Abstract
Stem cells persist throughout life by self-renewing in numerous tissues including the central1 and peripheral2 nervous systems. This raises the issue of whether there is a conserved mechanism to effect self-renewing divisions. Deficiency in the polycomb family transcriptional repressor Bmi-1 leads to progressive postnatal growth retardation and neurological defects3. Here we show that Bmi-1 is required for the self-renewal of stem cells in the peripheral and central nervous systems but not for their survival or differentiation. The reduced self-renewal of Bmi-1-deficient neural stem cells leads to their postnatal depletion. In the absence of Bmi-1, the cyclin-dependent kinase inhibitor gene p16Ink4a is upregulated in neural stem cells, reducing the rate of proliferation. p16Ink4a deficiency partially reverses the self-renewal defect in Bmi-1−/− neural stem cells. This conserved requirement for Bmi-1 to promote self-renewal and to repress p16Ink4a expression suggests that a common mechanism regulates the self-renewal and postnatal persistence of diverse types of stem cell. Restricted neural progenitors from the gut and forebrain proliferate normally in the absence of Bmi-1. Thus, Bmi-1 dependence distinguishes stem cell self-renewal from restricted progenitor proliferation in these tissues.
Bmi-1 is an oncogene that causes neoplastic proliferation when overexpressed in lymphocytes4,5. Deletion of Bmi-1 leads to defects in axial skeleton patterning and haematopoiesis, and to ataxia and seizures3. Bmi-1-deficient mice can survive into adulthood but show progressive postnatal growth retardation (ref. 3 and Supplementary Fig. 1), raising the possibility that Bmi-1 regulates stem cell function. Two studies have shown that Bmi-1 is required for the postnatal maintenance of haematopoietic stem cells6,7. In those studies, however, it was not technically possible in the haematopoietic system to discern directly whether the failure of stem cell maintenance was caused by a defect in self-renewal or survival.
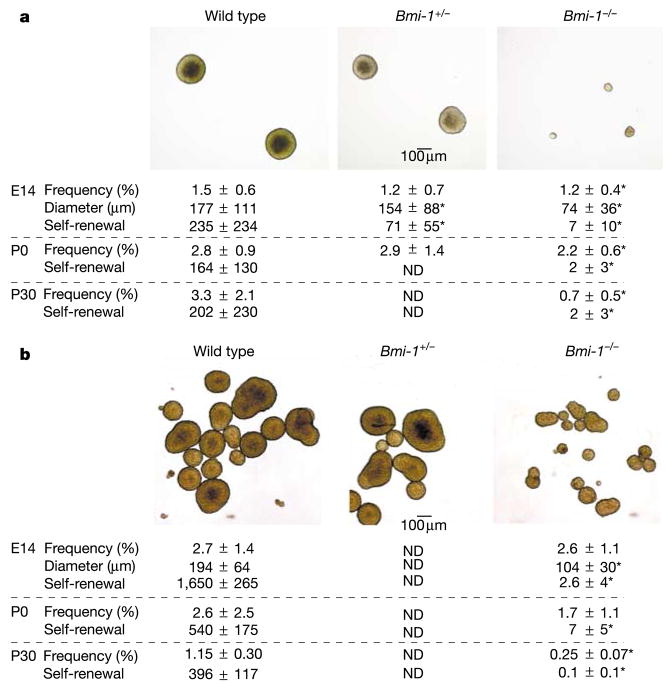
To determine whether Bmi-1 regulates the self-renewal of mouse neural stem cells, we examined the effect of Bmi-1 deficiency on the self-renewal of central nervous system (CNS) stem cells from the telencephalon at embryonic day 14.5 (E14.5)8 and neural crest stem cells (NCSCs) from the gut at E14.5 (enteric nervous system)9. CNS stem cells form CNS neurospheres, whereas gut NCSCs form PNS neurospheres in non-adherent cultures (Fig. 1 and Supplementary Fig. 3). CNS and PNS neurospheres and purified uncultured rat gut NCSCs9 expressed Bmi-1 (Supplementary Fig. 2 and data not shown). Bmi-1−/− telencephalon cells formed multipotent neurospheres, but at a significantly lower frequency (P < 0.05) than did wild-type telencephalon cells (Fig. 1). This suggests that there are significantly fewer stem cells in the Bmi-1−/− telencephalon. The Bmi-1−/− neurospheres were also roughly 14-fold smaller in volume (P < 0.01) and gave rise to 33-fold fewer (P < 0.01) secondary neurospheres on subcloning, indicating a defect in self-renewal. In the E14.5 PNS, the frequency of cells capable of forming neurospheres did not differ between wild-type and Bmi-1−/− guts, but the Bmi-1−/− neurospheres were significantly smaller and gave rise to 60-fold fewer secondary multipotent neurospheres (P < 0.01) on subcloning (Fig. 1). Thus, Bmi-1 deficiency reduced the self-renewal of both CNS stem cells and NCSCs in culture.
Figure 1.
CNS stem cells and gut neural crest stem celcgls (NCSCs) require Bmi-1 to self-renew normally. Images show typical neurospheres that formed after 10 d in non-adherent cultures from E14.5 CNS neural stem cells (a) or PNS NCSCs (b). a, The frequency of Bmi-1 −/− E14 telencephalon cells, P0 SVZ cells or P30 SVZ cells that formed multipotent CNS neurospheres was significantly reduced relative to wild-type cells (*P < 0.05). The diameter and self-renewal of the Bmi-1 −/− neurospheres were also significantly reduced. Self-renewal capacity is expressed as the number of secondary neurospheres generated per primary neurosphere on subcloning. b, Similar results were obtained for multipotent PNS neurospheres generated by E14.5, P0 or P30 wild-type and Bmi-1 −/− gut cells, except that the frequency of neurosphere-forming cells was not significantly reduced until P30. Values are the mean ± s.d. for 3–6 independent experiments. Bmi-1 deficiency also consistently reduced self-renewal when the data were normalized to control for differences in the size of neurospheres: 3.5 ± 0.8% of dissociated wild-type P0 PNS neurosphere cells formed secondary neurospheres on subcloning, as compared with 0.3 ± 0.1% of Bmi-1 −/− cells (P < 0.001). ND, not determined.
If neural stem cells require Bmi-1 for normal self-renewal in vivo, the stem cells in Bmi-1-deficient mice should become depleted postnatally. In the absence of Bmi-1, the frequency of lateral ventricle subventricular zone (SVZ) cells and gut cells that were capable of forming multipotent neurospheres in culture was modestly reduced at postnatal day 0 (P0) and strongly reduced at P30 (Fig. 1). Similar trends were observed in terms of the ability of the cells to form multilineage adherent colonies in culture (data not shown). The extent to which Bmi-1 deficiency reduced the self-renewal potential of stem cells increased over time (Fig. 1). Thus, Bmi-1 is required for the self-renewal of stem cells in diverse tissues, and Bmi-1−/− stem cells rarely persist into adulthood.
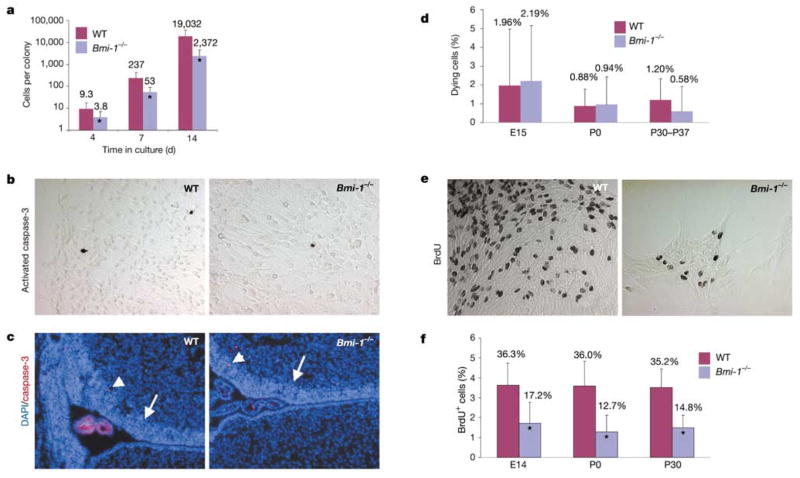
Consistent with the reduction in neurosphere size (Fig. 1), Bmi-1−/− neural stem cell colonies contained one-third to one-tenth as many cells as did wild-type colonies (Fig. 2a). We were unable to detect any increase in cell death in Bmi-1−/− stem cell colonies or in the SVZ of the lateral ventricle in vivo (Fig. 2b–d). By contrast, the proliferation of cells in stem cell colonies was significantly reduced (Fig. 2e, f). Thus, the reduced self-renewal of Bmi-1−/− neural stem cells was caused at least partly by reduced proliferation.
Figure 2.
Bmi-1 deficiency reduces proliferation but does not increase cell death in CNS stem cell colonies. P0 SVZ cells were dissociated and plated in adherent cultures, and the number of cells per colony was counted after 4, 7 and 14 d (a). All colonies were counted after 4 d, but only colonies that contained neurons and glia were counted after 7 and 14 d. At all time points, wild-type (WT) colonies contained significantly more cells than the Bmi-1 −/− colonies (*P < 0.01). Similar results were obtained at E14 and P30 (not shown). Only a few cells in adherent CNS stem cell colonies (b) or in the P0 SVZ (c) stained with antibodies against activated caspase-3 (a marker of cell death). Arrows and arrowheads indicate the SVZ and caspase-3-positive cells, respectively. Note that the choroid plexus in the lateral ventricle is highly autofluorescent (c). No statistically significant difference in the frequency of dying cells was observed between wild-type and Bmi-1 −/− CNS stem cell colonies on the basis of the frequency of condensed, fragmented nuclei identified by 4′, 6-diamidino-2-phenylindole dihydrochloride (DAPI) staining (d), or activated caspase-3 (not shown). Bmi-1 deficiency was associated with a significant decrease in the rate of BrdU incorporation into CNS stem cell colonies (e, f; *P < 0.01), indicating reduced proliferation. A similar reduction in proliferation was observed in Bmi-1 −/− NCSC colonies in culture (not shown).
To assess the effect of Bmi-1 on proliferation in vivo, we examined the rate of 5-bromodeoxyuridine (BrdU) incorporation into P0 and P30 SVZ cells. As compared with wild-type SVZ cells, Bmi-1−/− SVZ cells showed a significantly lower rate of BrdU incorporation at both ages (17.8 ± 0.5% (Bmi-1−/−) versus 19.9 ± 2.0% (wild type) of SVZ cells were BrdU-positive at P0; P < 0.05), although the effect was greater at P30 (13.1 ± 2.7% versus 20.1 ± 0.5%; P < 0.01).
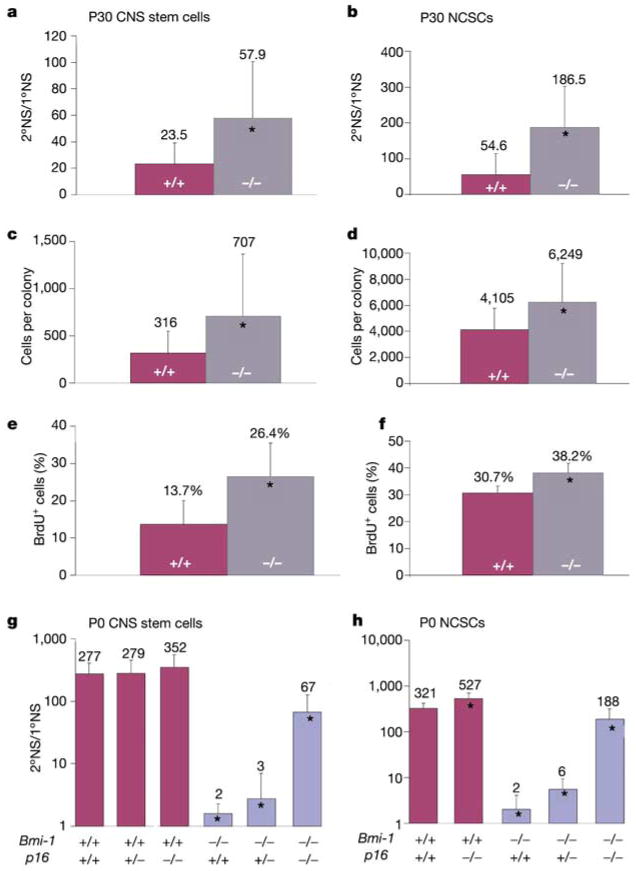
Bmi-1 deficiency is associated with increased expression of the p16Ink4a and p19Arf genes encoding cyclin-dependent kinase inhibitors, and deletion of these genes partially rescues the growth of Bmi-1−/− mice10–12. Analysis by quantitative real-time polymerase chain reaction (qRT–PCR) showed that p16Ink4a expression increased 5–21-fold, whereas p19Arf expression increased 1.4–3-fold, in Bmi-1−/− CNS and PNS neurospheres of all ages (Supplementary Table 1 and Supplementary Figs 4f and 5f). To test whether p16Ink4a regulates neural stem cell self-renewal, we examined neurospheres cultured from p16−/− adult mice (ref. 13 and Fig. 3). p16−/− neurospheres from both the CNS and PNS were larger and self-renewed to a significantly greater extent than did wild-type neurospheres (Fig. 3a, b). Adherent CNS and PNS p16−/− stem cell colonies contained more cells and incorporated BrdU at a higher rate (Fig. 3c–f). These data indicate that p16Ink4a inhibits neural stem cell self-renewal in culture, although the magnitude of this effect may depend on culture conditions, because p16Ink4a can be induced in response to stress14.
Figure 3.
p16Ink4a negatively regulates the self-renewal of CNS stem cells and gut NCSCs in culture. SVZ and gut cells from adult p16 −/− and wild-type mice were dissociated and cultured to generate neurospheres (a, b) or adherent stem cell colonies (c–f). After 7 d (a, c, e) or 10 d (b, d, f) in culture, self-renewal (a, b), the total number of cells per colony (c, d), and the percentage of BrdU-positive cells per colony (e, f) were assayed. In each case, the p16 −/− adult neural stem cell colonies self-renewed more, proliferated more and contained more cells (*P < 0.05). No differences were observed between p16 −/− and wild-type colonies in the frequency of apoptotic cells (0.59 ± 0.61% versus 0.35 ± 0.48% respectively). Bmi-1 +/−p16 +/− mice were also bred to generate P0 pups, from which SVZ CNS stem cells (g) and gut NCSCs (h) were cultured. For both types of cell, p16Ink4a deficiency significantly increased the self-renewal of Bmi-1 −/− stem cells (*P < 0.01), although it did not fully restore self-renewal to wild-type levels (*P < 0.01 relative to wild type). Note that p16Ink4a deficiency in Bmi-1+/+ stem cells significantly increased the self-renewal of P0 NCSCs (h) but not P0 CNS stem cells (g).
To address directly whether Bmi-1 promotes neural stem cell self-renewal by suppressing the expression of p16Ink4a, we generated Bmi-1−/−p16−/− mice. p16Ink4a deficiency significantly increased the self-renewal of Bmi-1−/− CNS stem cells and NCSCs in culture (P < 0.01), but did not fully restore self-renewal to wild-type levels (Fig. 3g, h). This indicates that there are additional pathways downstream of Bmi-1 that regulate self-renewal, perhaps including p19Arf. The increased self-renewal of Bmi-1−/−p16−/− CNS stem cells and NCSCs was associated with a significant increase in the rate of proliferation in stem cell colonies (23.5 ± 11.1% BrdU- positive cells versus 8.9 ± 11.6% in Bmi-1−/−p16+/+ CNS stem-cell colonies, P < 0.01). Therefore, the increase in p16Ink4a expression in the absence of Bmi-1 contributed to the reduced self-renewal of Bmi-1−/− neural stem cells in culture.
Because p16Ink4a and p19Arf expression can increase in cultured cells15, we examined whether these genes were also upregulated in uncultured progenitors (Supplementary Table 1). Expression of p16Ink4a RNA and protein was increased in the P0 and P30 SVZ of Bmi-1−/− mice but was not detected in the E15 telencephalon (Supplementary Table 1 and Supplementary Fig. 4e). p16Ink4a was also upregulated in uncultured Bmi-1−/− P30 SVZ FSChiS-SEA-1hiCD24−/lo cells, which are enriched for CNS stem cells16,17. Expression of p19Arf followed similar trends but was upregulated to a lesser extent than p16Ink4a (Supplementary Table 1). In the PNS, p16Ink4a and p19Arf followed trends similar to those observed in the CNS. Expression of p16Ink4a was also increased in P0 and P30 uncultured Bmi-1−/− p75+ gut cells, which are enriched for NCSCs2. Consistent with the increased expression of p16Ink4a in the nervous system of Bmi-1-deficient mice in vivo, p16 deficiency partially rescued at least some aspects of nervous system development in Bmi-1−/− mice. For example, the number of neurons per section through the P30 distal small intestine was 92 ± 30 (mean ± s.d.) for wild-type, 47 ± 19 for Bmi-1−/− and 78 18 for Bmi-1 p16−/− mice (all differences P < 0.05).
These data suggest that Bmi-1 is required in the nervous system in vivo to repress p16Ink4a and p19Arf postnatally but not during fetal development. The lack of p16Ink4a or p19Arf expression in the Bmi-1−/− telencephalon may be a reason why forebrain development seems relatively normal in Bmi-1−/− newborns despite the poor proliferation of E14.5 Bmi-1−/− neurospheres, which do express p16Ink4a in culture. Nonetheless, p16Ink4a expression was increased in vivo in the Bmi-1−/− SVZ at P0 and P30, consistent with the proliferation defect observed in the postnatal SVZ and with the postnatal depletion of stem cells.
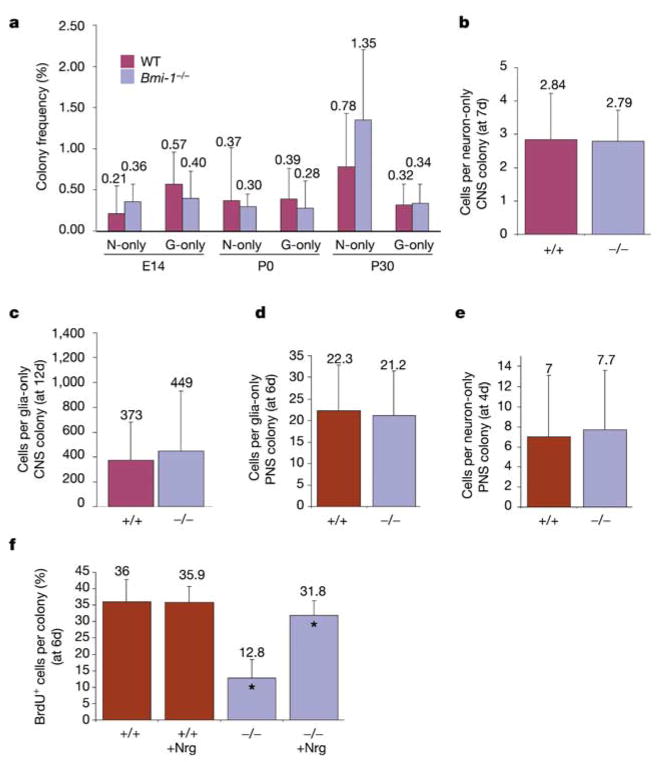
The proliferation of restricted neural progenitors might be regulated differently from that of stem cells because restricted progenitors are not multipotent and divide a limited number of times before becoming postmitotic. To test whether these cells are dependent on Bmi-1, we cultured E14 telencephalon cells, or P0 or P30 SVZ cells, at clonal density under adherent conditions. In contrast to the significantly reduced frequency of stem cells in Bmi-1−/− samples (Fig. 1), the frequency of cells that formed neuron-only or glia-only colonies did not significantly differ between wild-type and Bmi-1−/− samples (Fig. 4a). There were also no significant differences in the number of cells per neuron-only or glia-only colony from the CNS or PNS (Fig 4b–e). Thus, Bmi-1−/− restricted neural progenitors were present in relatively normal numbers and proliferated normally in culture.
Figure 4.
Restricted neural progenitors from the CNS and PNS proliferate normally in the absence of Bmi-1. E14 telencephalon cells or P0 or P30 SVZ cells were dissociated and cultured at clonal density under adherent conditions. a–c, The frequency of CNS cells that formed neuron-only or glia-only colonies after 12–14 d in culture did not significantly differ between wild-type (WT) and Bmi-1 −/− mice (a), nor did the number of cells per neuron-only colony (b) or glia-only colony (c). d, The number of cells per glia-only colony formed by dissociated P0 gut cells did not differ between wild-type and Bmi-1 −/− mice. e, In BMP4-treated cultures of E14.5 gut p75+ cells, no difference was observed in the number of cells per neuron-only colony. f, Bmi-1 −/− neural progenitor colonies in the absence of Nrg proliferated at a significantly lower rate than did wild-type colonies (*P < 0.01), but Bmi-1 −/− colonies in the presence of Nrg proliferated similarly to wild-type colonies (although the difference was still statistically significant, *P < 0.05).
To test whether lineage commitment caused the proliferation of neural progenitors to become Bmi-1 independent, we cultured E14 gut p75+ neural crest cells at clonal density for 4 d with or without bone morphogenetic protein 4 (BMP4), which instructs gut NCSCs to undergo neurogenesis9,18,19. In the absence of BMP4 no neuron-only colonies were observed in these cultures, but in the presence of BMP4 significant numbers of neuron-only colonies emerged. The number of cells per neuron-only colony did not differ between wild-type and Bmi-1−/− cultures (Fig. 4e).
Neuregulin (Nrg) promotes the proliferation of glial progenitors in addition to instructing postnatal gut NCSCs to acquire a glial fate2,19,20. To test whether Bmi-1−/− glial progenitors proliferated normally when stimulated by Nrg, we cultured dissociated P0 gut cells in adherent cultures at clonal density with or without Nrg for 6 d. In the absence of Nrg, Bmi-1−/− colonies incorporated BrdU (pulsed on day 6) at only a third of the rate of wild-type colonies; however, in the presence of Nrg, Bmi-1−/− colonies incorporated BrdU at almost the same rate as wild-type colonies (Fig. 4f). These data indicate that lineage determination factors change the cell-cycle regulation of stem cells in addition to restricting their developmental potential, causing the proliferation of neural progenitors to become Bmi-1 independent.
Bmi-1 was expressed in restricted neural progenitors and p16Ink4a RNA and protein expression remained increased in Bmi-1−/− restricted neural progenitor colonies (Supplementary Fig. 5f); however, p16Ink4a deficiency did not affect the frequency or proliferation of restricted neural progenitors (Supplementary Fig. 5). Thus, lineage restriction was associated with a change in cell-cycle regulation that caused restricted neural progenitors to proliferate in culture in a way that was not affected by Bmi-1 or p16Ink4a deficiency.
The reduced stem cell frequency in the Bmi-1−/− telencephalon (Fig. 1) indicates that other pathways downstream of Bmi-1 may also regulate self-renewal, because neither p16Ink4a nor p19Arf was expressed in the telencephalon (Supplementary Table 1). To identify additional pathways that are regulated directly or indirectly by Bmi-1, we compared the gene expression profiles of wild-type and Bmi-1−/− neurospheres that had been cultured from the P0 SVZ or from the P0 gut. Because Bmi-1 is a repressor21,22, and the function of Bmi-1 seems to be conserved between CNS and PNS stem cells, we examined whether any genes were significantly upregulated (fold change >2; P < 0.05) in Bmi-1−/− neurospheres from both the CNS and PNS. We found 11 genes that met these criteria and that were confirmed to be expressed differentially in independent samples by qRT–PCR (out of 36,701 probe sets examined; Supplementary Table 2). In addition to p16Ink4a and p19Arf, these included genes that were previously identified as being regulated by Bmi-1, such as Hox genes (hoxD8, hoxD9 and hoxC9)7,23,24, and several genes that have not been previously identified as being regulated by Bmi-1, such as the cyclin-dependent kinase inhibitor gene p21, which regulates haematopoietic stem cell self-renewal25, and Gas6, which encodes a ligand for the Axl receptor tyrosine kinase26. More work will be required to determine whether any of these genes regulate neural stem cell self-renewal.
Although Bmi-1 was found to be consistently required for the self-renewal of haematopoietic stem cells6,7, CNS stem cells and NCSCs, it was not required for the proliferation of restricted neuronal and glial progenitors from the forebrain or from the enteric nervous system (Fig. 4). It is not the case that all restricted progenitors proliferate normally in the absence of Bmi-1, because Bmi-1−/−lymphocytes are also impaired in their proliferation3. It is also possible that other types of restricted neural progenitor (such as from other regions of the nervous system) might require Bmi-1 for proliferation as the intrinsic properties of neural progenitors vary among regions of the nervous system9. Nonetheless, Bmi-1 dependence distinguishes stem cell self-renewal from the proliferation of at least some types of restricted progenitor, providing insight that will help to elucidate these pathways further.
Methods
Isolation of CNS and PNS progenitors
Bmi-1+/− mice that had been backcrossed at least six times onto a C57BL/6 background were mated to generate E14.5 embryos, P0 pups, or 4–5-week-old adults that were genotyped by PCR (see Supplementary Information). p16−/− mice were on an FVB background and were also genotyped by PCR. For CNS preparations, we dissected embryonic telencephalons or P0 or P30 SVZs as described16,27. CNS progenitors were dissociated and plated as described in the Supplementary Information. We processed embryonic gut tissues as described9. Dissociated embryonic gut cells were stained with an antibody against p75 (Chemicon International), and p75+ cells (which include NCSCs) were sorted by a FACSVantage dual-laser flow cytometer (Becton-Dickinson) into culture plates. Guts from P0 and P30 mice were dissected in ice-cold PBS, and the outer muscle/ plexus layers were peeled free from the underlying epithelium as described2, dissociated and plated by pipette.
Cell culture
Cell suspensions were plated on adherent plates at clonal density, which means that cells were plated at a low density such that individual cells could form spatially distinct colonies19. This allowed us to infer the developmental and proliferative potential of single cells on the basis of the types and numbers of cells that comprised each colony. For proliferation studies, 10μM BrdU was added to the cultures for 1 h at 37 °C before the cells were fixed and stained with an antibody against BrdU. Culture conditions are described in the Supplementary Information.
For the neurosphere formation assays (non-adherent cultures) we used ultra-low binding plates (Corning). To test the self-renewal capacity of the neurospheres, individual CNS neurospheres were selected, centrifuged at 210g for 4 min, and then mechanically dissociated and replated. PNS neurospheres were plated for 24 h into plates treated with poly-D-lysine, and then trypsin-digested, mechanically dissociated, and replated. We quantified self-renewal as the number of secondary neurospheres generated per primary neurosphere (2°NS/1°NS).
Tissue fixation and immunohistochemistry
Mice were injected intraperitoneally with 50 mg per kg (body weight) BrdU and killed after 2 h for perinatal mice and after 3 h for adult mice. The brains were fixed immediately in 4% paraformaldehyde and immunostained as described in the Supplementary Information.
The methods used for qRT–PCR, western blot analysis and microarray analysis are described in the Supplementary Information.
Supplementary Material
Supplementary Information accompanies the paper on www.nature.com/nature.
Acknowledgments
We thank M. Kukuruga, A. M. Deslaurier, M. Kiel and the University of Michigan Flow-Cytometry Core Facility (supported by University of Michigan Comprehensive Cancer and Multipurpose Arthritis Center NIH grants); D. Qian for mouse breeding; D. Misek, R. Koenig and R. Kuick for microarray analysis; E. Smith in the Hybridoma Core Facility (supported through the Michigan Diabetes Research and Training Center, and the Rheumatic Disease Center); M. van Lohuizen for the Bmi1−/− mice; and R. DePinho and D. Scadden for the p16−/− mice. This work was supported by the NIH, the Searle Scholars Program and the Howard Hughes Medical Institute. A.V.M. was supported by a University of Michigan MSTP training grant. R.P. was the recipient of a postdoctoral fellowship from the Spanish Ministry of Science and Technology.
Footnotes
Competing interests statement The authors declare that they have no competing financial interests.
References
- 1.Morshead CM, Craig CG, van der Kooy D. In vivo clonal analyses reveal the properties of endogenous neural stem cell proliferation in the adult mammalian forebrain. Development. 1998;125:2251–2261. doi: 10.1242/dev.125.12.2251. [DOI] [PubMed] [Google Scholar]
- 2.Kruger GM, et al. Neural crest stem cells persist in the adult gut but undergo perinatal changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35:657–669. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Lugt NMT, et al. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 1994;8:757–769. doi: 10.1101/gad.8.7.757. [DOI] [PubMed] [Google Scholar]
- 4.Haupt Y, Bath ML, Harris AW, Adams JM. BMI-1 transgene induces lymphomas and collaborates with Myc in tumorigenesis. Oncogene. 1993;8:3161–3164. [PubMed] [Google Scholar]
- 5.Alkema MJ, Jacobs H, van Lohuizen M, Berns A. Perturbation of B and T cell development and predisposition to lymphomagenesis in Eu-Bmi1 transgenic mice require the Bmi1 RING finger. Oncogene. 1997;15:899–910. doi: 10.1038/sj.onc.1201262. [DOI] [PubMed] [Google Scholar]
- 6.Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- 7.Park IK, et al. Bmi-1 is required for the maintenance of adult self-renewing hematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 8.Davis A, Temple S. A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature. 1994;372:263–266. doi: 10.1038/372263a0. [DOI] [PubMed] [Google Scholar]
- 9.Bixby S, Kruger GM, Mosher JT, Joseph NM, Morrison SJ. Cell-intrinsic differences between neural stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 2002;35:643–656. doi: 10.1016/s0896-6273(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs JJL, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 11.Itahana K, et al. Control of the replicative life span of human fibroblasts by p16 and the polycomb protein Bmi-1. Mol Cell Biol. 2003;23:389–401. doi: 10.1128/MCB.23.1.389-401.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs JJL, et al. Senescence bypass screen identifies TBX2, which represses Cdkn2a (p19ARF) and is amplified in a subset of human breast cancers. Nature Genet. 2000;26:291–298. doi: 10.1038/81583. [DOI] [PubMed] [Google Scholar]
- 13.Sharpless NE, et al. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature. 2001;413:86–91. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- 14.Lowe SW, Sherr CJ. Tumor suppression by Ink4a–Arf: progress and puzzles. Curr Opin Genet Dev. 2003;13:77–83. doi: 10.1016/s0959-437x(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 15.Zindy F, Quelle DE, Roussel MF, Sherr CJ. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene. 1997;15:203–211. doi: 10.1038/sj.onc.1201178. [DOI] [PubMed] [Google Scholar]
- 16.Rietze RL, et al. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001;412:736–739. doi: 10.1038/35089085. [DOI] [PubMed] [Google Scholar]
- 17.Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 18.Shah NM, Groves A, Anderson DJ. Alternative neural crest cell fates are instructively promoted by TGFβ superfamily members. Cell. 1996;85:331–343. doi: 10.1016/s0092-8674(00)81112-5. [DOI] [PubMed] [Google Scholar]
- 19.Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–749. doi: 10.1016/s0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- 20.Shah NM, Marchionni MA, Isaacs I, Stroobant PW, Anderson DJ. Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell. 1994;77:349–360. doi: 10.1016/0092-8674(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 21.Bunker CA, Kingston RE. Transcriptional repression by Drosophila and mammalian polycomb group proteins in transfected mammalian cells. Mol Cell Biol. 1994;14:1721–1732. doi: 10.1128/mcb.14.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs JJL, van Lohuizen M. Polycomb repression: from cellular memory to cellular proliferation and cancer. Biochim Biophys Acta. 2002;1602:151–161. doi: 10.1016/s0304-419x(02)00052-5. [DOI] [PubMed] [Google Scholar]
- 23.Alkema MJ, van der Lugt NMT, Bobeldijk RC, Berns A, van Lohuizen M. Transformation of axial skeleton due to overexpression of bmi-1 in transgenic mice. Nature. 1995;374:724–727. doi: 10.1038/374724a0. [DOI] [PubMed] [Google Scholar]
- 24.van der Lugt NMT, Alkema MJ, Berns A, Deschamps J. The Polycomb-group homolog Bmi-1 is a regulator of murine Hox gene expression. Mech Dev. 1996;58:153–164. doi: 10.1016/s0925-4773(96)00570-9. [DOI] [PubMed] [Google Scholar]
- 25.Cheng T, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 26.Stritt TN, et al. The anticoagulation factor protein S and its relative Gas6, are ligands for the Tyro3/ Axl family of receptor tyrosine kinases. Cell. 1995;80:661–670. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- 27.Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information accompanies the paper on www.nature.com/nature.