Summary
Pancreatitis is a severe debilitating disease with high morbidity and mortality. Treatment is mostly supportive, and until now there are no clinically useful strategies for anti-inflammatory therapy. Although omega-3 polyunsaturated fatty acids (n-3 PUFA) are known to have anti-inflammatory effects, the utility of these fatty acids in the alleviation of pancreatitis remained to be investigated. The aim of this study was to examine the effect of n-3 PUFA on both acute and chronic pancreatitis in a well-controlled experimental system. We used the fat-1 transgenic mouse model, characterised by endogenously increased tissue levels of n-3 PUFA, and their wild-type littermates to examine the effect of n-3 PUFA on both acute and chronic cerulein induced pancreatitis. Disease activity and inflammatory status were assessed by both histology and molecular methods. In acute pancreatitis, fat-1 mice showed a trend towards decreased necrosis and significantly reduced levels of plasma IL-6 levels as well as reduced neutrophil infiltration in the lung. In chronic pancreatitis there was less pancreatic fibrosis and collagen content accompanied by decreased pancreatic stellate cell activation in the fat-1 animals with increased n-3 PUFA tissue levels as compared to wild-type littermates with high levels of omega-6 (n-6) PUFA in their tissues. Our data provide evidence for a reduction of systemic inflammation in acute pancreatitis and of tissue fibrosis in chronic pancreatitis by increasing the tissue content of omega-3 polyunsaturated fatty acids. These results suggest a beneficial potential for n-3 PUFA supplementation in acute and particularly chronic pancreatitis.
Introduction
Acute pancreatitis (AP) is characterised by severe abdominal pain and oedematous or necrotic organ changes that can lead to a severe systemic inflammation with severe morbidity in 20% of cases and approximately 4% mortality [1]. There are no specific therapies for acute pancreatitis. Medical management is aimed at the control of symptoms, the prevention of severe complications, and possibly endoscopic stone removal if common bile duct stones are present or suspected [2]. In acute pancreatitis organ damage is usually initiated by autodigestion of the pancreas. This process begins in the pancreatic acinar cell with intracellular proteolytic activation of pancreatic enzymes leading to the initiation of autodigestion. This is followed by a massive inflammatory reaction with infiltration of neutrophils and macrophages. While the role of these inflammatory cells in the removal of necrotic material is important, there is also evidence that the massive inflammatory reaction exacerbates damage by secretion of pro-inflammatory cytokines with subsequent release of proteases and reactive oxygen species [1]. Indeed, several antioxidants were shown in experimental models to protect from tissue damage in acute pancreatitis (AP) [3]. In addition there is experimental evidence that COX-2 inhibition [4–8] as well as genetic deletion of COX-2 [9] can protect from tissue damage in AP. This suggests involvement of the pro-inflammatory arachidonic acid cascade in AP.
Chronic pancreatitis (CP) affects 5.6 to 24.2 million people in the United States [10, 11] and can be a debilitating chronic medical condition characterized by bouts of severe pain and progressive organ dysfunction with endocrine and exocrine organ insufficiency. Chronic pancreatitis can be due to genetic factors, and correlations with mutations in PRSS1 and PRSS2 as well as SPINK1 and CFTR are well characterised by now [11]. The disease is often associated with alcohol abuse, in which case the most important part of clinical management is total abstinence. So far, the only medical treatment options for chronic pancreatitis (CP) are control of symptoms (pain), the treatment of the resulting endocrine (diabetes mellitus) and exocrine pancreas insufficiency as well as alleviation of bile duct stenosis arising from chronic inflammatory and fibrotic changes in the head of the pancreas [11]. There are different theories to explain the pathogenesis of chronic pancreatitis, including pancreatic duct damage by obstruction or primary autoimmune or inflammatory events [10], followed by multiple bouts of acute inflammation and necrotic changes of the pancreas with progressive organ fibrosis [11]. Common consequence of all these pathological processes is the chronic damage of pancreatic tissue with persisting presence of fibrogenic pancreatic stellate cells that secrete collagen and cytokines [12–15]. Approaches used so far to alleviate the course of CP [16] include immunomodulation [17, 18], Cox-2 inhibitors [19], and antioxidants [20].
Omega-3 polyunsaturated fatty acids (n-3 PUFA) have been implicated in inflammation dampening [21]. Recent research has identified potent anti-inflammatory mediators derived from n-3 PUFA and elucidated the mechanisms of their action [22]. The n-3 PUFA derived mediators are known to play a key role in the resolution of inflammation and therefore termed resolvins and protectins [23]. Our previous results in the fat-1 mouse model showed that an increased tissue status of n-3 PUFA led to formation of resolvins and protectins and to protection from acute DSS induced colitis [24]. Recent studies have implicated a role of n-3 PUFA in the dampening of acute pancreatic inflammation [25–28].
The cerulein-induced pancreatitis is an experimental mouse model for human acute and chronic pancreatitis characterized by histological changes (inflammatory infiltrate, oedema, cell necrosis), increased serum amylase and lipase, release of pro-inflammatory cytokines (TNF-α, IL-1β), and activation of trypsinogen in acinar cells [29].
The study presented here was designed to examine the effect of an endogenously increased n-3 PUFA status on the induction and development of acute and chronic cerulein-induced pancreatitis in fat-1 mice versus wild-type (wt) control animals. These transgenic mice express a Caenorhabditis elegans desaturase, leading to the formation of endogenously high levels of n-3 PUFA from n-6 PUFA, changing the n-6/n-3 PUFA ratio from values around 30/1 to ratios of approximately 1-5/1 [30]. In contrast to feeding studies supplementing n-3 PUFA, the fat-1 model eliminates confounding factors of diet (content of trace elements, fibres, antioxidants etc.) that could have significant effects on the course of pancreatitis itself.
Materials and Methods
Mice
Transgenic fat-1 mice were generated and maintained as described previously [30]. The heterozygous female offspring from several breeding pairs was used for induction of acute pancreatitis, while the male offspring from these matings was used for induction of chronic pancreatitis.
PUFA analysis
Fatty acid profiles were analysed using gas chromatography as described previously [31].
Induction of pancreatitis
Cerulein was purchased from Research plus Inc. NJ. For acute pancreatitis (AP), mice received 12 hourly intraperitoneal injections of 50µg/kg bodyweight cerulein in 200ul saline or only 200ul saline (control group). Animals were sacrificed 1h after the last injection (Figure 1A). Chronic pancreatitis (CP) was induced by 6 hourly intraperitoneal injections of 50 µg/kg cerulein (Sigma) in saline or only saline; three days a week, for 6 weeks. One week after the last injection all mice were sacrificed (Figure 3A). Mice were anesthetized with isoflurane and killed by cardiopuncture to maximize blood yield. Parts of the pancreas and the lungs were snap-frozen in liquid nitrogen and stored at −70°C, while other parts were fixed in 10% buffered formalin and embedded in paraffin.
Evaluation of pancreatitis severity
Pancreatitis was evaluated by measurement of serum amylase and lipase activity and by histopathological assessment of morphological changes. Hematoxylin-eosin (H&E) stainings were performed of pancreatic sections and lung sections. For acute pancreatitis, an experienced pathologist blinded to the experimental treatment examined edema, acinar cell necrosis, and inflammatory cell infiltration. For chronic pancreatitis lobular atrophy, acinar atrophy, fibrosis and inflammation were examined by a blinded observer.
Immunohistochemistry
Fresh pancreatic or lung tissue was fixed in 10% neutral buffered formalin overnight, followed by automated processing and embedding in paraffin. MPO-staining was performed on formalin-fixed paraffin embedded tissue using the avidin-biotin-peroxidase complex technique. A rabbit polyclonal antibody to Myeloperoxidase RTU (Abcam) was used as primary antibody in 1:100 dilutions. The sections were incubated overnight at 4°C. As secondary antibody a biotinylated Goat anti rabbit IgG (Vector Laboratories) was used in a 1:250 dilution. The tissue was counterstained with hematoxylin, negative controls were performed without the primary antibody. For α-SMA staining the slides were exposed to a 1:25 diluted (equals 8 υl/ml) anti-α-SMA antibody (Thermo-Fischer-Scientific) for 10 min at room temperature. After washing with PBS, the primary anti-α-SMA antibody was detected using an Alexa fluor 595 antibody. Stainings were quantified by counting the number of α-SMA-positive cells in 1–3 HPFs per mouse by two different blinded observers, positive cells located in or near blood vessel walls were ignored, and the mean value of these counts was used for further analysis. Data are expressed as the number of α-SMA-positive cells per 100 pancreatic cells. For visualization of pancreatic cell nuclei, cells were co-stained with DAPI (4,6-diamine-2-phenylindole dihydrochloride) in an aqueous dilution of 1:10.000 for 5–10 min.
Quantification of intrapancreatic collagen
Paraffin section were de-waxed and hydrated and stained in a solution of 0.1% Sirius red in saturated picric acid for 45 min at room temperature, as described previously [32]. Slices were then rinsed with acidified water and afterwards in 100% ethanol. The sections were then cleared in Xylene and subsequent mounted in mounting medium. The total amount of collagen stained on each submitted section was calculated in a blinded fashion as follows and as described previously [33]. In the first step, pancreas was distinguished from the background according to a difference in light density, and a measurement of the total pancreatic tissue area was performed. In the second step, the amount of collagen (stained in red) was measured and was finally expressed as a percentage of the total pancreatic surface. The mean of the values in 3 fields per mouse was used for further analysis.
Semiquantitative real-time PCR
Total RNA was isolated from whole pancreas tissue using RNeasy mini kit (Qiagen) following the manufacturer’s instructions and rtPCR performed as described previously [24, 34].
Determination of MPO-activity
Myeloperoxidase (MPO) activity as a reflection of neutrophil sequestration within the pancreas or the lung was assessed as described previously [33]. Briefly, pancreatic and lung tissue was homogenized in 50 mM potassium phosphate (ph 6), containing 0.5g/dl centrimonium bromide, followed by 3 freeze-thaw circles and sonication. We then incubated the samples for 2 hours at 60°C and centrifuged for 20 minutes at maximum speed. The supernatant was added to 50 mM potassium phosphate (pH 6) containing 0.167 mg/ml o-dianisidine dihydrochloride and 0.0005% hydrogen peroxide and absorbance was measured at 460 nm for 3 minutes every 30 seconds.
IL-6-ELISA
For evaluation of the systemic inflammatory activity, serum IL-6 was measured in wt and fat-1 treated and control mice. The ELISA was purchased from eBioscience and performed according to the manufacturer's protocol.
Statistical analysis
All results are presented as mean ± SEM, except where stated otherwise. Student’s t test was used to evaluate the difference between two groups. rtPCR was analyzed by using the 2ΔCt method. Statistical significance was accepted at the level of p < 0.05 and Prism 4 for Windows Software (Graph Pad) was used for all calculations.
Results
Fatty acid profiles in the pancreas
There was a significant difference in the n-3 and n-6 PUFA tissue content between pancreas tissue from untreated wt and fat-1 animals, with a AA/EPA+DHA ratio in fat-1 mice of 0.1 (with predominantly EPA present) as compared to a very high ratio of 61.3 in wild-type mice. These differences led us to our study of pancreatitis in this model.
Increased n-3 PUFA tissue content reduces necrosis in acute pancreatitis
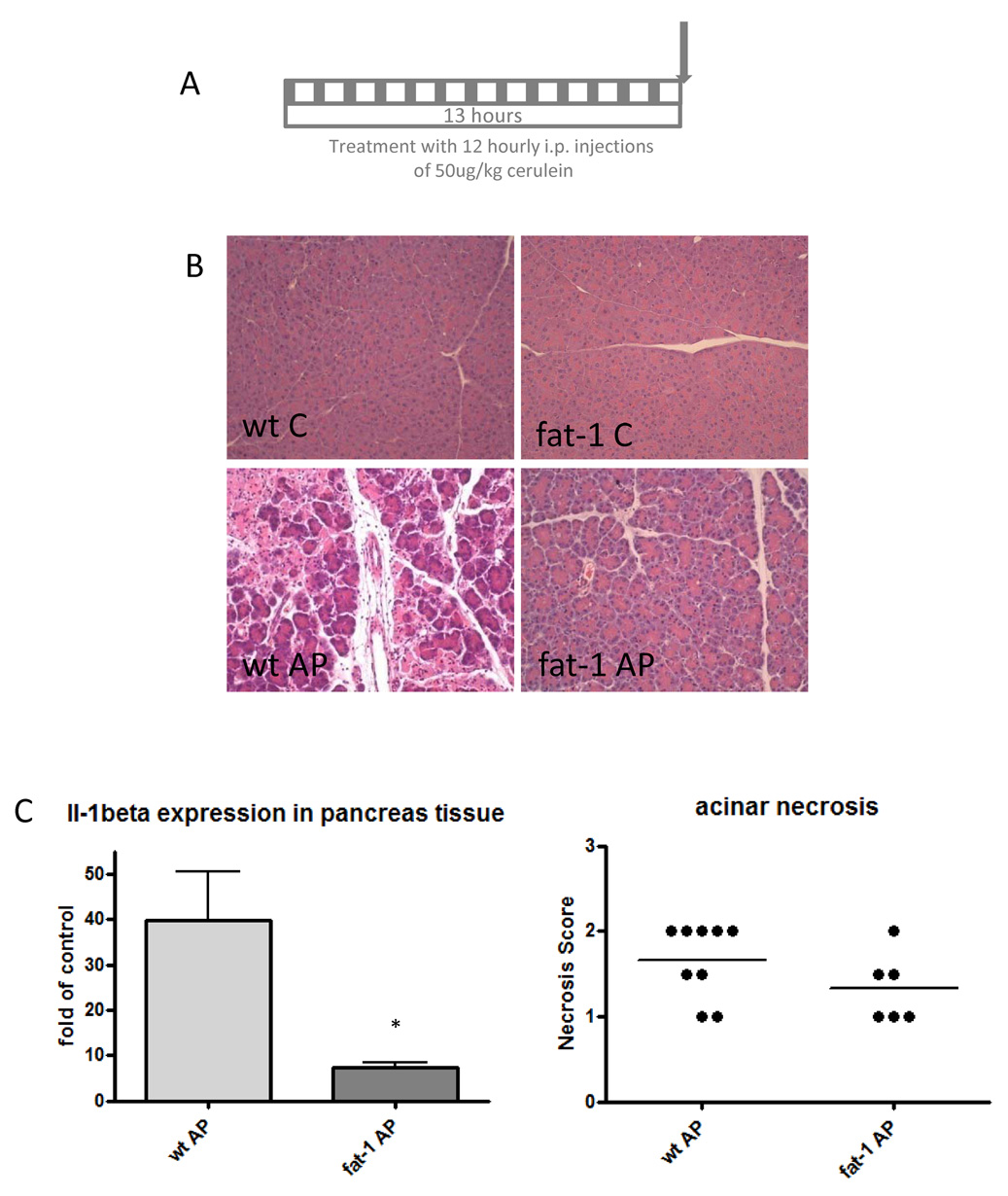
We treated fat-1 and wild-type mice with repeated cerulein injections over one day to induce acute pancreatitis (Figure 1A). All animals developed severe lipasemia and amylasemia with no significant differences between the fat-1 and the wt animals. Histological scoring of pancreas tissue revealed a trend towards decreased pancreatic necrosis in the fat-1 mice (Figure 1B and 1D). In rtPCR analysis of pancreatic tissue we found decreased expression of the pro-inflammatory cytokine IL-1β in fat-1 mice (Figure 1C). There were no significant differences in the mRNA expression levels of TGF-β and IL-6 in the pancreas (data not shown). Unfortunately, more extensive rtPCR analysis was limited by the small amounts of tissue material.
Figure 1. Acute pancreatitis in fat-1 and wild-type mice.
A. Acute pancreatitis was induced by 12 hourly intraperitoneal injections of cerulein.
B. Fat-1 treated mice are less susceptible to injury caused by the cerulein-induced pancreatitis. As compared to saline-injected wild-type (upper left panel) and fat-1 (upper right panel) control animals, the pancreas of wild-type mice injected with cerulein show acinar cell destruction and necrosis (lower left). These histological signs of acute pancreatitis are reduced in fat-1 mice treated with cerulein (lower right). Hematoxylin-Eosin stains (200×) are shown for the different groups.
C. Expression of IL-1β m-RNA, measured by quantitative real time RT-PCR, in mice with acute pancreatitis, n=3 for both groups. *p<0.05 versus wild-type mice with acute pancreatitis.
D. Pancreatic necrosis score in mice with acute pancreatitis. Differences were not significant.
Reduced systemic inflammation in fat-1 mice with acute pancreatitis
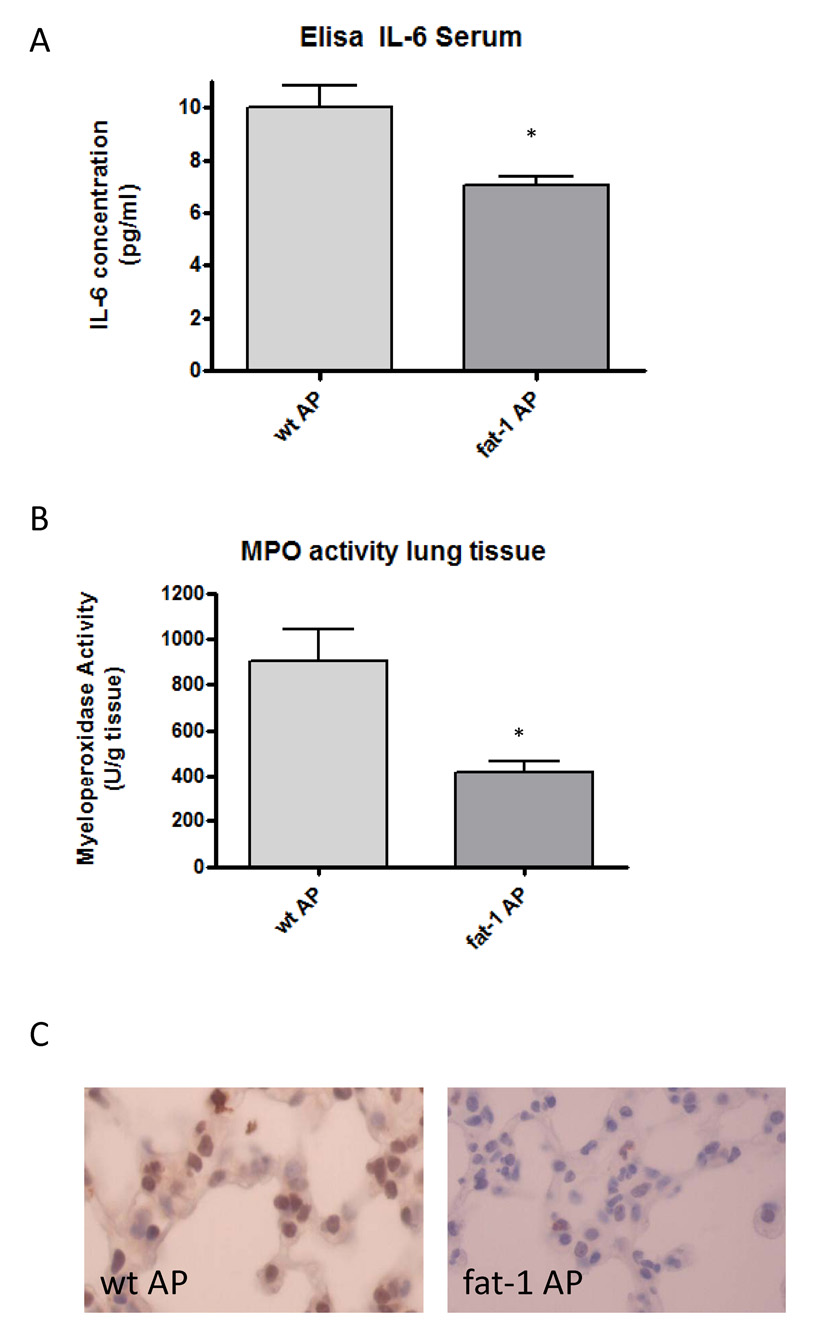
To test for the systemic inflammatory reaction in acute pancreatitis, we examined IL-6 in the plasma which is regarded as a sensitive parameter for the severity of acute pancreatitis. IL-6 levels were significantly lower in fat-1 mice with AP as compared to wt animals with AP (p < 0.05) (Figure 2A). A similar change was observed in the myeloperoxidase (MPO) activity in lung tissue, showing significantly lower MPO-activity in fat-1 mice with AP as compared to wt mice with AP (Figure 2B). Identifying neutrophils in the lung by staining for MPO supported these data (Figure 2C).
Figure 2. Signs of systemic inflammation in acute pancreatitis.
A. Serum IL-6 levels in wild-type mice with acute pancreatitis (wt AP, n=6) compared to fat-1 mice with acute pancreatitis (fat-1 AP, n=5). *p<0.05 compared to wild-type mice with acute pancreatitis.
B. Myeloperoxidase activity in lung tissue demonstrating lower levels in fat-1 animals with acute pancreatitis (fat-1 AP, n=5) as compared to wild-type animals with acute pancreatitis (wt AP, n=4), *p<0.05 versus wt animals with acute pancreatitis.
C. Myeloperoxidase (MPO) antibody staining in lung tissue (400× magnification), reflecting neutrophil infiltration. Tissue from a wild-type mouse with acute pancreatitis showed marked neutrophil infiltration (left panel). In comparison, lung tissue of a fat-1 mouse with acute pancreatitis showed less infiltration (right panel).
Decreased fibrosis in fat-1 mice with chronic pancreatitis
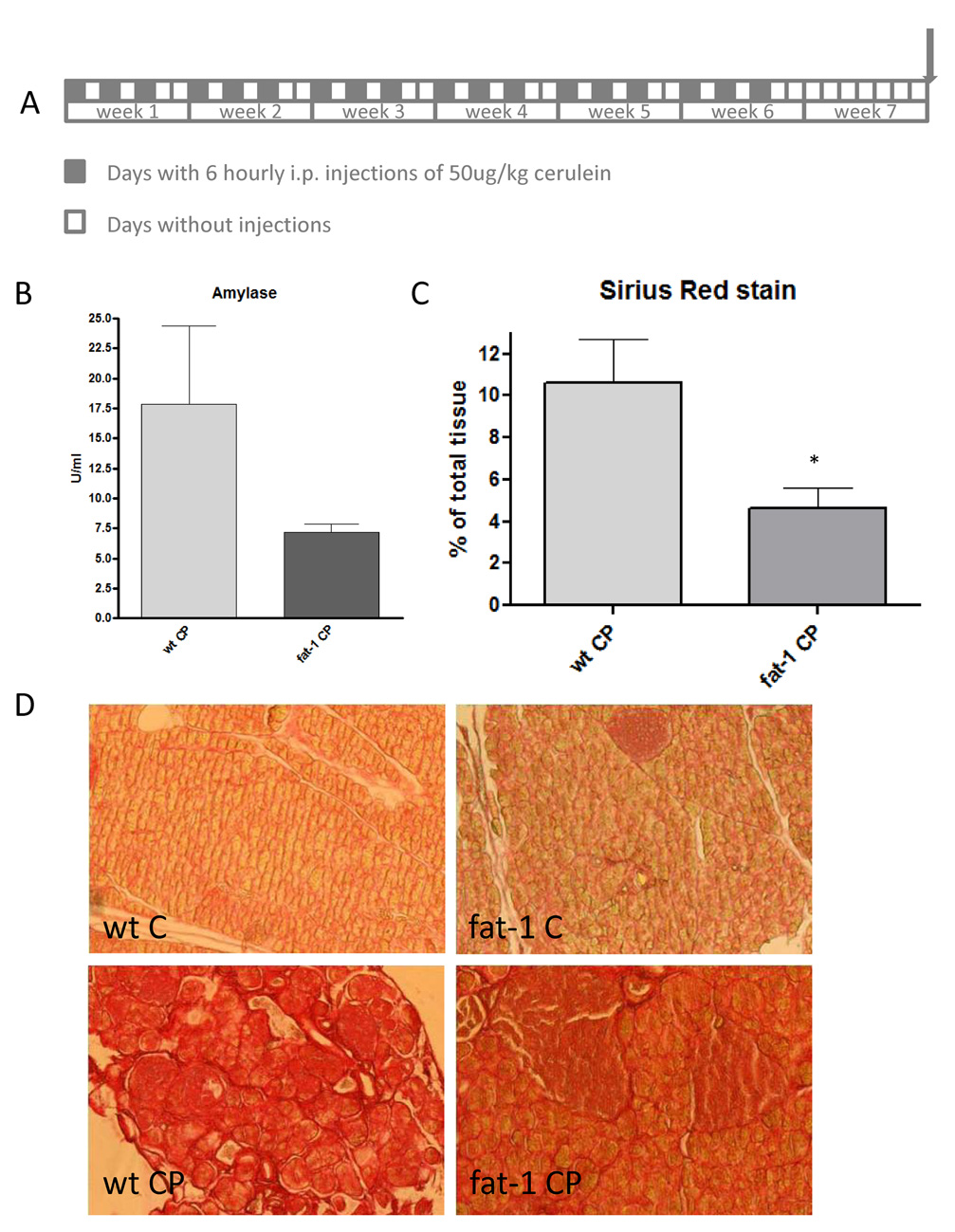
The decreased activity of AP in fat-1 mice suggested the possibility of a beneficial effect on chronic inflammation dampening in this context. We therefore studied chronic cerulein induced pancreatitis (Figure 3A) in fat-1 mice to examine the effects of the endogenously increased n-3 PUFA tissue status on organ atrophy and fibrosis. Fat-1 mice with chronic pancreatitis lost less body weight than their wt littermates (27.0 g ± 0.4 versus 25.5 g ± 0.9). In addition there were slightly lower amylase levels in fat-1 mice with chronic pancreatitis as compared to wt mice (Figure 3B), but both of these differences were not significant. There was, however, a significant difference in the amount of fibrosis as evidenced by Sirius red staining for collagen content of pancreatic tissue. Image analysis of representative samples demonstrated a lower tissue collagen content in pancreatic tissue from fat-1 mice with CP as compared to the collagen content in wt mice with CP (p < 0.05) (Figure 3C and 3D).
Figure 3. Chronic pancreatitis and collagen content in wt and fat-1 mice.
A. Chronic pancreatitis was induced over seven weeks with six intraperitoneal injections of cerulein every second day for six weeks. Mice were then sacrificed one week later.
B. Serum Amylase levels of wt animals with chronic pancreatitis (wt CP, n=8), and fat-1 animals with chronic pancreatitis (fat-1 CP, n=9). Differences were not significant.
C. Quantification of collagen as area of collagen per total pancreatic tissue in percent. Wild-type animals with chronic pancreatitis (wt CP, n=9) and fat-1 animals with chronic pancreatitis (fat-1 CP, n=13) are shown. *p<0.05 versus wild-type animals with chronic pancreatitis.
D. Pancreas tissue samples of wild-type (upper left) and fat-1 (upper right) controls and of wild-type (lower left) and fat-1 (lower right) mice with chronic pancreatitis stained with Sirius Red for the visualisation of collagen. Magnification is 200×.
Increased n-3 PUFA tissue content decreased IL-6 and pancreatic stellate cell activation in chronic pancreatitis
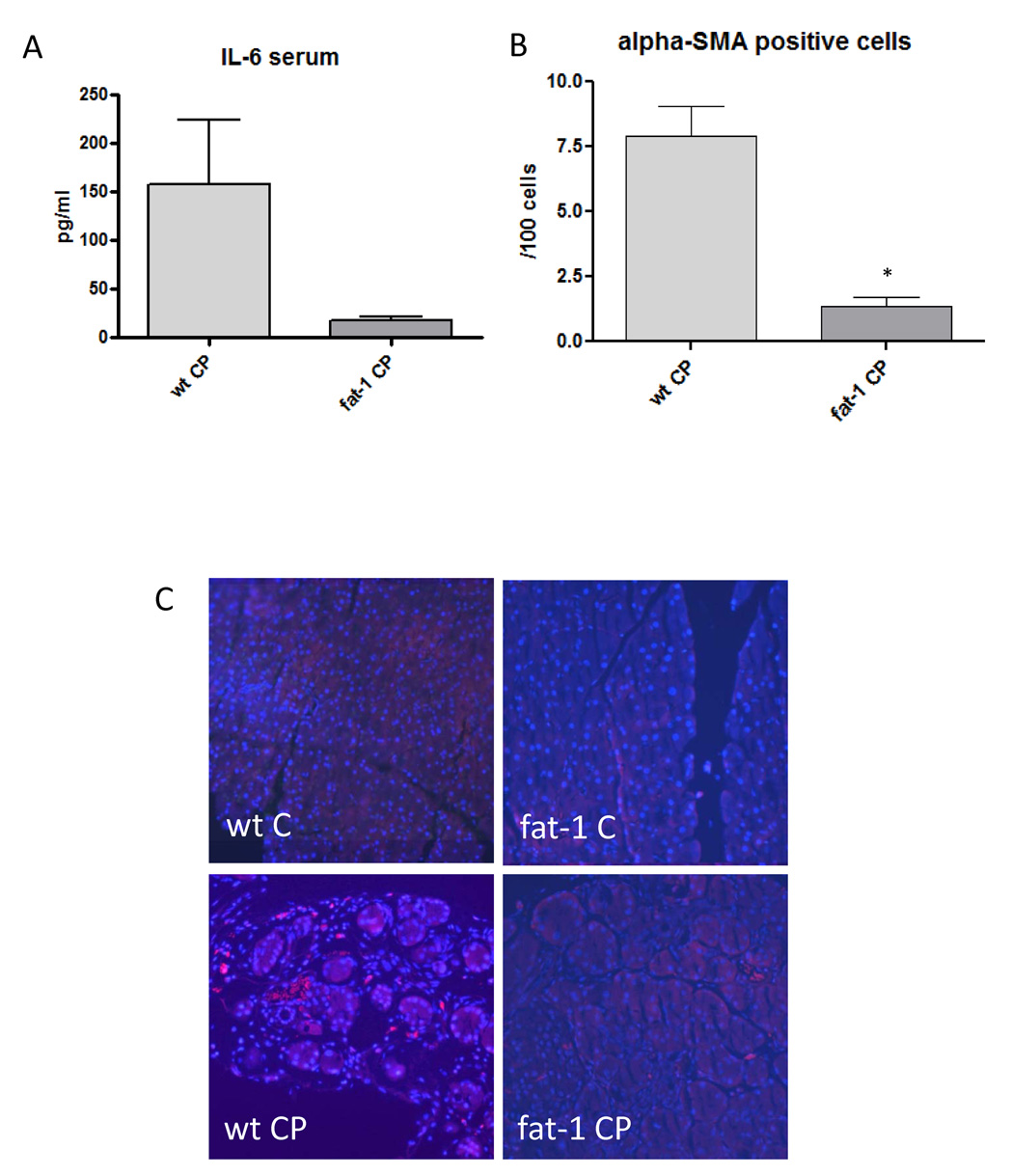
As an indication for the decreased inflammatory activity plasma levels of IL-6 were also lower in fat-1 mice with CP as compared to wild-type mice (Figure 4A). Recent research has implicated pancreatic stellate cells (PSC), myofibroblast-like cells found in the exocrine pancreas, as potent producers of interleukins in the context of pancreatic inflammation [35]. Pancreatic stellate cells also contribute to excess synthesis of extracellular matrix, leading to fibrotic organ changes commonly associated with CP and also with pancreatic cancer [12]. We examined the presence of activated pancreatic stellate cells in fat-1 and wt mice with cerulein-induced CP and found a significant difference. Staining with an antibody against alpha smooth muscle actin (α-SMA), a sensitive method to demonstrate activated pancreatic stellate cells, showed a ten times lower content of activated PSC in fat-1 mice with CP than in wild-type animals with CP (Figure 4B and 4C).
Figure 4. IL-6 and Pancreatic stellate cells in chronic pancreatitis.
A. Level of IL-6 in the serum of wt mice with chronic pancreatitis (wt CP, n=6) and fat-1 mice with chronic pancreatitis (fat-1 CP, n=4). The difference was not significant (p=0.13).
B. Quantification of activated pancreatic stellate cells (PSC) in pancreatic tissue of wild-type (wt CP, n=6) and fat-1 mice (fat-1 CP n=3) with chronic pancreatitis. *p<0.05 versus wt CP.
C. Pancreas tissue double-stained with DAPI and anti-α-SMA antibody for detection of activated PSC in wild-type and fat-1 animals with chronic pancreatitis and respective controls. Magnification 200×.
Discussion
The results presented here show that an increased n-3 PUFA tissue status in the pancreas can alleviate acute as well as chronic inflammatory changes in the organ. Particularly noteworthy in this context is the reduction of fibrosis in chronic pancreatitis, as fibrosis is important for morbidity and prognosis of the disease.
In this study we observed a trend towards decreased necrotic changes in pancreas tissue with high n-3 PUFA tissue content. A decreased necrosis in fat-1 mice with AP could contribute to the decreased acute inflammation by reducing the rupture and leakage of intracellular contents into the extracellular environment, thereby reducing the ensuing inflammatory tissue reactions. There was a lower expression of IL-1β observed in the fat-1 mice with AP. On a systemic level, inflammation dampening was evident by lower IL-6 serum levels in fat-1 mice with AP as compared to their wt littermates with AP. Indeed, IL-6 levels have been implicated as important prognostic parameter in human pancreatitis [36, 37]. Our data demonstrate that the increased n-3 PUFA tissue content also lowers neutrophil infiltration in the lung and thus show a lowered pulmonary inflammatory reaction as a further sign of decreased systemic inflammation and multiorgan involvement. These results are consistent with previous studies showing beneficial effects of n-3 PUFA on acute pancreatitis [25–27].
The dampening of acute pancreatitis episodes could be the basis for the alleviated course of CP in fat-1 animals. Our data demonstrate significantly less fibrotic changes in fat-1 mice with CP as compared to their wt littermates with the disease. Most hypotheses for the development of chronic pancreatitis favour a repeated hit mechanism involving many bouts of acute pancreatitis, leading to the changes of chronic pancreatitis that are associated with significant morbidity and high healthcare expenditure in the western world. In this study we demonstrate for the first time that there could be a role for n-3 PUFA in the reduction of the tissue damage typical for chronic pancreatitis. This was reflected in a significantly lower collagen content in fat-1 mice with CP as compared to wt mice with CP, as well as in a significantly less intensive activation of pancreatic stellate cells, which have an important role in the pathogenesis of pancreatic fibrosis and are also involved in the pancreatic inflammatory reaction [12].
The lipid data in fat-1 mice demonstrate significantly decreased levels of particularly AA in the pancreas of fat-1 animals. Interestingly, the most important n-3 PUFA present in pancreas tissues of these animals is eicosapentaenoic acid (EPA), also a 20-carbon PUFA that is metabolized to lipid mediators by the same enzymes metabolizing AA [38, 39]. EPA is also precursor of resolvin E1 and resolvin E2, substances with potent anti-inflammatory effects that were described and characterised recently [22, 40, 41]. Therefore the decreased inflammatory activity observed here could be a result of a shift from AA-derived lipid mediators to EPA-derived lipid mediators. Particularly the EPA and DHA derived resolvins have been implicated in the processes involved in inflammation resolution [23, 42]. Clearance of inflammatory cells and resolution of inflammation could thus be significantly promoted by the increased presence of n-3 PUFA in fat-1 mice. Thus, protection from pancreatic fibrosis could be a consequence of enhanced resolution that is mediated by lipid mediators formed from EPA in the context of repeated bouts of acute pancreatitis. Future studies are needed to address this question, as the sparse pancreas tissue available from the mice did not allow detailed analyses of lipid mediators in this study.
Taken together, the results presented here show that an increased n-3 PUFA tissue status in the pancreas decreases the systemic inflammatory response in acute pancreatitis and reduce fibrotic changes in chronic pancreatitis. These data now need verification in other animal models as well as in the context of human disease. Together with the previous animal [25–27], as well as human studies [43, 44] examining acute pancreatitis and n-3 PUFA supplementation, our data support a potential benefit for n-3 PUFA supplementation particularly in chronic pancreatitis.
Acknowledgements
This work was supported by grants from the Biomedical Exchange Program (to L.K.), from the American Cancer Society (RSG-03-140-01-CNE) and the NIH (NIH R01 113605) (both to J.X.K.) and from Charite Medical School Research Grants (to K.H.W.). We thank Dr. Van Westerloo in Amsterdam for his help with the planning of experimental procedures, Lisa Sun for help with the gas chromatography and Candice Romany for support with the stainings.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest exist.
References
- 1.Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143–152. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 2.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 3.Pandol SJ. Acute pancreatitis. Curr Opin Gastroenterol. 2006;22:481–486. doi: 10.1097/01.mog.0000239861.89209.5f. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien G, Shields CJ, Winter DC, Dillon JP, Kirwan WO, Redmond HP. Cyclooxygenase-2 plays a central role in the genesis of pancreatitis and associated lung injury. Hepatobiliary Pancreat Dis Int. 2005;4:126–129. [PubMed] [Google Scholar]
- 5.Slogoff MI, Ethridge RT, Rajaraman S, Evers BM. COX-2 inhibition results in alterations in nuclear factor (NF)-kappaB activation but not cytokine production in acute pancreatitis. J Gastrointest Surg. 2004;8:511–519. doi: 10.1016/j.gassur.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 6.Song AM, Bhagat L, Singh VP, Van Acker GG, Steer ML, Saluja AK. Inhibition of cyclooxygenase-2 ameliorates the severity of pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1166–G1174. doi: 10.1152/ajpgi.00370.2001. [DOI] [PubMed] [Google Scholar]
- 7.Foitzik T, Hotz HG, Hotz B, Wittig F, Buhr HJ. Selective inhibition of cyclooxygenase-2 (COX-2) reduces prostaglandin E2 production and attenuates systemic disease sequelae in experimental pancreatitis. Hepatogastroenterology. 2003;50:1159–1162. [PubMed] [Google Scholar]
- 8.Alhan E, Kalyoncu NI, Ercin C, Kural BV. Effects of the celecoxib on the acute necrotizing pancreatitis in rats. Inflammation. 2004;28:303–309. doi: 10.1007/s10753-004-6055-x. [DOI] [PubMed] [Google Scholar]
- 9.Ethridge RT, Chung DH, Slogoff M, Ehlers RA, Hellmich MR, Rajaraman S, Saito H, Uchida T, Evers BM. Cyclooxygenase-2 gene disruption attenuates the severity of acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 2002;123:1311–1322. doi: 10.1053/gast.2002.35951. [DOI] [PubMed] [Google Scholar]
- 10.DiMagno MJ, Dimagno EP. Chronic pancreatitis. Curr Opin Gastroenterol. 2006;22:487–497. doi: 10.1097/01.mog.0000239862.96833.89. [DOI] [PubMed] [Google Scholar]
- 11.Witt H, Apte MV, Keim V, Wilson JS. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007;132:1557–1573. doi: 10.1053/j.gastro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–59. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu K, Kobayashi M, Tahara J, Shiratori K. Cytokines and peroxisome proliferator-activated receptor gamma ligand regulate phagocytosis by pancreatic stellate cells. Gastroenterology. 2005;128:2105–2118. doi: 10.1053/j.gastro.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu K, Shiratori K, Kobayashi M, Kawamata H. Troglitazone inhibits the progression of chronic pancreatitis and the profibrogenic activity of pancreatic stellate cells via a PPARgamma-independent mechanism. Pancreas. 2004;29:67–74. doi: 10.1097/00006676-200407000-00058. [DOI] [PubMed] [Google Scholar]
- 15.Fitzner B, Brock P, Nechutova H, Glass A, Karopka T, Koczan D, Thiesen HJ, Sparmann G, Emmrich J, Liebe S, Jaster R. Inhibitory effects of interferon-gamma on activation of rat pancreatic stellate cells are mediated by STAT1 and involve down-regulation of CTGF expression. Cell Signal. 2007;19:782–790. doi: 10.1016/j.cellsig.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Talukdar R, Tandon RK. Pancreatic stellate cells: New target in the treatment of chronic pancreatitis. J Gastroenterol Hepatol. 2008;23:34–41. doi: 10.1111/j.1440-1746.2007.05206.x. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto T, Yamada T, Kuno A, Ogawa K, Tang M, Sano H, Ohara H, Nakao H, Kataoka H, Shirai T, Itoh M. FTY720, an immunosuppressant, attenuates chronic pancreatitis in rats by suppressing T-cell infiltration. Pancreas. 2005;30:e64–e70. doi: 10.1097/01.mpa.0000157386.15898.3a. [DOI] [PubMed] [Google Scholar]
- 18.Zhao HF, Ito T, Gibo J, Kawabe K, Oono T, Kaku T, Arita Y, Zhao QW, Usui M, Egashira K, Nawata H. Anti-monocyte chemoattractant protein 1 gene therapy attenuates experimental chronic pancreatitis induced by dibutyltin dichloride in rats. Gut. 2005;54:1759–1767. doi: 10.1136/gut.2004.049403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reding T, Bimmler D, Perren A, Sun LK, Fortunato F, Storni F, Graf R. A selective COX-2 inhibitor suppresses chronic pancreatitis in an animal model (WBN/Kob rats): significant reduction of macrophage infiltration and fibrosis. Gut. 2006;55:1165–1173. doi: 10.1136/gut.2005.077925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo BM, Oh TY, Kim YB, Yeo M, Lee JS, Surh YJ, Ahn BO, Kim WH, Sohn S, Kim JH, Hahm KB. Novel antioxidant ameliorates the fibrosis and inflammation of cerulein-induced chronic pancreatitis in a mouse model. Pancreatology. 2005;5:165–176. doi: 10.1159/000085268. [DOI] [PubMed] [Google Scholar]
- 21.Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, Cannon JG, Rogers TS, Klempner MS, Weber PC, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320:265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 22.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, Serhan CN, Kang JX. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci U S A. 2006;103:11276–11281. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharif S, Broman M, Babcock T, Ong E, Jho D, Rudnicki M, Helton WS, Espat NJ. A priori dietary omega-3 lipid supplementation results in local pancreatic macrophage and pulmonary inflammatory response attenuation in a model of experimental acute edematous pancreatitis (AEP) JPEN J Parenter Enteral Nutr. 2006;30:271–276. doi: 10.1177/0148607106030004271. [DOI] [PubMed] [Google Scholar]
- 26.Alhan E, Turkyilmaz S, Ercin C, Kaklikkaya N, Kural BV. Effects of omega-3 fatty acids on acute necrotizing pancreatitis in rats. Eur Surg Res. 2006;38:314–321. doi: 10.1159/000094019. [DOI] [PubMed] [Google Scholar]
- 27.Foitzik T, Eibl G, Schneider P, Wenger FA, Jacobi CA, Buhr HJ. Omega-3 fatty acid supplementation increases anti-inflammatory cytokines and attenuates systemic disease sequelae in experimental pancreatitis. JPEN J Parenter Enteral Nutr. 2002;26:351–356. doi: 10.1177/0148607102026006351. [DOI] [PubMed] [Google Scholar]
- 28.Lasztity N, Hamvas J, Biro L, Nemeth E, Marosvolgyi T, Decsi T, Pap A, Antal M. Effect of enterally administered n-3 polyunsaturated fatty acids in acute pancreatitis--a prospective randomized clinical trial. Clin Nutr. 2005;24:198–205. doi: 10.1016/j.clnu.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 29.van Westerloo DJ, Maris NA, Bruno MJ, van der Poll T. Caerulein induced pancreatitis. Gut. 2003;52:452–453. doi: 10.1136/gut.52.3.452-a. author reply 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- 31.Kang JX, Wang J. A simplified method for analysis of polyunsaturated fatty acids. BMC Biochem. 2005;6:5. doi: 10.1186/1471-2091-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demols A, Van Laethem JL, Quertinmont E, Degraef C, Delhaye M, Geerts A, Deviere J. Endogenous interleukin-10 modulates fibrosis and regeneration in experimental chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1105–G1112. doi: 10.1152/ajpgi.00431.2001. [DOI] [PubMed] [Google Scholar]
- 33.van Westerloo DJ, Florquin S, de Boer AM, Daalhuisen J, de Vos AF, Bruno MJ, van der Poll T. Therapeutic effects of troglitazone in experimental chronic pancreatitis in mice. Am J Pathol. 2005;166:721–728. doi: 10.1016/S0002-9440(10)62293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nowak J, Weylandt KH, Habbel P, Wang J, Dignass A, Glickman JN, Kang JX. Colitis-associated colon tumorigenesis is suppressed in transgenic mice rich in endogenous n-3 fatty acids. Carcinogenesis. 2007;28:1991–1995. doi: 10.1093/carcin/bgm166. [DOI] [PubMed] [Google Scholar]
- 35.Vonlaufen A, Apte MV, Imhof BA, Frossard JL. The role of inflammatory and parenchymal cells in acute pancreatitis. J Pathol. 2007;213:239–248. doi: 10.1002/path.2231. [DOI] [PubMed] [Google Scholar]
- 36.Stimac D, Fisic E, Milic S, Bilic-Zulle L, Peric R. Prognostic values of IL-6, IL-8, and IL-10 in acute pancreatitis. J Clin Gastroenterol. 2006;40:209–212. doi: 10.1097/00004836-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Mayer J, Rau B, Gansauge F, Beger HG. Inflammatory mediators in human acute pancreatitis: clinical and pathophysiological implications. Gut. 2000;47:546–552. doi: 10.1136/gut.47.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang P, Chan D, Felix E, Cartwright C, Menter DG, Madden T, Klein RD, Fischer SM, Newman RA. Formation and anti-proliferative effect of prostaglandin E3 from eicosapentaenoic acid in human lung cancer cells. J Lipid Res. 2004 doi: 10.1194/jlr.M300455-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci U S A. 2003;100:1751–1756. doi: 10.1073/pnas.0334211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 41.Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, Arita M, Serhan CN. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Campbell EL, Louis NA, Tomassetti SE, Canny GO, Arita M, Serhan CN, Colgan SP. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. Faseb J. 2007 doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 43.Petrov MS, Atduev VA, Zagainov VE. Advanced enteral therapy in acute pancreatitis: is there a room for immunonutrition? A meta-analysis. Int J Surg. 2008;6:119–124. doi: 10.1016/j.ijsu.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Li W, Li N, Li J. {omega}-3 Fatty Acids-Supplemented Parenteral Nutrition Decreases Hyperinflammatory Response and Attenuates Systemic Disease Sequelae in Severe Acute Pancreatitis: A Randomized and Controlled Study. JPEN J Parenter Enteral Nutr. 2008;32:236–241. doi: 10.1177/0148607108316189. [DOI] [PubMed] [Google Scholar]