Peak ratio of CK | ||||
| Tertile 1, % | 33 | 81 | 22 | |
| Tertile 2, % | 33 | 18 | 37 | <0.01 |
| Tertile 3, % | 34 | 1 | 41 | |
| CKMB, median (25th–75th percentile), u/L | 21 (8–89) | 6 (4–9) | 41 (14–125) | <0.01 |
| Peak TnT, median (25th–75th percentile), ng/mL | 0.7 (0.2–2.6) | 0.2 (0.1–0.4) | 1.3 (0.3–3.8) | <0.001 |
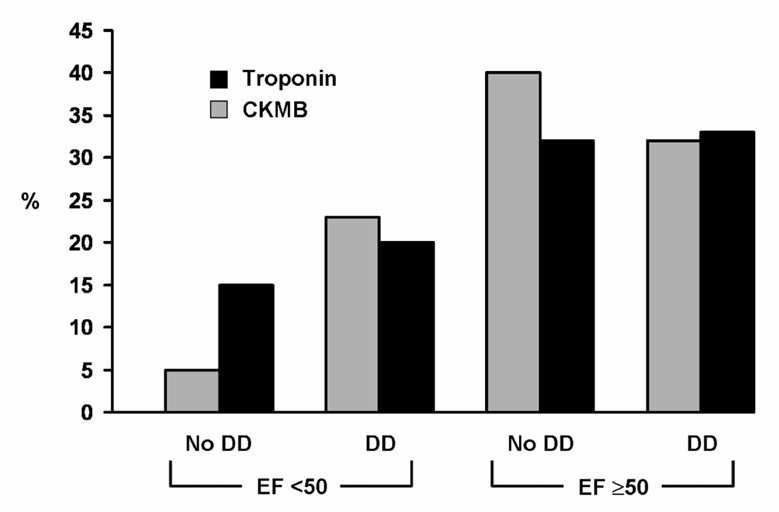
Echocardiograms were performed at a median (Q1, Q3) of 1 day (1–2 days) post-MI. Systolic dysfunction was present in 33% of the MI patients, while moderate or severe diastolic dysfunction was present in 53% of them. Diastolic dysfunction with preserved left ventricular systolic function was present in 33%. Patients meeting only troponin-based criteria had higher EF (55 ± 14% versus 53 ± 13%, p value 0.01), lower wall motion score index, and smaller end diastolic diameter than cases diagnosed by CK-MB criteria (Table 2). Moderate or severe diastolic dysfunction was equally prevalent in both groups. Other echocardiographic characteristics were similar in both groups. The distributions of left ventricular systolic and diastolic functions differed by MI type (Figure 1).
Table 2.
Echocardiographic characteristics stratified by the type of biomarker used for diagnosis
| Overall (N=482) | Troponin T only (N=119) | CK-MB (N=363) | P value | |
|---|---|---|---|---|
| Wall motion score index, mean ± sd | 1.4 ± 0.5 | 1.3 ± 0.5 | 1.5 ± 0.5 | <0.01 |
| Left ventricular mass index, mean ± sd, g/m2 | 104 ± 33 | 110 ± 39 | 102 ± 30 | 0.29 |
| Left ventricular end-diastolic diameter, mean ± sd, mm | 49 ± 6 | 48 ± 7 | 50 ± 6 | 0.02 |
| EF >= 50, % | 67 | 71 | 65 | 0.20 |
| EF, mean ± sd, % | 53 ± 13 | 55 ± 14 | 53 ± 13 | <0.01 |
| Diastolic dysfunction moderate/severe, % | 53 | 55 | 53 | 0.66 |
| Presence of mitral regurgitation, % | 44 | 50 | 42 | 0.12 |
Figure 1. Systolic and diastolic function by MI type.
Legend: DD for moderate or severe diastolic dysfunction.
HF after MI
During a mean follow-up of 0.8 ± 0.7 years, 142 patients developed HF. This corresponds to a one year survival free of HF of 71% (95%CI 67%–75%) with 87% of the HF episodes occurring within the first month post-MI. Among cases meeting only troponin-based criteria, the survival free of HF at one year was 75% (95%CI 68%–84%) compared to 70% (95%CI 65%–75%) among those meeting CK-MB criteria (p= 0.15).
After adjustment for clinical characteristics (age, female sex and comorbidity), MIs meeting solely troponin-based criteria were associated with a large reduction in the risk of post-MI HF (Table 3). The association between troponin-only MI and post-MI HF was unchanged by the addition of EF≥ 50% and moderate or severe diastolic dysfunction. With the addition of wall motion score index or left ventricular end diastolic dimension, this association was slightly attenuated.
Table 3.
Clinical and echocardiographic predictors of post MI HF
| Adjustment | CKMB | Troponin | P-value |
|---|---|---|---|
| Unadjusted | 1 (reference) | 0.75 (0.50, 1.13) | 0.17 |
| Age, sex, comorbidity | 1 (reference) | 0.56 (0.37, 0.85) | 0.01 |
| Age, sex, comorbidity, EF ≥ 50% | 1 (reference) | 0.60 (0.39, 0.91) | 0.02 |
| Age, sex, comorbidity, moderate or severe diastolic dysfunction | 1 (reference) | 0.57 (0.38, 0.86) | <0.01 |
| Age, sex, comorbidity and WMSI | 1 (reference) | 0.66 (0.43, 1.01) | 0.06 |
| Age, sex, comorbidity left ventricular end-diastolic dimension | 1 (reference) | 0.65 (0.41, 1.03) | 0.06 |
WMSI= wall motion score index
In ancillary analyses, we examined the impact of EF as a continuous variable. This analysis yielded similar trends as the main analyses presented herein, thereby attesting to the robustness of our findings.
Discussion
The present study reports the experience of a community with the prospective application of the new MI definition, focusing on left ventricular function and HF. Patients meeting only troponin-based criteria were more likely to have better systolic function than cases diagnosed with the CK-MB criteria. More than half of the patients who experienced an MI had moderate or severe diastolic dysfunction and the presence of moderate or severe diastolic dysfunction was similar in both groups. Compared to persons meeting CK-MB criteria, cases of MI meeting only troponin-based criteria had a substantially lower risk of post-MI HF independent of age, sex, comorbidities, and echocardiographic parameters.
Left ventricular function after MI
The recent literature has underscored the dearth of information on left ventricular function post-MI because ventricular function, by non-invasive or invasive methods, has not been evaluated after MI in routine clinical practice (23). This important caveat notwithstanding, the prevalence of left ventricular systolic dysfunction, when reported, ranged from 27% to 60%. The definitions of left ventricular systolic dysfunction varied greatly across studies thereby explaining the wide ranges of prevalence. However, in studies that used an EF less than or equal to 50% as the cut-off for left ventricular systolic dysfunction, the prevalence of left ventricular systolic dysfunction averaged 50% (23). Importantly, these reports all predated the implementation of the new MI criteria and consisted mainly of clinical trials with their inherent selection biases, such that it is not known whether these numbers are applicable to contemporary cases of MI diagnosed in the community and how this may have changed due to the implementation of the new MI definition. The present population-based study, which defined left ventricular systolic dysfunction as EF less than 50% (17), addresses this gap in knowledge by indicating that in a contemporary cohort that represents the comprehensive experience of a community; one third of MIs have left ventricular systolic dysfunction.
Left ventricular EF may be an imprecise indicator of the degree of myocardial damage as it may be quasi normal despite extensive myocardial damage due to compensatory hyperkinesis in non-infarcted regions (24). Thus, the semi-quantitative assessment of regional systolic function using wall motion score index is important to assess left ventricular systolic function (25, 26). Herein, patients diagnosed with MI based on the troponin-only criteria had lower wall motion score index than patients meeting CK-MB criteria.
To the best of our knowledge, there is no published epidemiological data on the prevalence of diastolic dysfunction after MI in the community. In the present community study, more than half of the subjects who experienced an MI had moderate or severe diastolic dysfunction, and the prevalence of moderate or severe diastolic dysfunction was similar between persons with troponin-only MI versus those with CK-MB MI. An informal comparison to prior reports from Olmsted County indicated that overall the prevalence of moderate or severe diastolic dysfunction after MI was higher than noted in a random sample of residents from the population (27) but lower than that reported among persons with HF (28).
HF after MI
Composite evidence from the literature suggests that, after an MI, 30% to 40% of patients will develop HF following admission for the acute event (23). Prior reports from Olmsted County including all patients who experienced an incident MI from 1979 to 1994 diagnosed by the CK criteria indicated that at 5 years 36% had HF (22). In contemporary MI cases, 28% of cases experienced HF at one year of follow up (29), which was similar to that observed in the present series. The present study extends previous reports by indicating that patients with MI meeting only troponin-based criteria experienced less HF than their counterparts with MI meeting CK-MB criteria.
In the present study, the vast majority of the episodes of HF occurred within the first month post-MI irrespective of the type of MI criteria met. This timing may be related to the early onset of left ventricle remodeling that occurs in the post-MI period (30). Patients meeting troponin-only MI criteria had a lower risk for post-MI HF than patients meeting CK-MB criteria. This observation is congruent with the fact that patients meeting only troponin criteria have better left ventricular systolic function than patients meeting CK-MB criteria, evident by the lower wall motion score index among those with troponin MI compared with the CK-MB MI. However, we reported herein a similar degree of diastolic dysfunction regardless of the type of MI. This suggests that systolic function likely plays a predominant role in the genesis of post-MI HF. These findings are important for risk stratification among these newly identified MIs.
Our data are congruent with the concept that the larger the infarction, the greater the reduction in LV function and the greater the frequency of HF (23). There is evidence in the literature that troponin measurements correlate with infarct size measured with various imaging techniques (31–34). Troponin is acknowledged to be substantially more sensitive than CK-MB (1, 2). This will enable the detection of smaller MIs (1, 2). Our data indicate that MIs associated with elevations in CKMB are larger than those associated with troponin elevations alone (5). We previously reported(5) for all participants on the parent study, mortality rates which were higher among patients with MI meeting CK-MB criteria compared to cases meeting only troponin-based criteria. The results seen on the present group were similar (data not shown)
Strengths and limitations
A focus on left ventricular function post-MI as intended in the present study requires that patients undergo such an assessment. However, in clinical practice in most centers in the US, a substantial proportion of patients with MI do not undergo evaluation of ventricular function early after MI (14, 23). Thus, most, if not all, the studies that report on subjects who underwent an imaging examination during the hospitalization for MI reflect this selective practice. This should be considered, when interpreting the data.
The internal validity of the present data is quite robust as our study identified all consecutive patients who were then classified according to rigorous validation criteria independently of clinical practice. The racial and ethnic composition of Olmsted County may limit the generalizability of these data to groups under-represented in the population. While no single community can represent the nation, studies of chronic diseases in Olmsted County indicate that results from the county can be extrapolated to a large part of the population.(7) Nonetheless, the present study will need replication in other racial and ethnic groups.
The ability to prospectively examine the impact of the new definition using standardized criteria and consistent assays throughout the study and to evaluate the clinical implications of the redefinition within the same population are unique strengths of the present study. This addresses the stated need for “sentinel centers” that was deemed necessary by the panel of experts to understand the implications of the new definition (4). Indeed, the clinical impact of the redefinition of MI cannot be assessed without the use of standardized, highly reliable criteria.
Conclusions
In the community, the prospective application of the new MI definition indicates that patients meeting only troponin-based criteria had better systolic function than patients diagnosed with the CK-MB criteria. Overall, moderate or severe diastolic dysfunction was present in 53% and similar in both biomarker groups. Compared to persons meeting CK-MB criteria, MI patients meeting only troponin-based criteria had a lower risk of post-MI HF.
Acknowledgments
We are indebted to Diane Batzel for study coordination, Susan Stotz R.N. for assistance in data collection and Kristie Shorter for manuscript preparation. We are grateful to Ellen Koepsell, R.N., the study manager.
This study was supported by a Clinician Investigator Fellowship Award from the Mayo Clinic and grants from the Public Health Service and the National Institutes of Health (AR30582, R01 HL 59205 and R01 HL 72435). Dr Roger is an Established Investigator of the American Heart Association.
Abbreviations list
- ACC/ESC
American College of Cardiology/ European Society of Cardiology
- CK-MB
creatinine kinase and its MB fraction
- CI
confidence interval
- EF
ejection fraction
- HR
hazard ratio
- HF
heart failure
- MI
myocardial infarction
- Q1
25th percentile
- Q3
75th percentile
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure None
References
- 1.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959–969. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 2.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50(22):2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Sabatine MS, Morrow DA, de Lemos JA, Gibson CM, Murphy SA, Rifai N, et al. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation. 2002;105(15):1760–1763. doi: 10.1161/01.cir.0000015464.18023.0a. [DOI] [PubMed] [Google Scholar]
- 4.Alpert JS, Thygesen K. A call for universal definitions in cardiovascular disease. Circulation. 2006;114(8):757–758. doi: 10.1161/CIRCULATIONAHA.106.648030. [DOI] [PubMed] [Google Scholar]
- 5.Roger VL, Killian JM, Weston SA, Jaffe AS, Kors J, Santrach PJ, et al. Redefinition of myocardial infarction: prospective evaluation in the community. Circulation. 2006;114(8):790–797. doi: 10.1161/CIRCULATIONAHA.106.627505. [DOI] [PubMed] [Google Scholar]
- 6.Richards AM, Lainchbury JG, Nicholls MG. Unsatisfactory redefinition of myocardial infarction. Lancet. 2001;357(9269):1635–1636. doi: 10.1016/S0140-6736(00)04858-3. [DOI] [PubMed] [Google Scholar]
- 7.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 8.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 9.Hellermann JP, Reeder GS, Jacobsen SJ, Weston SA, Killian JM, Roger VL. Longitudinal trends in the severity of acute myocardial infarction: a population study in Olmsted County, Minnesota. American Journal of Epidemiology. 2002;156(3):246–253. doi: 10.1093/aje/kwf034. [DOI] [PubMed] [Google Scholar]
- 10.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 11.Kors JA, Crow RS, Hannan PJ, Rautaharju PM, Folsom AR. Comparison of computer-assigned Minnesota Codes with the visual standard method for new coronary heart disease events. Am J Epidemiol. 2000;151(8):790–797. doi: 10.1093/oxfordjournals.aje.a010279. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Jimenez F, Goraya TY, Hellermann JP, Jacobsen SJ, Reeder GS, Weston SA, et al. Measurement of ejection fraction after myocardial infarction in the population. Chest. 2004;125(2):397–403. doi: 10.1378/chest.125.2.397. [DOI] [PubMed] [Google Scholar]
- 15.Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WL, Jr, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64(4):744–753. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 16.Amico AF, Lichtenberg GS, Reisner SA, Stone CK, Schwartz RG, Meltzer RS. Superiority of visual versus computerized echocardiographic estimation of radionuclide left ventricular ejection fraction. Am Heart J. 1989;118(6):1259–1265. doi: 10.1016/0002-8703(89)90018-5. [DOI] [PubMed] [Google Scholar]
- 17.Yturralde RF, Gaasch WH. Diagnostic criteria for diastolic heart failure. Prog Cardiovasc Dis. 2005;47(5):314–319. doi: 10.1016/j.pcad.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 19.Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography) J Am Coll Cardiol. 2003;42(5):954–970. doi: 10.1016/s0735-1097(03)01065-9. [DOI] [PubMed] [Google Scholar]
- 20.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102(15):1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 21.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88(1):107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 22.Hellermann JP, Goraya TY, Jacobsen SJ, Weston SA, Reeder GS, Gersh BJ, et al. Incidence of heart failure after myocardial infarction: is it changing over time? Am J Epidemiol. 2003;157(12):1101–1107. doi: 10.1093/aje/kwg078. [DOI] [PubMed] [Google Scholar]
- 23.Weir RA, McMurray JJ, Velazquez EJ. Epidemiology of heart failure and left ventricular systolic dysfunction after acute myocardial infarction: prevalence, clinical characteristics, and prognostic importance. Am J Cardiol. 2006;97(10A):13F–25F. doi: 10.1016/j.amjcard.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Kjoller E, Kober L, Jorgensen S, Torp-Pedersen C. Long-term prognostic importance of hyperkinesia following acute myocardial infarction. TRACE Study Group. TRAndolapril Cardiac Evaluation. Am J Cardiol. 1999;83(5):655–659. doi: 10.1016/s0002-9149(98)00962-x. [DOI] [PubMed] [Google Scholar]
- 25.Thune JJ, Kober L, Pfeffer MA, Skali H, Anavekar NS, Bourgoun M, et al. Comparison of regional versus global assessment of left ventricular function in patients with left ventricular dysfunction, heart failure, or both after myocardial infarction: the valsartan in acute myocardial infarction echocardiographic study. J Am Soc Echocardiogr. 2006;19(12):1462–1465. doi: 10.1016/j.echo.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 26.Moller JE, Hillis GS, Oh JK, Reeder GS, Gersh BJ, Pellikka PA. Wall motion score index and ejection fraction for risk stratification after acute myocardial infarction. Am Heart J. 2006;151(2):419–425. doi: 10.1016/j.ahj.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 27.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. Jama. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 28.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, et al. Systolic and diastolic heart failure in the community. Jama. 2006;296(18):2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 29.Bursi F, Weston SA, Killian JM, Gabriel SE, Jacobsen SJ, Roger VL. C-reactive protein and heart failure after myocardial infarction in the community. Am J Med. 2007;120(7):616–622. doi: 10.1016/j.amjmed.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 30.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35(3):569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 31.Giannitsis E, Steen H, Kurz K, Ivandic B, Simon AC, Futterer S, et al. Cardiac magnetic resonance imaging study for quantification of infarct size comparing directly serial versus single time-point measurements of cardiac troponin T. J Am Coll Cardiol. 2008;51(3):307–314. doi: 10.1016/j.jacc.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 32.Steen H, Giannitsis E, Futterer S, Merten C, Juenger C, Katus HA. Cardiac troponin T at 96 hours after acute myocardial infarction correlates with infarct size and cardiac function. J Am Coll Cardiol. 2006;48(11):2192–2194. doi: 10.1016/j.jacc.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Licka M, Zimmermann R, Zehelein J, Dengler TJ, Katus HA, Kubler W. Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart. 2002;87(6):520–524. doi: 10.1136/heart.87.6.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panteghini M, Cuccia C, Bonetti G, Giubbini R, Pagani F, Bonini E. Single-point cardiac troponin T at coronary care unit discharge after myocardial infarction correlates with infarct size and ejection fraction. Clin Chem. 2002;48(9):1432–1436. [PubMed] [Google Scholar]