Abstract
The brainstem reticular formation is an area important to the control of REM sleep. The antagonist of GABAA receptors, bicuculline methiodide (BMI), injected into the rat nucleus pontis oralis (PnO) of the reticular formation resulted in a long-lasting increase in REM sleep. Thus, one factor controlling REM sleep appears to be the number of functional GABAA receptors in the PnO. The long-lasting effect produced by BMI may result from secondary influences on other neurotransmitter systems known to have long-lasting effects. To study this question, rats were surgically prepared for chronic sleep recording and additionally implanted with guide cannulas aimed at sites in the PnO. Multiple, 60 nl, unilateral injections were made either singly or in combination. GABAA receptor antagonists, BMI and gabazine (GBZ), produced dose-dependent increases in REM sleep with GBZ being approximately 35 times more potent than BMI. GBZ and the cholinergic agonist, carbachol, produced very similar results, both increasing REM sleep for about eight-hours, mainly through increased period frequency, with little reduction in REM latency. Pre-injection of the muscarinic antagonist, atropine, completely blocked the REM sleep-increase by GBZ. GABAergic control of REM sleep in the PnO requires the cholinergic system and may be acting through presynaptic modulation of acetylcholine release.
Keywords: muscarinic, gabazine, bicuculline, intracerebral injection, nucleus pontis oralis, sublaterodorsal nucleus
Introduction
The oral pontine reticular formation (PnO) is one region of brain considered a REM sleep induction zone because of increased REM sleep following the intracerebral injection of several different neurotransmitter-receptor ligands directly into this area (Ahnaou et al., 1999; Ahnaou et al., 2000; Baghdoyan et al., 1987; Bier and McCarley, 1994; Bourgin et al., 1999; Bourgin et al., 1995; Bourgin et al., 1997; Marks and Birabil, 1998; Marks et al., 2003; Yamuy et al., 1995). The ability of pharmacological agents interacting with these brainstem mechanisms to significantly alter the expression of state strongly implicates the PnO as one region concerned with the natural regulation of REM sleep. The heterogeneous cell population and complex inter-connectivity of the PnO has made it difficult to discover how interactions within and among other brain regions permit the PnO to perform this function. A behavioral-pharmacological approach has aided in the resolution of this problem by helping to identify putative mechanisms involved. One of the most recent additions to the list of local mechanisms operating in the PnO in the control of REM sleep is γ-aminobutyric acid-type A (GABAA) receptors. The GABAA receptor antagonist, bicuculline methiodide (BMI), microinjected into the PnO induced REM sleep (Sanford et al., 2003; Xi et al., 1999).
GABAergic neurotransmision is a prominent mechanism in the pontine reticular formation. It is estimated, based on ultra-structural examination, that 30% of all synapses in the ventral PnO of the cat are GABAergic (De La Roza and Reinoso-Suarez, 2006). GABAA receptor subunit immunoreactivity also is observed on neuron plasma membrane domains in the pontine reticular formation not associated with glutamic acid decarboxylase-positive boutons (De La Roza and Reinoso-Suarez, 2006; Fritschy et al.,1992). This suggests GABAA receptors play a role not only at a high proportion of synapses, but also at extrasynaptic sites. The PnO is densely innervated by GABAergic fibers (De La Roza and Reinoso-Suarez, 2006; Ford et al., 1995) whose sources have yet to be identified. The absence of Golgi-type II cells, short axoned interneurons, in the reticular formation does not preclude an innervation by axon collaterals of local neurons. Unidentified afferents of the PnO also are from widespread regions along the neuraxis with more than half originating from other areas of the brain reticular formation, including the contralateral PnO (Shammah-Lagnado et al., 1987). The ability of GABA to influence activity in the PnO could derive from many structures, and diversity of local sites of action within the PnO could invest GABA with several different functions. The action of GABAA receptor blockade in the PnO to induce REM sleep is consistent with one function of GABA at this site to be to control REM sleep.
In the cat, unilateral injections of 250 nl, 10−2 M BMI, in what was labeled the PnO, resulted in a short latency appearance of REM sleep with very long episode durations. The effect was short lived lasting approximately 1 hour (Xi et al., 1999). In the rat a somewhat different response was reported. Bilateral injections of 200 nl, 10−4 or 10−3 M BMI into the PnO resulted in significantly increased REM sleep expression over the 6 Hr period of the post-injection recordings. Higher doses induced stereotyped motor activity and resulted in decreased REM sleep. Latency to REM sleep onset was little affected compared to vehicle-control injections (Sanford et al., 2003).
As with BMI, the nature of increased REM sleep differs in rat and cat following intracerebral injection of other receptor-ligands into the PnO. For example, the cholinergic agonist carbachol, 44 × 10−3 M in 500 nl, unilaterally injected in the cat induced a short latency appearance of REM sleep with long episode durations (Baghdoyan et al., 1984b). Unlike following BMI, carbachol increased REM sleep in the cat lasted many hours (Baghdoyan et al., 1984b). In the rat, there was a narrow range of doses of carbachol, 60 nl, 10−4−10−3 M, that resulted in lower magnitude increased REM sleep than in cat, smaller reductions in latency, but also lasted for long durations, 8 hours (Marks and Birabil, 2007). Higher doses reduced REM sleep and increased wakefulness, and bilateral carbachol injections in PnO lost its effectiveness. The basis of these species differences are unknown.
Here, we address several aspects of REM sleep-induction by GABAA receptor blockade in the PnO of the rat. Inasmuch as only bilateral injections have been reported (Sanford et al., 2003), and laterality is a relevant variable in REM sleep-induction by carbachol (Marks and Birabil, 1998), unilateral injections might result in effects in the rat more similar to the effects of unilateral injections the cat. Another issue is the use of BMI as a GABAA receptor antagonist. It is known that bicuculline, and especially its quaternary N-methyl derivatives, such as BMI, have many non-GABAA receptor-mediated actions (reviewed in, Seutin and Johnson, 1999). Of central concern, is the action of BMI as a potent inhibitor of acetylcholinesterase (Olsen et al., 1976). Injection of an acetylcholinesterase inhibitor, neostigmine, into the PnO of the cat induced REM sleep (Baghdoyan et al., 1984a). Use of a different and more selective competitive GABAA antagonist, such as gabazine (GBZ), may support or fail to support GABAA receptor-blockade as the mechanism mediating the induction of REM sleep in PnO.
The short-lasting action of BMI in cat is consistent with the time course of receptor occupancy produced by a single injection, while the long-lasting effect in rat indicates BMI may only initiate events that maintain high levels of REM sleep for longer periods of time. This is consistent with the blockade of the inhibitory action of GABA resulting in liberation of other neurotransmitters, such as cholinergic agonists whose actions in the PnO of the rat have been documented to have long-lasting effects in the increase of REM sleep (Marks and Birabil, 2007). Such indirect actions would be revealed by antagonizing the effect of GABAA receptor blockade with receptor antagonists of a different neurotransmitter system.
We find unilateral injections do not produce effects in rat similar to those reported in cat. The GABAA antagonist, GBZ, is effective at producing long-lasting increases in REM sleep with potency compared to BMI relative to their range of affinities for GABAA receptors. And, the action of GBZ to increase REM sleep is blocked by the muscarinic antagonist, atropine. These results are discussed with respect to the role of GABA in the PnO in the control of REM sleep in the rat.
Experimental procedures
Animal preparation
All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the local Institutional Animal Care and Use Committees at UT Southwestern Medical Center and the Dallas VA. Male, Long-Evans Hooded rats (Harlan, Indianapolis, IN, USA) weighing between 255 and 446 gms (N=11) were instrumented for chronic sleep-recording under ketamine/acepromazine anesthesia (80/2.5 mg/kg). As previously described (Marks and Birabil, 1998), this consisted of electrodes for recording: cortical electroencephalogram, two screws tapped ipsilaterally into skull respectively over somatosensory and motor cortex; hippocampal electroencephalogram, a stainless steel wire (200 µm dia.), insulated except at the tip, implanted in the dorsal hippocampus referenced to a cortical screw over the cerebellum; and the nuchal electromyogram, two spring electrodes sutured under the trapezious muscle. In addition, guide cannulas with stylettes inserted (Plastics One, Inc., Roanoke, VA, USA) were stereotaxicaly, bilaterally aimed at sites in the PnO previously shown to support increased REM sleep in rat after carbachol infusion (bregma −8.3 to −8.8, L 1.0, 7.7 mm from skull) (Bourgin et al., 1995; Marks and Birabil, 1998; Marks and Birabil, 2007). Lateral to medial angles of approach were used (14° from vertical).
After one week recovery from surgery, animals were placed individually in sound-attenuated, video-monitored, light- (12 h light/12 h dark) and temperature- (22±1°C) controlled enclosures. A cable connected to a swing-arm and commutator tethered each rat to the recording equipment while allowing unrestricted movement in the cage. Animals remained in the recording environment except when removed for adaptation to handling (each day for the first week).
Intracerebral injection
Injections were performed unilaterally within 30 min of the start of the dark period by inserting a cannula (28-gauge), back-filled with drug solution, connected to a 1.0-µl syringe (Hamilton, Reno, NV, USA) through, oil-filled, polyethylene tubing (Plastics One, Inc.). All solutions were filter sterilized (0.2 µm, pore size) before injection. The volume injected was held constant at 60 nl, of which 20 nl were injected every 45 s followed by a 3-min wait before cannula was removed. Under conditions in which two injections were carried out consecutively at the same site (double injection), a second pre-filled cannula was inserted immediately following removal of the first and the procedure was repeated ending with stylette reinsertion. The rat was then returned to its cage. Each pontine site received multiple single or double injections with a minimum of seven days between injection-procedures.
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in normal saline. Each rat received four saline-vehicle injections as control distributed randomly throughout the course of the drug injections. The drugs and concentrations used were: (−)-bicuculline methiodide (BMI), 10−3 M; gabazine (SR-95531, GBZ), 10−5 and 10−4 M; carbamoylcholine chloride, carbachol, 10−4 and 10−3 M; atropine sulfate, 4 × 10−3 M.
Polygraphic recordings were obtained for 24 hrs following each injection-procedure. Electrical potentials were digitized (256 S/s) and stored on optical disks. The recordings were visually scored (Rodent sleep stager, Grass Instruments, West Warwick, RI, USA) without knowledge of the experimental condition utilizing standard criteria. Each 15-s epoch was assigned a score of wake, slow wave (SW) sleep or REM sleep. From these data, stage totals, bout frequency, and mean bout duration were determined for each hour. Bout frequency was a count of the number of times an epoch was scored a particular stage that was preceded by an epoch scored a different stage. Mean bout duration was computed by dividing the time in stage by the bout frequency. REM sleep and SW sleep latencies were computed based on the interval from stylette reinsertion after injection to the first continuous 30-s (2 epochs) episode of each respective state.
After completion of the injection experiments, rats were killed by decapitation under an overdose of pentobarbital and injection sites were determined in coronal sections (50 µm) stained with cresyl violet and mapped with the aid of the atlas of Paxinos and Watson (1986).
Statistical Analyses
All statistical analyses were performed in SigmaStat 3 (Systat Software, Richmond, CA, USA) using a probability of less than 0.05 to determine significance. One-way repeated measures ANOVAs were used for each dependent variable in experiments based on 8-hour recordings with individuals receiving multiple drug injections. In instances where main effects were found significant, all pair-wise multiple comparisons (Holm-Sidak method) were conducted to determine mean differences between drug conditions. An overall significance level of 0.05 was used in these tests of simple effects.
For the determination of the distribution of state in time following injections of GBZ 10−4 M, measures were divided into 2 hr-blocks. The 2 hr-blocks taken from different times of day had unequal variances in every measure tested. The violation of the homogeneity of variance assumption precluded the use of a parametric statistical analyses. Instead, the Kruskal-Wallis one-way analysis of variance was used to determine whether the 2 hr-blocks significantly differed from baseline values in the 24 hr study.
Results
Comparison of GBZ to BMI
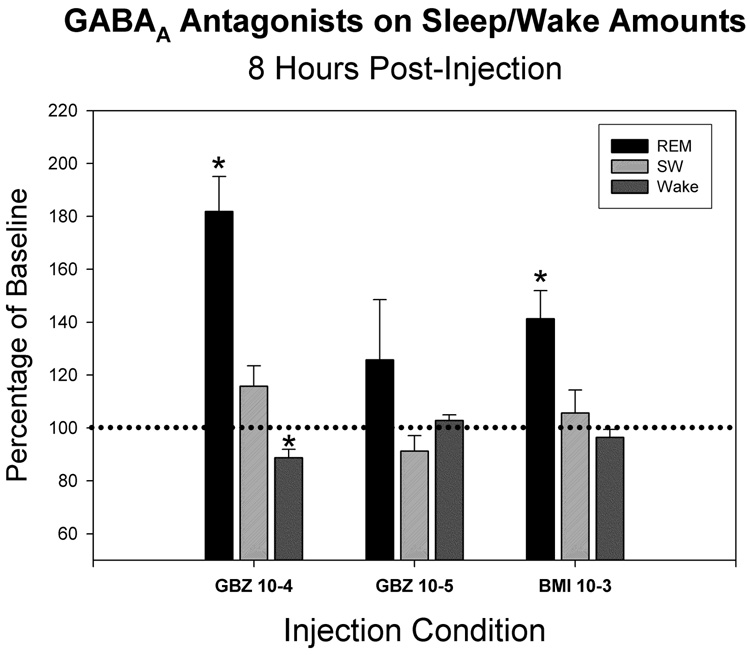
To test the specificity of GABAA receptor-mediated actions of BMI to induce REM sleep, 10−3 M solutions of BMI were unilaterally injected and the effects compared to those of two doses of a different GABAA receptor antagonist, GBZ, at 10−5 and 10−4 M at four injection sites in the PnO. Both agents were found to increase REM sleep amounts in the eight hours following injection compared to REM sleep amounts following the mean of four vehicle-control injections (fig. 1). BMI at 10−3 M resulted in a significant increase in REM sleep of 141±10.6 (mean±SEM) percent of control. GBZ at 10−4 M resulted in a greater, significant increase of 181.9±13.1%, but that produced by 10−5 M GBZ was lower, 125.8±22.7%, and did not reach statistical significance. For all agents and doses, increases in the frequency of REM sleep bouts were greater than increases in mean REM bout durations. In 3 of the 4 cases, the order of administration was GBZ-10−4, BMI- 10−3, GBZ-10−5. In the fourth case the order was reversed. Receiving GBZ-10−4 last instead of first did not change the response to GBZ-10−4 M, which maintained the greatest magnitude of all drug injections. This indicates order was not the factor responsible for the differential responses to drug injection. Approximating the equivalent dose of BMI by linearly interpolating between the effects of the two doses of GBZ, resulted in a concentration of GBZ at 2.8×10−5 M to produce the equivalent effect on REM sleep of 10−3 M BMI. This computation applied to this region of the dose-response curve yielded a potency of GBZ 35-fold greater than BMI to increase REM sleep. Neither BMI nor GBZ significantly altered SW sleep amounts. GBZ at the 10−4 M dose produced a small but statistically significant reduction in wake, 88.7±3.2 percent of control.
Figure 1.
Mean time spent in different arousal states for the 8-hrs following intracerebral injection of GABAA receptor antagonists expressed as a percentage of control values (100%). The states are: REM sleep (REM), slow-wave sleep (SW) and wakefulness (Wake). Gabazine (GBZ) was administered at two concentrations (10−4 and 10−5 M) and bicuculline methiodide (BMI) at a single concentration (10−3 M). * indicates values significantly different from control (p<0.05, N=4). Error bars are +SEM.
We have previously found latency-measures following the injection procedure to be highly variable both across and within individual rats (Marks and Birabil, 2001). Nonetheless, a repeated-measures analysis of the current data revealed both BMI and GBZ-10−4, but not GBZ-10−5, produced significant reductions in the latency to REM sleep onset, 79.6±3.8%, 48.9±9.7% and 95.4±13.0% of control, respectively. Even in the individual case of the greatest reduction in drug-induced latency, 87.6 compared to 33.8 min (control compared to following GBZ-10−4), this was not a triggering of REM sleep (fig. 2).
Figure 2.
Mean latency of different sleep parameters following intracerebral injection of GABAA receptor antagonists. The parameters are onset of: REM sleep (REM), slow-wave sleep (SW) and REM sleep following SW sleep (R/SW). Gabazine (GBZ) was administered at two concentrations (10−4 and 10−5 M) and bicuculline methiodide (BMI) at a single concentration (10−3 M). * indicates values significantly different from control (p<0.05, N=4). Error bars are +SEM.
Time course of GBZ effects
To characterize the time course of the action of GABAA receptor antagonism on REM sleep, 24-hour recordings were analyzed in two-hr-bins for the GBZ 10−4 M dose (N=8). Immediately following the injection, stereotyped, continual circling in the direction ipsilateral to the side of the injection was observed in 3 of the 8 rats. Within minutes or seconds, circling would resolve into expressions of normal behavior except that any turning was always to the side ipsilateral to the injection. Two additional rats showed this ipsilateral turning without stereotyped circling. The mean time from injection to the first turn to the contralateral direction for these five rats was 15.2 min. Neither the induction of ipsilateral turning nor its duration was related to the amount of REM sleep-increase induced by the GBZ at the dose used. Attempts at utilizing higher doses of GBZ invariably resulted in circling behavior that could be both violent and protracted (data not shown). The strong possibility of injury to the rat prohibited the use of GBZ at a dose greater than 10−4 M.
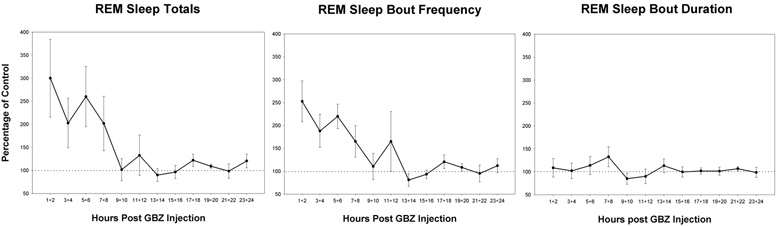
Figure 3a shows the mean REM sleep-totals for 2-hr blocks following GBZ injection in PnO as a percentage of each rat’s respective mean, vehicle-control REM sleep-totals. The trend was for means to remain elevated for the first eight hours. The main effect of block values with respect to control was statistically significantly. Increased REM sleep in individual cases were not consistently elevated for each block over the 8 hrs. These differences among animals in the times that were elevated resulted in high variability in the grouped data (see error bars, ± SEM, fig. 3a and b). Variability across animals was greatly reduced when data consisted of the aggregate over the eight hours.
Figure 3.
Time course effects on the means of REM sleep parameters following intracerebral injection of the GABAA receptor antagonist, gabazine (GBZ), at a concentration of 10−4 M. The 24-hours are divided into 12 consecutive 2-hr blocks starting at the time of injection. Means for each block are expressed as a percentage of the mean control value for each respective block (100%). The parameters are: total time in REM sleep (a), REM sleep-bout frequency (b) and mean REM sleep-bout duration (c). Significant differences in blocks are found in a and b, but not c (p<0.05, N=8). Error bars are ± SEM.
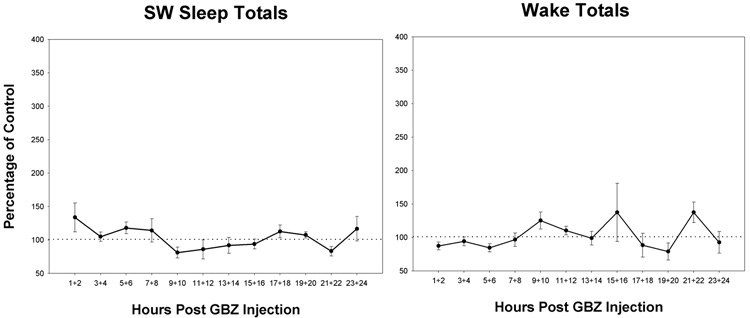
The GBZ effect on REM sleep-bout frequency was similar to that on total REM sleep amounts (fig, 3b). The first 8-hrs appeared elevated and block values with respect to control also was statistically significant. The increased REM sleep by GBZ was predominantly due to increased bout frequency inasmuch as the effect on bout duration was not significant (see fig. 3c). Although wake tended to be reduced and SW sleep increased in the first 8 hrs, the effects were small and not significant for either state of arousal (fig 4a and b).
Figure 4.
Time course effects on the means of sleep/wake parameters following intracerebral injection of the GABAA receptor antagonist, gabazine (GBZ), at a concentration of 10−4 M. Representation is the same as Figure 3 except the parameters are: total time in slow-wave (SW) sleep (a), and total time in wake (b). No significant differences in blocks are found (p<0.05, N=8). Error bars are ± SEM.
Interaction of GBZ with muscarinic receptors
The long-lasting increased REM sleep induced by the GABAA receptor antagonists raised the possibility of effects being mediated through a secondary neuromodulatory system. Inasmuch as it has been shown that BMI dialysed into the PnO of the cat resulted in increased levels of acetylcholine (Vasquez and Baghdoyan, 2004), we tested dependence on the cholinergic system for GBZ to increase REM sleep in the rat when unilaterally injected in the PnO.
In addition to the vehicle-control injections, rats received injections of: GBZ, 10−4 M; the cholinergic agonist, carbachol, 10−4 and 10−3 M; double injections of the muscarinic antagonist atropine, 4 × 10−3 M, followed by GBZ, 10−4 M; and atropine followed by vehicle. All these rats (N=5) were used in the 24 hr-assessment of GBZ, 10−4 M, and one rat received BMI and the lower dose of GBZ following this experiment and was used in the study comparing GABAA receptor antagonists. The final injection of the series produced a significant increase in REM sleep in all cases (4 of the 5 were carbachol). This indicated that the ability to induce the physiological response at the injection site was maintained through the course of the entire series of injections.
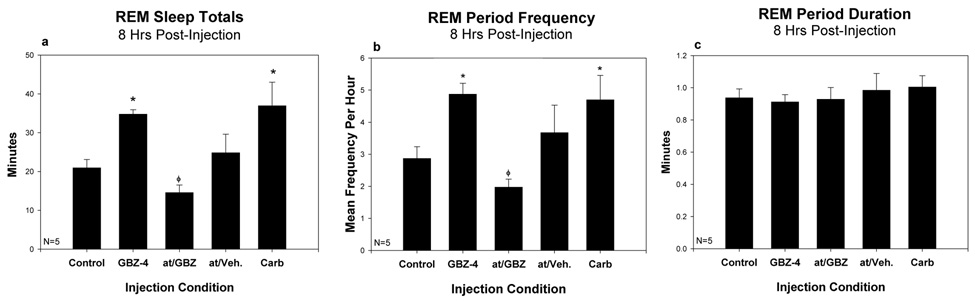
The actions of carbachol and GBZ on sleep/wake were very similar. Replicating previous findings (Bourgin et al., 1995; Marks and Birabil, 1998; Marks and Birabil, 2007), carbachol induced a significant increase in REM sleep in the eight hours following injection. The mean time in REM sleep following the dose of carbachol producing the greater effect (3 at 10−4 M and 2 at 10−3 M) was 173.0 ± 11.7 percent of mean control. GBZ 10−4 M significantly increased REM sleep time to 170.9 ± 12.6 percent of mean control (fig. 5a). Both carbachol and GBZ significantly increased the mean number of REM episodes per hour, 162.6 ± 9.7 and 174.6±10.9 percent of control, respectively (fig. 5b). Neither carbachol nor GBZ had a significant effect on mean REM sleep-bout durations, 108.9±10.2 and 98.7±7.9 percent of control, respectively (fig. 5c). In this group of rats, neither carbachol nor GBZ significantly reduced the latency to REM sleep-onset, 83.3±11.0 and 95.2±8.6 percent of control. Neither drug produced a significant change in SW sleep or wake amounts, though there were trends towards increased SW sleep and decreased wake (fig. 6a and b).
Figure 5.
Effects of cholinergic agents and gabazine on the means of REM sleep parameters for the 8-hours following intracerebral injection. The injection conditions consist of: mean of four vehicle-controls (Ctrl); gabazine 10−4 M (GBZ-4); atropine 4 × 10−3 M followed by gabazine 10−4 M (at/GBZ); atropine 4 × 10−3 M followed by vehicle (at/veh); and the most effective dose of carbachol, either 10−4 or 10−3 M (Carb) (see text for explanation). The parameters are: total time in REM sleep (a), REM sleep-bout frequency per hour (b) and mean REM sleep-bout duration (c). * indicates values significantly different from control (p<0.05, N=8). φ indicates significant difference between the means of the two atropine conditions (p<0.05, N=8). Error bars are +SEM.
Figure 6.
Effects of cholinergic agents and gabazine on the means of sleep/wake parameters for the 8-hours following intracerebral injection. Representation is the same as Figure 5 except the parameters are: total time in slow-wave (SW) sleep (a), and total time in wake (b). No statistical differences are found in these data (p<0.05, N=8).
The increased REM sleep produced by GBZ was totally blocked by a preinjection of atropine, which also tended to further decrease time in REM sleep below the control value, 71.4±11.0 percent of control (fig. 5a). This was accomplished through suppressing the increase in the number of REM episodes, 70.0±8.0 percent of control, with no effect on episode durations, 98.8±3.8 percent of control (fig. 5b and c). The dose of 4 × 10−3 M atropine preceding GBZ significantly reduced the REM sleep time and episode frequency produced by atropine preceding vehicle injection. This dose of atropine preceding a vehicle injection resulted in a small mean increase in REM sleep time, 116.6±16.9 percent of control. The combined effect of atropine and GBZ was not just an additive effect of their individual actions, supporting a dependence of the actions of GBZ on functional muscarinic receptors in the PnO.
Atropine had no consistent effect upon the stereotyped circling behavior induced by GBZ. Of the two rats with induced ipsilateral circling by GBZ 10−4 M, preinjection of atropine resulted in eliminating circling in one rat and greatly exacerbated circling in the other. In one other rat, atropine/GBZ injection was followed by exclusive turning to the ipsilateral side while no bias in turning was observed following GBZ alone. The consistent effect of atropine to block increased REM sleep induced by GBZ, but not the motor behavior induced by GBZ, indicated a further independence of these two actions of GABAA receptor antagonism in the PnO.
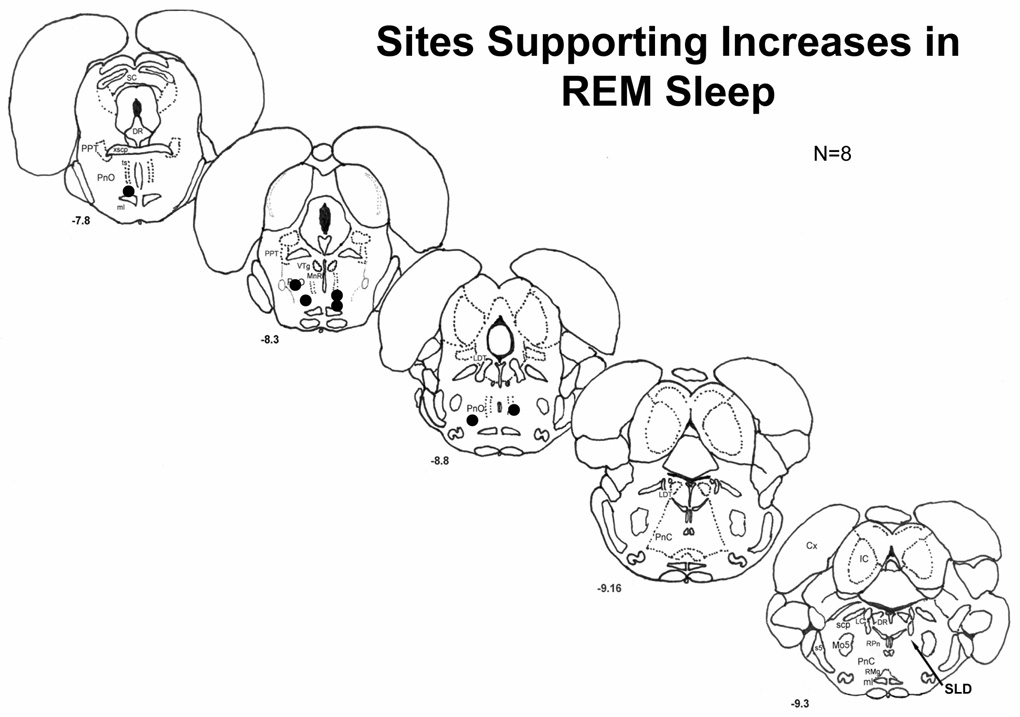
All injection-sites supporting increased REM sleep were histologically verified to be located in the PnO (see schematic map, fig. 7). With one exception, sites were distributed through the dorsoventral extent of the caudal region of the PnO betweenβ-8.3 and −8.8 mm. One site was found in the ventral portion of the rostral PnO. Within the PnO, injection-site location was not related to eliciting circling behavior with GBZ at the 10−4 M dose.
Figure 7.
Representation of the distribution of sites injected on a schematic illustration of coronal sections of the rat brainstem. Numbers in the lower left of each section indicate distance from the bregma suture in mm. Each site is indicated by a circle except that in the lower left of section −8.3, which are two sites (N=8). For reference, the arrow on section −9.3 points to the location of the SLD (see text).
Discussion
Laterality does not account for apparent species differences
We confirmed the finding that microinjection of BMI into the PnO of the rat resulted in a significant increase in REM sleep. We found long-lasting increased time in REM sleep, with minor changes in latency to onset, comparable to the previous study utilizing the same concentration of BMI at 10−3 M. This similar effect was produced despite use of bilateral injections in a volume of 200 nl in the previous study (Sanford et al., 2003). The current procedure utilized unilateral injection and 60 nl volumes. Unilateral versus bilateral injection did not account for any apparent species differences between rat and cat in the induction of REM sleep by injection of BMI into the pontine reticular formation (Xi et al., 1999). Inasmuch as the dose of BMI used unilaterally in cat was larger (250 nl, 10−2 M) than the unilateral dose used here, this may account for differences. In rat, however, such high concentrations of BMI injected in PnO elicited severe motor activation and reductions in REM sleep (Sanford et al., 2003).
In both cat and rat, two areas have been implicated as REM sleep induction zones. In addition to the PnO, the other site in cat has been termed the peri-LCα lying in the dorsal pontine reticular formation ventral to the locus coeruleus (Vanni-Mercier et al., 1989). The corresponding locus in rat has been termed both the dorsal subcoeruleus nucleus (Paxinos and Watson, 1986) and the sublaterodorsal nucleus (SLD) (Swanson, 1998). The term SLD will be used here. The SLD assumes a similar position in rat lying just ventral to the locus coeruleus, but caudal to the PnO, and dorsal to the caudal pontine nucleus (see arrow in fig. 7). In rat, GABAA receptor blockade in the SLD induced a short-latency REM sleep-like state similar to what has been reported for cat (Boissard et al., 2002; Pollock and Mistlberger, 2003). Based on the stereotaxic coordinates and schematic maps presented by authors of the cat studies (P:3, L:2, H:−4) (Xi et al. 1999), the microinjections appeared to be in the peri-LCα not the PnO (Vanni-Mercier et al., 1989; Berman, 1968). If the site injected in cat is homologous to the rat SLD, then there would not be a species difference with respect to the effect of GABAA receptor antagonists on REM sleep. The results of dialyzing BMI in what may be the cat homologue of the rat PnO (P:1.5 to 3, L:1 to 2, H:−5 to −7) induced a “REM sleep enhancement” having similarity to effects of injecting GABAA receptor antagonists into PnO of rat (Vazquez and Baghdoyan, 2004). This indicates there may be little difference between cat and rat, with respect to the effects of antagonizing GABAA receptors at homologous sites in the pons.
Antagonism of GABAA receptors is the mechanism of REM sleep induction
Inasmuch as BMI has been demonstrated to have many non-GABA receptor-mediated actions on neurons (Olsen et al., 1976; Seutin and Johnson, 1999), the specificity of BMI acting as a GABAA receptor antagonist was tested by comparison with the actions of GBZ, a more highly selective competitive antagonist (Heaulme et al., 1986). Qualitatively, the results on REM sleep when injected into the PnO were similar. Both had a long lasting effect to increase REM sleep mainly through increased bout-frequency and not bout-duration, and both had similarly small effects on reducing REM sleep latency. GBZ has higher affinity for GABAA receptors than BMI (Lüddens and Korpi, 1995) and this is consistent with finding increased potency of GBZ to increase REM sleep. Taken together, these results support GABAA receptor-antagonism being the common, mediating factor underlying BMI and GBZ inductions of REM sleep in PnO. GBZ also has been shown to be effective in inducing REM sleep in the SLD (Boissard et al., 2002).
Differential potencies between antagonists may relate to GABAA receptor-subtype
GABAA receptors consist of a large number of receptor-subtypes based on their subunit composition and this gives rise to a multiplicity of functional and pharmacological properties (Fritschy and Brünig, 2003). A study of binding affinities of bicuculline and GBZ to a variety of human recombinant GABAA receptors of different subunit constituencies in a heterologous expression system found a mean 12-fold higher affinity of GBZ over bicuculline (Ebert et al., 1997). In another study based on modulation of 35S-TBPS binding, GBZ was 3-fold more potent at most receptor subunit combinations examined, but as high as 30-fold for certain subunit combinations (Lüddens and Korpi, 1995).
Utilizing immunohistochemistry, a variety of GABAA receptor-subunits have been identified in the PnO (Fritschy et al., 1992, Pirker et al., 2000). The differential affinities of bicuculline and GBZ have not been determined for all possible subunit combinations or is it known what the subunit composition is of the GABAA receptors mediating increased REM sleep produced by antagonists. The approximately 35-fold difference found here in the potency to induce REM sleep was unusually high and may be indicative of mediation through a specific subunit combination present in the PnO. The magnitude of the differential response to BMI and GBZ may be useful in identification of a specific subtype receptor in the control of REM sleep.
The effect of antagonism of GABAA receptors in PnO is dependent on muscarinic receptors
Analysis of the 24-hr recordings following GBZ (10−4 M) determined the extent of the long-lasting effect on REM sleep, which was limited to about eight hours. It is unlikely the local concentration of GBZ after a single 60 nl injection was maintained at effective levels for eight hours. This indicates GBZ initiated a process that continued after it diffused away. This suggests the possibility that GBZ may be acting through control of release of another neurotransmitter whose modulatory action is long-lasting (MacDermott et al., 1999). Inasmuch as it has been shown that BMI resulted in increased acetylcholine levels in the PnO of the cat (Vazquez and Baghoyan, 2004) and injections of muscarinic agonists in PnO of rat induced increased REM sleep for durations of about 8-hrs and can be antagonized by atropine (Marks and Birabil, 2007,Bourgin et al., 1995), we sought to test whether the effect of GBZ was dependent upon activation of muscarinic receptors.
We found the cholinergic agonist, carbachol, increased REM sleep at each site that supported a REM sleep increase by GBZ. The dose-response relationship of carbachol to increase REM sleep in the PnO of the rat is an inverted “U” with a narrow range of effective dose (Marks and Birabil, 2001). A small change in the sensitivity to carbachol from one site to another can shift the dose-response curve right or left making it impossible to predict before hand which dose, 10−4 or 10−3 M, would more effectively increase REM sleep at a specific site in PnO (Marks and Birabil, 2007). At an individual site, repeated administrations of a single dose under the same conditions produced reliable results (Marks and Birabil, 1998).
The action of GBZ was very similar to the action of the most effective dose of carbachol at each site tested. This was so for the increased REM sleep-time and episode frequency, and the lack of effect on episode duration and latency. This shared response supports the possibility of a shared mechanism of action. Dependence of the effect of GBZ on the cholinergic system was demonstrated by the ability of the muscarinic receptor antagonist, atropine, to completely block the REM sleep increase when preinjected in PnO. Atropine alone, at the dose used, 4 × 10−3 M, did not reduce REM sleep. Additionally, the tendency for the combination of GBZ and atropine to reduce REM sleep below control levels, may indicate an unmasking of a competing, but normally less effective, mechanism in PnO whereby GABAA receptor antagonism reduces REM sleep. This may be, at least partly, responsible for the reductions in REM sleep reported for high doses of BMI (Sanford et al., 2003), although induction of motor activity also could play a role in promoting arousal at high doses.
At individual PnO sites tested, the magnitude of the REM sleep increase by GBZ was related to the dose-response relationship of carbachol to increase REM sleep. GBZ produced larger REM sleep increases at sites in which the 10−3 M dose of carbachol produced a response greater than the 10−4 M dose (data not shown). One can speculate the GBZ resulted in a level of acetylcholine release at sites very sensitive to acetylcholine (10−4 M sites) that was too large to induce an optimal increase in REM sleep. In other words, on the downward slope of the inverted “U” dose-response curve. Based on the current data, however, it is speculation that acetylcholine release is the mechanism by which GABAA receptor antagonism in the PnO increases REM sleep, though it is arguably likely. BMI dialyzed into the PnO of the cat resulted in increased levels of acetylcholine (Vazquez and Baghdoyan, 2004). Further, presynaptic GABAA receptors have been shown to have a role in control of release of a variety of neurotransmitters including acetylcholine (reviewed in MacDermott et al., 1999). Alternative mechanisms are possible, such as a postsynaptic GABAA receptor shunting of muscarinic receptor currents, which would be removed by GBZ. Alterations in the activity of PnO neurons projecting to the source of cholinergic afference is another possibility. Additional investigation is required to determine the specific mechanism in the PnO for GABAA receptor blockade’s dependence on the cholinergic system to increase REM sleep.
The pontine injections of BMI in cat that induced REM sleep at short latency was not blocked by the muscarinic antagonist scopolamine (Xi et al., 2004). This is consistent with the pontine BMI injections in cat acting at the homologue of SLD, the peri-LCα BMI facilitating release of acetylcholine has been shown for the cat PnO ventral to, but not, peri-LCα (Vazquez and Baghdoyan, 2004). The extent of the homology between rat SLD and cat peri-LCα is limited at least by the actions of cholinergic agonists to increase REM sleep. Cholinergic agonists injected into the cat peri-LCα induce REM sleep (Vanni-Mercier et al., 1989), but these ligands injected into the rat SLD do not (Boissard et al., 2002). The basis of these species differences is unclear. With greater certainty, it is highly unlikely the agents injected into the PnO, in this study, were diffusing away to act in the SLD. The SLD does not support REM sleep increases by cholinergic agonists or atropine blocking the effects of GABAA receptor antagonists. These are effects demonstrated here for each PnO site injected.
Mechanisms of GABA in the control of REM sleep
The sources of GABA to the REM sleep induction zones have yet to be determined. To be consistent with the effects of antagonizing GABAA receptors, it is expected that these GABAergic neurons have a REM-off state-related pattern of firing. To the extent that one source of GABA is the GABAergic neurons of the ipsi- and contralateral PnO itself, it has been reported, based on c-Fos expression, that rats sacrificed during a period of high propensity for REM sleep, following REM sleep deprivation, showed a reduced number of c-Fos expressing GABAergic neurons (Maloney et al., 2000). This is consistent with a lack of activation, or inhibition, of these GABAergic neurons in REM sleep. Utilizing the same procedures, local GABAergic neurons in the SLD appeared to be activated in REM sleep (Maloney et al., 1999).
GABAergic neurons in the area of the caudal midbrain reticular formation project to the SLD (Boissard et al., 2003). We have presented preliminary data showing GABAergic neurons in this area also project to PnO (Marks et al., 2006). Bilateral injection of muscimol, a GABAA receptor agonist that presumably silences neuronal activity, and ibotenic acid lesions that include this area both resulted in increased REM sleep (Lu et al., 2006; Luppi et al., 2007). These findings are consistent with removal of GABAergic input as a mechanism in the control of REM sleep. Additional investigation is needed to identify the neuronal populations giving rise to the GABAergic inputs to PnO and SLD, and determine their role in control of REM sleep.
Increased REM sleep produced by antagonism of GABA transmission in the PnO leads to the prediction that the concentration of GABA, at the relevant receptors in PnO, would be low during REM sleep or low when there is a high propensity to express REM sleep. Attempts to demonstrate low levels of GABA in the PnO of rat and cat by in vivo microdialysis have yielded mixed results in several preliminary reports (Thakkar et al. 2004; Vanini et al. 2006; Marks et al., 2007). This may be due to the inability of this method to measure the relevant pool of GABA (Timmerman and Westerink, 1997).
The functional role of GABAergic neurotransmission in the PnO not only concerns the availability of GABA, but the availability of functional receptors as well. The point of control may not only include up- and down-regulation of the relevant GABAA receptor subtypes, but may be dominated by it. Whether controlled by an activity-dependent mechanism, signal transduction-cascade initiated by other neurotransmitters, or in response to local GABA concentrations, a reduction in functional GABAA receptors can have the same effect as reducing availability with a competitive antagonist. In the PnO, this would result in increased REM sleep.
It has been shown that GABAA receptor-subtypes have heterogeneous distributions, can be enriched in one or another membrane domain on single neuronal cell types in a brain region or network circuit, and can selectively mediate specific functions (reviewed in, Fritschy and Brünig, 2003). This may be the case in the PnO. A specific GABAergic input may be associated with cholinergic synapses on a selected cell-type or process in the reticular formation and through the release of GABA binding to a specific GABAA receptor-subtype either inhibit acetylcholine release or shunt post synaptic currents. Withdrawal of this GABA inhibition by either, or both, decreased GABA release and down-regulation of the specific receptor-subtype would result in disinhibiting cholinergic action in the PnO and inducing increased REM sleep expression.
The present work raises many questions to be explored with a variety of techniques. These questions include: a) Is there a specific neuronal source of the GABA innervation of PnO involved in REM sleep control? b) how selective is this innervation with respect to its targets? c) is mediation through a specific GABAA receptor-subtype, and how is receptor expression regulated? d) what is the mechanism of the GABAcholinergic interaction? and d) ultimately, what are the properties of the entire network in PnO permitting control of REM sleep?
Conclusion
A behavioral-pharmacological approach has aided in the identification of many mechanisms in the PnO involved in the control of REM sleep. Although not an exclusive finding, modulation of the cholinergic system is a recurrent theme of several mechanisms studied. Based on the action of atropine to block REM sleep induction in PnO, the following receptor systems have been shown to require the cholinergic system: agonists at A2A adenosine receptors (Marks et al., 2003), vasoactive intestinal polypeptide (Bourgin et al., 1999), pituitary adenylyl cyclase activating polypeptide (Ahnaou et al., 2000), and antagonists at α2 adrenergic receptors (Bier and McCarley, 1994). Now added to this list are antagonists at GABAA receptors. There are mechanisms independent of the cholinergic system operating in the PnO, for example agonists at A1 adenosine receptors, not blocked by atropine (Marks et al., 2003), but the multiplicity of mechanisms modulating acetylcholine implicate the cholinergic system in the PnO as a nexus of numerous influences. REM sleep expression is subject to modulation by a host of varied factors including: recent past expression, time of day, light, temperature, learning and stress. The PnO may be an area serving to integrate environmental and experiential factors in the control of REM sleep. One mechanism to accomplish this appears to be through modulation of local cholinergic neurotransmision. To the extent different systems capable of this modulation constitute independent influences, another important question relevant here are what specific influences may GABA convey to the PnO in the modulation of REM sleep?
Acknowledgments
These studies were supported by NIH grant MH57434 and Veterans Administration Merit Review.
List of abbreviations
- BMI
bicuculline methiodide
- GABAA
γ-aminobutyric acid-type A
- GBZ
gabazine
- PnO
nucleus pontis oralis
- REM
rapid eye movement
- SLD
sublaterodorsal nucleus
- SW
slow wave
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahnaou A, Basille M, Gonzalez B, Vaudry H, Hamon M, Adrien J, Bourgin P. Long-term enhancement of REM sleep by the pituitary adenylyl cyclase-activating polypeptide (PACAP) in the pontine reticular formation of the rat. Eur J Neurosci. 1999;11:4051–4058. doi: 10.1046/j.1460-9568.1999.00811.x. [DOI] [PubMed] [Google Scholar]
- Ahnaou A, Laporte AM, Ballet S, Escourrou P, Hamon M, Adrien J, Bourgin P. Muscarinic and PACAP receptor interactions at pontine level in the rat: significance for REM sleep regulation. Eur J Neurosci. 2000;12:4496–4504. doi: 10.1046/j.0953-816x.2000.01345.x. [DOI] [PubMed] [Google Scholar]
- Baghdoyan HA, Monaco AP, Rodrigo-Angulo ML, Assens F, McCarley RW, Hobson JA. Microinjection of neostigmine into the pontine reticular formation of cats enhances desynchronized sleep signs. J Pharmacol Exp Ther. 1984a;231:173–180. [PubMed] [Google Scholar]
- Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions. Brain Res. 1984b;306:39–52. doi: 10.1016/0006-8993(84)90354-8. [DOI] [PubMed] [Google Scholar]
- Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. A neuroanatomical gradient in the pontine tegmentum for the cholinoceptive induction of desynchronized sleep signs. Brain Research. 1987;414:245–261. doi: 10.1016/0006-8993(87)90005-9. [DOI] [PubMed] [Google Scholar]
- Berman AL. The Brain Stem of the Cat: a Cytoarchitectonic Atlas with Stereotaxic Coordinates. Madison: The University of Wisconsin Press; 1968. [Google Scholar]
- Bier MJ, McCarley RW. REM-enhancing effects of the adrenergic antagonist idazoxan infused to the medial pontine reticular formation of the freely moving cat. Brain Research. 1994;634:333–338. doi: 10.1016/0006-8993(94)91939-9. [DOI] [PubMed] [Google Scholar]
- Boissard R, Fort P, Gervasoni D, Barbagli B, Luppi H-P. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep. Eur J Neurosci. 2003;18:1627–1639. doi: 10.1046/j.1460-9568.2003.02861.x. [DOI] [PubMed] [Google Scholar]
- Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi H-P. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16:1959–1973. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- Bourgin P, Ahnaou A, Laporte AM, Hamon M, Adrien J. Rapid eye movement sleep induction by vasoactive intestinal peptide infused into the oral pontine tegmentum of the rat may involve muscarinic receptors. Neuroscience. 1999;89:291–302. doi: 10.1016/s0306-4522(98)00290-5. [DOI] [PubMed] [Google Scholar]
- Bourgin P, Escourrou P, Gaultier C, Adrien J. Induction of rapid eye movement sleep by carbachol infusion into the pontine reticular formation in the rat. NeuroReport. 1995;6:532–536. doi: 10.1097/00001756-199502000-00031. [DOI] [PubMed] [Google Scholar]
- Bourgin P, Lebrand C, Escourrou P, Gaultier C, Franc B, Hamon M, Adrien J. Vasoactive intestinal polypeptide microinjections into the oral pontine tegmentum enhance rapid eye movement sleep in the rat. Neuroscience. 1997;77:351–360. doi: 10.1016/s0306-4522(96)00455-1. [DOI] [PubMed] [Google Scholar]
- De La Rosa C, Reinoso-Suarez F. GABAergic structures in the ventral part of the oral pontine reticular nucleus: an ultrastructural immunogold analysis. Neuroscience. 2006;142:1183–1193. doi: 10.1016/j.neuroscience.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Ebert B, Thompson SA, Saounatsou K, McKernan R, Krogsgaard-Larsen P, Wafford KA. Differences in agonist/antagonist binding affinity and receptor transduction using recombinant human γ-aminobutyric acid type A receptors. Mol Pharmacol. 1997;52:1150–1156. [PubMed] [Google Scholar]
- Ford B, Holmes CJ, Mainville L, Jones BE. GABAergic Neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior hypothalamus. J Comp Neural. 1995;363:177–196. doi: 10.1002/cne.903630203. [DOI] [PubMed] [Google Scholar]
- Fritschy J-M, Benke D, Mertens S, Oertel WH, Bachi T, Möhler H. Five subtypes of type A γ-aminobutyric acid receptors identified in neurons by double and triple immunofluorescence staining with subunit-specific antibodies. Proc Natl Acad Sci USA. 1992;89:6726–6730. doi: 10.1073/pnas.89.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy J-M, Brünig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol Ther. 2003;98:299–323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Heaulme M, Chambon JP, Leyris R, Molimard JC, Wermuth CG, Biziere K. Biochemical characterization of the interaction of three pyridazinyl-GABA derivatives with the GABAA receptor site. Brain Research. 1986;384:224–231. doi: 10.1016/0006-8993(86)91158-3. [DOI] [PubMed] [Google Scholar]
- Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- Lüddens H, Korpi ER. GABA antagonists differentiate between recombinant GABAA/benzodiazepine receptor subtypes. J Neurosci. 1995;15:6957–6962. doi: 10.1523/JNEUROSCI.15-10-06957.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi P-H, Gervasoni D, Verret L, Goutagny R, Peyron C, Salvet D, Leger L, Fort P. Paradoxical (REM) sleep genesis: The switch from an aminergic-cholinergic to a GABAergic-glutamatergic hypothesis. J Physiol (Paris) 2007;100:271–283. doi: 10.1016/j.jphysparis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- Maloney KJ, Mainville L, Jones BE. Differential c-Fos expression in cholinergic, monoaminergic, and GABAergic cell groups of the pontomesencephalic tegmentum after paradoxical sleep deprivation and recovery. J Neurosci. 1999;19:3057–3072. doi: 10.1523/JNEUROSCI.19-08-03057.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney KJ, Mainville L, Jones BE. C-Fos expression in GABAergic, serotonergic, and other neurons of the pontomedullary reticular formation and raphe after paradoxical sleep deprivation and recovery. J Neurosci. 2000;20:4669–4679. doi: 10.1523/JNEUROSCI.20-12-04669.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks GA, Birabil CG. Enhancement of rapid eye movement sleep in the rat by cholinergic and adenosinergic agonists infused into the pontine reticular formation. Neuroscience. 1998;86:29–37. doi: 10.1016/s0306-4522(98)00005-0. [DOI] [PubMed] [Google Scholar]
- Marks GA, Birabil CG. Comparison of three muscarinic agonists injected into the medial pontine reticular formation of rats to enhance REM sleep. Sleep Res Online. 2001;4:17–24. [Google Scholar]
- Marks GA, Shaffery JP, Speciale SG, Birabil CG. Enhancement of rapid eye movement sleep in the rat by actions at A1 and A2a adenosine receptor subtypes with a differential sensitivity to atropine. Neuroscience. 2003;116:913–920. doi: 10.1016/s0306-4522(02)00561-4. [DOI] [PubMed] [Google Scholar]
- Marks GA, Birabil CG. Carbachol induction of REM sleep in the rat is more effective at lights-out than lights-on. Brain Research. 2007;1142:127–134. doi: 10.1016/j.brainres.2007.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks GA, Birabil CG, Liang C-L. Neuroscience Meeting Planner. Vol. 458.16. Atlanta, GA: Society for Neuroscience, CD-ROM; 2006. Extra-reticular sources of GABA implicated in control of REM sleep and wakefulness. [Google Scholar]
- Marks GA, Sachs OW, Liang C-L, Birabil CG. Neuroscience Meeting Planner. Vol. 631.17. San Diego, CA: Society for Neuroscience, CD-ROM; 2007. GABAergic control of REM sleep in the pontine reticular formation of the rat. [Google Scholar]
- Olsen RW, Ban M, Miller T. Studies on the neuropharmacological activity of bicuculline and related compounds. Brain Research. 1976;102:283–299. doi: 10.1016/0006-8993(76)90883-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Orlando: Academic Press; 1986. [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Serk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Pollock MS, Mistlberger RE. Rapid eye movement sleep induction by microinjection of the GABA-A antagonist bicuculline into the dorsal subcoeruleus area of the rat. Brain Research. 2003;962:68–77. doi: 10.1016/s0006-8993(02)03956-2. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Tang X, Xiao J, Ross RJ, Morrison AR. GABAergic regulation of REM sleep in reticularis pontis oralis and caudalis in rats. J Neurophysiol. 2003;90:938–945. doi: 10.1152/jn.00993.2002. [DOI] [PubMed] [Google Scholar]
- Seutin V, Johnson SW. Recent advances in the pharmacology of quaternary salts of bicuculline. Trends Pharmacol Sci. 1999;20:268–270. doi: 10.1016/s0165-6147(99)01334-6. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Negrao B, Silva A, Ricardo JA. Afferent connections of the nuclei reticularis pontis oralis and caudalis: a horseradish peroxidase study in the rat. Neuroscience. 1987;20:961–989. doi: 10.1016/0306-4522(87)90256-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain: A Laboratory Guide. New York: Elsevier; 1998. [Google Scholar]
- Thakkar MM, Tao R, Ma Z, Yunren B, Winston S, McCarley RW. GABA release in the mPRF: role in regulation of sleep-wakefulness. Sleep. 2004;27:A7. Abstract Supplment. [Google Scholar]
- Timmerman W, Westerink BHC. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Vanini G, Watson CJ, Soto-Calderon H, Lydic R, Baghdoyan HA. Neuroscience Meeting Planner. Vol. 458.15. Atlanta, GA: Society for Neuroscience, CD-ROM; 2006. GABA levels in cat pontine reticular formation (PRF) and pedunculopontine tegmental nucleus (PPT) vary with arousal state. [Google Scholar]
- Vanni-Mercier G, Sakai K, Lin JS, Jouvet M. Mapping of cholinoceptive brainstem structures responsible for the generation of paradoxical sleep in the cat. Archs ital Biol. 1989;127:133–164. [PubMed] [Google Scholar]
- Vazquez J, Baghdoyan HA. GABAA receptors inhibit acetylcholine release in cat pontine reticular formation: implications for REM sleep regulation. J Neurophysiol. 2004;92:2198–2206. doi: 10.1152/jn.00099.2004. [DOI] [PubMed] [Google Scholar]
- Xi M-C, Morales FR, Chase MH. Evidence that wakefulness and REM sleep are controlled by a GABAergic pontine mechanism. J Neurophysiol. 1999;82:2015–2019. doi: 10.1152/jn.1999.82.4.2015. [DOI] [PubMed] [Google Scholar]
- Xi M-C, Morales FR, Chase MH. Interactions between GABAergic and cholinergic processes in the nucleus pontis oralis: neuronal mechanisms controlling active (rapid eye movement) sleep and wakefulness. J Neurosci. 2004;24:10670–10678. doi: 10.1523/JNEUROSCI.1987-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamuy J, Morales FR, Chase MH. Induction of rapid eye movement sleep by the microinjection of nerve growth factor into the pontine reticular formation of the cat. Neuroscience. 1995;66:9–13. doi: 10.1016/0306-4522(95)00052-k. [DOI] [PubMed] [Google Scholar]