Abstract
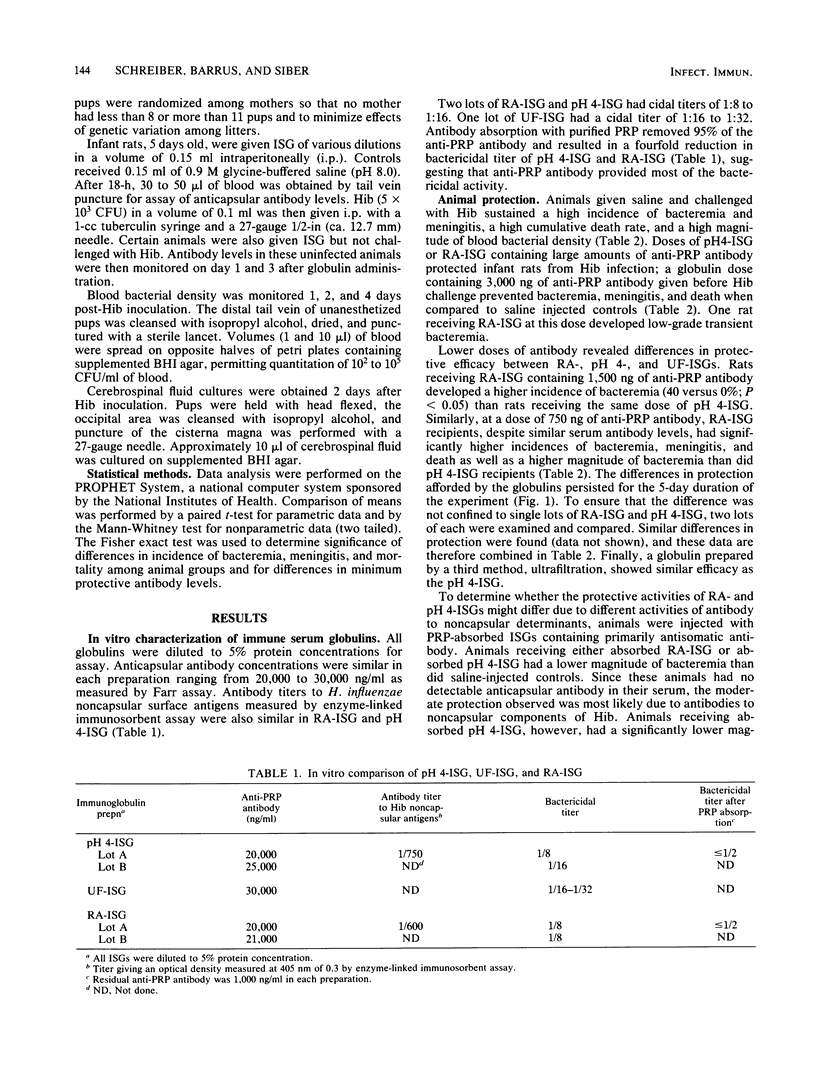
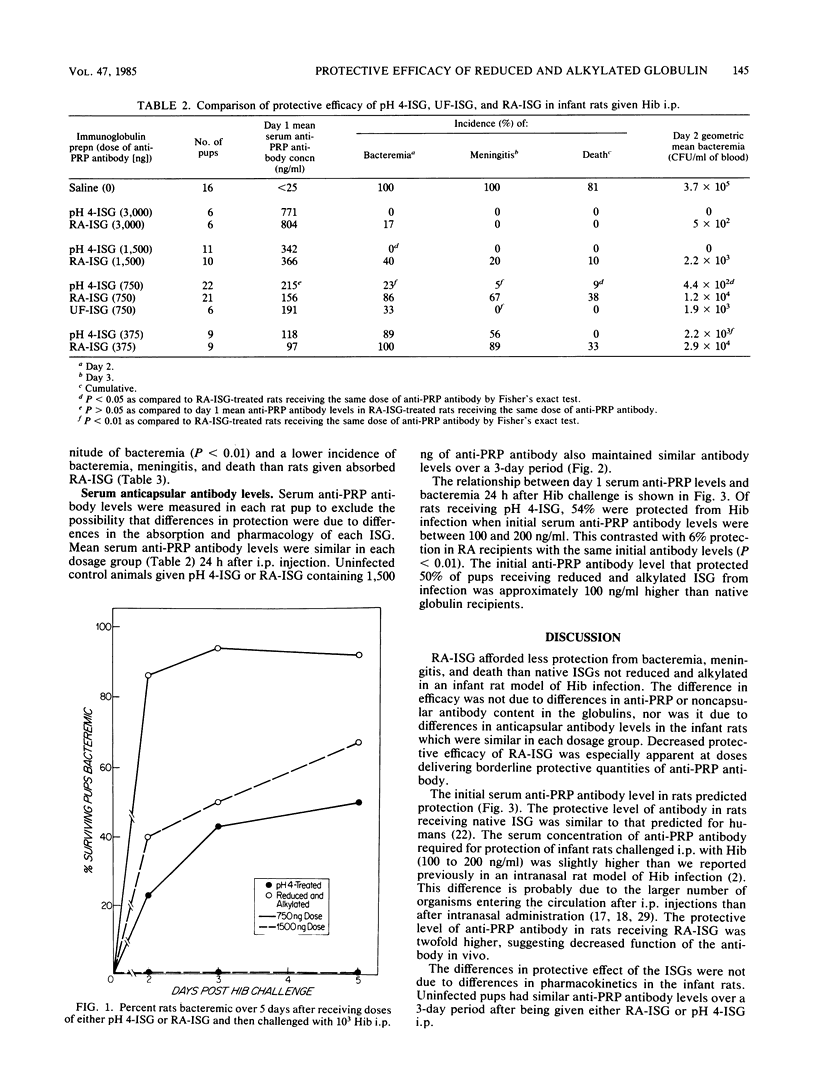
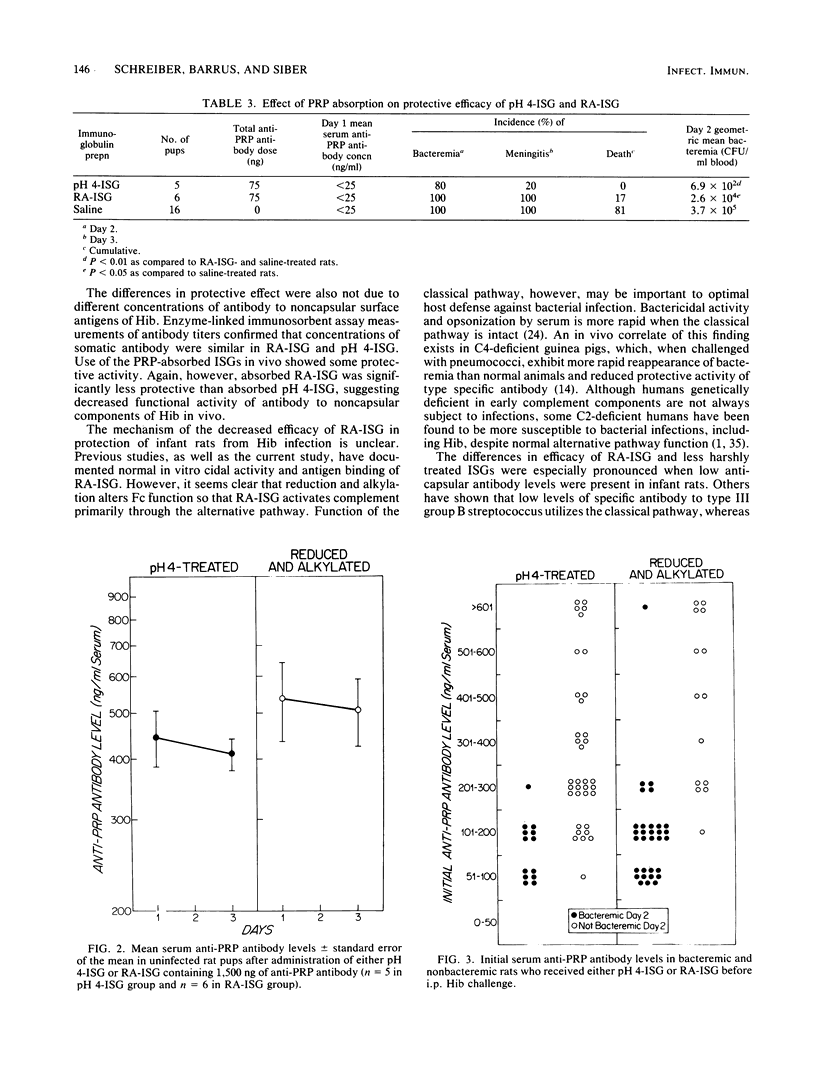
Conventionally prepared immune serum globulin frequently produces severe side effects when administered intravenously. A modified preparation in which 4 to 5 interchain disulfide bonds have been reduced and alkylated has been made for intravenous use. However, reduction and alkylation may affect Fc-mediated functions of immunoglobulin G, particularly its ability to fix complement by the classical pathway. To determine whether reduction and alkylation alters the protective activity of immune serum globulin in vivo we compared it with two less harshly prepared globulins (pH 4 treated or ultrafiltered) in an infant rat model of Haemophilus influenzae b infection. Antibody binding to the capsular and noncapsular components of H. influenzae b and in vitro bactericidal activity were similar in the globulin preparations. Infant rats were treated with various doses of globulins adjusted to provide identical concentrations of anticapsular antibodies as measured by the Farr radioactive antigen binding assay. At high doses of anticapsular antibody (greater than 1,500 ng per pup), all preparations protected well. At marginal doses (750 ng per pup), however, rats given reduced and alkylated globulin had a significantly greater incidence of bacteremia (P less than 0.05), meningitis (P less than 0.01), and death (P less than 0.05) and a higher magnitude of bacteremia (P less than 0.02) than rats who received pH4-treated or ultrafiltered globulins. These differences were not due to differences in anticapsular antibody concentrations achieved in the serum. The 50% protective serum concentrations of anticapsular antibody in this model were 200 to 300 ng/ml for reduced and alkylated globulin and 100 to 200 ng/ml for acid-treated globulin. Absorption of the globulins with purified H. influenzae b capsule reduced in vitro bactericidal activity and rat protective activity. However, the magnitude of bacteremia was lower in rats receiving absorbed pH 4-treated globulin than in those receiving absorbed reduced and alkylated globulin (P less than 0.05). We conclude that reduced and alkylated immunoglobulin G provides significantly less protective activity against H. influenzae b infection in this model than globulins not so modified, and we suggest that the altered Fc function of the immunoglobulin G, such as the decreased ability to fix complement by the classical pathway or decreased Fc-mediated opsonization, may be responsible for this impairment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnello V. Complement deficiency states. Medicine (Baltimore) 1978 Jan;57(1):1–23. doi: 10.1097/00005792-197801000-00001. [DOI] [PubMed] [Google Scholar]
- Ambrosino D., Schreiber J. R., Daum R. S., Siber G. R. Efficacy of human hyperimmune globulin in prevention of Haemophilus influenzae type b disease in infant rats. Infect Immun. 1983 Feb;39(2):709–714. doi: 10.1128/iai.39.2.709-714.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. Intrinsic tritium labeling of the capsular polysaccharide antigen of Haemophilus influenzae type B. J Immunol. 1978 Mar;120(3):866–870. [PubMed] [Google Scholar]
- BARANDUN S., KISTLER P., JEUNET F., ISLIKER H. Intravenous administration of human gamma-globulin. Vox Sang. 1962;7:157–174. doi: 10.1111/j.1423-0410.1962.tb03240.x. [DOI] [PubMed] [Google Scholar]
- Barandun S., Skvaril F., Morell A. Prophylaxis and treatment of diseases by means of immunoglobulins. Monogr Allergy. 1975;9:39–60. [PubMed] [Google Scholar]
- Bing D. H. Complement interaction with immune serum globulin and immune globulin intravenous. Am J Med. 1984 Mar 30;76(3A):19–24. doi: 10.1016/0002-9343(84)90315-2. [DOI] [PubMed] [Google Scholar]
- Collins M. S., Dorsey J. H. Comparative anti-Pseudomonas aeruginosa activity of chemically modified and native immunoglobulin G (human), and potentiation of antibiotic protection against Pseudomonas aeruginosa and group B Streptococcus in vivo. Am J Med. 1984 Mar 30;76(3A):155–160. doi: 10.1016/0002-9343(84)90335-8. [DOI] [PubMed] [Google Scholar]
- Edwards M. S., Nicholson-Weller A., Baker C. J., Kasper D. L. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980 May 1;151(5):1275–1287. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrand R. J. Recurrent haemophilus septicaemia and immunoglobulin deficiency. Arch Dis Child. 1970 Aug;45(242):582–584. doi: 10.1136/adc.45.242.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes P. M., Lundblad J. L. Preparation of a stable intravenous gamma-globulin: process design and scale-up. Vox Sang. 1980 Aug;39(2):101–112. doi: 10.1111/j.1423-0410.1980.tb01844.x. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Rockwell R. Experimental Haemophilus influenzae type b meningitis: immunological investigation of the infant rat model. Infect Immun. 1978 Jun;20(3):705–713. doi: 10.1128/iai.20.3.705-713.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosea S. W., Brown E. J., Frank M. M. The critical role of complement in experimental pneumococcal sepsis. J Infect Dis. 1980 Dec;142(6):903–909. doi: 10.1093/infdis/142.6.903. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Rosen F. S. The gamma globulins. IV. Therapeutic uses of gamma globulin. N Engl J Med. 1966 Oct 13;275(15):826–831. doi: 10.1056/NEJM196610132751508. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moxon E. R., Murphy P. A. Haemophilus influenzae bacteremia and meningitis resulting from survival of a single organism. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1534–1536. doi: 10.1073/pnas.75.3.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ONCLEY J. L., MELIN M. The separation of the antibodies, isoagglutinins, prothrombin, plasminogen and beta1-lipoprotein into subfractions of human plasma. J Am Chem Soc. 1949 Feb;71(2):541–550. doi: 10.1021/ja01170a048. [DOI] [PubMed] [Google Scholar]
- Ostrow P. T., Moxon E. R., Vernon N., Kapko R. Pathogenesis of bacterial meningitis. Studies on the route of meningeal invasion following Hemophilus influenzae inoculation of infant rats. Lab Invest. 1979 Jun;40(6):678–685. [PubMed] [Google Scholar]
- Press E. M. Fixation of the first component of complement by immune complexes: effect of reduction and fragmentation of antibody. Biochem J. 1975 Jul;149(1):285–288. doi: 10.1042/bj1490285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B., Parke J. C., Jr, Schneerson R., Whisnant J. K. Quantitative measurement of "natural" and immunization-induced Haemophilus influenzae type b capsular polysaccharide antibodies. Pediatr Res. 1973 Mar;7(3):103–110. doi: 10.1203/00006450-197303000-00001. [DOI] [PubMed] [Google Scholar]
- Root R. K., Ellman L., Frank M. M. Bactericidal and opsonic properties of C4-deficient guinea pig serum. J Immunol. 1972 Sep;109(3):477–486. [PubMed] [Google Scholar]
- Römer J., Späth P. J., Skvaril F., Nydegger U. E. Characterization of various immunoglobulin preparations for intravenous application. II. Complement activation and binding to staphylococcus protein A. Vox Sang. 1982 Feb;42(2):74–80. doi: 10.1159/000460851. [DOI] [PubMed] [Google Scholar]
- Santos J. I., Shigeoka A. O., Rote N. S., Hill H. R. Protective efficacy of a modified immune serum globulin in experimental group B streptococcal infection. J Pediatr. 1981 Dec;99(6):873–879. doi: 10.1016/s0022-3476(81)80009-1. [DOI] [PubMed] [Google Scholar]
- Schroeder D. D., Tankersley D. L., Lundblad J. L. A new preparation of modified immune serum globulin (human) suitable for intravenous administration. I. Standardization of the reduction and alkylation reaction. Vox Sang. 1981;40(6):373–382. doi: 10.1111/j.1423-0410.1981.tb00725.x. [DOI] [PubMed] [Google Scholar]
- Smith A. L., Smith D. H., Averill D. R., Jr, Marino J., Moxon E. R. Production of Haemophilus influenzae b meningitis in infant rats by intraperitoneal inoculation. Infect Immun. 1973 Aug;8(2):278–290. doi: 10.1128/iai.8.2.278-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele N. P., Munson R. S., Jr, Granoff D. M., Cummins J. E., Levine R. P. Antibody-dependent alternative pathway killing of Haemophilus influenzae type b. Infect Immun. 1984 May;44(2):452–458. doi: 10.1128/iai.44.2.452-458.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. I., Siber G. R., Scheifele D. W., Smith D. H. Rapid diagnosis of Hemophilus influenzae type b infections by latex particle agglutination and counterimmunoelectrophoresis. J Pediatr. 1978 Jul;93(1):37–42. doi: 10.1016/s0022-3476(78)80596-4. [DOI] [PubMed] [Google Scholar]
- Weller P. F., Smith A. L., Smith D. H., Anderson P. Role of immunity in the clearance of bacteremia due to Haemophilus influenzae. J Infect Dis. 1978 Oct;138(4):427–436. doi: 10.1093/infdis/138.4.427. [DOI] [PubMed] [Google Scholar]
- Wright J. K. Reduced immunoglobulin G activates complement system with decreased cooperativity. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1284–1290. doi: 10.1016/0006-291x(78)91360-8. [DOI] [PubMed] [Google Scholar]
- van Furth R., Leijh P. C., Klein F. Correlation between opsonic activity for various microorganisms and composition of gammaglobulin preparations for intravenous use. J Infect Dis. 1984 Apr;149(4):511–517. doi: 10.1093/infdis/149.4.511. [DOI] [PubMed] [Google Scholar]


