Abstract
It has been known for some time that the application of ultrasound can enhance the efficacy of thrombolytic medications such as recombinant tissue plasminogen activator (rt-PA). Potential clinical applications of this ultrasound enhanced thrombolysis (UET) include the treatment of myocardial infarction, acute ischemic stroke, deep venous thrombosis, and other thrombotic disorders. It may be possible to reduce the dose of rt-PA while maintaining lytic efficacy, however there is little data on the rt-PA concentration dependence of UET. In this work, the rt-PA concentration dependence of clot lysis resulting from 120 kHz UET exposure was measured in an in-vitro human clot model. Clots were exposed to rt-PA for 30 minutes, with (UET treated) or without 120 kHz ultrasound (rt-PA treated) at 37° C, and the clot width measured as a function of time. The rt-PA concentration ranged from 0 to 10 µg/ml. The initial lytic rate for the UET treated group was greater than that of the rt-PA group at almost all rt-PA concentrations, and exhibited a maximum over concentration values of 1 to 3 µg/ml.
Keywords: Ultrasound enhanced thrombolysis, tissue plasminogen activator, acute ischemic stroke
Introduction
Ultrasonic enhancement of recombinant tissue plasminogen activator (rt-PA) thrombolysis has been a subject of active investigation for some time (Behrens et al 1999, Holland et al 2007, Kimura et al 1994, Lauer et al 1992, Saguchi et al 2008, Suchkova et al 1998). These studies are motivated by the idea that combining ultrasound and rt-PA may lead to more effective lytic treatment than rt-PA alone for thrombotic diseases such as myocardial infarction (Cohen et al 2003, Siegel et al 2001), acute ischemic stroke (Alexandrov et al 2004), other thrombotic vascular disorders such as peripheral arterial occlusion (Culp et al 2001, Wissgott et al 2007) and deep venous thrombosis (Castaneda et al 2002, Sugimoto et al 2003). Currently, the only FDA approved therapy for acute ischemic stroke treatment is the intravenous administration of rt-PA within 3 hours of symptom onset. However, many contraindications, and substantial side effects such as intracerebral hemorrhage (Tanne et al 1999) limit the clinical use of this treatment. These limitations have led to increasing interest in ultrasound enhanced thrombolysis (UET) as a potential therapy for stroke and other thrombotic disorders.
Previous in-vitro and in-vivo studies demonstrating increased lytic efficacy of UET encompass a wide range of center frequencies from 20 kHz (Dhond et al 2000) to 2 MHz (Cintas et al 2004). The motivation for studies utilizing low-frequency UET (~ kHz) is that such signals penetrate the skull with less attenuation than higher frequencies (Ammi et al 2008, In Press, Coussios et al 2002, White et al 2006). Studies of higher frequency UET (~MHz) were motivated by the well-established clinical use of transcranial Doppler ultrasound in measuring blood flow in cerebral vessels. However, these higher frequencies may not be able to penetrate the temporal bone in up to 12% of patients (Chabria et al 1998, Marinoni et al 1997, Poster et al 1997). This finding, and the increased attenuation of MHz frequencies by the human temporal bone ((White et al 2006) may limit the clinical utility of UET at these frequencies for stroke treatment.
A wide range of ultrasound intensities have been demonstrated to be efficacious in in-vitro studies of UET. In the work of Suchkova (Suchkova et al 2002), UET efficacy was demonstrated in an in-vitro human clot model for continuous-wave ultrasound (CWUS) exposures of 27, 40 and 100 kHz for intensities ranging from 0.25 to 1.0 W/cm2. Assuming that the acoustic behavior of the incident ultrasound approximates that of a “plane wave,” this roughly corresponds to pressure amplitudes of 0.08 to 0.16 MPa (Kinsler et al 2000). Overall, low frequency (less than 1 MHz) in-vitro studies utilized acoustic pressure amplitudes of 0.08 to 0.2 MPa (Akiyama et al 1998, Behrens et al 2001, Daffertshofer et al 2004). Most of the higher frequency (1 MHz or greater) UET studies utilize pressure amplitudes of approximately 0.15 to 0.49 MPa (Francis et al 1992, Lauer et al 1992).
The success of these in-vitro studies has motivated several clinical trials of UET in acute ischemic stroke. Recently, the CLOTBUST trial using 2 MHz transcranial Doppler ultrasound and rt-PA treatment showed an increased recanalization rate and a trend towards improved neurologic outcome in UET treated stroke patients, as compared with rt-PA treatment alone (Alexandrov et al 2004). For purposes of comparison, the maximum ultrasound power utilized in this study was 0.75 W, yielding a pressure amplitude greater than or equal to 0.1 MPa (GmbH 2003). Similar results were found in a trial employing 2 MHz UET and the ultrasound contrast agent Levovist (Molina et al 2006). However, a clinical trial utilizing 300 kHz UET showed no improvement in recanalization rate or patient outcome compared to rt-PA alone (Daffertshofer et al 2005). In addition, this trial (TRUMBI) was terminated due to a substantially increased intracerebral hemorrhage rate in UET treated patients. In the TRUMBI trial, the ultrasound exposures used an average intensity of 0.7 W/cm2, a pulse repetition frequency (PRF) of 100 Hz, and a 5% duty cycle. This yields an estimated pressure amplitude of 0.64 MPa. This is substantially larger than pressure amplitudes used in much of the in-vitro work performed in low-frequency UET studies (Akiyama et al 1998, Behrens et al 2001, Daffertshofer et al 2004) and as discussed in the work of Saguchi (Saguchi et al 2008), such a large pressure amplitude could lead to increased bioeffects. Overall, it is clear that the optimal parameters for the treatment of acute ischemic stroke with UET are not known.
Mechanisms suggested as causing UET include thermal heating (Sakharov et al 2000), acoustic streaming (Lauer et al 1992), and cavitation (Everbach et al 2000). In a recent study by S. Datta (Datta et al 2006) a significant correlation of UET with the presence of stable cavitation was demonstrated. In stable cavitation, a long-lived population of microbubbles is created in the fluid media, and these bubbles oscillate in size in response to the driving ultrasound field, potentially resulting in substantial fluid flows. In their in-vitro work, porcine clots were exposed to rt-PA and 120 kHz ultrasound. It was found that stable cavitation was induced in the sample clot at a threshold pressure amplitude of 0.2±0.02 MPa. Substantial enhancement of rt-PA lytic efficacy was also observed at this pressure. Similar results were observed in an in-vitro human clot model by others (Prokop et al 2007), strongly suggesting that stable cavitation is likely responsible for much of the ultrasonic enhancement of rt-PA lytic efficacy. In addition, it is known that the bleeding complications of rt-PA lytic therapies are dose related (Turi et al 1993). This suggests that it may be possible to optimize the lytic and acoustic parameters of UET therapy to minimize rt-PA dosage and ultrasound bioeffects, while maximizing lytic efficacy. However, there is little data on the rt-PA concentration dependence of UET for any ultrasound frequency.
In this work, we present the effect of varying rt-PA concentration on the lytic efficacy of 120 kHz UET in an in-vitro human clot model. The ultrasound treatment used a pressure amplitude of 0.175 MPa, a duty cycle of 50% and a pulse repetition frequency of 1667 Hz. The pressure amplitude was chosen to approximate the 120 kHz stable cavitation threshold pressure as determined by S Datta (Datta et al 2006), and the remaining acoustic parameters have been demonstrated to yield substantial enhancement of rt-PA lytic efficacy in previous work (Cheng et al 2005, Meunier et al 2007a, Shaw et al 2006). Lytic efficacy was determined by measuring sample thrombus size during rt-PA or rt-PA and 120 kHz ultrasound treatment. Clots were imaged using a novel microscopic imaging technique to measure clot diameter as a function of time.
Materials and Methods
Materials
Human whole blood was drawn from 21 volunteers by sterile venipuncture following protocol approval by the local Institutional Review Board and written informed consent. Samples of 0.5–2 ml were placed in sterile glass tubes (Model Number 14-962-25, Fisher Scientific, Pittsburgh, PA, USA) and allowed to form clots in and around a small diameter (~600 µm) micropipette (Model 20λ, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) through which a segment of 7-0 silk suture (Ethicon Industries, Cornelia, GA, USA) had been threaded. This is similar to clot production methods used in imaging studies by Cheng et al (Cheng et al 2005), and Winter and Yu (Winter et al 2005, Yu et al 2000). The clots were incubated for three hours at 37°C, and refrigerated at 5°C for 3 days ensuring maximal clot retraction, lytic resistance and stability (Francis et al 1995, Loren et al 1989, Meunier et al 2007, Shaw et al 2006). Before each experiment, the micropipette was removed to produce a cylindrical clot adherent to the suture. The clot was typically 5–8 µl in volume and approximately 300 µm in width. At this size the clots were similar in diameter to the intracerebral segments of the middle cerebral arteries (80 to 840 µm in diameter) or other cerebral vessels such as the recurrent artery of Heubner and its perforators (643 ± 237 µm in diameter) (Marinkovic et al 1958, Tao et al 2006).
The rt-PA was obtained from the manufacturer (Activase®, Genentech, San Francisco, CA, USA) as a lyophilized powder. Each vial was mixed with sterile water to a concentration of 1 mg/ml as per manufacturer’s instructions, aliquoted into 1.0 ml centrifuge tubes (Model 04-978-145, Fisher Scientific, Pittsuburgh, PA, USA), and stored at −80°C. The enzymatic activity of rt-PA is stable for at least 1 year when stored in this fashion (Jaffe et al 1989). Human fresh-frozen plasma (hFFP) was procured from a blood bank in 250–300 ml units. Each unit was briefly thawed, aliquoted into 50 ml centrifuge tubes (Model Number 05-539-9, Fisher Scientific, Pittsburgh, PA, USA), and stored at −80°C. Aliquots of rt-PA and plasma were allowed to thaw for experiments, and the remaining amounts discarded following completion of each experiment.
Methods
For each experiment, the clot attached to the suture was placed in a clean micropipette (Model Number 5-000-2200, Drummond Scientific Company, Broomall, PA, USA), and inserted into a U-shaped sample holder composed of hollow luer lock connectors and silicone tubing (OD 0.125”, Cole Parmer, Vernon Hills, IL, USA). The sample holder was placed in an acrylic water tank with a microscope slide at the bottom.
The water in the tank was maintained at a temperature of 37±1°C during all experiments using a heating element (Model Number A721, Hagen, Mansfield, MA, USA). The tank was placed over the objective of an inverting microscope (Model: Olympus IM, Olympus, Melville, NY, USA) to visualize the clot. The field of view in the image was 340 µm × 260 µm (640 pixels × 480 pixels). The entire apparatus was placed on top of a vibration isolation table (Model XL-G, Newport, Irvine, CA, USA) for mechanical isolation. Images were recorded at 6 frames/minute using a CCD camera (Model KP-MIA, Hitachi, Woodbury, USA), and data was stored for later analysis on a computer (Dell, Round Rock, TX; Intel Pentium). A complete description of the imaging apparatus has been previously provided (Cheng et al 2005, Meunier et al 2007).
The ultrasound signal was provided by a custom made 120 kHz transducer (Sonic Concepts Inc, Bothell, WA, USA). This transducer was initially calibrated using a PVDF hydrophone (Model TC-4038, Reson, Goleta, CA, USA) and the −3dB beamwidth was measured to be 1.0 cm with a focal length of 3.2 cm. Note that the ultrasound beam width is substantially larger than the diameter of the sample clots, ensuring uniform insonation of the clot. All of the ultrasound irradiations were performed without any interposed acoustic barriers such as temporal bone in this study, so that the acoustic field incident on the sample clot is well-defined. Further details of this apparatus can be found in Cheng et al (2005), and Meunier et al (2007).
Clots were exposed to hFFP and rt-PA at one of seven concentrations: 0 (control), 0.25, 0.50, 1.00, 3.15, 6 and 10 (µg/ml). In addition, clots were exposed to either rt-PA alone (rt-PA) or to rt-PA and 120 kHz ultrasound (UET). Note that these treatment groups include two control groups; the first in which clots were exposed to only hFFP, and the second whereby clots were exposed to hFFP and ultrasound alone.
The rt-PA concentrations were chosen to contain the therapeutic concentration range in humans (Seifried et al 1989, Tanswell et al 1991). Individual trials began by slowly injecting 1 ml of hFFP (control trials) or 1 ml of hFFP containing rt-PA (all other trials) into the sample holder. At time t equal to zero, the solution was in contact with the clot. Removing the syringe exposed the ends of the sample holder to atmospheric pressure, and the clot surface to a static fluid column. Clots were exposed to a specific treatment regimen for 30 minutes. Each regimen used an average of n = 18 clots (range 6–44), from at least two (range 2 to 15) donors.
The UET treated clots were exposed to one of seven concentrations of rt-PA (0–10 µg/ml), and 120-kHz ultrasound with a peak-to-peak pressure of 0.35 MPa. The pulse repetition frequency (PRF) was 1667 Hz, and the duty cycle (DC) was held constant at 50%. These parameters were chosen as substantial rt-PA lytic enhancement has previously been demonstrated with these ultrasound settings (Datta et al 2006, Holland et al 2007, Meunier et al 2007)
Data Analysis and Statistical Methods
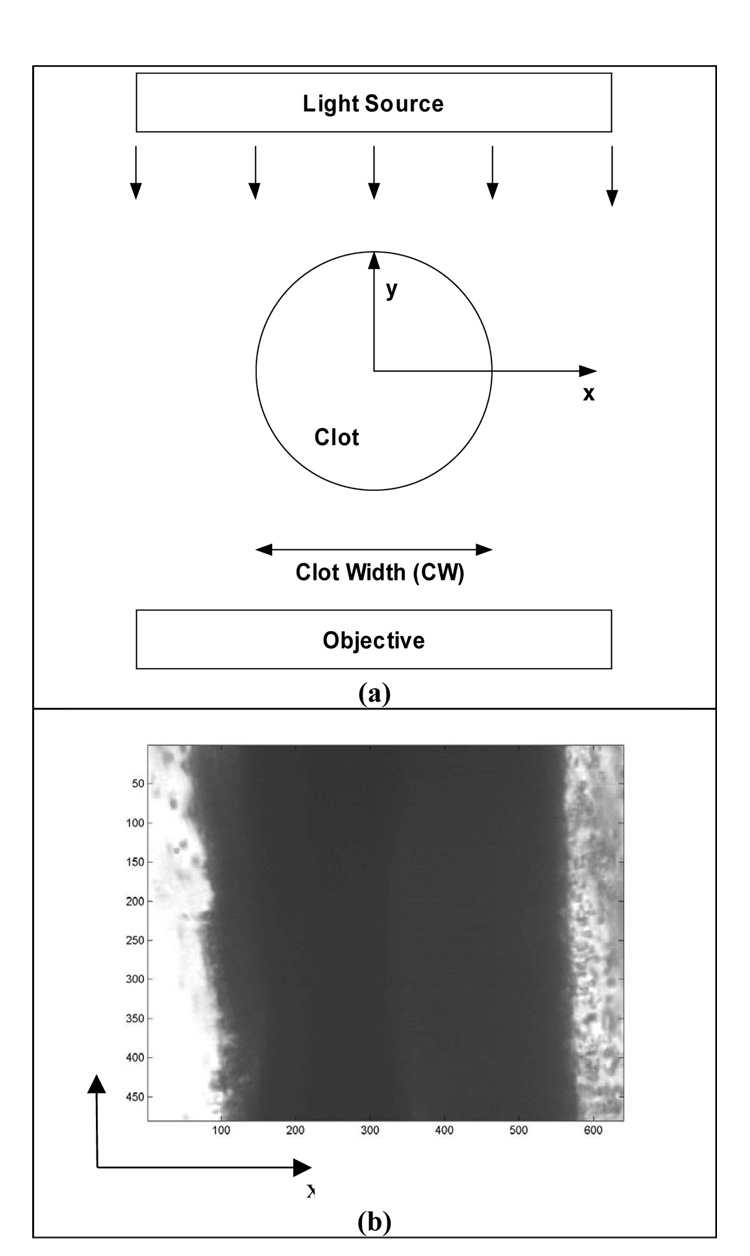
Clot width was determined by measuring the light intensity transmitted through the sample clot. The CCD camera records image light intensity I(x,z) at each pixel (x,z), where z is parallel to the suture and the long axis of the clot. Figure 1 exhibits the clot orientation relative to the imaging apparatus. In this figure, z is along the long axis of the clot, x is transverse across the width of the clot, and the light illuminating the clot is incident along the –y direction. By analyzing the light intensity in each pixel, the clot edges can be identified, thus enabling measurement of clot width (Meunier et al 2007).
Figure 1.
Illustration of the geometry of the clot and imaging apparatus. The light source of the inverting microscope is incident on the clot in the –y direction, and the z axis is parallel to the suture. CW is the clot width.
An image from the CCD camera was stored on a desktop computer for each frame as a function of time t. The average clot width as a function of time CW(t) was calculated using a computer program written in Matlab 6.5 R13 (Mathworks, Inc., Natwick, MA, USA). First, the spatial gradient of the light intensities ∂I(x,z,t)/∂x was calculated for each row (constant z) of pixels. The positions of the two clot-plasma interfaces were determined via an edge-detection routine that finds the values of x(z,t),
| (1a) |
at fixed z and t, such that x1 and x2 satisfy the condition,
| (1b) |
where Γ is a constant representing the abrupt change in light intensity at the clot-plasma interface. A Γ of 2.5 was previously found to be sufficient to detect well-defined clot edges (Cheng et al 2005, Meunier et al 2007). The width W(z,t) of the clot at each z was then
| (1c) |
The average clot width CW(t) for a given image obtained at time t could then be calculated using the expression,
| (2) |
where Nz is the number of rows in the image; thus yielding the average clot width as a function of time.
For each treatment group, the CW(t) values were averaged over all trials for that given treatment yielding
| (3) |
where NTRIAL is the number of trials for a given treatment group. For purposes of comparison among treatment groups, the CWAVG (t) values were then normalized to the average clot width during the first minute (, six frames) or;
| (4) |
This average was performed to improve the accuracy of the value for for each of the respective treatment groups (Cheng et al 2005, Meunier et al 2007).
Non-linear regression was used to model the clot width over time (SPSS V14.0, SPSS Inc., Chicago, IL, USA). Boostrapping was used to obtain the standard errors of parameter estimates, which were determined with a sequential quadratic programming algorithm. The impact of treatment variables on the lytic rate and efficacy was estimated using a mixed-model analysis of variance. This method allows testing of the hypothesis while taking into account the repeated measures nature of the data and any within-subject correlations. The interaction between US and rt-PA concentration was considered, a p-value of less than 0.05 was considered statistically significant. PROC MIXED, SAS v8.02 (SAS Institute, Cary, NC, USA), was used.
Results
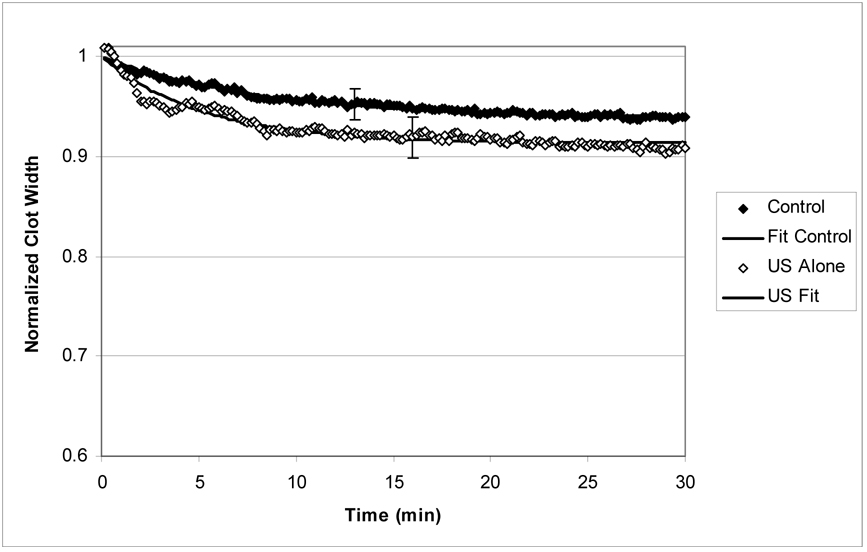
Figure (2a) exhibits the normalized average clot width versus time for the control and ultrasound treated groups. These clots were not exposed to exogenous rt-PA. Note that data exhibit an initial rapid decrease in clot width over the first few minutes, followed by a more gradual decline. However, overall there is very little clot lysis resulting from these two treatments. After 30 minutes of treatment, the clot width has decreased 4% and 9% for the control and ultrasound treated groups respectively.
Figure 2.
Figure 2a: Normalized clot width (CWNORM) versus time for the ultrasound only and control treated clots. The error bars are representative standard deviations for the data. The solid lines are fits to equation (5) and the description is quite good.
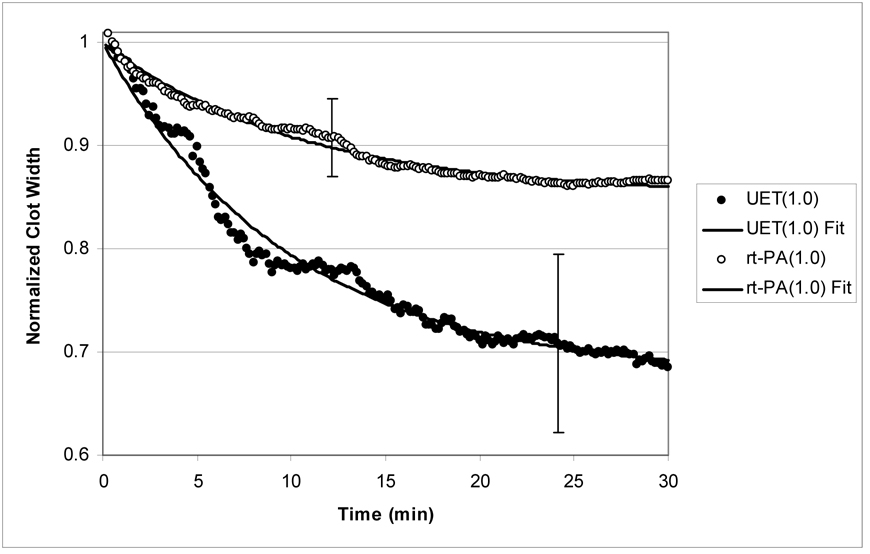
Figure 2b: Normalized clot width (CWNORM) versus time for clots treated with rt-PA(1µg/ml) and UET(1µg/ml). As before, the lines are fits to equation (5), and the description is good. Note that there is significantly more clot lysis resulting from UET treatment as compared with rt-PA alone.
As a representative example of the data for rt-PA and UET treated clots, Figure (2b) exhibits the normalized average clot width for clots exposed to rt-PA at a concentration of 1 µg/ml. The open circles (○) denote clots exposed to rt-PA alone (rt-PA group), and the closed circles (●) denote clots exposed to rt-PA and 120 kHz ultrasound (UET group). Note that there is a substantial increase in clot lysis for these two groups compared with that of the controls (Figure 2a). In addition, the UET treated clots exhibit a substantially greater decline in clot width at all times as compared with the rt-PA treated clots.
It was found that the normalized clot width data for all treatment groups were well-described (R2≥0.93 for all groups) by the empirical two-parameter expression
| (5) |
In this expression, B and k are parameters derived from the fit, and t is time. The parameter k is the decay constant for clot lysis, and the parameter B can be considered as the ‘final’ clot with as a result of treatment. This follows from noting that as the normalized average clot width becomes approximately equal to B in equation (5) for large values of t. Also note that at time zero, CWNORM is equal to one, thus equation (5) reproduces the qualitiative behavior of the normalized clot width as a function of time.
Note that for small values of time t, equation (5) can be approximately written as,
| (6) |
Equation (6) exhibits a linear dependence on t. This expression allows one to define the ‘initial lytic rate,’ LR as
| (7) |
Values for the initial lytic rate LR can thus be calculated for each treatment group from the values of B and k obtained for that group. The B and k values are obtained by fitting the normalized average clot width data to equation (5). Overall, the model of equation (5)–equation (7) is similar to the treatment of JM Meunier et al (2007).
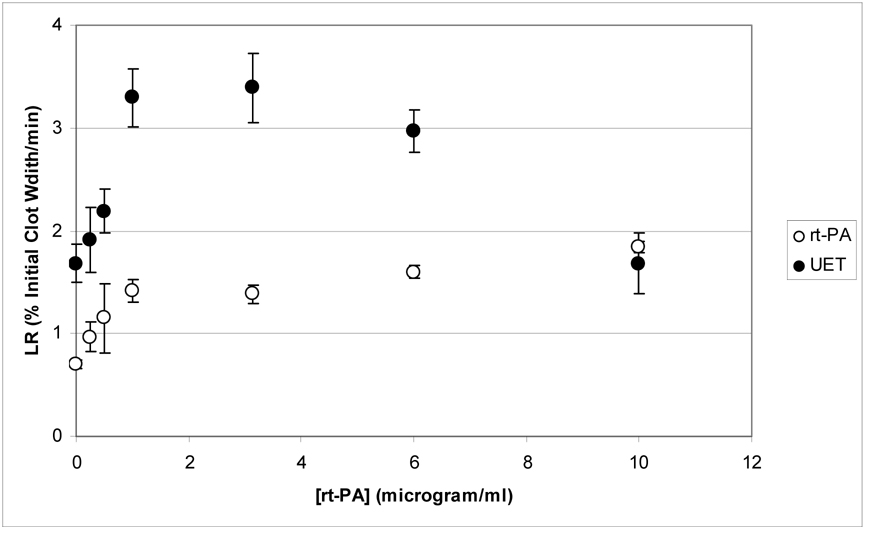
Figure (3) shows the LR values as a function of rt-PA concentration for clots in both the rt-PA group and the UET group. Note that there is a monotonic increase in LR for rt-PA treated clots up to a concentration of 1 µg/ml, after which there is little or no increase for higher rt-PA concentrations. In contrast, LR for the UET treated clots exhibits a maximum for rt-PA concentrations in the range of 1 to 3 µg/ml, with a decline in efficacy for higher concentrations of rt-PA. In addition, the initial lytic rate for the UET group is greater than that of the rt-PA treated group for almost all rt-PA concentrations.
Figure 3.
Initial lytic rate (LR), as determined from equation (7), as a function of rt-PA concentration for rt-PA and UET treated clots. For both the rt-PA and UET groups, there is an increase in LR up to an rt-PA concentration of 1 µg/ml. The UET treated group exhibits a peak in LR for [rt-PA] ≈ 1–3 (µg/ml) while there is no increase in LR for the rt-PA group for higher rt-PA concentrations.
As previously discussed, the normalized average clot width in equation (5) becomes approximately equal to the parameter B for long times. If there is little or no clot lysis, B is large. If there is substantial clot lysis, B is small. This suggests that a reasonable measure of overall clot lysis resulting form lytic treatment is the ‘fractional clot loss,’ FCL which we define as
| (8) |
where values for B are obtained from fitting the normalized average clot width data to equation (5) as before, for a given treatment group. Qualitatively, FCL becomes large for increased clot lysis, and is small if there is little lysis. Therefore, this parameter is a reasonable measure of lytic efficacy for these experiments.
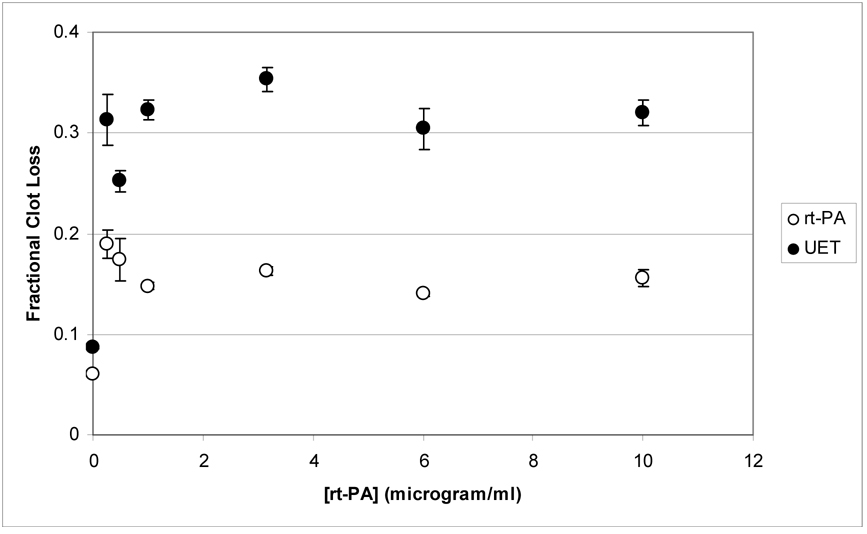
Figure (4) exhibits the fractional clot loss as a function of rt-PA concentration for the rt-PA and UET treatment groups. There is an initial increase in FCL with increasing rt-PA concentration, with little subsequent increase for rt-PA concentrations greater than or equal to 1 µg/ml. The UET treated group also shows a greater degree of fractional clot lysis than the rt-PA treated group at all concentrations. Averaging over all concentrations, FCL for the UET group was 1.8 ± 0.4 times larger than that for the rt-PA group.
Figure 4.
Mean fractional clot loss (FCL, see equation 8) for UET and rt-PA treated clots, as a function of rt-PA concentration. The error bars are the standard errors. Note that FCL exhibits little or no increase for both groups for [rt-PA] ≥ 1 (µg/ml).
Discussion
It has been shown that the lytic efficacy of tissue plasminogen activator is increased by the addition of 120 kHz ultrasound for rt-PA concentrations ranging from 0 to 10 (µg/ml) in an in-vitro human clot model. Both the initial lytic rate, LR, and the fractional clot loss, FCL, are higher for almost all concentrations by the addition of 120 kHz ultrasound. Overall the results presented here imply that the rt-PA concentration may be optimized in clinical applications of UET.
The maximum initial lytic rate was found in the UET group at an rt-PA concentration of 1 to 3 µg/ml (Figure 3). In contrast, the data shown in Figure 4 demonstrate an apparent saturation in fractional clot loss for rt-PA concentrations greater than 1 µg/ml in both rt-PA and UET treated groups. Since the bleeding complications of lytic therapy are dose related ( Adams et al 1996, Haley et al 2005, Turi et al 1993), it would be reasonable to suggest using the lowest concentration of rt-PA that yields the greatest UET lytic enhancement. The data shown in Figure 3 and Figure 4 would lead one to conclude that using an rt-PA concentration of ~1 (µg/ml) satisfies these criteria. At this concentration, both LR and FCL are maximized. Further increasing the rt-PA dose results in an eventual decrease in LR and does not yield any additional increase in FCL in this in-vitro human clot system. Although these results are preliminary, they indicate that optimization of 120 kHz UET to maximize lytic efficacy while minimizing rt-PA exposure may be feasible.
Others have observed a similar concentration dependence of rt-PA lytic efficacy shown in Figure 4. Onundarsen et al (1992) measured clot lysis in radiolabelled human whole-blood clots as a function of rt-PA concentration. In their work, radioactive 125I was incorporated into human whole blood clots, and clot lysis was determined by measuring the percentage of solubilized 125I after 4 hours. They observed a gradual increase in clot lysis up to an rt-PA concentration of 1 µg/ml, followed by a decrease for increasing rt-PA concentration. They suggested that higher rt-PA concentrations depleted the available plasminogen thus reducing the amount of active plasmin available to lyse the sample clot.
In a similar study, Blinc et al (Blinc et al 1993) measured the lytic efficacy of 1 MHz UET in human plasma clots as a function of rt-PA concentration. Their sample clots were treated for 1 hour, using an ultrasound intensity of 4 W/cm2. For purposes of comparison, the 120 kHz ultrasound intensity used here was approximately 0.47 W/cm2. Using a radiolabeled fibrin technique similar to Onundarsen et al (Onundarsen et al 1992), they found that 1 MHz ultrasound enhanced the lytic efficacy of rt-PA by a factor of 2 to 3 as compared with rt-PA treatment alone for rt-PA concentrations from 0 to 1 µg/ml. Overall, this group demonstrated a monotonic increase in 1 MHz UET lytic enhancement for the 0 to 1 (µg/ml) rt-PA concentration range, similar to the results presented here.
In a recent study, Datta et al (Datta et al 2006) showed a correlation between the presence of stable cavitation, and increased lytic efficacy of 120 kHz ultrasound enhanced thrombolysis in an in-vitro porcine clot model. They also found that stable cavitation increased rt-PA penetration into the sample porcine clot. Others have found similar results for human clots (Francis et al 1995, Kondo et al 1999). In the absence of ultrasound, exogenous tPA diffuses into the clot down the concentration gradient (Blinc et al 1993, Shaw et al 2007), and converts plasminogen to plasmin. The plasmin then cleaves the fibrin, thus lysing the clot. The rt-PA concentration dependence of the lytic efficacy for rt-PA treated clots (Figure 3 and Figure 4) is likely due to plasminogen depletion in the sample clot and plasma. As rt-PA concentration increases, it converts plasminogen contained and bound in the clot, but an in the surrounding plasma into plasmin. It has been demonstrated by others that the plasminogen contained within the plasma surrounding the clot plays a substantial role in rt-PA mediated clot lysis (Sakharov et al 1995). Plasmin itself has a short lifetime in-vivo due to inhibitory proteins such as α2 antiplasmin (Robbie et al 1996). If a large portion of the available plasminogen is converted into plasmin, much of it will be inhibited or destroyed by these inhibitory proteins, limiting its availability for thrombolytic action.
Such plasminogen depletion at high rt-PA concentrations could also explain the behavior of the lytic rate for the 120 kHz UET treated clots. Given that ultrasound increases the penetration of rt-PA into the clot, more of the clot’s fibrin mesh is exposed to the lytic activity of plasmin, thus increasing the total amount of clot lysis (Figure 4). However, at higher concentrations of rt-PA, the amount of available plasminogen might be reduced due to plasminogen depletion via inhibitory proteins. Therefore, although ultrasound increases the volume of clot exposed to rt-PA, it does not increase the total amount of the various chemical constituents involved in thrombolysis. As a result, UET lytic efficacy may be limited by plasminogen depletion at higher rt-PA concentrations, resulting in the observed maximum lytic rate for the UET-treated clots (Figure 3).
There has been overall a very substantial amount of in-vitro work in UET. However, this work is unique in the choice of pressure amplitude 0.175 MPa to approximate the 120 kHz stable cavitation threshold, as observed by Datta et al ((Datta et al 2006). This amplitude is substantially lower than some of the higher values used in in-vitro work and some clinical studies ((Daffertshofer et al 2005). Since both thermal and mechanical bioeffects of ultrasound can be substantial ((Daffertshofer et al 2005, Holland et al 1996), it is reasonable to use the lowest possible pressure amplitude needed for UET. This current work suggests that this approach of minimizing ultrasound exposure while accomplishing lytic enhancement of rt-PA is possible, although much work remains to optimize UET for clinical treatment of acute ischemic stroke and other thrombotic disorders.
This study was conducted using in-vitro clots, which limits the clinical applicability of these findings, although both human clots and human plasma were used. Also, in the clinical scenario of an occluding thrombus, the blood pressure and blood flow might contribute to the removal of the clot. Neither of these phenomena are modeled here. However, the addition of a pressure gradient would increase rt-PA clot lysis, as has been demonstrated by others (Diamond et al 1993). As a result, the in-vitro model used here is experimentally conservative in the sense that clot lysis is minimized for both rt-PA and UET treated clots.
120 kHz ultrasound substantially increases the lytic efficacy of rt-PA in this in-vitro human clot model for almost all rt-PA concentrations, as determined by measuring sample clot width as a function of time and lytic treatment. Both the initial lytic rate and the fractional clot loss were found to increase montonically for rt-PA concentrations ranging for 0 to 1 (µg/ml). Above 1 (µg/ml), there was little or no increase in fractional clot loss in both the rt-PA and UET groups, whereas the lytic rate exhibited a maximum at rt-PA concentrations from 1 to 3 µg/ml. The authors speculate that these results suggest that 120 kHz UET therapy can be optimized to reduce rt-PA exposure while maintaining lytic enhancement. Further work is needed to determine the optimal ultrasound parameters for the clinical use of ultrasound enhanced thrombolysis.
Acknowledgements
This work was supported by NIH/NINDS through grants K02-NS056253 and R01-NS047603. Useful conversations with Dr. S. Datta are gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams HP, Jr, Brott TG, Furlan AJ, Gomez CR, Grotta J, Helgason CM, Kwiatkowski T, Lyden PD, Marler JR, Torner J, Feinberg W, Mayberg M, Thies W. Guidelines for Thrombolytic Therapy for Acute Stroke: a Supplement to the Guidelines for the Management of Patients with Acute Ischemic Stroke. A statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Stroke. 1996;27:1711–1718. [PubMed] [Google Scholar]
- Akiyama M, Ishibashi T, Yamada T, Furuhata H. Low-frequency ultrasound penetrates the cranium and enhances thrombolysis in vitro. Neurosurgery. 1998;43:828–832. doi: 10.1097/00006123-199810000-00062. [DOI] [PubMed] [Google Scholar]
- Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, Montaner J, Saqqur M, Demchuk AM, Moye LA, Hill MD, Wojner AW. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. New England Journal of Medicine. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- Ammi AY, Mast TD, Huang I-H, Abruzzo TA, Coussios C-C, Shaw GJ, Holland CK. Characterization of the ultrasound propagation through ex-vivo human temporal bone. Ultrasound in medicine & biology. 2008 doi: 10.1016/j.ultrasmedbio.2008.02.012. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens S, Daffertshofer M, Spiegel D, Hennerici M. Low-frequency, low-intensity ultrasound accelerates thrombolysis through the skull. Ultrasound in Medicine and Biology. 1999;25:269–273. doi: 10.1016/s0301-5629(98)00158-6. [DOI] [PubMed] [Google Scholar]
- Behrens S, Spengos K, Daffertshofer M, Schroeck H, Dempfle CE, Hennerici M. Transcranial ultrasound-improved thrombolysis: Diagnostic vs. therapeutic ultrasound. Ultrasound in Medicine and Biology. 2001;27:1683–1689. doi: 10.1016/s0301-5629(01)00481-1. [DOI] [PubMed] [Google Scholar]
- Blinc A, Francis C, Trudnowski J, Carstensen E. Characterization of ultrasound-potentiated fibrinolysis in vitro. Blood. 1993;81:2636–2643. [PubMed] [Google Scholar]
- Castaneda F, Li R, Young K, Swischuk JL, Smouse B, Brady T. Catheter-directed thrombolysis in deep venous thrombosis with use of reteplase: immediate results and complications from a pilot study. Journal of Vascular Interventional Radiology. 2002;13:577–580. doi: 10.1016/s1051-0443(07)61650-9. [DOI] [PubMed] [Google Scholar]
- Chabria N, Torbey MT. A reason for failure to obtain transcranial doppler flow signals: Hyperostosis of the skull. Stroke. 1998;19:274. [PubMed] [Google Scholar]
- Cheng JY, Shaw GJ, Holland CK. In vitro microscopic imaging of enhanced thrombolysis with 120-kHz ultrasound in a human clot model. Acoustic Research Letters Online. 2005;6:25–29. doi: 10.1121/1.1815039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JY, Shaw GJ, Holland CK. In vitro microscopic imaging of enhanced thrombolysis with 120 kHz ultrasound in a human clot model. Acoustic Research Letters Online. 2005;6:25–29. doi: 10.1121/1.1815039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintas P, Nguyen F, Boneu B, Larrue V. Enhancement of enzymatic fibrinolysis with 2-MHz ultrasound and microbubbles. J Thromb Haemost. 2004;2:1163–1166. doi: 10.1111/j.1538-7836.2004.00746.x. [DOI] [PubMed] [Google Scholar]
- Cohen MG, E ET, Bluguermann J, Kevorkian R, Berrocal DH, Carlevaro O, Picabea E, Hudson MP, Siegel RJ, Douthat L, B.Greenbaum A, Echt D, Weaver ED, Grinfeld LR. Transcutaneous ultrasound-facilitated coronary thrombolysis during acute myocardial infarction. American Journal of Cardiology. 2003;92:454–457. doi: 10.1016/s0002-9149(03)00666-0. [DOI] [PubMed] [Google Scholar]
- Coussios CC, Holland CK, Shaw GJ. Transmission of a large unfocussed 120 kHz and 1 MHz ultrasound beam through the human skull. Journal of the Acoustic Society of America. 2002;112:2433. [Google Scholar]
- Culp WC, Porter TR, Xie F, Goertzen TC, McCowan TC, Vonk BN, Baxter BT. Microbubble potentiated ultrasound as a method declotting thrombosed dialysis grafts: Experimental study in dogs. Cardiovascular and Interventional Radiology. 2001;24:407–412. doi: 10.1007/s00270-001-0052-4. [DOI] [PubMed] [Google Scholar]
- Daffertshofer M, Gass A, Ringleb P, Sitzer M, Sliwka U, Els T, Sedlaczek O, Koroshetz WJ, Hennerici MG. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: Increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator - Results of a phase II clinical trial. Stroke; a journal of cerebral circulation. 2005;36:1441–1446. doi: 10.1161/01.STR.0000170707.86793.1a. [DOI] [PubMed] [Google Scholar]
- Daffertshofer M, Huang Z, Fatar M, Popolo M, Schroeck H, Kuschinsky W, Moskowitz MA, Hennerici MG. Efficacy of sonothrombolysis in a rat model of embolic ischemic stroke. Neuroscience Letters. 2004;361:115–119. doi: 10.1016/j.neulet.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Datta S, Coussios CC, McAdory LE, Tan J, Porter T, Courten-Meyers G, Holland CK. Correlation of cavitation with ultrasound enhancement of thrombolysis. Ultrasound in Medicine and Biology. 2006;32:1257–1267. doi: 10.1016/j.ultrasmedbio.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Coussios CC, McAdory LE, Tan J, Porter T, De Courten-Myers G, Holland CK. Correlation of cavitation with ultrasound enhancement of thrombolysis. Ultrasound in Medicine and Biology. 2006;32:1257–1267. doi: 10.1016/j.ultrasmedbio.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhond MR, Nguyen TT, Dolan C, Pulido G, Bommer WJ. Ultrasound-enhanced thrombolysis at 20 kHz with air-filled and perfluorocarbon-filled contrast bispheres. Journal of the American Society of Echocardiography. 2000;13:1025–1029. doi: 10.1067/mje.2000.107006. [DOI] [PubMed] [Google Scholar]
- Diamond SL, Anand S. Inner clot diffusion and permeating during fibrinolysis. Biophysical Journal. 1993;65:2622–2643. doi: 10.1016/S0006-3495(93)81314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everbach EC, Charles WF. Cavitational mechanisms in ultrasound-accelerated thrombolysis at 1 MHz. Ultrasound in medicine & biology. 2000;26:1153–1160. doi: 10.1016/s0301-5629(00)00250-7. [DOI] [PubMed] [Google Scholar]
- Francis C, Blinc A, Lee S, Cox C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound in Medicine and Biology. 1995;21:419–424. doi: 10.1016/0301-5629(94)00119-x. [DOI] [PubMed] [Google Scholar]
- Francis CW, Onundarson PT, Carstensen EL, Blinc A, Meltzer RS, Schwarz K, Marder VJ. Enhancement of fibrinolysis in vitro by ultrasound. Journal of Clinical Investigation. 1992;90:2063–2068. doi: 10.1172/JCI116088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GmbH DES. Operating Instructions EZ-Dop(R) Singen. 2003 [Google Scholar]
- Haley EC, Lyden PD, C.Johnston K, Hemmen TM. A pilot dose-escalation safety study of tenecteplase in acute ischemic stroke. Stroke. 2005;37:607–612. doi: 10.1161/01.STR.0000154872.73240.e9. [DOI] [PubMed] [Google Scholar]
- Holland CK, Deng CX, Apfel RE, Alderman JL, Fernandez LA, Taylor KJW. Direct evidence of cavitation in vivo from diagnostic ultrasound. Ultrasound in Medicine and Biology. 1996;22:917–925. doi: 10.1016/0301-5629(96)00083-x. [DOI] [PubMed] [Google Scholar]
- Holland CK, Vaidya SS, Datta S, Coussios CC, Shaw GJ. Ultrasound enhanced tissu eplasminogen activator thrombolysis in an in-vitro porcine clot model. Thrombosis Research. 2007 doi: 10.1016/j.thromres.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe GJ, Green GD, Abrams GH. Stability of recombinant tissue plasminogen activator. American Journal of Ophthalmology. 1989;108:90–91. doi: 10.1016/s0002-9394(14)73272-6. [DOI] [PubMed] [Google Scholar]
- Kimura K, Lijima S, Kobayashi K, Furuhata H. Evaluation of the thrombolytic effect of tissue-type plasminogen activator with ultrasonic irradiation: in-vitro experiment involving assay of the fibrin degradation products from the clot. Biological and Pharmaceutical Bulletin. 1994;17:126–130. doi: 10.1248/bpb.17.126. [DOI] [PubMed] [Google Scholar]
- Kinsler LE, Frey AR, Coppens AB, Sanders JV. Fundamentals of Acoustics. 4th Edition. New York: John Wiley & Sons, Inc; 2000. [Google Scholar]
- Kondo I, Mizushige K, T TU, Masugata H, Ohmori K, Matsuo H. Histological observations and the process of ultrasound contrast agent enhancement of tissue plasminogen activator thrombolysis with ultrasound exposure. Japanese Journal of Circulation. 1999;63:478–484. doi: 10.1253/jcj.63.478. [DOI] [PubMed] [Google Scholar]
- Lauer CG, Burge R, Tang DB, Bass BG, Gomez ER, Alving BM. Effect of ultrasound on tissue-type plasminogen activator-induced thrombolysis. Circulation. 1992;86:1257–1264. doi: 10.1161/01.cir.86.4.1257. [DOI] [PubMed] [Google Scholar]
- Lauer G, Burge RB, Tang DB, Bass BG, Gomez ER, Alving BM. Effect of ultrasound on tissue-type plasminogen activator induced thrombolysis. Circulation. 1992;86:1257–1264. doi: 10.1161/01.cir.86.4.1257. [DOI] [PubMed] [Google Scholar]
- Loren M, Fade-Garcia LJ, Toorado MC, Navarro JL. Thrombus age and tissue plasminogen activator mediated thrombolysis in rats. Thrombosis Research. 1989;56:67–76. doi: 10.1016/0049-3848(89)90009-1. [DOI] [PubMed] [Google Scholar]
- Marinkovic SV, Miliasavljevic MM, Kovacevic MS, Stevia ZD. Perforating branches of the middle cerebral artery: Microanatomy and clinical significance of their intracerebral segments. Stroke. 1958;15:1022–1029. doi: 10.1161/01.str.16.6.1022. [DOI] [PubMed] [Google Scholar]
- Marinoni M, Ginanneschi A, Forleo P, Amaducci L. Technical limits in transcranial Doppler recording: Inadequate acoustic windows. Ultrasound in Medicine and Biology. 1997;23:1275–1277. doi: 10.1016/s0301-5629(97)00077-x. [DOI] [PubMed] [Google Scholar]
- Meunier JM, Holland CK, Lindsell CJ, Shaw GJ. Duty cycle dependence of 120 kHz ultrasound enhanced thrombolysis in human clot. Ultrasound in Medicine and Biology. 2007;33:576–783. doi: 10.1016/j.ultrasmedbio.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JM, Holland CK, Lindsell CJ, Shaw GJ. Duty Cycle Dependence of Ultrasound Enhanced Thrombolysis in a Human Clot Model. Ultrasound in Medicine and Biology. 2007a;33:576–583. doi: 10.1016/j.ultrasmedbio.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina CA, Ribo M, Rubiera M, Montaner J, Santamarina E, Delgado-Mederos R, Arenillas JF, Huertas R, Purroy F, Delgado P, Alvarez-Sabin J. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke. 2006;37:425–429. doi: 10.1161/01.STR.0000199064.94588.39. [DOI] [PubMed] [Google Scholar]
- Onundarsen PT, W.Francis C, J.Marder V. Depletion of plasminogen in vitro or during thrombolytic therapy limits fibrinolytic potential. Journal of Laboratory and Clinical Medicine. 1992;120:120–128. [PubMed] [Google Scholar]
- Poster T, Federlein J, Przuntek H, Bütner T. Insufficient and absent acoustic temporal bone window: Potential and limitations of transcranial contrast-enhanced color-coded sonography and contrast-enhanced power-based sonography. Ultrasound in Medicine and Biology. 1997;23:857–862. doi: 10.1016/s0301-5629(97)00047-1. [DOI] [PubMed] [Google Scholar]
- Prokop AF, Soltani A, Roy RA. Cavitational Mechanisms in Ultrasound-Accelerated Fibrinolysis. Ultrasound in Medicine and Biology. 2007;33:924–933. doi: 10.1016/j.ultrasmedbio.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Robbie LA, Bennett B, M.Croll A, Brown PA, Booh NA. Proteins of the fibrinolytic system in human thrombi. Thrombosis and Haemostasis. 1996;75:127–133. [PubMed] [Google Scholar]
- Saguchi T, Onoue H, Urashima M, Ishibashi T, Abe T, Furuhata H. Effective and Safe Conditions of Low-Frequency Transcranial Ultrasonic Thrombolysis for Acute Ischemic Stroke. Neurologic and Histologic Evaluation in a Rat Middle Cerebral Artery Stroke Model. Stroke; a journal of cerebral circulation. 2008 doi: 10.1161/STROKEAHA.107.496117. [DOI] [PubMed] [Google Scholar]
- Sakharov DV, C.Rijken D. Superficial accumulation of plasminogen during plasma clot lysis. Circulation. 1995;92:1883–1890. doi: 10.1161/01.cir.92.7.1883. [DOI] [PubMed] [Google Scholar]
- Sakharov DV, Hekkenberg RT, Rijken DC. Acceleration of fibrinolysis by high-frequency ultrasound: The contribution of acoustic streaming and temperature rise. Thrombosis Research. 2000;100:333–340. doi: 10.1016/s0049-3848(00)00319-4. [DOI] [PubMed] [Google Scholar]
- Seifried E, Tanswell P, Ellbruck D, Haerer W, Schmidt A. Pharmacokinetics and haemostatic status during consecutive infusion of recombinant tissue-type plasminogen activator. Thrombosis and Haemostasis. 1989;61:497–501. [PubMed] [Google Scholar]
- Shaw GJ, Bavani N, Dhamija A, Lindsell CJ. Effect of mild hypothermia on the thrombolytic efficacy of 120 kHz ultrasound enhanced thrombolysis in an in-vitro human clot model. Thrombosis Research. 2006;117:603–608. doi: 10.1016/j.thromres.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Shaw GJ, Dhamija A, Bavani N, Lindsell CJ. Effect of mild hypothermia on the thrombolytic efficacy of 120 kHz ultrasound enhanced thrombolysis in an in-vitro human clot model. Thrombosis Research. 2006;117:603–608. doi: 10.1016/j.thromres.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Shaw GJ, Dhamija A, Bavani N, Wagner KR, Holland CK. Arrhenius temperature dependence of in vitro tissue plasminogen activator thrombolysis. Physics in Medicine and Biology. 2007;52:2953–2967. doi: 10.1088/0031-9155/52/11/002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RJ, Atar S, Fishbein MC, Brasch AV, Peterson TM, Nagai T, Pal D, Nishioka T, Chae JS, Birnbaum Y, Zanelli C, Luo H. Noninvasive transcutaneous low frequency ultrasound enhances thrombolysis in peripheral and coronary arteries. Echocardiography. 2001;18:247–257. doi: 10.1046/j.1540-8175.2001.00247.x. [DOI] [PubMed] [Google Scholar]
- Suchkova V, Carstensen EL, Francis CW. Ultrasound enhancement of fibrinolysis at frequencies of 27 to 100 kHz. Ultrasound in Medicine and Biology. 2002;28:377–382. doi: 10.1016/s0301-5629(01)00522-1. [DOI] [PubMed] [Google Scholar]
- Suchkova VN, Siddiqi FN, Carstensen EL, Dalecki D, Child S, Francis CW. Enhancment of thrombolysis with 40 kHz ultrasound. Circulation. 1998;98:1030–1035. doi: 10.1161/01.cir.98.10.1030. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Hoffman LV, Razavi MK, Kee ST, Sze DY, Dake MD, Semba CP. The safety, efficacy, and pharmacoeconomics of low-dose alteplase compared with urokinase for catheter-directed thrombolysis of arterial and venous occlusions. Journal of Vascular Surgery. 2003;37:512–517. doi: 10.1067/mva.2003.41. [DOI] [PubMed] [Google Scholar]
- Tanne D, Bates VE, Verro P, Kasner SE, Binder JR, Patel SC, Mansbac HH, Daley S, Schultz LR, Scott P, Dayno JM, Verecskey-Porter K, Benesch C, Book D, Coplin WM, Dulli D, Levine SR. Initial clinical experience with IV tissues plasminogen activator for acute ischemic stroke: a multicenter survey. The t-PA Stroke Survey Group. Neurology. 1999;53:424–427. doi: 10.1212/wnl.53.2.424. [DOI] [PubMed] [Google Scholar]
- Tanswell P, Seifried E, Stang E, Krause J. Pharmacokinetics and hepatic catabolism of tissue-type plasminogen activator. Arzneimittelforschung. 1991;41:1310–1319. [PubMed] [Google Scholar]
- Tao X, Yu XJ, Bhattarai B, Li TH, Jin H, Wei GW, Ming JS, Ren W, Jiong C. Microsurgical anatomy of the anterior communicating artery complex in adult Chinese heads. Surgical Neurology. 2006;65:151–161. doi: 10.1016/j.surneu.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Turi ZG, Goldber S, LittleJohn JK, Ark CV, Shadoff N, Karlsberg R, Williams J, Butman J, Stadius ML, Wise K, Buchbinder M, Genton E, Laskey WK, DeMaria A, White C, Sheehan F, Comp PC, Wynne J, Batson-Fowler G, Edwards S. Dose-related efficacy and bleeding complications of double-chain tissue plasminogen activator in acute myocardial infarction. American Journal of Cardiology. 1993;71:1009–1014. doi: 10.1016/0002-9149(93)90564-s. [DOI] [PubMed] [Google Scholar]
- White PJ, Clement GT, Hynynen K. Local frequency dependence in transcranial ultrasound transmission. Physics in Medicine and Biology. 2006;51:2293–2305. doi: 10.1088/0031-9155/51/9/013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter PM, Shukla HP, D.Caruthers S, Scott MJ, Fuhrhop RW, Robertson JD, Gaffney PJ, Wickline SA, Lanza` GM. Molecular imaging of human thrombus with computed tomography. Academic Radiology. 2005;12:S9–S13. doi: 10.1016/j.acra.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Wissgott C, Richter A, Kamusella P, Steinkamp HJ. Treatment of critical limb ischemia using ultrasound-enhanced thrombolysis (PARES Trial): final results. Journal of Endovascular Therapy. 2007;14:438–443. doi: 10.1177/152660280701400402. [DOI] [PubMed] [Google Scholar]
- Yu X, Song SK, Chen J, Scott MJ, Fuhrhop RJ, Hall CS, Gaffney PJ, Wickline SA, Lanza GM. High-resolution MRI characterization of human thrombus using a novel fibrin-targeted paramagnetic nanoparticle contrast agent. Magnetic Resonance in Medicine. 2000;44:867–872. doi: 10.1002/1522-2594(200012)44:6<867::aid-mrm7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]