Abstract
The neuropeptide calcitonin gene-related peptide (CGRP), expressed by nociceptive sensory afferents in joints, is an important mediator in the pathogenesis of arthritis. Capsaicin causes neurons in the dorsal root ganglia (DRG) to release CGRP from their central and/or peripheral axons, suggesting a functional link between CGRP and the capsaicin receptor TRPV1. The expression of both TRPV1 and CGRP have been reported to increase in several models of arthritis but the specific involvement of TRPV1-expressing articular afferents that can release CGRP remains unclear. We here wanted to ascertain whether the increase in the number of CGRP-positive primary afferents during arthritis may be affected by genetic deletion of TRPV1. For this, we quantified the expression of CGRP in primary afferent neurons in DRG in wild type mice (WT) vs. TRPV1-KO mice with adjuvant-induced arthritis (AIA), using immunohistochemistry. We found that the fraction of DRG neurons that were immunopositive for CGRP 1) was higher in naïve TRPV1-KO mice than in naïve WT mice, 2) increased progressively 3–21 days after induction of AIA, and 3) this increase was bilateral but significantly greater on the CFA-injected side than on the IFA-injected side in TRPV1-KO mice. The increased expression of CGRP in AIA may reflect a phenotypic switch of primary afferents from non-peptidergic to peptidergic and the larger increase in TRPV1-KO mice may represent a plastic change to compensate for the missing receptor in a major sensory circuit.
Keywords: TRPV1, Calcitonin gene-related peptide, Dorsal root ganglion, Adjuvant-induced arthritis, Mouse
1. Introduction
The neuropeptide calcitonin gene-related peptide (CGRP), a marker for peptidergic nociceptive afferents (Lawson et al., 2002), is an important mediator in the pathogenesis of arthritis (Schaible et al., 2002). CGRP has been shown in articular afferents at the level of the dorsal root ganglia (DRG, O’Brien et al., 1989) and in peripheral nerve fibers in normal and arthritic joints (Donaldson et al., 1992; Larsson et al., 1991; Sluka and Westlund, 1993). Fine articular afferents may not only mediate the slow burning pain reported by arthritic patients but, through the release of CGRP into the joint, may facilitate inflammation and angiogenesis (Ferrell et al., 1997; Konttinen et al., 1994), two closely related pathogenetic components of arthritis that are the key determinants of disease progression (Bonnet and Walsh, 2005). In experimental arthritis, the release of CGRP into the joint is increased (Ahmed et al., 1995a) and CGRP gene knockout mice (CGRP-KO) have reduced behavioral pain responses, consistent with a pro-inflammatory and pro-nociceptive role for CGRP in joint inflammation (Zhang et al., 2001).
CGRP is expressed by C- and Aδ-fiber primary afferents, the majority of which express the vanilloid receptor TRPV1 (Guo et al., 1999). TRPV1, a non-selective cation channel, is important for thermal nociception and inflammatory hyperalgesia and allodynia (Caterina et al., 1997). In vitro, TRPV1-expressing cells can be activated by noxious heat as well as by vanilloids, including capsaicin. In mice, knocking out the TRPV1 gene prevented the occurrence of thermal hyperalgesia in an experimental model of hindpaw inflammation (Caterina et al., 2000; Davis et al., 2000). TRPV1-KO mice have thus been used to study the involvement of capsaicin-sensitive sensory afferents in several models of painful inflammatory conditions, including arthritis (Bolcskei et al., 2005); we and others have found that the severity of adjuvant-induced arthritis (AIA) is significantly reduced in TRPV1-KO mice (Barton et al., 2006; Chen et al., 2008; Szabo et al., 2005).
Noxious stimulation causes capsaicin-sensitive DRG neurons to release CGRP from their central and/or peripheral axons (Averbeck and Reeh, 2001; Bernardini et al., 2004; Nanayama et al., 1989), suggesting a functional link between CGRP and TRPV1, the receptor for capsaicin. This is further supported by observations that the peripheral CGRP release is reduced in TRPV1-KO mice and that blockade of the CGRP receptor abolishes TRPV1-mediated effects on the circulatory system (Wang et al., 2006a; Wu et al., 2006). Furthermore, mice were shown to exhibit variable sensitivity to noxious heat proportional to the level of genetic expression of CGRP (Mogil et al., 2005). In a recent study using CGRP-KO and TRPV1-KO mice, Szabo et al. (2008) reported that the protective role of CGRP in a model of scleroderma, a disease that shares common pathogenetic mechanisms with rheumatoid arthritis, is mediated by TRPV1.
Both CGRP and TRPV1 expression in DRG has been reported to increase in a rat model of arthritis (Fernihough et al., 2005; Staton et al., 2007). Moreover, pretreatment with capsaicin was found to mitigate the upregulation of CGRP in the rat AIA model (Ahmed et al., 1995b), supporting previous observations that capsaicin reduces the expression of neuropeptides in AIA (Levine et al., 1986), and suggesting that the effect may be mediated by capsaicin-sensitive afferents. However, the mechanism through which TRPV1 participates in the pathogenesis of arthritis and the specific involvement of TRPV1-expressing articular afferents that can release CGRP remains unclear. The goal of the present study was to ascertain whether the increase in the number of CGRP-positive primary afferents during arthritis is affected by the genetic deletion of TRPV1. For this, we quantified the expression of CGRP in primary afferent neurons in TRPV1-KO mice vs. wild type mice with AIA, using immunohistochemistry.
2. Materials and methods
2.1. Animal model, euthanasia and tissue handling
A total of 32 male mice, ages 4–6 months and weighing 25–30 g, were used in this study. These included 16 each of TRPV1-KO and wild-type C57BL/6 mice (WT, Jackson Labs, Bar Harbor, ME). All experimental procedures involving mice were carried out in compliance with the National Research Council’s Guide for the Care and Use of Laboratory Animals, and according to a protocol approved by the Institutional Animal Care and Use Committee at University of North Carolina.
For AIA, mice were lightly anesthetized with a mixture of ketamine and xylazine (90 mg/kg and 10 mg/kg, i.p.) and injected with 10 μl of complete Freund’s adjuvant (CFA, 20 mg/ml suspension of heat-killed Mycobacterium tuberculosis in vehicle, Difco Lab, Detroit, MI) in each of two sites, front and back of the left ankle, using a 30-gauge needle attached to a 10 μl Hamilton syringe. The right ankle of each mouse was injected with the same volume of vehicle (paraffin oil containing mannide monooleate; Freund’s incomplete adjuvant, IFA). Matching numbers of TRPV1-KO and WT mice were sacrificed at 3d (n=4), 7d (n=4), 14d (n=8), and 21d (n=12) after induction of arthritis; two each of TRPV1-KO and WT mice without injections were sacrificed for collecting data in naïve animals.
For tissue collection, mice under deep anesthesia with sodium pentobarbital (80 mg/kg, i.p.) were perfused intracardially with 30 ml freshly prepared solution of 1% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 (PB), followed by 100 ml solution of 4% paraformaldehyde in PB. L4-L5 DRG bilaterally were dissected out, postfixed in 4% paraformaldehyde for 4 hours, cryoprotected in 30% sucrose in PB for 24–48 hours, and sectioned on a cryostat at 10 μm.
2.2. Immunohistochemistry
For immunohistochemistry, sections were permeabilized with 0.1% Triton X-100 in phosphate-buffered saline (PBS; 0.01 M, pH 7.4) for 15 minutes, blocked with 5% normal donkey serum in 0.1% Triton X-100 in PBS (NDS; Jackson Immunoresearch, West Grove, PA) for 30 minutes, and incubated overnight with a guinea pig anti-CGRP antibody (1:2,000, Peninsula, San Carlos, CA) in NDS. After several rinses with PBS and incubation with 5% NDS for 30 minutes, sections were incubated with donkey anti-guinea pig antibody (1:200; Jackson) for 2 hours, rinsed, and coverslipped with Vectashield (Vector, Burlingame, CA). Digital micrographs were obtained with a Retiga EX cooled CCD camera (Q-Imaging, Surrey, CA) attached to a Leitz DMR fluorescent microscope (Leitz, Wetzlar, Germany) and saved as TIFF files; contrast and brightness were adjusted with Photoshop CS2 (Adobe Systems, San Jose, CA). The primary antibody employed here was characterized and its selectivity was confirmed using Western blots and preadsorption with CGRP, and is in common use in our laboratory. As a routine control, we processed sections according to the above protocol, except that primary or secondary antibodies were omitted; omission of primary or secondary antibodies completely abolished specific staining.
2.3. Quantification and statistical analysis
Digital images were analyzed using Image J 1.38x software (NIH, Bethesda, MD). All counts and measurements were performed by an investigator blinded to the source material, including the genotype of the mouse and the side. Twelve DRG sections were analyzed per side per mouse (4–8 sections of each of L4 and L5 ganglia, 60 μm apart); neuronal profiles (NP) were identified by their typical cellular morphology. The cut-off brightness level (labeling density threshold) was determined by averaging the integral brightness of three NP per section that were judged to be minimally positive. All profiles whose mean labeling density exceeded this threshold were counted as positive. The “background” immunofluorescence was high enough to allow visualization of immunonegative NP, in some cases these were verified on images acquired with DIC optics. The fraction of immunopositive NP in each section were expressed as a percentage of total counted NP [% = (positive NP/total counted NP) × 100]. Data were analyzed with SPSS 11.5x (SPSS, Chicago, IL) and graphed using Kaleidagraph (Synergy Software, Reading, PA). Differences between animal groups were studied for significance with one-way analysis of variance (ANOVA), which assessed the overall influence of genotype, side (left, CFA-injected or right, IFA-injected), and time after induction of arthritis as main factors, followed by a post hoc general contrast comparison using Tukey’s test. Significance was set at p<0.05.
3. Results
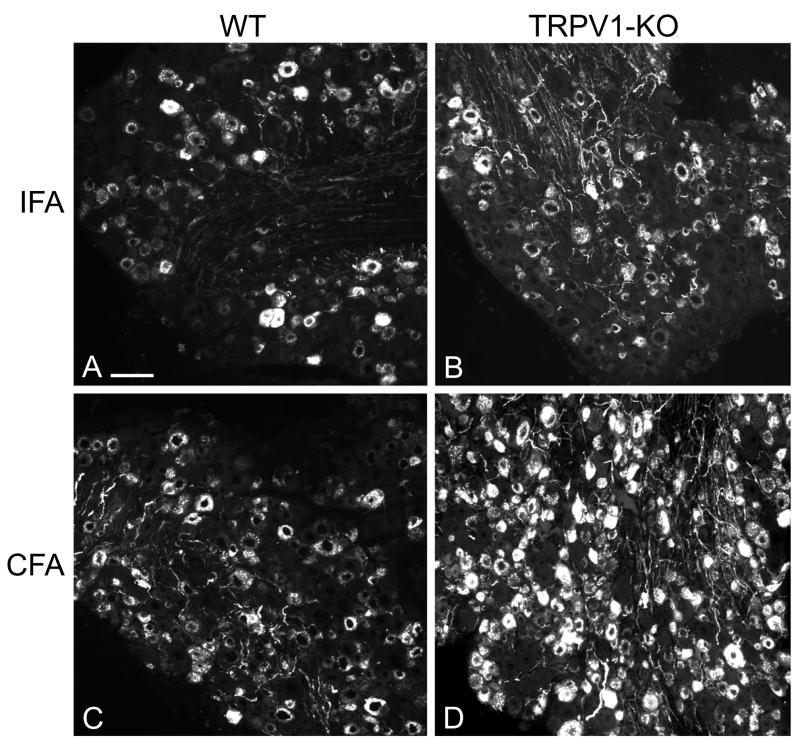
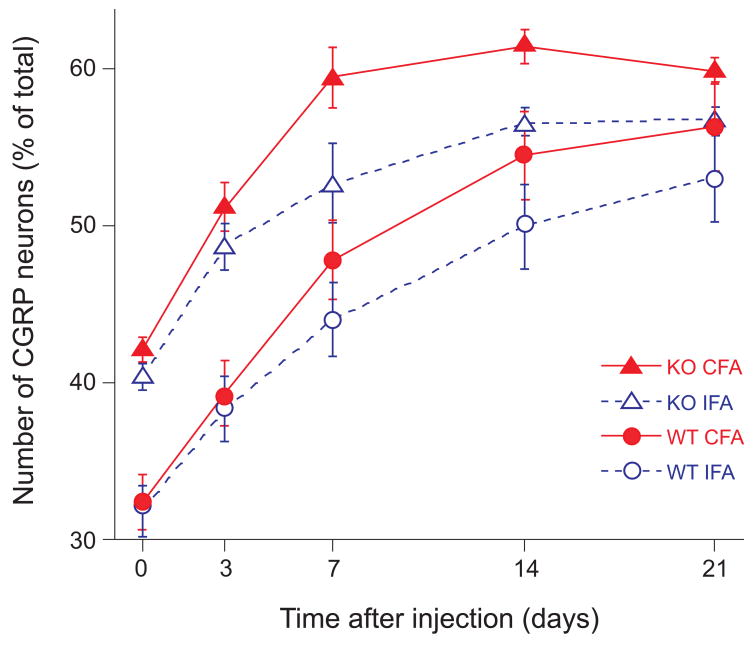
In material from all mice, multiple NP and axons in the DRG and dense terminal fibers in the superficial laminae of the spinal dorsal horn were immunostained for CGRP. In DRG of mice with AIA, the number of stained NP appeared greater on the CFA-injected side than on the IFA-injected side (Fig. 1). Counts in DRG at L4-L5 revealed that in uninjected (naïve) WT mice, 32.6±0.9% (mean value ± standard error of the mean, time point “0” in Fig. 2) of all NP were CGRP-positive; in naïve KO mice this percentage was significantly greater (41.6±0.5%). After induction of AIA, the level of expression of CGRP was significantly greater in TRPV1-KO than in WT mice on the CFA-injected (51.3±1.5% vs. 39.6±1.0% after 3 days, 59.4±1.8% vs. 48.0±1.3% after 7 days, and 61.3±1.0% vs. 54.5±0.8% after 14 days, comparative data with mean difference at the .05 level of significance are listed here, all data are graphed in Fig. 2). On the IFA-injected side, expression of CGRP was also greater in TRPV1-KO than in WT mice (48.8±1.4% vs. 38.7±1.3% after 3 days, 52.8±2.5% vs. 44.4±1.1% after 7 days, and 56.6±0.8% vs. 50.1±0.8% after 14 days).
Figure 1.
Immunofluorescent staining for CGRP in DRG from sections of wild type (WT) and TRPV1-KO mice: the number of labeled neuronal profiles appears greater on the CFA-injected side than on the IFA-injected side 21 days after induction of AIA. In every group (WT and TRPV1-KO), the sections of are from the left and right L5 DRG of the same mouse. Scale bar, 100 μm.
Figure 2.
The expression of CGRP was higher in TRPV1-KO (KO) than in wild type (WT) mice with AIA: the fraction (mean ± standard error of the mean) of immunostained neuronal profiles was higher in naïve KO mice than in naïve WT mice (time point “0”), increased progressively 3–21 days after induction of AIA, and at days 7 and 14, was significantly greater on the CFA-injected side than on the IFA-injected side in TRPV1-KO mice.
Thus, AIA caused bilateral increase in the fraction of CGRP-positive NP in DRG in both WT and KO mice. This increase was more pronounced during the early stages of AIA and appeared to plateau between days 7 and 14. Although naïve KO mice had a higher fraction of CGRP-positive NP than naïve WT mice, 3 and 7 days after induction of AIA, the trend of increase in the level of expression of CGRP was similar in the two groups on both CFA and IFA sides (the curves of increase with time are roughly parallel). At the later stages of arthritis, both the rate of increase and the differences between KO and WT mice were smaller at day 14 and insignificant at day 21. However, at both 7 and 14 days, the increase on the CFA-injected side over the IFA-injected side was greater in KO mice (59.4±1.8% vs. 52.8.0±2.5% after 7 days, and 61.3±1.0% vs. 56.6±0.8% after 14 days) than in WT mice (Fig. 2).
4. Discussion
The main findings of this study are that the fraction of DRG neurons that were immunopositive for CGRP 1) was higher in naïve TRPV1-KO mice than in naïve WT mice, 2) increased progressively 3–21 days after induction of AIA, and 3) this increase was bilateral but significantly greater on the CFA-injected side than on the IFA-injected side in TRPV1-KO mice.
We here report for the first time that TRPV1-KO mice have higher levels of expression of CGRP in primary afferents than WT mice, presumably reflecting a compensatory change during development. The percentage of CGRP-positive DRG neurons in naïve WT mice in the present study is in general agreement with the published data in C57BL6 mice (30%, (Zwick et al., 2002) and BALB/c mice (27%, Robinson et al., 2004). We also reported previously that of all articular afferents from the mouse ankle, identified by retrograde axonal tracing, 44% immunostained for CGRP and 31% co-stained for CGRP and TRPV1 (Cho and Valtschanoff, 2008). In the rat, 25% of trigeminal afferents that innervate the temporomandibular joint express TRPV1 and 73% of them coexpress CGRP (Ichikawa et al., 2004). In addition, the expression of CGRP in articular afferents of naïve C57BL6 mice has been found to increase with age: Salo et al. (2002) reported that 22% of knee afferents in young mice expressed CGRP, in older mice, this number increased to 31% at 52 weeks and to 33% by 96 weeks, whereas the expression of TRPV1 decreased with age (Wang et al., 2006b).
In mice with AIA, the changes in expression of CGRP in DRG and spinal cord correlated with the severity of arthritis, as verified by behavioral and histopathological assessment (Chen et al., 2008). In particular, the immunostaining for CGRP in DRG reaching its maximum at 14 days and then beginning to decline is analogous to the expression of the nociceptive marker p-ERK and the temporal pattern of change in the pain hypersensitivity observed in these mice (Chen et al., 2008). This is also in agreement with the observation that the level of angiogenesis (a major pathogenetic component of arthritis, see Introduction) in rats increased 14 days after induction of osteoarthritis and then decreased to control levels by day 28 (Mapp et al., 2008). The bilateral increase of CGRP in DRG that we observed in mice with AIA may reflect the arthritogenic potential of IFA (Cannon et al., 1993) and/or the ability of CFA, when injected in one joint, to evoke systemic inflammation and arthritis in the contralateral joint (Kelly et al., 2007).
In a rat model of arthritis, the fraction of CGRP-positive neurons was reported to increase from 46% to 86% of identified knee afferents in L4 DRG (Fernihough et al., 2005) and from 45% to 66% (Ochiai et al., 2007); the fraction of TRPV1-positive neurons also increased from 54% to 78% in this model. Upregulation or de novo expression of CGRP in sensory neurons may represent one mechanism of pain hypersensitivity in arthritis (Staton et al., 2007). The behavioral response of arthritic CGRP-KO mice was antinociceptive, suggesting that the release of CGRP plays a significant role in the central sensitization in these animals (Zhang et al., 2001).
Upregulation of CGRP may occur in parallel with the increase in expression of TRPV1 in DRG neurons (Amaya et al., 2003; Aoki et al., 2004) or in the peripheral nerve fibers at the site of inflammation (Carlton and Coggeshall, 2001; Yiangou et al., 2001). Also in parallel with TRPV1, the changes in CGRP expression may be bilateral and may depend on the particular model of arthritis, survival time, and animal species (Bar et al., 2004).
That CGRP-positive articular afferents co-express TRPV1 (Cho and Valtschanoff, 2008) is consistent with the possibility that synovial release of CGRP in the course of arthritis (Lotz et al., 1987; Saito and Koshino, 2000) is triggered by depolarization of these afferents via TRPV1. We showed in the rat that TRPV1-positive primary afferents, the majority of which co-express CGRP, contact ascending nociceptive neurons in the spinal dorsal horn (Hwang et al., 2004) and trigeminal dorsal horn (Bae et al., 2004). Perhaps these are the same neurons in the superficial dorsal horn that express the CGRP receptor (Cottrell et al., 2005) and may thus be targets for centrally-released CGRP. Whether the increased immunostaining for CGRP in TRPV1-KO mice represents an overexpression of the peptide, whether this translates into an increased central release of CGRP in arthritis, and whether it reflects an attempt at functional compensation for the missing TRPV1 in the same or a parallel afferent input channel to the spinal nociceptive neurons, remains to be established. The issue could be addressed by a functional study using knockout mice and/or pharmacologic antagonists.
Acknowledgments
This work was supported by NIH grant AR053721.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed M, Bjurholm A, Schultzberg M, Theodorsson E, Kreicbergs A. Increased levels of substance P and calcitonin gene-related peptide in rat adjuvant arthritis. A combined immunohistochemical and radioimmunoassay analysis. Arthritis Rheum. 1995a;38:699–709. doi: 10.1002/art.1780380519. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Bjurholm A, Srinivasan GR, Lundeberg T, Theodorsson E, Schultzberg M, Kreicbergs A. Capsaicin effects on substance P and CGRP in rat adjuvant arthritis. Regul Pept. 1995b;55:85–102. doi: 10.1016/0167-0115(94)00095-f. [DOI] [PubMed] [Google Scholar]
- Amaya F, Oh-hashi K, Naruse Y, Iijima N, Ueda M, Shimosato G, Tominaga M, Tanaka Y, Tanaka M. Local inflammation increases vanilloid receptor 1 expression within distinct subgroups of DRG neurons. Brain Res. 2003;963:190–196. doi: 10.1016/s0006-8993(02)03972-0. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Takahashi Y, Ohtori S, Moriya H, Takahashi K. Distribution and immunocytochemical characterization of dorsal root ganglion neurons innervating the lumbar intervertebral disc in rats: a review. Life Sci. 2004;74:2627–2642. doi: 10.1016/j.lfs.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Averbeck B, Reeh PW. Interactions of inflammatory mediators stimulating release of calcitonin gene-related peptide, substance P and prostaglandin E(2) from isolated rat skin. Neuropharmacology. 2001;40:416–423. doi: 10.1016/s0028-3908(00)00171-4. [DOI] [PubMed] [Google Scholar]
- Bae YC, Oh JM, Hwang SJ, Shigenaga Y, Valtschanoff JG. Expression of vanilloid receptor TRPV1 in the rat trigeminal sensory nuclei. J Comp Neurol. 2004;478:62–71. doi: 10.1002/cne.20272. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Schaible HG, Brauer R, Halbhuber KJ, von Banchet GS. The proportion of TRPV1 protein-positive lumbar DRG neurones does not increase in the course of acute and chronic antigen-induced arthritis in the knee joint of the rat. Neurosci Lett. 2004;361:172–175. doi: 10.1016/j.neulet.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Barton NJ, McQueen DS, Thomson D, Gauldie SD, Wilson AW, Salter DM, Chessell IP. Attenuation of experimental arthritis in TRPV1R knockout mice. Exp Mol Pathol. 2006;81:166–170. doi: 10.1016/j.yexmp.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Bernardini N, Neuhuber W, Reeh PW, Sauer SK. Morphological evidence for functional capsaicin receptor expression and calcitonin gene-related peptide exocytosis in isolated peripheral nerve axons of the mouse. Neuroscience. 2004;126:585–590. doi: 10.1016/j.neuroscience.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Bolcskei K, Helyes Z, Szabo A, Sandor K, Elekes K, Nemeth J, Almasi R, Pinter E, Petho G, Szolcsanyi J. Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain. 2005;117:368–376. doi: 10.1016/j.pain.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford) 2005;44:7–16. doi: 10.1093/rheumatology/keh344. [DOI] [PubMed] [Google Scholar]
- Cannon GW, Woods ML, Clayton F, Griffiths MM. Induction of arthritis in DA rats by incomplete Freund’s adjuvant. J Rheumatol. 1993;20:7–11. [PubMed] [Google Scholar]
- Carlton SM, Coggeshall RE. Peripheral capsaicin receptors increase in the inflamed rat hindpaw: a possible mechanism for peripheral sensitization. Neurosci Lett. 2001;310:53–56. doi: 10.1016/s0304-3940(01)02093-6. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Chen Y, Willcockson HH, Valtschanoff JG. Vanilloid receptor TRPV1-mediated phosphorylation of ERK in murine adjuvant arthritis. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2008.06.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WG, Valtschanoff JG. Vanilloid receptor TRPV1-positive sensory afferents in the mouse ankle and knee joints. Brain Res. 2008;1219:59–65. doi: 10.1016/j.brainres.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell GS, Roosterman D, Marvizon JC, Song B, Wick E, Pikios S, Wong H, Berthelier C, Tang Y, Sternini C, Bunnett NW, Grady EF. Localization of calcitonin receptor-like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. J Comp Neurol. 2005;490:239–255. doi: 10.1002/cne.20669. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Donaldson LF, Harmar AJ, McQueen DS, Seckl JR. Increased expression of preprotachykinin, calcitonin gene-related peptide, but not vasoactive intestinal peptide messenger RNA in dorsal root ganglia during the development of adjuvant monoarthritis in the rat. Brain Res Mol Brain Res. 1992;16:143–149. doi: 10.1016/0169-328x(92)90204-o. [DOI] [PubMed] [Google Scholar]
- Fernihough J, Gentry C, Bevan S, Winter J. Regulation of calcitonin gene-related peptide and TRPV1 in a rat model of osteoarthritis. Neurosci Lett. 2005;388:75–80. doi: 10.1016/j.neulet.2005.06.044. [DOI] [PubMed] [Google Scholar]
- Ferrell WR, McDougall JJ, Bray RC. Spatial heterogeneity of the effects of calcitonin gene-related peptide (CGRP) on the microvasculature of ligaments in the rabbit knee joint. Br J Pharmacol. 1997;121:1397–1405. doi: 10.1038/sj.bjp.0701265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Burette A, Rustioni A, Valtschanoff JG. Vanilloid receptor VR1-positive primary afferents are glutamatergic and contact spinal neurons that co-express neurokinin receptor NK1 and glutamate receptors. J Neurocytol. 2004;33:321–329. doi: 10.1023/B:NEUR.0000044193.31523.a1. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Fukunaga T, Jin HW, Fujita M, Takano-Yamamoto T, Sugimoto T. VR1-, VRL-1- and P2X3 receptor-immunoreactive innervation of the rat temporomandibular joint. Brain Res. 2004;1008:131–136. doi: 10.1016/j.brainres.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Kelly S, Dunham JP, Donaldson LF. Sensory nerves have altered function contralateral to a monoarthritis and may contribute to the symmetrical spread of inflammation. Eur J Neurosci. 2007;26:935–942. doi: 10.1111/j.1460-9568.2007.05737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konttinen YT, Kemppinen P, Segerberg M, Hukkanen M, Rees R, Santavirta S, Sorsa T, Pertovaara A, Polak JM. Peripheral and spinal neural mechanisms in arthritis, with particular reference to treatment of inflammation and pain. Arthritis Rheum. 1994;37:965–982. doi: 10.1002/art.1780370701. [DOI] [PubMed] [Google Scholar]
- Larsson J, Ekblom A, Henriksson K, Lundeberg T, Theodorsson E. Concentration of substance P, neurokinin A, calcitonin gene-related peptide, neuropeptide Y and vasoactive intestinal polypeptide in synovial fluid from knee joints in patients suffering from rheumatoid arthritis. Scand J Rheumatol. 1991;20:326–335. doi: 10.3109/03009749109096808. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Crepps B, Perl ER. Calcitonin gene-related peptide immunoreactivity and afferent receptive properties of dorsal root ganglion neurones in guinea-pigs. J Physiol. 2002;540:989–1002. doi: 10.1113/jphysiol.2001.013086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Dardick SJ, Roizen MF, Helms C, Basbaum AI. Contribution of sensory afferents and sympathetic efferents to joint injury in experimental arthritis. J Neurosci. 1986;6:3423–3429. doi: 10.1523/JNEUROSCI.06-12-03423.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M, Carson DA, Vaughan JH. Substance P activation of rheumatoid synoviocytes: neural pathway in pathogenesis of arthritis. Science. 1987;235:893–895. doi: 10.1126/science.2433770. [DOI] [PubMed] [Google Scholar]
- Mapp PI, Avery PS, McWilliams DF, Bowyer J, Day C, Moores S, Webster R, Walsh DA. Angiogenesis in two animal models of osteoarthritis. Osteoarthritis Cartilage. 2008;16:61–69. doi: 10.1016/j.joca.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Miermeister F, Seifert F, Strasburg K, Zimmermann K, Reinold H, Austin JS, Bernardini N, Chesler EJ, Hofmann HA, Hordo C, Messlinger K, Nemmani KV, Rankin AL, Ritchie J, Siegling A, Smith SB, Sotocinal S, Vater A, Lehto SG, Klussmann S, Quirion R, Michaelis M, Devor M, Reeh PW. Variable sensitivity to noxious heat is mediated by differential expression of the CGRP gene. Proc Natl Acad Sci U S A. 2005;102:12938–12943. doi: 10.1073/pnas.0503264102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanayama T, Kuraishi Y, Ohno H, Satoh M. Capsaicin-induced release of calcitonin gene-related peptide from dorsal horn slices is enhanced in adjuvant arthritic rats. Neurosci Res. 1989;6:569–572. doi: 10.1016/0168-0102(89)90045-x. [DOI] [PubMed] [Google Scholar]
- O’Brien C, Woolf CJ, Fitzgerald M, Lindsay RM, Molander C. Differences in the chemical expression of rat primary afferent neurons which innervate skin, muscle or joint. Neuroscience. 1989;32:493–502. doi: 10.1016/0306-4522(89)90096-1. [DOI] [PubMed] [Google Scholar]
- Ochiai N, Ohtori S, Sasho T, Nakagawa K, Takahashi K, Takahashi N, Murata R, Moriya H, Wada Y, Saisu T. Extracorporeal shock wave therapy improves motor dysfunction and pain originating from knee osteoarthritis in rats. Osteoarthritis Cartilage. 2007;15:1093–1096. doi: 10.1016/j.joca.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil. 2004;16:113–124. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- Saito T, Koshino T. Distribution of neuropeptides in synovium of the knee with osteoarthritis. Clin Orthop Relat Res. 2000:172–182. doi: 10.1097/00003086-200007000-00024. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Westlund KN. Behavioral and immunohistochemical changes in an experimental arthritis model in rats. Pain. 1993;55:367–377. doi: 10.1016/0304-3959(93)90013-F. [DOI] [PubMed] [Google Scholar]
- Staton PC, Wilson AW, Bountra C, Chessell IP, Day NC. Changes in dorsal root ganglion CGRP expression in a chronic inflammatory model of the rat knee joint: differential modulation by rofecoxib and paracetamol. Eur J Pain. 2007;11:283–289. doi: 10.1016/j.ejpain.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Szabo A, Helyes Z, Sandor K, Bite A, Pinter E, Nemeth J, Banvolgyi A, Bolcskei K, Elekes K, Szolcsanyi J. Role of transient receptor potential vanilloid 1 receptors in adjuvant-induced chronic arthritis: in vivo study using gene-deficient mice. J Pharmacol Exp Ther. 2005;314:111–119. doi: 10.1124/jpet.104.082487. [DOI] [PubMed] [Google Scholar]
- Szabo A, Czirjak L, Sandor Z, Helyes Z, Laszlo T, Elekes K, Czompoly T, Starr A, Brain S, Szolcsanyi J, Pinter E. Investigation of sensory neurogenic components in a bleomycin-induced scleroderma model using transient receptor potential vanilloid 1 receptor- and calcitonin gene-related peptide-knockout mice. Arthritis Rheum. 2008;58:292–301. doi: 10.1002/art.23168. [DOI] [PubMed] [Google Scholar]
- Wang LH, Luo M, Wang Y, Galligan JJ, Wang DH. Impaired vasodilation in response to perivascular nerve stimulation in mesenteric arteries of TRPV1-null mutant mice. J Hypertens. 2006a;24:2399–2408. doi: 10.1097/01.hjh.0000251900.78051.56. [DOI] [PubMed] [Google Scholar]
- Wang S, Davis BM, Zwick M, Waxman SG, Albers KM. Reduced thermal sensitivity and Nav1.8 and TRPV1 channel expression in sensory neurons of aged mice. Neurobiol Aging. 2006b;27:895–903. doi: 10.1016/j.neurobiolaging.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Komori N, Qin C, Farber JP, Linderoth B, Foreman RD. Sensory fibers containing vanilloid receptor-1 (VR-1) mediate spinal cord stimulation-induced vasodilation. Brain Res. 2006;1107:177–184. doi: 10.1016/j.brainres.2006.05.087. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Dyer NH, Chan CL, Knowles C, Williams NS, Anand P. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet. 2001;357:1338–1339. doi: 10.1016/s0140-6736(00)04503-7. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hoff AO, Wimalawansa SJ, Cote GJ, Gagel RF, Westlund KN. Arthritic calcitonin/alpha calcitonin gene-related peptide knockout mice have reduced nociceptive hypersensitivity. Pain. 2001;89:265–273. doi: 10.1016/s0304-3959(00)00378-x. [DOI] [PubMed] [Google Scholar]
- Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, Albers KM. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci. 2002;22:4057–4065. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]